- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board

What Is Down Syndrome?
Causes and risk factors, living with down syndrome.
- Complications
- Next in Down Syndrome Guide Down Syndrome: Symptoms and Intellectual and Physical Traits
Down syndrome is a lifelong genetic condition that begins to have effects before birth and can significantly impact many aspects of a person’s life.
People who have Down syndrome can experience physical effects as well as cognitive challenges. However, the severity of the condition varies from person to person, and many people living with Down syndrome can lead happy, productive, and healthy lives.
This article describes the types of Down syndrome, its causes, risk factors, symptoms, screening tests, and long-term outlook.
manonallard / Getty Images
Types of Down Syndrome
Down syndrome is congenital, which means that it is present at birth. In fact, Down syndrome is present at conception. The condition occurs when a fertilized egg has an extra copy of chromosome 21. Down syndrome is also called trisomy 21.
The types of Down syndrome differ based on chromosomal patterns, as follows:
- Trisomy 21 : This is the most common type of Down syndrome. It occurs when a person has three copies of chromosome 21 in each cell of their body. A person's typical number of chromosomes is 46 (in 23 pairs). A person with Down syndrome has 47 chromosomes if all other chromosome pairs are typical.
- Mosaic Down syndrome : This type of Down syndrome occurs when there is a mixture of some cells in the body with trisomy 21 and some cells in the body without an extra chromosome 21. The symptoms can be similar to symptoms of full trisomy 21, but sometimes the effects are milder. It is seen in about 2% of people diagnosed with Down syndrome.
- Translocation : This type occurs when a person has extra chromosome 21 genetic material attached to another chromosome. With this type of Down syndrome, the person may have 46 chromosomes in their cells. About 3% of people diagnosed with Down syndrome have this type.
The extra genetic material from the third copy of chromosome 21 causes the body to develop differently. This occurs whether a person has full trisomy 21, mosaic Down syndrome, or translocation.
This produces changes in the developing fetus's physical features. Many of the changes are present at birth, and some can develop as the child grows into adolescence and adulthood.
Risk factors for Down syndrome include:
- Advanced age of the parents, especially females age 35 and older at the time of conception. It's important to understand that if the genetic parents are in an at-risk age, a surrogate who is below the at risk age does not reduce the risk.
- A family history of Down syndrome or another chromosomal disorder in a parent or sibling
Down Syndrome and Genetics
The genetic pattern of Down syndrome occurs due to the presence of an extra copy of chromosome 21 in the parents' egg cell or sperm cell. A child should normally receive only one copy of each chromosome from each parent—resulting in two copies of each chromosome in every one of the child’s cells.
When cells from either parent have two copies of any chromosome, this results in trisomy (the presence of a third copy) in all of the growing baby’s cells throughout their life. Trisomy cannot be repaired with any type of medical intervention.
The process that causes an egg cell or a sperm cell to have an extra copy of a chromosome is called nondisjunction. It occurs during the formation of the egg and the sperm cell, prior to the embryo's conception.
Down syndrome is not usually inherited—most people who have Down syndrome are the first in the family to have it, and their parents do not have the condition. However, it can be passed from parent to child if a person who has Down syndrome becomes pregnant or impregnates someone.
How Common Is Down Syndrome?
Down syndrome affects approximately 1 out of every 675 live births.
What Are the Symptoms of Down Syndrome?
Several characteristic physical changes and symptoms occur due to Down syndrome. These changes are often recognizable at birth, but some children might not have obvious features until early childhood.
Many of the physical features—short stature, heavy build, and prominent eyelids—might resemble other family members who do not have Down syndrome, potentially making the condition less recognizable.
Effects of Down syndrome include:
- Skeletal differences
- Short stature
- Heart malformations
- Abnormal lung development
- Intestinal malformations
- Learning difficulties
- Weak immune system
- Hypothyroidism (low function of the thyroid gland)
People who have Down syndrome have a higher-than-average risk of developing Alzheimer’s dementia later in life. Sometimes people who have Down syndrome have blood cell abnormalities affecting white blood cells and red blood cells.
Screening for Down Syndrome During Pregnancy
It is possible to identify Down syndrome during pregnancy. Testing and screening are not a standard part of prenatal care, but they can be done at the pregnant person's request.
Sometimes people who are at risk of having a baby with Down syndrome may request a screening test during pregnancy. But some people do not want to screen, even if they know that their baby is at high risk. And some people may ask for a screening test even when they do not have an elevated risk.
First Trimester
A pregnant person can have a quad screen early in pregnancy. This blood test measures hormones in the pregnant person’s blood that might be abnormal if the growing baby has Down syndrome or other congenital problems. The test cannot rule in or rule out Down syndrome.
Second Trimester
Ultrasound testing can examine the fetus’s physical features, potentially identifying some characteristics that can occur with Down syndrome—such as heart malformations. This test can reliably identify developmental differences, but it does not rule in or rule out Down syndrome.
A chromosomal examination can be done with amniocentesis or chorionic villi sampling. These minimally invasive procedures involve collecting a sample of cells that are genetically identical to the baby’s cells. The cells are collected with a needle using ultrasound guidance.
This test can definitively diagnose Down syndrome and specifically identify the type of Down syndrome.
Living with Down syndrome is a challenge for the whole family. Accommodations are often necessary to achieve learning goals and physical development during the toddler and school-age years. Many school districts offer accommodations for people who are living with Down syndrome,
Additionally, parents should seek the assistance of a multidisciplinary healthcare team to get the testing, therapy, and assistive devices needed to optimize quality of life.
Socializing is possible for people who have Down syndrome. Many people with Down syndrome can develop friendships and a supportive community.
People with Down syndrome can enjoy hobbies and other interests and often pursue those interests with lessons or classes. Many people with Down syndrome also have talents that they work to improve, such as art, music, acting, and more.
Complications of Down Syndrome
Several complications can occur as a result of having Down syndrome. These are not caused directly by the chromosomal abnormality, but they can develop due to the physical changes that occur as a result of the chromosomal abnormality.
Examples of complications include:
- Scoliosis : This abnormal curve of the spine can develop due to spine differences and altered body positioning.
- Heart failure : The heart does not pump enough blood to meet the body's needs. Can be a result of heart defects, high body weight, and low physical activity
- Bowel obstruction : A blockage of the intestine can occur due to intestinal malformation and dietary factors.
- Lung aspiration : Drawing foreign substances into the lungs may occur due to contributing factors such as lung disease, scoliosis, and general weakness.
- Depression : This mood disorder can occur as a result of the impact of coping with physical and cognitive limitations.
- Infections : People who have Down syndrome can have a higher risk of infections, including severe effects of COVID-19 .
Long-Term Outlook for Down Syndrome
In general, many people with Down syndrome can live long and healthy lives. The life expectancy is improving and is now over age 55.
Many people with Down syndrome can work in a job compatible with their physical and cognitive abilities. Support groups and advocacy organizations can often help find resources for job placement, recreational activities, transportation, and financial aid.
In general, people with Down syndrome are not able to live independently. Some may live with their families. Others may live in a group home or assisted living facility equipped to support their limitations and provide appropriate day-to-day help.
Centers for Disease Control and Prevention. Facts about Down syndrome .
National Institute for Child Health and Human Development. Who is at risk for Down syndrome?
Antonarakis SE, Skotko BG, Rafii MS, et al. Down syndrome . Nat Rev Dis Primers . 2020;6(1):9. doi:10.1038/s41572-019-01437
Hendrix JA, Amon A, Abbeduto L, et al. Opportunities, barriers, and recommendations in down syndrome research . Transl Sci Rare Dis . 2021;5(3-4):99-129. doi:10.3233/trd-200090
Chicoine B, Rivelli A, Fitzpatrick V, Chicoine L, Jia G, Rzhetsky A. Prevalence of common disease conditions in a large cohort of individuals with Down syndrome in the United States . J Patient Cent Res Rev . 2021;8(2):86-97. doi:10.17294/2330-0698.1824
Hamaguchi Y, Kondoh T, Fukuda M, et al. Leukopenia, macrocytosis, and thrombocytopenia occur in young adults with Down syndrome . Gene. 2022;835:146663. doi:10.1016/j.gene.2022.146663
Nikjoo S, Rezapour A, Moradi N, Nassiri S, Kabir A. Willingness to pay for Down syndrome screening: a systematics review . Med J Islam Repub Iran . 2022;36:149. doi:10.47176/mjiri.36.149
Lemoine L, Benoît Schneider. Family support for (increasingly) older adults with Down syndrome: factors affecting siblings' involvement . J Intellect Disabil. 2022:17446295221082725. doi:10.1177/17446295221082725
By Heidi Moawad, MD Dr. Moawad is a neurologist and expert in brain health. She regularly writes and edits health content for medical books and publications.
Home — Essay Samples — Nursing & Health — Down Syndrome — The Causes and Physical and Mental Effects of Down Syndrome
The Causes and Physical and Mental Effects of Down Syndrome
- Categories: Down Syndrome
About this sample

Words: 551 |
Published: Sep 25, 2018
Words: 551 | Page: 1 | 3 min read

Cite this Essay
To export a reference to this article please select a referencing style below:
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr Jacklynne
Verified writer
- Expert in: Nursing & Health

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
2 pages / 1193 words
3 pages / 1361 words
3 pages / 1410 words
5 pages / 2415 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Down syndrome, also known as Trisomy 21, is a genetic disorder that occurs when there is an extra copy of chromosome 21. This extra genetic material can cause delays in physical growth, intellectual development, and [...]
Down syndrome is a genetic disorder that affects the mind and causes physical disability. It is caused by irregular division of cells which causes formation of a full or extra chromosome. A normal person should have a 21 pairs [...]
The association of advanced maternal age with chromosomal aneuploidies has been widely discussed and debated over decades. The effect of paternal age was underreported and left room for analysis and discussion. In a [...]
The death penalty has been a capital offense of significance across many years. The decision to put someone to death has been productive over the years. The nature of the Death penalty punishment brings the worst fate to [...]
Suffering from any kind of allergy is a psychological burden. You constantly have to avoid your allergen which could have a lot of impact on your habits and living your life to the fullest. In cases where its totally impossible [...]
Programs that revolve around exercise training have emerged as useful measures for the management of type 2 diabetes mellitus. Most studies have revealed that exercise plays an effective role in the management of type 2 diabetes [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
93 Down Syndrome Essay Topic Ideas & Examples
🏆 best down syndrome topic ideas & essay examples, ⭐ good research topics about down syndrome, 📃 simple & easy down syndrome essay titles, ❓ research questions about down syndrome.
- Down Syndrome, Symptoms, Prevention and Treatment This extra copy of chromosome affects the development of the body and brain of the children born with this condition. Down syndrome is a genetic disorder and the probability of having a child with this […]
- Down’s Syndrome Recurrence Discussion A 30-year-old mother has a 1 in 1000 chance of giving birth to a child with Down’s syndrome. When reducing the risk of 1 in 1000 by three, it is possible to calculate an increasing […]
- Prenatal Testing for Down Syndrome The key points of contention in the discussion are the justification of the risks, the ethical choice on the principle of health, and the responsibility for stigmatization.
- The Down Syndrome Impacts on the Body Face morphology and upper nasal mucosa infections are the primary causes of chronic ear problems in newborns with Down syndrome. Developmental delays and behavioral issues in youngsters with Down syndrome are common.
- Challenges of Families with Down Syndrome Children The first challenge that the White family encountered was the decision of what to do with the fetus. It was only after the birth that Herzenbergs knew that the child had Down syndrome, while Whites […]
- Down Syndrome Genetics and Behaviors Using current research literature on behavioral issues and novel treatments for Down syndrome, this paper explores and discusses behavioral inflexibility, restrictive and repetitive behaviors, and Down syndrome’s neurogenetic nature.
- Down Syndrome: Congenital Heart Disease and Prenatal Testing Its purpose is to “test the validity and reliability of a scale that measures pregnant women’s attitudes and decision-making concerning prenatal Down syndrome screening and diagnosis in urban areas of Taiwan”.
- Comprehensive Care Plan for Patients with Down Syndrome It may also be highly beneficial to evaluate significant events that may influence the physical and mental health of the patient.
- Maternal Serum and Down Syndrome The main purpose of the research is to identify the authors who considered the problem of Down’s syndrome causes and analyze the results of the research conducted by those scholars.
- Down Syndrome: Implications for Learning and Development At the same time, the woman’s age of 35 and older is considered a risk factor that increases the chance of Down syndrome in the baby.
- The Great Down-Aging Syndrome: Why 40 Is the New 20 It has also been observed that the middle-aged people are fervent consumers of those products that are mainly used by the young. Not all middle-aged consumers are attracted to products that are meant for the […]
- Blunt Abdominal Trauma in Down Syndrome Patient Delving into the case, we could also admit the fact that the usage of the given examination procedure helped to determine the signs of Systemic inflammatory response syndrome, which could be extremely dangerous for the […]
- Down Syndrome: Coping and Supporting Individuals with DS DS is one of the foremost causes of cognitive impairment in children; however, with early interventions and medical advances, the potential for individuals with DS is expanding by the day.
- Pregnancy Termination in Down’s Syndrome Case One of the reasons why women of different ages decide to terminate their pregnancy is any genetic disease of the fetus and the risk of having an unhealthy child.
- Down Syndrome as the Most Common Genetic Condition in the US Firstly, to describe Down syndrome and the life of people with this disorder, it is necessary to give a scientific definition to this condition and underline the causes. People with Down syndrome are also people, […]
- Living with Down Syndrome: A Case Study from the UAE Healthy influence of these mammals is proved and the UAE tries to create the best conditions for people who suffer from Down Syndrome.
- Child with Down’s Syndrome – Life Story After playing for a while, he followed us to the living room, and I was moved to tears when he took my hand into his and asked my name.
- Down Syndrome and Dementia: Theories and Treatment The genetic material in the chromosome 21 is responsible for the development of the disorder, and its symptoms appear at the infantry stage of development.
- Down Syndrome: How to Lead Normal Lives with This Condition Mental development in children with Down syndrome varies greatly and at birth, it is not possible to predict the extent to which the child will be affected in terms of physical symptoms and cognitive development.
- Learners with Down Syndrome: A Handbook for Teaching Professionals The research premises on a set of research studies to provide experimental evaluation of current programs that address education of children with disabilities.
- Types of Tests Identifying Down Syndrome The major cause of the syndrome is associated with the existence of extra copy of the 21st chromosome. The triple screen test serves to identify Down syndrome in cases the level of AFP is low […]
- Living With the Down Syndrome: Causes and Symptoms
- Corrigendum: Pioglitazone Improves Mitochondrial Organization and Bioenergetics in Down Syndrome Cells
- Microstate Changes Associated With Alzheimer’s Disease in Persons With Down Syndrome
- Low-Resolution Place and Response Learning Capacities in Down Syndrome
- Genetics and Evolution: Cystic Fibrosis and Down Syndrome
- The Speech and Language Deficits of Children With Down Syndrome
- Inclusive Classrooms With Down Syndrome Students
- Autism, Down Syndrome and Equal Rights: A Look at the Past and Present of Diverse Populations and Sport
- Down Syndrome and Spina Bifida – Cause, Effects and Treatment
- Down Syndrome and the Value of Inclusive Education
- Response Inhibition and Interference Suppression in Individuals With Down Syndrome Compared to Typically Developing Children
- Semantic Verbal Fluency Pattern, Dementia Rating Scores and Adaptive Behavior Correlate With Plasma A 42 Concentrations in Down Syndrome Young Adults
- Music Therapy and Down Syndrome
- Between ‘Desperation’ and Disability Rights: Analysis of Alternative Medicine for Children With Down Syndrome
- Chromosomal Abnormalities: Down Syndrome
- Health Issues, Diabetes and Down Syndrome
- Problems Associated With Children With Down Syndrome
- Psychological and Physical Characteristics of Down Syndrome
- Most Successful People Who Have Down Syndrome
- Down Syndrome Affects Physical Growth, Facial Characteristics
- Cognitive Skills, Behavior and Learning Potential of Preschool Children With Down Syndrome
- The Causes, Symptoms, Diagnosis and Management of Down Syndrome
- Children With Down Syndrome: A Developmental Disorder
- Down Syndrome and the Formation of Reproductive Cells
- Down Syndrome Children Interaction With Family and Peers
- Causation and Developmental Course of Down Syndrome
- Dance Therapy for Down Syndrome Effects and Improvements
- Classroom Behaviour, Language Competence, and the Acceptance of Children With Down Syndrome by Their Mainstream Peers
- Learning Styles for Children With Down Syndrome
- The Negative and Positive Influence of the Media on People With Down Syndrome
- Parents and Children With Birth Defects: Down Syndrome
- Children With Intellectual Disabilities: Down Syndrome
- Down Syndrome: Causes, Symptoms, Diagnosis, & Treatment
- Development and Learning for People With Down Syndrome
- The Physical and Mental Characteristics of Children With Down Syndrome, Increased Risk Factors, and the Need for Medical Care and Stimulating Environments
- Maternal Line-1 DNA Methylation and Congenital Heart Defects in Down Syndrome
- Health Case History: Down Syndrome and Moderate Intellectual Disability
- Blood Beta-Amyloid and Tau in Down Syndrome: A Comparison With Alzheimers Disease
- Improving Working Memory Abilities in Individuals With Down Syndrome: A Treatment Case Study
- Allocentric Spatial Learning and Memory Deficits in Down Syndrome
- What Causes Down Syndrome?
- Why the Family Physician Patient Patients With Down Syndrome?
- How Does Down Syndrome Affect the One Who Has It?
- How Can Individuals With Down Syndrome Prosper in Life?
- How Do Individuals With Down Syndrome Process Faces and Words Conveying Emotions?
- Which Gender Is More Likely to Get Down Syndrome?
- What Is Down’s Syndrome Caused By?
- Can a Down Syndrome Girl Have a Baby?
- What Are the Five Characteristics of Down Syndrome?
- What Is the Life Expectancy of a Down Syndrome Person?
- Can Down Syndrome Be Cured?
- What Are the Three Types of Down Syndrome?
- How Can You Prevent Down Syndrome During Pregnancy?
- Can People With Down Syndrome Be Smart?
- What Happens if the Down Syndrome Test Is Positive?
- Does Down Syndrome Run in the Family?
- Is Autism a Form of Down Syndrome?
- Can Stress Cause Down Syndrome?
- Can Two Down Syndromes Have a Normal Baby?
- Is Down Syndrome a Disability?
- Which Organ Is Most Often Affected by Down Syndrome?
- Does a Mother’s Age Affect Down Syndrome?
- Can You Tell if a Baby Has Down Syndrome in an Ultrasound?
- What Is the Most Common Cause of Death in Down Syndrome?
- Can a Person With Down Syndrome Drive?
- Who Is the Oldest Person With Down Syndrome?
- What Are the Symptoms of Down Syndrome in Pregnancy?
- Can Down Syndrome Be Cured During Pregnancy?
- What Race Is Down Syndrome Most Common In?
- What Week of Pregnancy Does Down Syndrome Occur?
- Abnormal Psychology Paper Topics
- BPD Research Ideas
- Epigenetics Essay Titles
- Dyslexia Topics
- Bipolar Disorder Research Ideas
- Genetic Engineering Topics
- Nervous System Research Topics
- Parenting Research Topics
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 26). 93 Down Syndrome Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/down-syndrome-essay-topics/
"93 Down Syndrome Essay Topic Ideas & Examples." IvyPanda , 26 Feb. 2024, ivypanda.com/essays/topic/down-syndrome-essay-topics/.
IvyPanda . (2024) '93 Down Syndrome Essay Topic Ideas & Examples'. 26 February.
IvyPanda . 2024. "93 Down Syndrome Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/down-syndrome-essay-topics/.
1. IvyPanda . "93 Down Syndrome Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/down-syndrome-essay-topics/.
Bibliography
IvyPanda . "93 Down Syndrome Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/down-syndrome-essay-topics/.
IvyPanda uses cookies and similar technologies to enhance your experience, enabling functionalities such as:
- Basic site functions
- Ensuring secure, safe transactions
- Secure account login
- Remembering account, browser, and regional preferences
- Remembering privacy and security settings
- Analyzing site traffic and usage
- Personalized search, content, and recommendations
- Displaying relevant, targeted ads on and off IvyPanda
Please refer to IvyPanda's Cookies Policy and Privacy Policy for detailed information.
Certain technologies we use are essential for critical functions such as security and site integrity, account authentication, security and privacy preferences, internal site usage and maintenance data, and ensuring the site operates correctly for browsing and transactions.
Cookies and similar technologies are used to enhance your experience by:
- Remembering general and regional preferences
- Personalizing content, search, recommendations, and offers
Some functions, such as personalized recommendations, account preferences, or localization, may not work correctly without these technologies. For more details, please refer to IvyPanda's Cookies Policy .
To enable personalized advertising (such as interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. These partners may have their own information collected about you. Turning off the personalized advertising setting won't stop you from seeing IvyPanda ads, but it may make the ads you see less relevant or more repetitive.
Personalized advertising may be considered a "sale" or "sharing" of the information under California and other state privacy laws, and you may have the right to opt out. Turning off personalized advertising allows you to exercise your right to opt out. Learn more in IvyPanda's Cookies Policy and Privacy Policy .
- Patient Care & Health Information
- Diseases & Conditions
- Down syndrome
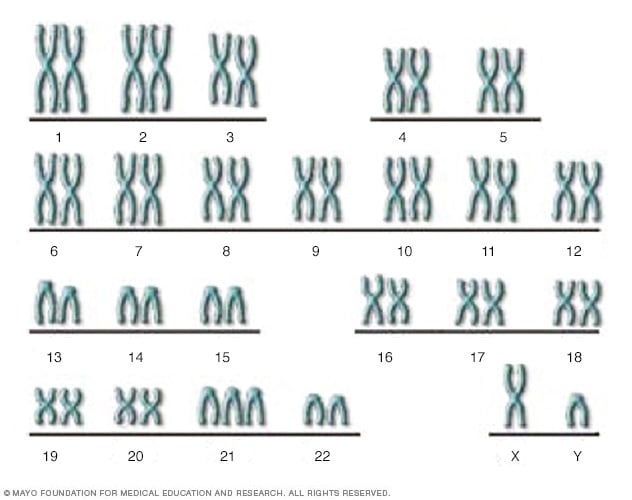
- The genetic basis of Down syndrome
There are 23 pairs of chromosomes, for a total of 46. Half the chromosomes come from the egg (the mother) and half come from the sperm (the father). This XY chromosome pair includes the X chromosome from the egg and the Y chromosome from the sperm. In Down syndrome, there is an additional copy of chromosome 21, resulting in three copies instead of the normal two copies.
Down syndrome is a genetic disorder caused when abnormal cell division results in an extra full or partial copy of chromosome 21. This extra genetic material causes the developmental changes and physical features of Down syndrome.
Down syndrome varies in severity among individuals, causing lifelong intellectual disability and developmental delays. It's the most common genetic chromosomal disorder and cause of learning disabilities in children. It also commonly causes other medical abnormalities, including heart and gastrointestinal disorders.
Better understanding of Down syndrome and early interventions can greatly increase the quality of life for children and adults with this disorder and help them live fulfilling lives.
Products & Services
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Each person with Down syndrome is an individual — intellectual and developmental problems may be mild, moderate or severe. Some people are healthy while others have significant health problems such as serious heart defects.
Children and adults with Down syndrome have distinct facial features. Though not all people with Down syndrome have the same features, some of the more common features include:
- Flattened face
- Protruding tongue
- Upward slanting eye lids (palpebral fissures)
- Unusually shaped or small ears
- Poor muscle tone
- Broad, short hands with a single crease in the palm
- Relatively short fingers and small hands and feet
- Excessive flexibility
- Tiny white spots on the colored part (iris) of the eye called Brushfield's spots
- Short height
Infants with Down syndrome may be average size, but typically they grow slowly and remain shorter than other children the same age.
Intellectual disabilities
Most children with Down syndrome have mild to moderate cognitive impairment. Language is delayed, and both short and long-term memory is affected.
When to see a doctor
Children with Down syndrome usually are diagnosed before or at birth. However, if you have any questions regarding your pregnancy or your child's growth and development, talk with your doctor.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Human cells normally contain 23 pairs of chromosomes. One chromosome in each pair comes from your father, the other from your mother.
Down syndrome results when abnormal cell division involving chromosome 21 occurs. These cell division abnormalities result in an extra partial or full chromosome 21. This extra genetic material is responsible for the characteristic features and developmental problems of Down syndrome. Any one of three genetic variations can cause Down syndrome:
- Trisomy 21. About 95 percent of the time, Down syndrome is caused by trisomy 21 — the person has three copies of chromosome 21, instead of the usual two copies, in all cells. This is caused by abnormal cell division during the development of the sperm cell or the egg cell.
- Mosaic Down syndrome. In this rare form of Down syndrome, a person has only some cells with an extra copy of chromosome 21. This mosaic of normal and abnormal cells is caused by abnormal cell division after fertilization.
- Translocation Down syndrome. Down syndrome can also occur when a portion of chromosome 21 becomes attached (translocated) onto another chromosome, before or at conception. These children have the usual two copies of chromosome 21, but they also have additional genetic material from chromosome 21 attached to another chromosome.
There are no known behavioral or environmental factors that cause Down syndrome.
Is it inherited?
Most of the time, Down syndrome isn't inherited. It's caused by a mistake in cell division during early development of the fetus.
Translocation Down syndrome can be passed from parent to child. However, only about 3 to 4 percent of children with Down syndrome have translocation and only some of them inherited it from one of their parents.
When balanced translocations are inherited, the mother or father has some rearranged genetic material from chromosome 21 on another chromosome, but no extra genetic material. This means he or she has no signs or symptoms of Down syndrome, but can pass an unbalanced translocation on to children, causing Down syndrome in the children.
Risk factors
Some parents have a greater risk of having a baby with Down syndrome. Risk factors include:
- Advancing maternal age. A woman's chances of giving birth to a child with Down syndrome increase with age because older eggs have a greater risk of improper chromosome division. A woman's risk of conceiving a child with Down syndrome increases after 35 years of age. However, most children with Down syndrome are born to women under age 35 because younger women have far more babies.
- Being carriers of the genetic translocation for Down syndrome. Both men and women can pass the genetic translocation for Down syndrome on to their children.
- Having had one child with Down syndrome. Parents who have one child with Down syndrome and parents who have a translocation themselves are at an increased risk of having another child with Down syndrome. A genetic counselor can help parents assess the risk of having a second child with Down syndrome.
Complications
People with Down syndrome can have a variety of complications, some of which become more prominent as they get older. These complications can include:
- Heart defects. About half the children with Down syndrome are born with some type of congenital heart defect. These heart problems can be life-threatening and may require surgery in early infancy.
- Gastrointestinal (GI) defects. GI abnormalities occur in some children with Down syndrome and may include abnormalities of the intestines, esophagus, trachea and anus. The risk of developing digestive problems, such as GI blockage, heartburn (gastroesophageal reflux) or celiac disease, may be increased.
- Immune disorders. Because of abnormalities in their immune systems, people with Down syndrome are at increased risk of developing autoimmune disorders, some forms of cancer, and infectious diseases, such as pneumonia.
- Sleep apnea. Because of soft tissue and skeletal changes that lead to the obstruction of their airways, children and adults with Down syndrome are at greater risk of obstructive sleep apnea.
- Obesity. People with Down syndrome have a greater tendency to be obese compared with the general population.
- Spinal problems. Some people with Down syndrome may have a misalignment of the top two vertebrae in the neck (atlantoaxial instability). This condition puts them at risk of serious injury to the spinal cord from overextension of the neck.
- Leukemia. Young children with Down syndrome have an increased risk of leukemia.
- Dementia. People with Down syndrome have a greatly increased risk of dementia — signs and symptoms may begin around age 50. Having Down syndrome also increases the risk of developing Alzheimer's disease.
- Other problems. Down syndrome may also be associated with other health conditions, including endocrine problems, dental problems, seizures, ear infections, and hearing and vision problems.
For people with Down syndrome, getting routine medical care and treating issues when needed can help with maintaining a healthy lifestyle.
Life expectancy
Life spans have increased dramatically for people with Down syndrome. Today, someone with Down syndrome can expect to live more than 60 years, depending on the severity of health problems.
There's no way to prevent Down syndrome. If you're at high risk of having a child with Down syndrome or you already have one child with Down syndrome, you may want to consult a genetic counselor before becoming pregnant.
A genetic counselor can help you understand your chances of having a child with Down syndrome. He or she can also explain the prenatal tests that are available and help explain the pros and cons of testing.
- What is Down syndrome? National Down Syndrome Society. http://www.ndss.org/down-syndrome/what-is-down-syndrome/. Accessed Dec. 16, 2016.
- Down syndrome fact sheet. National Down Syndrome Society. http://www.ndss.org/Down-Syndrome/Down-Syndrome-Facts/. Accessed Dec. 16, 2016.
- Messerlian GM, et al. Down syndrome: Overview of prenatal screening. http://www.uptodate.com/home. Accessed Dec. 16, 2016.
- National Library of Medicine. Down syndrome. Genetics Home Reference. https://ghr.nlm.nih.gov/condition/down-syndrome. Accessed Dec. 16, 2016.
- Facts about Down syndrome. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/birthdefects/downsyndrome.html. Accessed Dec. 16, 2016.
- Down syndrome. Eunice Kennedy Shriver National Institute of Child Health and Human Development. https://www.nichd.nih.gov/health/topics/down/conditioninfo/Pages/default.aspx. Accessed Dec. 16, 2016.
- Frequently asked questions. Prenatal genetic diagnostic tests. FAQ164. Pregnancy. American College of Obstetricians and Gynecologists. https://www.acog.org/-/media/For-Patients/faq164.pdf?dmc=1&ts=20161216T1208042192. Accessed Dec. 16, 2016.
- Ostermaier KK. Down syndrome: Management. http://www.uptodate.com/home. Accessed Dec. 22, 2016.
- Ostermaier KK. Down syndrome: Clinical features and diagnosis. http://www.uptodate.com/home. Accessed Jan. 10, 2017.
- Gabbe SG, et al., eds. Genetic screening and prenatal genetic diagnosis. In: Obstetrics: Normal and Problem Pregnancies. 7th ed. Philadelphia, Pa.: Saunders Elsevier; 2017.
- Rink BD, et al. Screening for fetal aneuploidy. Seminars in Perinatology. 2016;40:35.
- Bunt CW, et al. The role of the family physician in the care of children with Down syndrome. American Family Physician. 2014;90:851.
- Butler Tobah YS (expert opinion). Mayo Clinic, Rochester, Minn. Jan. 26, 2017.
Associated Procedures
- Amniocentesis
- Genetic testing
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
About Down Syndrome
In every cell in the human body there is a nucleus, where genetic material is stored in genes. Genes carry the codes responsible for all of our inherited traits and are grouped along rod-like structures called chromosomes. Typically, the nucleus of each cell contains 23 pairs of chromosomes, half of which are inherited from each parent. Down syndrome occurs when an individual has a full or partial extra copy of chromosome 21.
This additional genetic material alters the course of development and causes the characteristics associated with Down syndrome. A few of the common physical traits of Down syndrome are low muscle tone, small stature, an upward slant to the eyes, and a single deep crease across the center of the palm – although each person with Down syndrome is a unique individual and may possess these characteristics to different degrees, or not at all.
On this page:
Your donation will help people with Down syndrome, their families, and their caregivers.

How common is Down syndrome?
According to the Centers for Disease Control and Prevention, approximately one in every 775 babies in the United States is born with Down syndrome, making Down syndrome the most common chromosomal condition. About 5,000 babies with Down syndrome are born in the United States each year.
-De Graaf, G., Buckley, F., & Skotko, B. (2024, May 3). People living with Down syndrome in the USA: Births and Population . https://go.downsyndromepopulation.org/usa-factsheet
When was Down syndrome discovered?
For centuries, people with Down syndrome have been alluded to in art, literature, and science. It wasn’t until the late nineteenth century, however, that John Langdon Down, an English physician, published an accurate description of a person with Down syndrome. It was this scholarly work, published in 1866, that earned Down the recognition as the “father” of the syndrome. Although other people had previously recognized the characteristics of the syndrome, it was Down who described the condition as a distinct and separate entity.
In recent history, advances in medicine and science have enabled researchers to investigate the characteristics of people with Down syndrome. In 1959, the French physician Jérôme Lejeune identified Down syndrome as a chromosomal condition. Instead of the usual 46 chromosomes present in each cell, Lejeune observed 47 in the cells of individuals with Down syndrome. It was later determined that an extra partial or whole copy of chromosome 21 results in the characteristics associated with Down syndrome. In the year 2000, an international team of scientists successfully identified and catalogued each of the approximately 329 genes on chromosome 21. This accomplishment opened the door to great advances in Down syndrome research.
Are there different types of Down syndrome?
Trisomy 21 (nondisjunction).
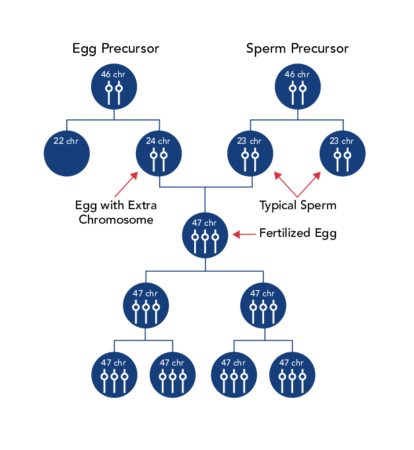
Down syndrome is usually caused by an error in cell division called “nondisjunction.” Nondisjunction results in an embryo with three copies of chromosome 21 instead of the usual two. Prior to or at conception, a pair of 21st chromosomes in either the sperm or the egg fails to separate. As the embryo develops, the extra chromosome is replicated in every cell of the body. This type of Down syndrome, which accounts for 95% of cases, is called trisomy 21.
Mosaicism (or mosaic Down syndrome) is diagnosed when there is a mixture of two types of cells, some containing the usual 46 chromosomes and some containing 47. Those cells with 47 chromosomes contain an extra chromosome 21.
Mosaicism is the least common form of Down syndrome and accounts for only about 2% of all cases of Down syndrome (Facts about Down syndrome, 2021). Research has indicated that individuals with mosaic Down syndrome may have fewer characteristics of Down syndrome than those with other types of Down syndrome. However, broad generalizations are not possible due to the wide range of abilities people with Down syndrome possess.
Translocation
In translocation, which accounts for about 3% of cases of Down syndrome, the total number of chromosomes in the cells remains 46; however, an additional full or partial copy of chromosome 21 attaches to another chromosome, usually chromosome 14 (Facts and Down syndrome, 2021). The presence of the extra full or partial chromosome 21 causes the characteristics of Down syndrome.
What causes Down syndrome?
Regardless of the type of Down syndrome, a person may have, all people with Down syndrome have an extra, critical portion of chromosome 21 present in all or some of their cells.
The cause of the extra full or partial chromosome is still unknown. Age is the only factor that has been linked to an increased chance of having a baby with Down syndrome resulting from nondisjunction or mosaicism. However, due to higher birth rates in younger women, 51% of children with Down syndrome are born to women under 35 years of age. (De Graaf et al., 2022).
There is no definitive scientific research that indicates that Down syndrome is caused by environmental factors or the parents’ activities before or during pregnancy.
The additional partial or full copy of the 21st chromosome which causes Down syndrome can originate from either parent. Approximately 5% of the cases have been traced to the father.
A Promising Future Together
This booklet includes sections on healthy starts, early intervention therapies, how to find support and care for your family, and what the future holds for your child.

What is the likelihood of having a child with Down syndrome?
Down syndrome occurs in people of all races and economic levels, though older women have an increased chance of having a child with Down syndrome. A 35-year-old woman has about a one in 350 chance of conceiving a child with Down syndrome, and this chance increases gradually to 1 in 100 by age 40. At age 45 the incidence becomes approximately 1 in 30. The age of the mother, or birthing parent, does not seem to be linked to the risk of translocation.
Since many couples are postponing parenting until later in life, the incidence of Down syndrome conceptions is expected to increase. Therefore, genetic counseling for parents is becoming increasingly important. Still, many physicians are not fully informed about advising their patients about the incidences of Down syndrome, advancements in diagnosis, and the protocols for care and treatment of babies born with Down syndrome.
Does Down syndrome run in families?
All three types of Down syndrome are genetic conditions (relating to the genes), but only 1% of all cases of Down syndrome have a hereditary component (passed from parent to child through the genes). Heredity is not a factor in trisomy 21 (nondisjunction) and mosaicism. However, in one-third of cases of Down syndrome resulting from translocation, there is a hereditary component – accounting for about 1% of all cases of Down syndrome (Facts about Down syndrome, 2021).
The age of the parent does not seem to be linked to the risk of translocation. Most cases are sporadic – chance – events. However, in about one-third of cases, one parent is a carrier of a translocated chromosome.
What is the likelihood of having a second child with Down syndrome?
Once a parent has given birth to a baby with trisomy 21 (nondisjunction) or translocation, it is estimated that the chances of having another baby with trisomy 21 is 1 in 100 up until age 40.
The risk of recurrence of translocation is about 3% if the father is the carrier and 10-15% if the mother is the carrier. Genetic counseling can determine the origin of translocation.
| Maternal Age | Incidence of Down syndrome |
|---|---|
| 20 | 1 in 2,000 |
| 21 | 1 in 1,700 |
| 22 | 1 in 1,500 |
| 23 | 1 in 1,400 |
| 24 | 1 in 1,300 |
| 25 | 1 in 1,200 |
| 26 | 1 in 1,100 |
| 27 | 1 in 1,050 |
| 28 | 1 in 1,000 |
| 29 | 1 in 950 |
| 30 | 1 in 900 |
| 31 | 1 in 800 |
| 32 | 1 in 720 |
| 33 | 1 in 600 |
| 34 | 1 in 450 |
| 35 | 1 in 350 |
| 36 | 1 in 300 |
| 37 | 1 in 250 |
| 38 | 1 in 200 |
| 39 | 1 in 150 |
| 40 | 1 in 100 |
| 41 | 1 in 80 |
| 42 | 1 in 70 |
| 43 | 1 in 50 |
| 44 | 1 in 40 |
| 45 | 1 in 30 |
| 46 | 1 in 25 |
| 47 | 1 in 20 |
| 48 | 1 in 15 |
| 49 | 1 in 10 |
How is Down syndrome diagnosed?
There are two categories of tests for Down syndrome that can be performed before a baby is born: screening tests and diagnostic tests. Prenatal screens estimate the chance of the fetus having Down syndrome. These tests do not tell you for sure whether your fetus has Down syndrome; they only provide a probability. Diagnostic tests, on the other hand, can provide a definitive diagnosis with almost 100% accuracy.
There is an extensive menu of prenatal screening tests now available for pregnant parents. Most screening tests involve a blood test and an ultrasound (sonogram). The blood tests (or serum screening tests) measure quantities of various substances in the blood of the parent. Together with age, these are used to estimate the chance of having a child with Down syndrome. These blood tests are often performed in conjunction with a detailed sonogram to check for “markers” (characteristics that some researchers feel may have a significant association with Down syndrome). New advanced prenatal screens are now able to detect chromosomal material from the fetus that is circulating in the maternal blood. These tests are not invasive (like the diagnostic tests below), but they provide a high accuracy rate. Still, all of these screens will not definitively diagnose Down syndrome. Prenatal screening and diagnostic tests are now routinely offered to all ages.
The diagnostic procedures available for prenatal diagnosis of Down syndrome are chorionic villus sampling (CVS) and amniocentesis. These procedures, which carry up to a 1% risk of causing a spontaneous termination (miscarriage), are nearly 100% accurate in diagnosing Down syndrome. Amniocentesis is usually performed in the second trimester between 15 and 20 weeks of gestation, CVS in the first trimester between 11 and 14 weeks (Chrionic villus sampling, 2020).
Down syndrome is usually identified at birth by the presence of certain physical traits: low muscle tone, a single deep crease across the palm of the hand, a slightly flattened facial profile, and an upward slant to the eyes. Because these features may be present in babies without Down syndrome, a chromosomal analysis called a karyotype is done to confirm the diagnosis. To obtain a karyotype, doctors draw a blood sample to examine the baby’s cells. They photograph the chromosomes and then group them by size, number, and shape. By examining the karyotype, doctors can diagnose Down syndrome. Another genetic test called fluorescence in situ hybridization (FISH) can confirm a diagnosis in a shorter amount of time by visualizing and mapping the genetic material in an individual's cells.

What impact does Down syndrome have on society?
Individuals with Down syndrome are becoming increasingly integrated into society and community organizations, such as schools, health care systems, work forces, and social and recreational activities. Individuals with Down syndrome possess varying degrees of cognitive delays, from very mild to severe. Most people with Down syndrome have cognitive delays that are mild to moderate.
Due to advances in medical technology, individuals with Down syndrome are living longer than ever before. In 1910, children with Down syndrome were expected to survive to age nine. With the discovery of antibiotics, the average survival age increased to 19 or 20. Now, with recent advancements in clinical treatment, most particularly corrective heart surgeries, as many as 80% of adults with Down syndrome reach age 60, and many live even longer (Down syndrome, 2018). More and more Americans are interacting with individuals with Down syndrome, increasing the need for widespread public education and acceptance.
Preferred Language Guide
Use this language when referring to down syndrome and people who have down syndrome:.
- People with Down syndrome should always be referred to as people first.
- Instead of “a Down syndrome child,” it should be “a child with Down syndrome.” Also avoid “Down’s child” and describing the condition as “Down’s,” as in, “He has Down’s.”
- Down syndrome is a condition or a syndrome, not a disease.
- People “have” Down syndrome, they do not “suffer from” it and are not “afflicted by” it.
- “Typically developing” or “typical” is preferred over “normal.”
- “Intellectual disability” or “cognitive disability” has replaced “mental retardation” as the appropriate term.
- NDSS strongly condemns the use of the word “retarded” in any derogatory context. Using this word is hurtful and suggests that people with disabilities are not competent.
Down vs. Down’s
- NDSS uses the preferred spelling, Down syndrome, rather than Down’s syndrome.
- Down syndrome is named for the English physician John Langdon Down, who characterized the condition, but did not have it. An “apostrophe s” connotes ownership or possession.
- While Down syndrome is listed in many dictionaries with both popular spellings (with or without an apostrophe s), the preferred usage in the United States is Down syndrome. The AP Stylebook recommends using “Down syndrome,” as well.
A downloadable version of the Preferred Language Guide is available to print and distribute.
Citations:
Chorionic villus sampling. Mayo Clinic. https://www.mayoclinic.org/tests-procedures/chorionic-villus-sampling/a… . Published November 12, 2020. Accessed June 28, 2022.
de Graaf G, Buckley F, Skotko B. People living with Down syndrome in the USA: BIRTHS AND POPULATION. May 2022. https://go.dselink.net/us-population-factsheet.  ;
Down syndrome. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/down-syndrome/symptoms-c… . Published March 8, 2018. Accessed June 28, 2022.
Facts about Down syndrome. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/birthdefects/downsyndrome.html . Published April 6, 2021. Accessed June 28, 2022.
Thank You For Your Support!
Gifts from supporters like you make our work possible.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Down syndrome
Stylianos e. antonarakis.
1 Department of Genetic Medicine and Development, University of Geneva Medical School, Geneva, Switzerland.
Brian G. Skotko
2 Down Syndrome Program, Division of Medical Genetics, Department of Pediatrics, Massachusetts General Hospital, Boston, MA, USA.
3 Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
Michael S. Rafii
4 Keck School of Medicine of University of Southern California, California, CA, USA.

Andre Strydom
5 Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK.
Sarah E. Pape
Diana w. bianchi.
6 Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
7 National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA.
Stephanie L. Sherman
8 Department of Human Genetics, Emory University School of Medicine, Atlanta, GA, USA.
Roger H. Reeves
9 Department of Physiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
10 McKusick-Nathans Department of Genetic Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Author contributions
Trisomy 21, the presence of a supernumerary chromosome 21, results in a collection of clinical features commonly known as Down syndrome (DS). DS is among the most genetically complex of the conditions that are compatible with human survival post-term, and the most frequent survivable autosomal aneuploidy. Mouse models of DS, involving trisomy of all or part of human chromosome 21 or orthologous mouse genomic regions, are providing valuable insights into the contribution of triplicated genes or groups of genes to the many clinical manifestations in DS. This endeavour is challenging, as there are >200 protein-coding genes on chromosome 21 and they can have direct and indirect effects on homeostasis in cells, tissues, organs and systems. Although this complexity poses formidable challenges to understanding the underlying molecular basis for each of the many clinical features of DS, it also provides opportunities for improving understanding of genetic mechanisms underlying the development and function of many cell types, tissues, organs and systems. Since the first description of trisomy 21, we have learned much about intellectual disability and genetic risk factors for congenital heart disease. The lower occurrence of solid tumours in individuals with DS supports the identification of chromosome 21 genes that protect against cancer when overexpressed. The universal occurrence of the histopathology of Alzheimer disease and the high prevalence of dementia in DS are providing insights into the pathology and treatment of Alzheimer disease. Clinical trials to ameliorate intellectual disability in DS signal a new era in which therapeutic interventions based on knowledge of the molecular pathophysiology of DS can now be explored; these efforts provide reasonable hope for the future.
Down syndrome (DS) is the most common genomic disorder of intellectual disability and is caused by trisomy of Homo sapiens chromosome 21 (HSA21). The eponym of the syndrome is from Down, who described the clinical aspects of the syndrome in 1866 (REF. 1 ). The DS phenotype involves manifestations that affect multiple bodily systems, in particular the musculoskeletal, neurological and cardiovascular systems. Individuals with DS commonly have short stature, muscle hypotonia, atlantoaxial instability, reduced neuronal density, cerebellar hypoplasia, intellectual disability and congenital heart defects (CHDs; particularly atrioventricular septal defects (AVSDs)). Individuals with DS are also more likely to develop certain health conditions, including hypothyroidism, autoimmune diseases, obstructive sleep apnoea, epilepsy, hearing and vision problems, haematological disorders (including leukaemia), recurrent infections, anxiety disorders and early-onset Alzheimer disease (AD) ( FIG. 1 ). Other conditions, such as most solid tumour types, show inverse comorbidity and seem to be less common in individuals with DS than in the general population 2 .

Individuals with trisomy 21 (the presence of a supernumerary chromosome 21; also known as Down syndrome (DS)) present with a distinct collection of symptoms and manifestations that affect multiple body systems, although variation exists between individuals. Individuals with DS are generally of short stature, with short fingers, hypotonia and atlantoaxial instability. Facial characteristics include the presence of epicanthic folds, flat nasal bridge and occiput, small mouth and ears, and up-slanting palpebral fissures. Congenital heart defects are common, particularly atrioventricular septal defect (AVSD). Individuals with DS are also more likely to develop certain health conditions compared with the general population, including hypothyroidism, obstructive sleep apnoea, epilepsy, hearing and vision problems, haematological disorders (including leukaemia), recurrent infections, anxiety disorders, and early-onset Alzheimer disease.
The discovery of a link between a supernumerary chromosome 21 and the DS phenotype was first reported in 1959 (REF. 3 ) and was an important landmark for the development of genetic medicine. Mouse models for the study of DS were first developed in 1990 (REF. 4 ), and the complete nucleotide sequence of the long arm of HSA21 was published in 2000 by a multinational consortium of investigators 5 . Substantial progress has been made in the ensuing 19 years in understanding the molecular pathophysiology of the different phenotypic manifestations of DS, which is currently considered a disorder of gene expression dysregulation. In addition, widely used screening methods have been introduced for the prenatal detection of DS. The management of the different symptoms and the quality of life of individuals with DS have improved. However, enormous challenges remain, including understanding the precise biological mechanism of each phenotypic component of the syndrome; treatment of the different symptoms, including cognitive dysfunction; and integration of individuals with DS into society in different parts of the world. Furthermore, studies in animal models to unravel the effects of triplication of the >200 protein-coding genes on HSA21, not to mention the triplicated non-coding genes and the downstream and indirect effects of these alterations, are extremely challenging.
In this Primer, we discuss the epidemiology of DS, current understanding of genetics and pathophysiology resulting from the extra chromosome 21, and advances in the diagnosis of trisomy 21. Furthermore, we review the management of the syndrome and the quality of life of individuals with DS, and offer an outlook for the future. Several reviews of DS and trisomy 21 have been published elsewhere and provide additional details about this common and challenging syndrome 6 – 9 .
Epidemiology
The lifetime prevalence of DS is increasing substantially as the global population grows. For example, in the USA, the population prevalence of DS increased from ~50,000 in 1950 (3.3 per 10,000 individuals) to ~212,000 in 2013 (6.7 per 10,000 individuals; based on unpublished data 10 ), mostly due to improvements in childhood survival of individuals with DS 11 ( FIG. 2 ). The life expectancy of individuals with DS in the USA increased from an estimated mean of 26 years and median of 4 years in 1950 to 53 years and 58 years, respectively, in 2010 (REF. 11 ). As of 2015, estimates of DS population prevalence have been reported for Europe (4.9 per 10,000 individuals) 12 , Europe excluding former Eastern Bloc countries (6.0 per 10,000 individuals), and former Eastern Bloc countries (3.3 per 10,000 individuals) 12 . However, a precise global estimate cannot be reliably calculated until more birth registries are created within countries and more data are available on historical and current survival of individuals with DS in different parts of the world.

a | Prevalence of Down syndrome (DS) in the USA for the period 1950–2013. This graph combines the prevalence data for 1950–2010 in the USA 9 with unpublished prevalence data for 2011–2013 for the same region 10 . b | Pregnancy outcomes in the USA for the period 1974–2013. This graph combines estimates of live births, natural losses and elective terminations in women carrying a fetus with DS after 10 weeks gestation for the period 1974–2010 in the USA 17 with unpublished data for 2011–2013 (REF. 10 ). GA, gestational age. Part a adapted from REF. 11 , Springer Nature Limited. Part b adapted with permission from REF. 17 , Wiley-VCH.
DS occurs in all populations, but differences in maternal age at conception between countries and ethnicities influence the number of live births 13 – 16 . As of 2013 in the USA, unpublished data suggest that ~1 in every 779 babies born had DS 10 (~12.8 per 10,000 live births) ( FIG. 2a ). The prevalence of DS is influenced by maternal age at conception, which varies among countries, and is estimated to be ~1 in 365 fetuses at 10 weeks gestation 17 ( FIG. 2b ). Some of these pregnancies will result in spontaneous miscarriage, ~32% between 10 weeks gestation and the expected date of birth and ~25% between 16 weeks gestation and the expected date of birth, the risk depending on maternal age, as estimated from reports from England and Wales 18 . The number of pregnancies that are electively terminated is influenced by the availability and accuracy of screening tests within each country, the number of people choosing prenatal screening and subsequently prenatal testing, and parental decisions once a prenatal diagnosis of DS is made. In 2013, an estimated 3,400 DS-related elective terminations were carried out in the USA, resulting in a 33% reduction in the number of babies with DS that would have been born that year. By comparison, the estimated percentage reduction was 55% in Australia for 2004 (REF. 19 ), and 54% in Europe, 66% in Europe excluding the former Eastern Bloc countries and 32% in former Eastern Bloc countries, for the period 2010–2015 (REF. 12 ). In China, during the period 2003–2011, the termination rate for fetuses with DS led to a 55% reduction in the overall perinatal prevalence 20 .
Studies from England and Wales 21 , Slovenia 22 , Australia 19 , 23 and EUROCAT regions 24 have shown that increasing maternal age has counterbalanced the uptake in prenatal screening, resulting in a stable or slightly decreasing prevalence of DS in live births from the 1990s until now. In Europe, DS prevalence in live births has been decreasing slightly since the 1990s, although there are substantial regional differences 12 . In southern Europe, DS prevalence in live births almost halved in the period 1980–2015 (REF. 12 ). By contrast, in the Netherlands, the live birth prevalence increased slightly in the period 1980–2000 but seems to have been decreasing slightly since 2005 (REF. 25 ). In the USA, the increased prevalence of DS in live births began in the 1980s and levelled off after 2005 (REF. 17 ). Sequencing cell-free placental DNA in maternal plasma, termed noninvasive prenatal screening (NIPS) or noninvasive prenatal testing (NIPT), has been available since 2011 in many countries; however, not enough time has elapsed for the full impact of surveillance programmes on birth rates to be measured. Furthermore, variability in access to prenatal care is also expected to affect the uptake of surveillance programmes.
Risk factors
Advanced maternal age at conception is a major risk factor for trisomy 21, as is true for all human autosomal trisomies 26 . This risk is associated with non-disjunction of homologous chromosomes or chromatids occurring during the meiotic divisions that occur in the formation of oocytes 27 . Advanced maternal age has been associated with HSA21 segregation errors in both maternal meiosis I and meiosis II 28 – 32 . In addition, specific altered recombination patterns have been observed for these types of maternal errors, only some of which are associated with maternal age 33 .
Meiotic processes, such as recombination, involve the action of many genes. Thus, there is a clear rationale for examining whether genetic variants predispose to meiotic nondisjunction in humans. A study including a candidate gene analysis and an untargeted genome-wide association study (GWAS) of HSA21 nondisjunction in mothers who gave birth to an infant with trisomy 21 derived from a maternal error was conducted using the mothers as cases and the fathers as controls 34 . These analyses were stratified by maternal MI or MII errors. Results from this study emphasize the heterogeneity of risk factors based on type of error. For example, variants in candidate genes coding for components of the synaptonemal complex showed an association limited to meiosis I errors (for example, SYCE2 ) while others were associated with MII errors (for example, SYCP2 ) 34 .
Environmental factors also influence the risk of non-disjunction but are difficult to identify due to the inherent problem of defining the exposure, dosage and timing of each factor. Again, studies must define the type of error (parental origin and the type of meiotic or mitotic error) leading to trisomy 21 due to their heterogeneous aetiology. Environmental factors that influence the risk of trisomy 21 include tobacco use, folic acid supplementation, oral contraceptive use and several others (the studies investigating environmental risk factors and their limitations, and possible biomarkers of exposure such as telomere length, have been reviewed elsewhere 35 ).
For example, maternal socioeconomic status (SES) is associated only with maternal meiosis II errors 36 – 39 . As SES is a proxy for specific exposures, a follow-up study examined maternal occupation as a risk factor and found that some job classification categories were more prevalent in mothers of infants with DS than in mothers of infants with no chromosomal abnormality or major birth defect, and were associated with specific types of meiotic errors 40 . A preliminary analysis revealed that these occupations involved exposure to solvents in the work environment. Further studies of this kind are needed to examine specific exposures to toxic agents in the work and home environments and their relationship with maternal and paternal HSA21 nondisjunction. This is emphasized by the finding that exposure to endocrine-disrupting chemicals affects meiosis and increases the prevalence of aneuploidy 41 . For example, exposure to the ubiquitous environmental contaminant bisphenol A (BPA) and other endocrine-disrupting chemicals affects the reproductive system in both sexes (including the ovaries, testes and reproductive tract) 41 , 42 .
It is important to point out that not only do different types of meiotic and mitotic errors leading to aneuploidy most likely have different susceptibility to specific environmental exposures but that exposures of multiple generations (for example, grandmother and mother) must also be considered 35 , 43 . For example, there is now mounting evidence of transgenerational effects of BPA exposure on sperm and oocytes in experimental models 42 .
Mechanisms/pathophysiology
Genetics of ds.
Partial or complete trisomy 21 (that is, the presence of part of or a complete supernumerary HSA21) is the genomic cause of DS 3 . A free trisomy 21 is present in 95% of individuals with DS and results from an error in maternal meiosis I (~66%) or meiosis II (~21%); paternal meiosis I (~3%) or meiosis II (5%); or mitosis, after the formation of the zygote (5%) 15 , 44 , 45 . Translocation accounts for trisomy 21 in ~5% of affected individuals, usually t(14;21) or t(21;21) 46 , 47 . Mosaicism for trisomy 21 occurs in ~2% of individuals with DS. Partial trisomy 21 is rare 48 , 49 and is associated with a range of symptoms that vary according to the length of the partial triplication of HSA21.
The first published sequence of HSA21 annotated 225 genes on chromosome 21q 5 . With increased knowledge of the elements of coding and non-coding genes and regulatory motifs, the number of gene structures recognized on HSA21 has increased considerably. The strengths and limitations of genome annotations available in existing public databases have been reviewed elsewhere 50 . The current version of GENCODE/ENSEMBL (GENCODE release 32) lists 233 protein-coding genes, 423 non-protein-coding genes (69 small, 330 long and 24 miscellaneous non-coding genes) and 188 pseudogenes. Of note, 48% of HSA21 has not been annotated 6 , the vast majority of which contains repetitive elements (as is the case for all human chromosomes).
Understanding the genetic aetiology of the increased susceptibility of individuals with DS to the multiple manifestations or conditions that are associated with the DS phenotype is an enormous challenge 51 . Compounding the difficulty of identifying the specific genetic and other effects of trisomy 21, studies of DS in humans are restricted to those conceptuses that survive to term; among pregnancies with a confirmed prenatal diagnosis of DS at 9–14 weeks, ~30–40% subsequently spontaneously miscarry 18 , 52 . Extrapolating to all pregnancies, ~80% of trisomy 21 conceptuses are lost during pregnancy 53 . Thus, it is essential to acknowledge that in studying any DS-associated condition, only the combinations of genetic variants that allow the conceptus to survive to term and to manifest the phenotype of interest are being considered.
Two main hypotheses have been proposed to explain the biological perturbations that underlie the phenotypic manifestations of DS: first, a specific HSA21 gene-dosage effect, which includes both the direct effects of overexpressed HSA21 genes and the downstream consequences of this overexpression; and second, developmental instability, in which nonspecific global disturbance of gene expression from the extra HSA21 results in disruption of overall biological homeostasis 54 , 55 . It is likely that the aetiology of DS-associated phenotypes involves both proposed mechanisms. Single-cell transcriptome analyses in trisomy 21 and other trisomies have suggested that the gene dosage effect for trisomic genes with low-to-average expression is mainly due to the higher fraction of trisomic cells expressing these genes 56 . This results in the expected 1.5-fold higher average expression of trisomic genes in the various tissues of individuals with trisomy 21.
Gene dosage effects.
The simplest effect of HSA21 trisomy is the direct effect of an increased dosage of a single HSA21 gene. For example, an increased dosage of APP , an HSA21 gene that encodes amyloid precursor protein (APP), increases susceptibility to early-onset AD in individuals with DS. Although APP is clearly an ‘effector’ gene, whether only the direct and downstream effects of APP overexpression affect the penetrance and severity of AD, or whether other aspects of trisomy 21 also have a role, remains to be determined. Of note, returning the App copy number to two copies in a mouse model of trisomy 21 (Ts65Dn) alleviates some but not all of the effects of App dosage 57 .
To define the direct and indirect consequences of increasing gene dosage, many studies have characterized the expression profile of HSA21 genes and the effect of overexpressing these genes on the rest of the genome. Common findings among these studies emerge. First, the expression of most but not all HSA21 genes is increased. It will be important to identify the mechanisms that underlie these discordant expression levels, which may include negative feedback, dosage compensation and epigenetic alterations. Second, in many affected individuals, the expression of some non-HSA21 genes is also altered, which suggests that trisomy 21 leads to perturbation of downstream transcription and signalling networks. A meta-analysis compared the expression profiles of trisomy 21 and euploid samples from various human tissues (such as brain and thymus) or cells (such as lymphoblastoid cell lines (LCLs), blood cells, fibroblasts and induced pluripotent stem cells (iPSCs)) 58 . This analysis found that the trisomy 21:euploid expression ratio was typically close to 3:2 for dysregulated genes and close to 1:1 for unaffected genes. These data are consistent with the hypothesis that upregulation of effector genes (that is, those that enhance or suppress gene expression) on HSA21 leads to similar changes in the expression of associated downstream genes, resulting in a similar altered ratio of 3:2 or 2:3 for these genes. There are exceptions to this pattern, with more extreme ratios that may result from gene–gene interactions or other types of chain effects that amplify the final expression levels. For example, JAKMIP3 is highly upregulated (256.13:1 ratio) in the thymus transcriptome map, whereas BEX5 is highly downregulated (0.07:1 ratio) in the brain transcriptome map.
Finally, as expected, the expression patterns of both HSA21 genes and non-HSA21 genes in trisomy 21 cells differ depending on the tissue being examined. For example, the interferon response is highly upregulated in trisomy 21 fibroblasts and LCLs, most likely due to increased expression of four interferon receptor genes ( IFNAR1 , IFNAR2 , INGR2 and IL10RB ) that are located on HSA21 (REF. 59 ). An increased interferon response was also present in iPSCs generated from trisomy 21 fibroblasts but not in neuronal cultures derived from these iPSCs 60 .
Disruption of homeostasis by genome-wide effects on transcription regulation.
Trisomy 21 may also affect global transcription, either directly if an HSA21 gene functions in transcription regulation or indirectly as a by-product of the additional genetic material 61 , 62 . For example, at least three HSA21 genes encode proteins involved in transcription regulation: ADARB1 (one of two proteins involved in adenosine-to-inosine RNA editing), the constitutive splicing factor U2AF1, and DYRK1A, a dual specificity kinase that phosphorylates splicing factors 60 . Altered ADARB1 transcript levels did not change global adenosine-to-inosine editing levels in trisomy 21 iPSC-derived neuronal cells, whereas trisomy 21-dependent splicing changes were observed in both iPSCs and the neuronal cultures derived from them 60 .
The possibility that increased genetic material could lead to altered genome-wide gene expression was suggested based on the observation that genes whose expression is similarly altered (that is, upregulated or downregulated) are clustered in regions termed gene expression dysregulation domains (GEDDs) in trisomic iPSCs and fibroblasts 61 . However, other studies using a range of methods found no evidence for the existence of GEDDs specifically in trisomy 21 cells 63 , 64 .
Studies of nuclear genome organization using three-dimensional fluorescence in situ hybridization (3D-FISH) have shown that chromosomes are preferentially localized to discrete regions within the nucleus, termed chromosome territories (reviewed elsewhere 65 ). Homeostasis in trisomic cells can be disrupted by the alteration of chromosome territories. Although studies of chromosome territories in trisomy 21 cells and their effects on cellular processes are just beginning, initial studies have found that an extra HSA21 does not change the overall organization of chromosome territories in the interphase nucleus 66 but does alter chromosome compaction and displaces other chromosome territories from their usual nuclear position 66 .
Trisomy 21 also alters regional and/or global methylation patterns (reviewed elsewhere 62 ), although the methylation changes are evenly distributed on all chromosomes and are not specifically enriched on HSA21. One observed pattern is an overall bias towards hypermethylation in DS cells compared with euploid cells, particularly in brain samples 67 – 69 . Furthermore, differential methylation in trisomy 21 cells seems to be localized to discrete regulatory regions of single genes and not to domain-like regions. A meta-analysis identified a small set of genes for which the methylation patterns were different between trisomy 21 and euploid cells in all tissues examined, suggesting that these altered methylation patterns are established early in development and thereby persist in multiple tissue types in DS 62 .
Two mechanisms have been proposed for the globally altered methylation patterns in trisomy 21. First, some triplicated HSA21 genes might function directly in methylation pathways, including SLC19A1 , FTCD , GART , CBS , PRMT2 , N6AMT1 , MIS18A and DNMT3L . SLC19A1 , FTCD , GART and CBS all have a role in the one-carbon metabolism pathway, which is central to DNA methylation. PRMT2 encodes a protein arginine methyltransferase with multiple targets, including histones 70 . N6AMT1 encodes a methyltransferase responsible for DNA N 6 -adenosine methylation 71 . The protein encoded by MIS18A is involved in maintaining the heterochromatic state of centromeres by recruiting the DNA methyltransferases DNMT3A and DNMT3B, thereby repressing the production of non-coding transcripts from centromeric satellite repeats 72 . DNMT3L also interacts with DNMT3A and DNMT3B and enhances their de novo methylation activity, but lacks DNA methyltransferase activity itself 73 . DNMT3L is overexpressed in DS neuroprogenitors, which leads to increased expression of APP and PSD95 in differentiating neurons 74 . These examples illustrate the various mechanisms by which overexpression of these HSA21 genes may lead to trans-epigenetic changes in DS.
The second proposed mechanism is that differential methylation is a result of altered transcription factor occupancy of their binding sites. For example, RUNX1, an HSA21-encoded transcription factor, is overexpressed in trisomy 21 T lymphocytes and differentially methylated sites in trisomy 21 cells are enriched for the RUNX1 binding motif 67 . Thus, the higher occupancy of RUNX1 binding sites in trisomy 21 cells affects CpG methylation at these sites. Additional support for this hypothesis comes from the observation that several types of sequence motifs, including the binding site for CTCF (an insulator protein that blocks the interaction between enhancers and promoters), are enriched at loci that are differentially methylated in all trisomy 21 tissues examined 67 , 75 . Because CTCF binding to its recognition site is sensitive to methylation, the pattern of CTCF occupancy may be particularly affected by DS-associated epigenetic perturbations, which may result in altered 3D conformation of chromatin in the nucleus.
Histone deacetylation and acetylation are other epigenetic mechanisms that are potentially influenced by triplication of some HSA21 genes. For example, DYRK1A , which is overexpressed in trisomy 21, promotes histone deacetylation by phosphorylation and activation of the deacetylase SIRT1 (REF. 76 ). Furthermore, DYRK1A affects chromatin remodelling through its interaction with the transcription repressor REST (also known as NRSF) 77 , resulting in neuronal gene dysregulation that might contribute to the neural phenotypic changes associated with DS.
Although genomic and transcriptomic data are important for understanding the consequences of trisomy 21 and how they relate to interindividual variation in clinical manifestations, integration with other ‘omics’ datasets is necessary to obtain a complete picture of the effects of trisomy 21. For example, in a proteomic study 78 using a method that analysed a large fraction of the proteome 79 of fibroblasts from a pair of monozygotic twins discordant for DS (with follow up on samples from 11 unrelated individuals with DS and matched controls), extensive changes were detected in the levels of proteins encoded by HSA21 genes and non-HSA21 genes. In transcriptome data from both of these twins 61 , steady-state transcript levels were moderately correlated with protein levels. However, the trisomy 21:euploid ratios for gene expression and protein levels were only weakly correlated (Spearman rank correlation ρ = 0.34–0.51), which was mostly due to incongruence of transcript and protein levels for non-HSA21 genes. Thus, substantial post-transcriptional regulation has a role in the differential effect of trisomy 21 on the expression levels of different genes. For example, for HSA21-encoded proteins that are components of heteromeric protein complexes with non-HSA21-encoded proteins, protein degradation seems to buffer against increased transcript levels. Gene enrichment set analyses have shown that protein levels are substantially altered for cell cycle-related functions, cell morphogenesis, lipoprotein metabolism and cellular respiration in mitochondria 78 .
In another study 80 using a proteomics method focused primarily on secreted proteins and those with extracellular domains 81 , plasma samples from 165 individuals with DS and 98 euploid individuals were compared. Among the proteins that were dysregulated in trisomy 21 samples, many are involved in immune control, the complement cascade and growth factor signalling. However, no clear link exists between the identity or levels of dysregulated proteins and specific clinical manifestations of DS.
Indicators of mitochondrial dysfunction have been observed in trisomy 21 cells (for example, fibroblasts) and organs, including the heart (their properties and consequences have been reviewed elsewhere 82 – 84 ). The mitochondrial phenotype in DS includes reduced ATP production by oxidative phosphorylation; decreased respiratory capacity; impaired generation of mitochondrial membrane potential; and alterations in mitochondrial structure. These altered functions result in perturbed mitochondrial energy metabolism and oxidative stress, which in turn could increase susceptibility of individuals with DS to a wide range of conditions, including intellectual disability and AD. The molecular basis for mitochondrial dysfunction involves effector HSA21 genes and key regulatory signalling pathways (reviewed elsewhere 82 ). Of 77 HSA21 genes consistently dysregulated identified by a meta-analysis of 45 DS gene expression studies 85 , NRIP1 , SUMO3 , DYRK1A , RCAN1 , SOD1 , APP and CBS are directly or indirectly involved in mitochondrial function and thus represent candidates for the observed DS-associated mitochondrial phenotypes.
Animal models
Animal models of DS, especially mouse models, have been instrumental in advancing DS research. However, a caveat when studying these models is that DS is a human condition that cannot be precisely replicated in other species. Nonetheless, insights into the mechanisms that underlie the developmental and functional consequences of trisomy 21 have been obtained by overexpression of groups of HSA21 genes or orthologues of HSA21 genes from other species. Important questions about the usefulness of animal models for studying trisomy 21 pathophysiology include the question as to what types of studies are informative in mice, whether results in mice are relevant to understanding human outcomes of trisomy 21 and whether mouse models of DS can be used as a translational platform for drug testing.
Mouse models have transformed basic research in DS, beginning with development of the Ts65Dn mouse, which contains a partial trisomy of Mus musculus chromosome 16 (MMU16) 4 , 86 . Prior to the introduction of this mouse model, the identification, cloning and expression of individual genes in transgenic mouse models was laborious, and studies using these approaches led to wide-ranging conclusions about individual genes that could ‘cause’ DS. However, overexpression of dozens or hundreds of genes simultaneously, supported by more precisely defined terminal phenotypes, has enabled a more realistic interpretation of studies of development, function and genome-wide perturbations in gene expression.
To understand the challenges of using mouse models, it is necessary to understand the complexity of trisomy 21. Trisomy 21 has profound phenotypic effects, as HSA21 contains >200 protein-coding genes and ~400 additional transcripts of known or presumed importance, which are present in an extra copy in every cell from conception onwards. Conversely, the gene overexpression changes in trisomy 21 are subtle, with an average increase in expression of ~1.5-fold in trisomic cells compared with euploid cells. Expression levels of individual genes vary widely and mRNA levels do not always correlate with the levels of the corresponding protein 78 . For genes expressed at a low level, this difference cannot be reliably measured with precision in many high-throughput assays. The consequences of this modest overexpression are substantial, as up to 80% of conceptuses with trisomy 21 do not survive to term 15 .
Relevance of mouse phenotypes to DS.
Several phenotypic features of DS manifest (to varying extents) in all individuals with trisomy 21, including cerebellar hypoplasia, retrusion of the midface skeleton and mandible, Alzheimer-disease-type histopathology at an early age, and intellectual impairment. The risk of CHDs is greatly increased in individuals with DS. Whereas adults with DS show an overall lower risk of developing solid tumour cancers, including breast cancers, when compared to the general population, children with DS have an increased incidence of leukaemia 2 . All of these features are present in some form in the animal models of DS that have been generated to date. For example, whereas the overt histopathology of AD (plaques and tangles) does not occur in mouse models of trisomy 21, accumulation of tau protein and endosomal changes have been reported in these mice 51 .
An overall finding from animal models of DS is that the penetrance (occurrence) and expressivity (severity) of a given phenotype is a function of overexpression of multiple genes; DS is not a collection of independent single gene effects. However, many mouse models that are trisomic for different subsets of mouse orthologues of HSA21 genes have been used effectively — especially in combination with individual gene knockouts that allow specific genes to be returned to the normal two copies in a trisomic background — to identify genes with major effects that are important for understanding a number of phenotypes 57 , 87 , 88 . Rather than asking the simplistic question of whether a gene causes DS, different mouse models provide information about whether altered expression of a specific gene or genes is necessary and/or sufficient to contribute to a phenotype. Interpretation is then limited by the ability to quantify the effects of trisomy in an animal model. Saying that a given model has incompletely penetrant CHDs is of little use in understanding the contributions of specific genes to specific outcomes; failure of ventricular or atrial septum development, AVSDs or outflow tract anomalies all occur in different developmental fields involving different cells at different stages of heart development with the contribution of different genes.
Genetic basis of animal models.
The extent of the triplicated region of HSA21 or the orthologous mouse genomic regions (that is, a segment of a mouse chromosome) is an important consideration in determining the relevance of a mouse model of DS. Trisomy for mouse genomic regions orthologous to HSA21 has the advantage that these regions are subject to endogenous transcription regulation, whereas genetic variation in, for example, promoter or enhancer sequences between mouse and human might be expected to affect transcription. Similarly, any normal human-specific variation in processing signals in DNA and RNA, or amino acid substitutions in proteins, could affect the stoichiometry of interactions between HSA21-encoded proteins and non-HSA21-encoded proteins. Data from GWAS and RNA sequencing suggest that these effects are small, but their potential importance cannot be dismissed. Conversely, although the gene content of HSA21 and the orthologous segments on MMU16, MMU17 and MMU10 are similar, they are not identical ( FIG. 3 ). Of the 233 predicted protein-coding genes on HSA21, only 168 are well conserved in mice. Furthermore, the difference in number and sequence between human and mouse in the non-coding transcripts of presumed function is even greater. The limitations of individual mouse models are emphasized in a comparative study of three of these models, Ts65Dn, Ts1Cje and Ts1Yey 89 .

Down syndrome (DS) results from the presence of a supernumerary Homo sapiens chromosome 21 (HSA21). More than 20 mouse models of DS have been created, which are designed to overexpress part of or a complete HSA21, or the orthologous mouse genomic regions 7 , 91 , 246 . Mouse orthologues of HSA21 genes occur on Mus musculus chromosome 16 (MMU16), MMU17 and MMU10. Tc1 mice carry a mutated HSA21 and are mosaic animals, with a mix of trisomic and euploid cells that is unique to each individual, apparently due to suboptimal function of the human centromere in mice. Ts65Dn animals contain a duplication of ~140 genes on MMU16, some of which are not orthologous to HSA21 247 , 248 (dashed line). TcMAC21 animals contain the long arm of HSA21 (HSA21q) as a mouse artificial chromosome (that is, with a mouse centromere to ensure that the chromosome is retained in every cell); however, this artificial chromosome contains deletions that affect ~8% of HSA21q genes. All of these models except Ts65Dn are direct duplications; that is, the genes in each are trisomic but they do not contain an extra chromosome or centromere. Ts1Rhr, Ts1Cje, Ts1Yey and Ts65Dn mouse models are discussed in the text.
These differences have not been emphasized in the past, as only one mouse model (Tc1) that contained a freely segregating HSA21 existed. To date, Ts65Dn is the only reported mouse model of DS that segregates an extra mouse chromosome; all other models are made by direct duplication of a mouse chromosome segment that is orthologous to HSA21 (detailed mapping of the partial mouse trisomies of segments orthologous to HSA21 has been reviewed elsewhere 6 , 7 ). Genome sequencing in Tc1 mice has revealed substantial deletions, mutations and duplications of HSA21 that compromise the expression of >20% of HSA21 genes in these mice 90 . However, the biggest drawback of Tc1 is the spontaneous loss of the entire HSA21 in a substantial proportion of cells, resulting in mosaic trisomy (that is, each animal is a unique mosaic of trisomic and euploid cells). As development is driven and regulated through cell–cell communication, many aspects of an individual are liable to vary if neighbouring cells suddenly change ploidy. As the loss of HSA21 is random, the development of every animal is unique. Consequently, these rearrangements and mosaicism necessitate cautious interpretation of studies using Tc1 mice. A new model containing 92% of the protein-coding genes on the long arm of HSA21 as a mouse artificial chromosome avoids the problem of mosaicism and has many of the DS manifestations of trisomy 21 that have been observed in previous mouse models 91 .
The demonstration that directed interchromosomal recombination could be achieved using the Cre–Lox system paved the way for the creation of new mouse models for dosage imbalance studies 92 , 93 . To date, >20 mouse models that are trisomic for different HSA21-homologous portions of MMU16, MMU17 and MMU10 have been created 7 ( FIG. 3 ). A set of three mouse strains that together contain a complete duplication of all mouse genomic regions orthologous to HSA21 has been described 94 . In theory, a three-way cross of these mouse strains should produce ‘full trisomy’ in one out of eight offspring. Although this low frequency is only acceptable for some applications, in practice, the frequency of full trisomy is far lower than the predicted Mendelian frequencies, making models such as Tc1 or the MAC21 mice the most viable options for maximum gene representation.
Whereas 95% of individuals with DS have an extra freely segregating HSA21 and therefore an extra centromere and telomeres, the segmental duplication mouse models of DS do not. In these models, trisomy occurs through direct intrachromosomal duplications so that chromosome (and centromere) number is unchanged. One effect of an extra centromere in mammals is reduced male fertility, which is observed in human males with DS and in mouse models of DS. The exact mechanism of this is not well understood 95 . Furthermore, trisomic cells seem to proliferate more slowly and/or have a prolonged cell cycle compared with euploid cells 96 , 97 . Although the results of many of these studies are contradictory, it is feasible that very small changes in the cell cycle could have a major effect on development. As human development requires 43 population doublings to progress from a single cell to the estimated one trillion cells in a fetus (if all of the cells survived — the actual number is clearly much higher), a very small increase in cell cycle length could result in recognizable phenotypes, such as small stature, short fingers, skeletal retrusion, decreased neuronal density in the brain and hypocellular tissues.
Pathophysiological insights from animal models.
In bio-medical research, mouse models provide an important translational tool to identify and prioritize therapeutic approaches to ameliorate the effects of a condition. Efforts to correlate DS-like phenotypes in mice with trisomy of specific genes (and groups of genes) have led to the development of more than 20 mouse models with partial trisomy 7 ( FIG. 3 ). Although the mouse provides a good mammalian genetic model for understanding DS pathogenetic mechanisms, it is important to keep in mind that mice do not develop DS. However, mouse models do illustrate several fundamental principles of gene effects in trisomy and preclinical pharmacological amelioration seems promising, especially for cognitive effects. Trisomic genes that contribute to a deleterious phenotype are targets for drug development to ameliorate features of DS. To illustrate the application of mouse models, examples of the use of mouse models to identify the genetic basis of trisomy effects in brain function, heart development and cancer risk are discussed.
Many preclinical studies emphasize improving cognitive function, possibly the most difficult target for pharmacological intervention. Ts65Dn and many subsequent models exhibit learning and memory deficits and electrophysiological changes consistent with altered synaptic function in the hippocampus 86 , 98 , 99 . Interestingly, Ts65Dn mice showed a significant learning deficit compared with Ts1Cje mice in a standard memory test 57 . Ts65Dn is trisomic for a larger number of genes than Ts1Cje ( FIG. 3 ), one of which is APP . Returning APP copy number to two by crossing a null allele of APP into the Ts65Dn background substantially improved the performance of these mice, although not to the level of a euploid mouse.
CHDs are present in ~50% of babies born with DS and mouse models display some analogous developmental deficits, albeit at a lower frequency than in human babies 100 . To identify a gene or genes that cause CHDs when present in three copies, seven (partial or complete) trisomies of the ~23 Mb region of MMU16 orthologous to HSA21 were studied 101 , which narrowed down the region responsible for causing CHDs (especially septal defects reminiscent of those in DS) to a 4.9 Mb portion of MMU16. However, three smaller trisomies subdividing the 4.9 MB segment did not have heart defects, suggesting that at least two genes must be trisomic to cause CHDs. In a different approach, interactions between trisomy and disomic modifiers of CHD risk were examined. A mouse hemizygous for Creld1 (a gene implicated in familial AVSD) was crossed with Ts65Dn and Ts1Cje mice 102 . Whereas Creld1 +/− mice had normal heart development, the occurrence of septal defects was markedly increased in Creld1 +/− Ts65Dn mice but not in Creld1 +/− Ts1Cje mice. Of the genes that are trisomic in Ts65Dn but not in Ts1Cje, 14 are expressed in heart development, including junctional adhesion molecule 2 ( Jam2 ), which consistently increases the frequency of heart abnormalities when overexpressed in zebrafish 103 . Restoration of two copies of Jam2 in Creld1 +/− Ts65Dn mice blocked the increased occurrence of CHD in these mice. Thus, trisomy for Jam2 is required for the disomic risk factor gene Creld1 to affect heart development.
Animal models have also been important in understanding cancer risk in individuals with DS. Epidemiological studies provide strong evidence that the incidence of many cancers is reduced in adults with DS 104 . Importantly, studies in mouse models of DS have confirmed these results, showing that partial trisomy 21 protects against various cancers. For example, familial adenomatous polyposis (FAP) is a congenital condition involving the formation of precancerous adenomatous polyps (predominantly in the colon) at an early age, and is caused by mutations in the tumour suppressor adenomatous polyposis coli ( APC ). Interestingly, Ts65Dn mice carrying an Apc mutation found in patients with FAP ( Apc Min ) develop 50% fewer adenomatous polyps than euploid mice 87 . Ts1Rhr mice, which are trisomic for just 33 of the genes triplicated in Ts65Dn, have a similar reduction in adenomatous polyp formation. This reduction is substantially reversed by restoring just one of these 33 genes, the proto-oncogene Ets2 , to two copies. Furthermore, Apc Min mice monosomic for these 33 genes (including Ets2 ) develop more tumours than euploid Apc Min mice. Thus, assessment in mouse models of trisomy and monosomy has uncovered a protective effect of Ets2 overexpression and a tumorigenesis-permissive effect of reduced Ets2 expression.
Preclinical studies and drug development.
Several clinical trials of pharmaceutical and nutraceutical agents to improve cognition in individuals with DS were initiated on the basis of preclinical observations in mouse models of DS (for example, NCT00748007 for rivastigmine, NCT02484703 and NCT02024789 for RG1662, and NCT01112683 for memantine). Although in-depth discussion of all of these trials is beyond the scope of this Primer, for illustrative purposes we discuss preclinical studies that led to phase I and II trials of RG1662 (also known as basmisanil or RO5186582), an inverse agonist of GABA A α5 receptors. Cognitive tests in Ts65Dn mice showed that these mice have deficits in learning and memory in tasks that depend on hippocampal function 86 , 105 , 106 , prompting electrophysiological studies demonstrating that long-term potentiation (LTP) is reduced in hippocampal slices from Ts65Dn mice 98 , 99 , 107 . The reduced LTP stemmed from excess GABA-mediated inhibition, which could be reversed by treatment with the GABA A inhibitor pentylenetetrazol (PTZ), restoring the inhibitory–excitatory balance and thereby improving performance in cognitive tests, even in young adult mice 108 .
These studies profoundly changed the understanding of cognitive dysfunction in DS and its treatment, by showing not only that treatment of cognitive deficits in DS is possible but that it is effective in adults, when previously it was widely thought that cognitive improvements could only be made during a small window early in life. Furthermore, this treatment had long-lasting effects, as the improved LTP was measured 3 months after cessation of PTZ treatment 108 . Thus, treatment for cognitive deficits is not a nebulous goal far in the future but can and should be addressed immediately. An inverse agonist of GABA A α5 receptors restores LTP and improves performance in cognitive tests in Ts65Dn mice 109 , 110 . RG1662 was assessed for safety and tolerability in individuals with DS in a successful phase I trial ( NCT01436955 ) that concluded in 2013. However, a phase II trial begun in 2014 was stopped prematurely in 2016 because RG1662 did not show efficacy.
Although the lack of efficacy in this specific trial design was disappointing, the trial represented several advances. First, it demonstrated that the pharmaceutical industry now had the confidence to treat intellectual disability, a first for the DS community. Second, the ability to enlist multiple centres throughout the USA and Europe that were capable of the complex measurements used in the trials, combined with strong support from the DS community in recruiting individuals for the trials, was a clear statement of interest by researchers, clinicians and individuals with DS. Finally, the application of cognitive tests designed specifically for DS was an important part of the trial. Although the official summary of results from this trial have not yet been published, the principles involved in treating individuals with DS have been reported by a number of the investigators involved in the trial 111 . Overall, the disappointment of the trial result was overlaid with optimism for improved treatment and trial design approaches in the future, and informed further trials such as a recent phase II trial using green tea extract containing epigallocatechin-3-gallate (EGCG) to improve cognitive outcomes 112 .
Mechanisms of Alzheimer disease
Almost all adults with DS develop AD-like neuropathology by 40 years of age. AD is characterized by a long asymptomatic preclinical stage in which amyloid pathology develops, ~15–20 years before any cognitive impairment is observed 113 . This preclinical stage is present in autosomal dominant forms of AD 114 and in early-onset AD associated with DS 115 . The evidence is compelling that an increased dosage of APP in trisomy 21 is necessary for increased risk of AD in individuals with DS, although the underlying mechanisms that link APP dosage to neurodegeneration are unknown. However, at least three molecular mechanisms have been proposed to explain the increased risk of AD in individuals with DS ( FIG. 4 ).

The increased gene dosage of APP (encoding amyloid precursor protein (APP)) and DYRK1A (encoding dual-specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A)) on Homo sapiens chromosome 21 (HSA21) in individuals with Down syndrome (DS; trisomy 21) increases their risk of developing Alzheimer disease (AD). APP can undergo non-amyloidogenic processing (not shown) and amyloidogenic processing by β-secretase 1 (β-sec) and γ-secretase (γ-sec) to produce the neurotoxic Aβ42 peptide. The increased APP levels result in higher levels of Aβ42, and phosphorylation of APP at Thr668 by DYRK1A increases the amyloidogenic processing of APP. DYRK1A has other targets in the cell, including splicing factors and the microtubule-binding protein tau. DYRK1A phosphorylation of splicing factors alters the splicing of the MAPT mRNA (encoding tau), resulting in increased levels of tau containing three microtubule-binding domains (three-repeat (3R) tau) and reduced levels of 4R tau. This imbalance leads to neurofibrillary tangle (NFT) formation, possibly due to the lower binding affinity of 3R tau for microtubules. Furthermore, DYRK1A phosphorylation of Thr212 in tau alters the conformation of the protein, reducing the affinity of tau for microtubules and leading to NFT formation. The presence of the ε4 allele of APOE ( APOE ⋆ ε4 ) alters the processing, deposition and clearance of Aβ 249 , and is therefore a major risk allele for AD. AICD, APP intracellular domain; APOE, apolipoprotein E; CNS, central nervous system; P i , inorganic phosphate; sAPP, soluble amyloid precursor protein.
APP and A β 42.
Increased APP expression resulting from the presence of an additional copy of the APP gene is strongly correlated with early-onset AD 116 , 117 . Genomic duplication of the APP locus is the cause of some cases of familial, early-onset AD 118 , 119 . In individuals with DS with complete HSA21 trisomy, the extra copy of HSA21-located APP results in increased levels of APP and its cleavage products, including the neurotoxic Aβ42 peptide, in the brain 120 . However, individuals with partial trisomy of HSA21 who are disomic for APP do not develop early-onset AD and have normal APP expression levels 121 .
A second mechanism involves multiple neuropathological effects of DYRK1A overexpression. HSA21-encoded DYRK1A phosphorylates APP at Thr668, resulting in increases phosphoAPP levels in cells from a mouse model of DS that overexpresses the human DYRK1A gene 122 . This phosphorylation facilitates the amyloidogenic processing of APP by β-secretase and γ-secretase, leading to increased production of neurotoxic Aβ42 peptide 123 , 124 . The levels of phosphoAPP and Aβ42 are increased in the brain of these mice, and phosphoAPP, DYRK1A and Aβ42 levels are increased in the brain of individuals with DS.
DYRK1A also has effects on another molecule implicated in the pathophysiology of AD, the microtubule-stabilizing protein tau. DYRK1A phosphorylates tau at Thr212, a residue that is hyperphosphorylated in individuals with AD 125 . Abnormal hyperphosphorylation of tau changes its conformation, leading to a reduced affinity for microtubules 126 and resulting in microtubule instability, neurofibrillary tangle (NFT) formation and cell death 127 . Furthermore, DYRK1A also phosphorylates splicing factors and thereby alters the splicing of the mRNA of the tau-encoding gene MAPT . Alternative splicing of MAPT results in the production of tau protein isoforms with three or four microtubule-binding domains (three-repeat (3R) tau and 4R tau, respectively). Equal levels of 3R tau and 4R tau are required for maintaining normal brain function and an imbalance in their levels has been detected in sporadic tauopathies 128 . DYRK1A overexpression increases the abundance of 3R tau at the expense of 4R tau, which is thought to lead to the formation of NFTs, as 3R tau has lower affinity for microtubules than 4R tau 129 , 130 .
Endosomal dysfunction.
An extra copy of APP is sufficient to cause endosomal enlargement and intracellular trafficking defects by an Aβ-independent mechanism 131 . RAB5 mediates the endocytosis of cell-surface proteins and the homotypic fusion of early endosomes 132 . Enlargement of RAB5-positive early endosomes, a phenotype consistent with excessive activation of RAB5 (REF. 133 ), is present in neurons in the brain of individuals with sporadic or familial AD 134 , 135 and in individuals with DS who develop AD 136 . In fact, endosomal size in circulating peripheral blood mononuclear cells might serve as a blood-based biomarker of AD-related endosomal pathology 137 .
Endosomal function is also affected by other factors in DS. The ε4 allele of APOE ( APOE ⋆ ε4 ) is the most important genetic risk factor for late-onset AD, as ~80% of all patients with AD have at least one APOE ⋆ ε4 allele 138 . APOE ⋆ ε4 is also thought to increase the risk of dementia in older adults with DS, albeit to a lower extent than for sporadic AD in euploid individuals 139 . Endosomal enlargement in trisomic neurons is thought to cause axonal trafficking defects that contribute to neuronal degeneration 140 . Furthermore, APOE regulates the endosomal trafficking of amyloid fibrils 141 and early endosomal enlargement is present in the brain of mice carrying APOE ⋆ ε4 (REF. 142 ). APP gene dose, Aβ42 and the C-terminal fragment of APP, C99 (also known as βCTF), also have important roles in the development of endosome dysfunction. An increased level of C99 is the only APP-related alteration associated with abnormal endosome enlargement and proliferation that is common to all forms of AD 143 – 146 .
As APP gene dosage is an important determinant of AD risk in individuals with DS, various therapies to reduce APP levels in individuals with DS might have efficacy in treating AD, including reducing APP expression (using anti-sense approaches) and APP production (using translational inhibitors such as Posiphen), blocking APP cleavage (such as by BACE or DYRK1A inhibition or using γ-secretase modulators) or removing Aβ (by active or passive Aβ immunization).
Diagnosis, screening and prevention
Prenatal screening.
In developed countries, laboratory-based prenatal screening for DS is offered as part of routine antenatal care. Screening is a way to identify pregnancies at high risk, thereby limiting diagnostic procedures to minimize the risk of an iatrogenic miscarriage. Since the 1980s, the primary prenatal screening approach has involved a combination of measuring maternal serum biochemical analytes and, more recently, the size of the fetal nuchal translucency (NT; a pouch of fluid behind the neck) in the first trimester 147 . Initially, levels of α-fetoprotein in maternal serum and amniotic fluid in the second trimester were measured and a level ~70% of that in a normal pregnancy indicated an elevated risk of a DS conceptus. More recently, additional maternal serum analytes have been measured, including β-human chorionic gonado-tropin, unconjugated oestriol, inhibin A and pregnancy-associated plasma protein A. The levels of all of these markers change with gestational age, and some are better at distinguishing between trisomy 21 and euploid fetuses in the first trimester and others in the second trimester. Thus, correct dating of the pregnancy by ultrasonography examination is needed for accurate interpretation of serum analyte test results. For each woman, the risk of DS in the fetus is calculated using a computer algorithm with inputs of raw analyte values, gestational age and demographic information, such as maternal age, geo-ethnic background, smoking status and whether or not she has diabetes. The numerical risk cut-off values used in clinical practice differ in their reference points (that is, the risk of an affected fetus versus a liveborn infant). The chance of having a liveborn infant with DS is lower than the chance of having a fetus with DS in the second trimester because some of the fetuses will spontaneously miscarry in the second trimester 18 . Professional guidelines recommend that pregnant women with positive screening results are offered post-test counselling and a diagnostic test, such as amniocentesis or chorionic villus sampling followed by genetic analysis.
In the late 1980s and early 1990s, prenatal ultrasonography was incorporated into routine care, and some abnormalities were demonstrated to be associated with DS. Of note, no fetal anatomical findings are diagnostic of DS and, in fact, many neonates with DS have apparently normal prenatal sonograms. First-trimester ultrasonography features that may indicate DS include an increased NT measurement for gestational age and four other first trimester markers: absent nasal bone, increased frontomaxillary angle, tricuspid valve regurgitation and absent or reduced flow in the ductus venosus 147 . A second trimester anomaly scan has become routine at 18–20 weeks of gestation and includes quantifiable markers, such as a thickened nuchal skin fold and femoral and humeral length measurements. Additional so-called ‘soft’ ultrasonography markers include cystic hygroma, prominent tongue, choroid plexus cysts, mild ventriculomegaly, heart defects, echogenic bowel, duodenal atresia, pyelectasis, bilateral fifth finger clinodactyly and a wide space between the great and second toes 148 .
Using a combination of either first or second trimester maternal serum analyte quantification and NT measurement, the positive predictive values (PPV) for positive screening results are ~3–5% (refs 149 , 150 ). Because of these low PPVs, there has been longstanding interest in a more precise NIPS for fetal chromosome aneuploidies. In 2011, sequencing of cell-free DNA in maternal serum became clinically available 151 . Using massively parallel sequencing, the DNA fragments are counted, mapped to specific areas of the genome, and compared to a reference value; an excess number of fragments that map to HSA21 is suggestive (but not diagnostic) of DS 151 . Within a very short time, NIPS has become the most clinically implemented example of genomic medicine, with ~10 million tests performed to date 152 . Currently, in the USA, a pregnant woman at high risk of having a fetus with DS (maternal age >35 years, positive family history, positive serum analyte and/or NT measurements, suggestive ultrasonographic abnormalities) is offered NIPS as a primary screen. In many countries in Europe, NIPS is beginning to be offered as a secondary test following a positive primary screen. At present, only in Belgium and the Netherlands is NIPS offered as a primary screen for all pregnant women, regardless of a priori risk.
A meta-analysis examined the PPV of NIPS in pregnant women at high or low risk of having a fetus with DS 153 . For DS, the PPVs were 91% and 82% for high-risk and low-risk pregnant women, respectively. Studies that directly compared the predictive performance of analyte and/or NT measurement to that of NIPS in the same pregnant woman have demonstrated a 10–20-fold increase in PPVs using sequencing 149 , 150 . Importantly, NIPS is not dependent on gestational age and can be performed at any time between 10 weeks of gestation and delivery. Because NIPS performs better than current standards of care, in some countries with nationalized health systems there is a move towards providing NIPS as a primary screen for DS 151 .
Because NIPS is noninvasive and has a very good PPV, there have been concerns about what effect this testing will have on the live birth rates of infants with DS. A preliminary study found no difference in the number of live births of babies with DS before and after NIPS became available in eight countries 154 . However, without access to all prenatal testing results, it is unclear how many more babies with DS would have been born during this time period. More extensive studies are needed to assess the effect of NIPS on the incidence of DS. Although many women choose not to have prenatal testing because it would not influence their reproductive decisions, in one study, women who knew prenatally that their babies had DS had better results on psychological tests than women who discovered at birth that their baby had DS 155 . A prenatal diagnosis of DS has been reported to have several benefits, including opportunity for parental education, meetings with paediatric subspecialists ahead of delivery, and change of the location of delivery so that qualified paediatric subspecialists are available for care, thereby enabling mother and baby to remain in the same hospital together 155 . A future benefit of accurate prenatal screening could include the opportunity to initiate treatment during fetal life to improve cognition (discussed later).
Each individual with DS has a distinct set of strengths and challenges that can change throughout his or her life 156 . Some individuals will require high levels of medical input from birth, whereas others may have few health complications. Similarly, some individuals will require social care and support throughout adulthood, whereas others live independently. Several health problems are more common in individuals with DS than in the general population, including CHDs, obstructive sleep apnoea, thyroid disease, dementia, epilepsy, gastrointestinal disease, hearing and vision problems, intellectual disability and developmental disorders, mental illness, immunological dysfunction, haematological disorders and musculoskeletal issues. Screening for these manifestations should be carried out regularly and there are consensus screening guidelines for children with DS (such as the guidelines of the American Academy of Pediatrics 157 ) but not yet for adults 158 – 160 . Services for adults are often more specialized than those for children, and care usually needs to be managed by a number of different medical teams. No consensus currently exists about who should oversee care in adults with DS, but primary care physicians often take on this role 100 ; occasionally, paediatricians may remain involved until early adulthood, and in some countries, such as the UK, intellectual disability psychiatrists are most likely to provide medical support within community teams. Due to the lack of consensus, adults with DS may miss out on regular screening and proactive treatments, with interventions only occurring when difficulties are clinically apparent 161 .
Illness and disease may present differently in people with DS. There is a risk of diagnostic overshadowing and misattribution of symptoms, which can be compounded by the high prevalence of communication difficulties in individuals with DS. Due to the multisystem involvement in DS, care requires multidisciplinary input from a range of medical, social care and educational teams.
Perinatal management
Pregnant women carrying a fetus with a confirmed DS diagnosis require regular monitoring and support throughout the perinatal period. Pregnant women carrying a fetus with DS are at increased risk of miscarriage, with an estimated rate of spontaneous fetal death after 12 weeks of 30%, which increases with maternal age 18 . Monitoring recommendations suggest that a detailed ultrasonography examination and fetal echocardiography should be performed at 18–20 weeks gestation, with further ultrasonography examinations at 28–30, 34–36 and 38 weeks to assess for evidence of upper gastrointestinal obstruction, chylothorax, fetal hydrops and intra-uterine growth restriction. If abnormalities are detected, then increased fetal surveillance is recommended. Local paediatric teams should be informed if abnormalities are detected so that they can be involved in planning postnatal care and treatment (for guidance see, for example, the Down’s Syndrome Association and the Down Syndrome Medical Interest Group fact sheet).
Congenital heart defects
CHDs occur in ~50% of individuals with DS, most commonly AVSD (42% of CHDs in individuals with DS), ventricular septal defect (22%) and atrial septal defect (16%) 162 . Although the frequency of the specific type of CHD depends on age and ethnicity, the primary point is that CHDs have a severe effect on the quality of life of the individual. During pregnancy, a fetal echocardiography examination is recommended. A cardiology examination should take place postnatally, and another echocardiography examination should be performed within the first month after birth. Management is the same as for the general population, including surgical repair 163 . The mortality rate after surgery in children with DS is equal to or lower than that in the general population 164 . All individuals with DS should have annual screening throughout life for signs of acquired valve disease and heart failure.
Sleep apnoea
Obstructive sleep apnoea is common in individuals with DS, with an estimated prevalence of 54–90% 159 , 165 . Screening for symptoms should be carried out at every health check; these symptoms include loud snoring, heavy breathing, restless nights and daytime sleepiness, as well as neurocognitive symptoms, such as irritability, depression, paranoia, cognitive decline and behavioural problems 166 . Overnight polysomnography is recommended for all children with DS before 4 years of age, regardless of symptoms 157 , 167 . Alternative approaches, such as home oximetry, have been suggested to enable identification of at-risk individuals and to reduce the number of children that require full diagnostic studies 168 . Management of sleep apnoea includes the use of continuous positive airway pressure (CPAP), mandibular advancement devices and weight loss. Surgical interventions, including tonsillectomy and adenoidectomy, can be considered, although sleep apnoea may persist after surgery 165 , 169 .
Thyroid dysfunction
Congenital hypothyroidism is present in ~1% of individuals with DS, and abnormal thyroid function tests have been reported in >50% of neonates with DS 170 – 172 . An increased risk of developing thyroid disease remains throughout life, and the risk of developing autoimmune-related thyroid dysfunction increases with age 173 . As clinical diagnosis can be difficult, it is important to perform regular blood screening. Thyroid-stimulating hormone (TSH) and thyroxine (T4) levels should be obtained postnatally and at 6 months and 12 months of age. Measurements of TSH should then be repeated annually 157 .
Alzheimer disease
A substantial proportion of individuals with DS develop early-onset AD, which is related to APP overproduction 120 , 174 , and dementia is the proximal cause of death in 70% of older adults with DS 175 . Clinical symptoms appear after 40 years of age, and 77% of individuals with DS aged 60–69 years, and up to 100% of those aged >70 years, will develop cognitive decline 176 – 178 ( FIG. 5 ). The cognitive decline needs to be understood in the context of the existing cognitive phenotype and individual risk, including APOE genotype, as the presence of the APOE ⋆ ε4 allele seems to increase the risk of earlier cognitive decline due to AD and of mortality 179 ( FIG. 5b ).

a | Distribution of age at dementia diagnosis in individuals with Down syndrome (DS). The risk of developing Alzheimer disease in individuals with DS is closely related to age. The mean age of dementia diagnosis is 55 years, although some individuals already show cognitive decline starting from 40 years of age, whereas others are not diagnosed until after 60 years of age. b | Cognitive decline in individuals with DS, measured by performance ( z score) in a memory test. Cross-sectional data from the London Down Syndrome Consortium showing the distribution of test scores on an object memory task by age and apolipoprotein E ( APOE ) genotype in individuals with DS. The task is a measure of short-term and delayed memory, adapted for individuals with DS. Participants are instructed to name and recall seven objects with two immediate recall trials, and one delayed recall trial after 5 minutes 250 . The data are split by APOE genotype to compare the effect of the ε4 allele of APOE ( APOE ⋆ ε4 ; which increases the risk of late-onset sporadic Alzheimer disease) with that of the APOE ⋆ ε2 allele (which decreases AD risk) and APOE ⋆ ε3 allele (which has a neutral effect on AD risk) on cognitive performance. Cognitive performance in individuals with an APOE ⋆ ε3 / APOE ⋆ ε4 or APOE ⋆ ε4 / APOE ⋆ ε4 genotype declines from 40 years of age, noticeably earlier than the average age of dementia diagnosis and earlier than in individuals with an APOE ⋆ ε2 / APOE ⋆ ε2 , APOE ⋆ ε2 / APOE ⋆ ε3 , APOE ⋆ ε2 / APOE ⋆ ε4 or APOE ⋆ ε3 / APOE ⋆ ε3 genotype. Part a is adapted from REF. 251 , CC-BY-4.0 ( https://creativecommons.org/licenses/by/4.0/ ). Part b adapted with permission from REF. 115 , Elsevier.
Deficits in memory and attention occur early in DS, as in individuals with sporadic AD 180 , but often go unnoticed until behavioural changes appear 181 . Some individuals with DS and dementia may present with seizures and, as the disease progresses, the development of neurological symptoms, such as myoclonus and seizures, is commonly seen 182 .
A baseline assessment of cognitive and adaptive functioning at 30 years of age is recommended in all individuals with DS, to aid future monitoring and diagnosis 183 . Management should focus on early detection and supportive measures. Positive benefit may be obtained from the use of acetylcholinesterase inhibitors 184 , 185 , although some individuals may develop a reduced heart rate and other adverse effects. Individuals with DS and dementia should have access to adequate support, as their needs will increase as the disease progresses. Efforts should be made to keep individuals within their familiar homes, and transfer to other providers should be based on individual circumstances and needs 186 .
Epilepsy is present in 8% of children with DS, with a bimodal age of onset (one peak before 3 years of age and the other after 30 years of age 160 ). Infant onset, most frequently infantile spasms, has been associated with a severe form of epilepsy termed West syndrome, which includes psychomotor regression and hypsarrhythmia (an abnormal pattern of activity between convulsions) on electroencephalography, and occurs in ~6–32% of seizures reported in infants 187 . Later onset of epilepsy is linked to the development of AD 136 . The management of epilepsy depends on the aetiology, but usually involves anticonvulsant medication, which is generally effective in reducing seizure activity 156 , 178 , 188 .
Hearing and vision
Conductive hearing loss is common in individuals with DS, with a high prevalence of otitis media with effusion 189 , 190 . Baseline hearing tests should be performed postnatally using, for example, brainstem auditory acoustic response, and then every 6 months until school age and at least annually thereafter 157 . Early recognition and treatment can reduce the risk of later hearing loss 191 . Sensorineural loss becomes increasingly common in adulthood. Treatment with hearing aids and cochlear implantation has been successful. The use of speech therapy, communication aids and sign language, including the language programme Makaton, can also be beneficial 160 .
Because there is increased risk of refractive errors, cataracts (congenital and developmental), keratoconus and amblyopia in individuals with DS, ophthalmological examination should be performed at birth and regularly throughout life, ideally every 1–2 years 157 . Treatment and corrective aids should be given promptly 157 . Cataract surgery is routinely performed, usually with good outcomes 192 , 193 .
Atlantoaxial instability
Atlantoaxial subluxation (misalignment of the first and second cervical vertebrae) develops in ~1–2% of children with DS 194 . If the condition is present, parents should be advised that participation in sports increases the risk of spinal cord injury in the child. Symptoms such as neck pain, weakness, spasticity, gait difficulties and hyperreflexia should be evaluated with cervical spine radiography 157 .
Mental health
Individuals with DS have increased prevalence of behavioural and mental health problems compared with the general population 195 , in particular, depressive and anxiety disorders 196 , 197 . A small proportion of adolescents and young adults with DS undergo acute regression (also known as Down syndrome disintegrative disorder), which involves a loss of skills and independence compared with their previous levels of functioning. At present, the cause of this decline is unknown, although it often seems to occur after exposure to emotional stressors 198 . To date, there is no definitive treatment for this presentation.
Diagnosis of mental health issues in individuals with DS can be complicated by the presence of intellectual disability, communication difficulties and atypical symptoms 199 . The effect of psychological stressors should not be underestimated, including transitions in accommodation, school or care arrangements, and bereavement 166 , 200 . Treatment for mental illness should be based on standard clinical guidelines, and individuals with DS show positive responses to behavioural interventions, psychotropic medication and psychological therapy.
Neurodevelopment
DS is the most common genetic cause of intellectual disability, with the majority of individuals with DS classified as having mild–moderate disability. Their cognitive profile demonstrates strengths in visual learning, but weaknesses in expressive language, verbal working memory, and episodic memory 201 . However, there is a wide range in cognitive function, with variations in IQ, language, attention, memory and functional abilities.
Individuals with DS often have autism spectrum disorder (~10–15%) and attention-deficit–hyperactivity disorder (ADHD; ~6%) and appropriate screens for these should be performed 160 , 202 . Clinical presentations may differ from those of the general population and assessments may require input from specialists. Standard treatments for ADHD are recommended, although there can be a less marked response to stimulant medication in individuals with intellectual disability than in those without intellectual disability 203 .
Prenatal therapy to improve neurocognition.
Increasing attention has been paid to using prenatal diagnosis of DS as an opportunity to provide antenatal therapy to improve neurocognition 204 , 205 . Impairment of neurogenesis in individuals with DS begins prenatally 206 – 208 and is one of several factors 209 that result in abnormalities in fetal brain growth and shape that are recognizable in third trimester prenatal ultrasound and MR images 210 . Thus, treatment initiated in fetal or early neonatal life is hypothesized to have maximal effect 211 (the ethical issues posed by treatment of fetuses with DS are discussed elsewhere 212 ).
To date, most experiments involving fetal therapy have been performed using mouse models of DS. Antenatal treatments that have resulted in postnatal improvement in learning and neurobehaviour in Ts65Dn mice include dosing with the vasoactive intestinal pep-tides NAPVSIPQ (NAP) and SALLRSIPA (SAL) 213 , maternal dietary choline supplementation 214 , and inhibition of DYRK1A. For example, prenatal treatment with the DYRK1A inhibitor EGCG, which crosses both the placental and blood–brain barriers in mice, improved some aspects of the cranial vault morphological defects in Ts65Dn mice 215 . Furthermore, oral administration of the novel DYRK1A inhibitor ALGERNON (altered generation of neurons) to pregnant Ts1Cje dams from embryonic day 10 (E10) to E15 improved neurogenesis, corticogenesis and behavioural performance of their pups, possibly by enhancing the proliferation of neural stem cells 216 .
Other potential therapeutic targets have been identified from studies of mouse models. For example, mitochondrial dysfunction in fibroblasts from human fetuses with DS is reversed by activation of the PGC1α pathway using metformin 217 . The mRNA and protein levels of PGC1α are markedly reduced in fibroblasts from fetuses with DS, and metformin treatment increased oxygen consumption and cellular ATP concentration, improved respiratory activity, increased mitochondrial membrane potential and reversed mitochondrial ultrastructural abnormalities in these cells 217 . Metformin already has FDA regulatory approval for the treatment of maternal gestational diabetes, so its use in clinical trials in pregnant women carrying fetuses with DS would potentially face fewer regulatory hurdles. Studies in mouse models of DS have also shown that prenatal treatment with the selective serotonin reuptake inhibitor fluoxetine restores hippocampal neurogenesis and connectivity, granule cell number and dendritic patterns, and hippocampus-dependent memory functions 218 . A clinical trial of high-dose fluoxetine in pregnant women (without psychiatric disorders) is ongoing at the University of Texas (USA) 211 , although no information about the trial is publicly available. However, fluoxetine use in the first trimester may have adverse effects, such as increasing the risk of congenital heart disease and other malformations 219 .
Apigenin, a natural flavone found in citrus fruits and leafy green vegetables, also shows promise in reducing oxidative stress and improving total oxidative capacity in human cells from fetuses with trisomy 21, as well as improving some behaviours in neonatal and adult Ts1Cje mice treated prenatally 204 . The recognition of the possibilities for improving fetal brain development, and testing of an increasing number of compounds for eventual use in clinical trials, make this a very exciting time in DS research, although recommendations for supplementation cannot be made owing to a paucity of evidence.
Immune and haematological systems
Individuals with DS are more prone to infections, especially of the respiratory tract 220 – 222 . Infections should be recognized and treated promptly 157 . There is no contraindication to immunizations, and the standard childhood schedule should be followed.
Individuals with DS also show a predisposition to autoimmune diseases, particularly coeliac disease 160 , 223 . Testing the levels of immunoglobulin A antibodies to tissue transglutaminase 2 is recommended if symptoms of coeliac disease are present 157 , but screening of asymptomatic individuals has not proved cost-effective 224 . Type 1 diabetes mellitus, alopecia areata and Addison disease have also been reported in individuals with DS at higher rates than in the general population.
Children with DS are at a markedly increased risk of developing acute leukaemia, compared with children without DS. Transient myeloproliferative disorder (TMD), also referred to as transient leukaemia of DS, is observed in 5–30% of individuals with DS before 3 months of age, and a blood count and film should be performed within 3 days of birth to enable the identification and monitoring of this disorder 225 . Although TMD usually resolves without treatment in the first few months of life, it seems to increase the risk of developing leukaemia by 5 years of age, and it is estimated that 20–30% of individuals with TMD will go on to develop myeloid leukaemia of DS 157 , 226 , 227 .
Quality of life
Research into the quality of life of individuals with DS is limited, although it is recognized that stigma and cultural norms can be barriers to the active participation of individuals with DS in the community. If adequately supported during their life, many individuals with DS can live fairly independently. The perspectives of family members of individuals with DS have been somewhat studied 228 . Support groups and DS networks can provide valuable input, and individuals with DS and their families should be aware of local and national organizations.
Children with DS are now regularly educated within mainstream schools, often with additional one-to-one or specific Special Educational Needs support 229 . Some children with DS can gain additional benefits in terms of language and literacy skills when educated in inclusion classrooms rather than in substantially separate classrooms, whereas others may require a specialist school 230 .
Many individuals with DS are employed and report fairly high levels of satisfaction, and many have become successful professionals, including artists, speakers and actors. However, there is a disparity between the number of individuals with DS who wish to work and those who have secured employment. They may access voluntary work and this seems to be more common than in the general adult population. Cultural assumptions, social care cuts and inadequate adjustments in work can make it difficult for individuals with DS to access employment 231 , 232 .
Quality of life can be affected by physical health, and proactive screening programmes and early intervention can reduce the negative effect of prolonged hospitalizations and ill health. Recognition and correction of hearing and vision problems can reduce the negative effect on communication and development 233 . Obesity, cervical spine abnormalities and muscular difficulties may contribute to the exclusion of individuals with DS from physical activity, and in the USA, children with DS have lower levels of physical activity than their peers 234 . However, the majority would be able to participate in exercise programmes if available, which can have a positive effect on health and wellbeing 235 .
Owing to early cognitive decline, many adults with DS have increased support needs as they age. Adequate proactive support, with regular reassessment of needs and services set up to anticipate functional impairments, is crucial for their quality of life.
Although the lives of individuals with DS have been much improved by the evolution of medical care and the benefits of societal changes, there is no treatment for DS at present that is based on the current understanding of the molecular pathophysiology of DS. Therefore, to design effective therapies, further research is needed to improve understanding of the biology of each symptom and feature of the syndrome. The most important topics for further research are discussed below.
Understanding the contribution of genetic variation to the different and variable phenotypic characteristics and manifestations of DS is an important goal that could be achieved by genome sequencing of thousands of individuals with DS, and linking the genetic variation with the detailed phenotypic characterization of each individual 6 . This effort is based on the reasonable hypothesis that genomic differences are major determinants of the extensive phenotypic variation. Furthermore, genes and other functional genomic elements that in three copies contribute to the phenotype of DS need to be identified. There is supportive evidence that genes causing disease states with haploinsufficiency, in which one functional copy is not sufficient, may also be important when present in three copies, as is the case with DS. For example, DYRK1A is haploinsufficient (probability of loss-of-function intolerant (pLI) score of 1), and the loss of one copy of DYRK1A causes intellectual disability 236 . Several studies have also shown that an extra copy of DYRK1A is involved in the biological dysfunction that results from trisomy 21 (REF. 237 ). However, the pLI score may not be an absolute discriminator of genes involved in the pathophysiology of DS. For example, APP has been strongly linked to AD in individuals with DS 120 but has a pLI score of 0.06, which is compatible with a haplosufficient gene. Furthermore, HMGN1 , a gene that is linked to the transcriptional dysregulation in trisomy 21(REF. 238 ), has a pLI score of 0.22. In addition to the causative contribution of specific genes on HSA21, the phenotypic contribution of the extra-chromosomal material, regardless of its gene content, needs to be investigated.
Experimental models, such as the mouse and other organisms 7 , need to be used to study the brain and other organs in the different stages of development. Advances in single-cell technologies 239 that enable study of the genome, transcriptome, histone modifications, chromatin contacts and protein levels in individual cells will certainly provide insights into the cellular mechanisms of the altered developmental pathways in DS and the effect of chromatin conformation on the dysregulation of gene expression.
Experimental efforts to modify the dysregulated expression of genes on HSA21 and other chromosomes may result in novel therapeutic approaches that should be explored further. For example, experimental silencing of large chromosomal regions by introducing the X chromosome-inactivation effector XIST on one copy of HSA21, or silencing one of the three alleles of HMGN1 , DYRK1A or APP , may rescue some phenotypic characteristics of DS.
Early-onset AD in individuals with DS is genetically analogous to some forms of autosomal dominant AD, and the amyloid pathway is strongly implicated in AD pathology due to APP triplication. Therefore, amyloid-targeting drugs should be trialled in individuals with DS, as they are at very high risk of early-onset AD, preferably before any symptoms develop in order to prevent or delay the onset of dementia 115 . PET imaging of amyloid or tau deposits in the brain 240 , 241 or measures of Aβ peptide ratios in cerebrospinal fluid 242 have been shown to be feasible biomarkers for patient stratification, whereas plasma levels of the neurodegeneration markers neurofilament light chain and neuronal pentraxin 2 show promise as biomarkers of treatment response 243 – 245 .
Population-based, longitudinal, large-scale cohort studies following individuals with DS would be helpful to the further understanding of the co-occurring medical conditions, family attitudes, educational achievement, individual experiences, and societal successes that now exist for individuals with DS. Increasingly sophisticated analytical methods combined with improved genetic models offer hope for more translation of promising therapies.
Acknowledgements
The authors thank the members of the London Down Syndrome (LonDownS) Consortium, G. de Graaf of the Dutch Down Syndrome Foundation and F. Buckley of Down Syndrome Education International for their review of the epidemiology section of this article. Work in the authors’ laboratories and clinics was supported by grants from the SNF, EU, ERC, Jerome Lejeune, and ChildCare Foundations to S.E.A.; a Wellcome Trust Strategic Award (grant number 098330/Z/12/Z) conferred upon the LonDownS Consortium, an MRC project grant (LonDownsPREVENT MR/S011277/1), and grants from the EU Joint Programme - Neuro-degenerative Disease Research (MR/R024901/1, as part of the HEROES consortium), Network of Centres of Excellence in Neurodegeneration (COEN) (MR/S005145/1), Lumind Foundation and Jerome Lejeune Foundation to A.S.; the Jerome Lejeune Foundation USA, Anna and John Sie Foundation, US National Institutes of Health (NIH; HD42053-10, UL1TR001064, ZIA HG200399-04) to D.W.B.; NIH and Lumind Foundation to S.L.S.; HD038384-20, HD098540 and the Lumind Foundation to R.H.R.; and the Alzheimer’s Society and BRC to S.P. The authors thank all past and present members of their laboratories, their collaborators and the patients and their families for their inspiration and support.
Competing interests
S.E.A. is the co-founder and CEO of MediGenome, a clinical and laboratory diagnostic company. B.G.S. occasionally consults on the topic of Down syndrome through Gerson Lehrman Group. B.G.S. receives remuneration from Down syndrome non-profit organizations for speaking engagements and associated travel expenses. B.G.S. receives annual royalties from Woodbine House, Inc., for the publication of his book, Fasten Your Seatbelt: A Crash Course on Down Syndrome for Brothers and Sisters . Within the past 2 years, B.G.S. has received research funding from F. Hoffmann-La Roche, Inc., and LuMind IDSC Down Syndrome Foundation to conduct clinical trials for people with Down syndrome. B.G.S. is occasionally asked to serve as an expert witness in legal cases where Down syndrome is discussed. B.G.S. serves in a non-paid capacity on the Honorary Board of Directors for the Massachusetts Down Syndrome Congress and the Professional Advisory Committee for the National Center for Prenatal and Postnatal Down Syndrome Resources. B.G.S. has a sister with Down syndrome. M.S.R. is a consultant to AC Immune SA. A.S. has consulted for Roche Pharmaceuticals, ONO Pharma, Aelis Farma and AC Immune, and he serves on the Scientific Advisory Board of ProMIS Neurosciences. A.S. and S.E.P. provide clinical services within the UK National Health Service to individuals with Down syndrome. The remaining authors declare no competing interests.
- Disorders Essay Topics Topics: 554
- Genetics Research Topics Topics: 213
- Therapy Essay Topics Topics: 313
- Metabolism Research Topics Topics: 48
- Hepatitis Essay Topics Topics: 57
- Gerontology Paper Topics Topics: 54
- Communicable Disease Research Topics Topics: 58
- Tuberculosis Paper Topics Topics: 133
- Myocardial Infarction Research Topics Topics: 52
- Pneumonia Research Topics Topics: 80
- Heart Attack Topics Topics: 54
- Dorothea Orem’s Theory Research Topics Topics: 85
- Ebola Topics Topics: 74
- Asthma Topics Topics: 155
- Microbiology Paper Topics Topics: 50
50 Down Syndrome Essay Topics
🏆 best essay topics on down syndrome, 🎓 most interesting down syndrome research titles, 💡 simple down syndrome essay ideas.
- Down Syndrome – Information
- Effect of Individualized Care of Adolescents Living With Down Syndrome
- Down’s Syndrome as a Genetic Disorder
- Speech Therapy for Children With Down Syndrome
- Down Syndrome: The Genetic Disorder
- Analysis of Down Syndrome Indiana
- Quality of Health Care According to People With Down Syndrome
- Down Syndrome: Review
- The Child Protection Policy: Case of Down Syndrome
- Fetus with Down Syndrome and Its Moral Status
- Neurological Phenotypes for Down Syndrome Across the Life Span
- Understanding the Challenges and Joys of Raising a Child With Down Syndrome
- The Emerging Phenotype in Infants With Down Syndrome
- Medical Technology for Down Syndrome: Advancements and Assistance
- Language Development for Children With Down Syndrome
- Daily Life and Planning for the Future of Ageing People With Down Syndrome
- Multiple Morbidity Across the Lifespan in People With Down Syndrome
- Opportunities, Barriers, and Recommendations in Down Syndrome Research
- Developmental Expectations and Medical Issues for Children With Down Syndrome
- Employment Opportunities and Benefits for People With Down Syndrome
- Down Syndrome: Prenatal Risk Assessment and Diagnosis
- Neuroanatomy of Down’s Syndrome: A High-Resolution MRI Study
- Resources to Support the Career Development of People With Down Syndrome
- Conducting Clinical Trials in Persons With Down Syndrome
- A First Clinical Trial for Down Syndrome Regression Disorder
- The Importance of Understanding Individual Differences in Down Syndrome
- Down Syndrome and Quality of Life: Some Challenges for Future Practice
- Social Skills Development in Children With Down Syndrome
- Down Syndrome: Knowledge and Attitudes Among Future Healthcare Providers
- Language Characteristics of Individuals With Down Syndrome
- Supporting People Who Have Down’s Syndrome to Overcome Communication Difficulties
- The Dynamics of Sibling Relationships With a Down Syndrome Child
- Making Inclusion Work for Children With Down Syndrome
- The Down Syndrome Brain in the Presence and Absence of Fibrillar β-Amyloidosis
- Profiles of Learning and Development in Down Syndrome
- Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis
- Embracing Abilities: Navigating Life With Down Syndrome
- Research Into the Link Between Alzheimer’s Disease and Down’s Syndrome
- Down Syndrome Developmental Milestones and Physical Activity
- Future Directions in Research on the Development of Persons With Down Syndrome
- Innovations in Down Syndrome: Support and Treatment
- Transitions for Youth With Down Syndrome: Adult Healthcare and Employment
- Premature Aging in Persons With Down Syndrome
- Gene Expression Studies in Down Syndrome: What Do They Tell Us About Disease Phenotypes?
- Resources for Parents of a New Baby With Down Syndrome
- Transition for Children With Down Syndrome From School to Community
- Molecular Changes in Human Down Syndrome Brains
- Health Care Information for Families of Children and Adolescents With Down Syndrome
- Screening for Down Syndrome and Other Conditions
- Comprehensive Volumetric Phenotyping of the Neonatal Brain in Down Syndrome
Cite this post
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2024, August 12). 50 Down Syndrome Essay Topics. https://studycorgi.com/ideas/down-syndrome-essay-topics/
"50 Down Syndrome Essay Topics." StudyCorgi , 12 Aug. 2024, studycorgi.com/ideas/down-syndrome-essay-topics/.
StudyCorgi . (2024) '50 Down Syndrome Essay Topics'. 12 August.
1. StudyCorgi . "50 Down Syndrome Essay Topics." August 12, 2024. https://studycorgi.com/ideas/down-syndrome-essay-topics/.
Bibliography
StudyCorgi . "50 Down Syndrome Essay Topics." August 12, 2024. https://studycorgi.com/ideas/down-syndrome-essay-topics/.
StudyCorgi . 2024. "50 Down Syndrome Essay Topics." August 12, 2024. https://studycorgi.com/ideas/down-syndrome-essay-topics/.
These essay examples and topics on Down Syndrome were carefully selected by the StudyCorgi editorial team. They meet our highest standards in terms of grammar, punctuation, style, and fact accuracy. Please ensure you properly reference the materials if you’re using them to write your assignment.
This essay topic collection was updated on September 12, 2024 .
| powered by | with any questions about these essays. I have also included some other pediatric items of interest for parents. For students writing reports, I have put together to help you. : My apologies, but the website was abandoned years ago due to a lack of time on my part. I've kept the website up in its original style as people keep coming to it for information. And yes, my coding skills are stuck from 20 years ago. But I wanted to make sure the information on Down Syndrome and the new Coronavirus were here. The link below goes to a specific file on my website and has everything we know as of this date. The Global Down Syndrome Organization will keep on this topic. | |||||||||||||||||||||||||
NEW:
Home / Essay Samples / Health / Mental Health / Down Syndrome Down Syndrome Essay ExamplesThe overview of down syndrome disease. Cause of Down syndrome is genetics. Any genetic disorder in chromosomes may emerge during pregnancy. This can be observed even if the family has no history of Down syndrome before. As an example, an ordinary mother may have a baby with Down syndrome with a... Inclusion: Primary Schools that Includes Children with Down SyndromeIn very cell in the human body there is a nucleus. In the nucleus our genetic material is stored in our genes, which carry all the information responsible for our traits inherited from our parents. They are grouped in rod like structures and are called... Down Syndrome: a Different DevelopmentThe word “retarded” has been used in many ways throughout this society. Once said out loud, people don’t understand how offensive it is to others. We all may know a couple of affected individuals, therefore, this word should not be in use by those who... The Impact of Down Syndrome on the Individual and Their FamilyThis paper explores seven published articles that report on the psychological and sociological impact of Down syndrome for the individual and their family. Parents discuss the many familial effects of having a child born with Down syndrome. Most parents report having a child with Down... An Overview of Common Genetic DisordersGenetic testing has been a popular trend lately due to the success of websites such as 23andMe, Ancestry, and many others; and the amount of information these testing services can provide by a simple swab or mouth rinse is amazing. While some use these services... Trying to find an excellent essay sample but no results? Don’t waste your time and get a professional writer to help! You may also like
samplius.com uses cookies to offer you the best service possible.By continuing we’ll assume you board with our cookie policy .--> --> | ||||||||||||||||||||||||||