- E-Submission
- Aims and scope
- About the journal
- Editorial board
- Open access
- Best practice
- Subscription information
- Current issue
- Most download
- Author index
- Research and publication ethics
- Peer review policy
- Advertising policy
- Copyright policy
- Open access policy
- Data sharing policy
- Archiving policy
- For Authors
- Instructions for authors
- Author’s checklist
- Article-processing charge
- Review process
- E-submission
- For Reviewers
- Instructions for reviewers
- Ethics regulation
- How to become a reviewer

Mini Review
June 30, 2024

Research Article

- CURRENT ISSUE
- AHEAD-OF PRINT
| 2024;30(2):103-113.
Jun 30, 2024 | and , and Antifungal Propineb and H30-3 against the Two Fungal Species">Peony Stem Rots by and , and Antifungal Propineb and H30-3 against the Two Fungal Species and , and Antifungal Propineb and H30-3 against the Two Fungal Species"> and , and Antifungal Propineb and H30-3 against the Two Fungal Species 2024;30(2):114-123.
Jun 30, 2024 |
| ">Evaluation of Garlic Germplasm for Resistance to Leaf Blight Caused by "> 2024;30(2):124-130.
Jun 30, 2024 | , a Carrot Black Leaf Blight Fungus, Using a Rezasulin-based Spore Survival Assay">Effects of Several Fungicides on the Spore Growth Period of , a Carrot Black Leaf Blight Fungus, Using a Rezasulin-based Spore Survival Assay , a Carrot Black Leaf Blight Fungus, Using a Rezasulin-based Spore Survival Assay"> Effects of Several Fungicides on the Spore Growth Period of , a Carrot Black Leaf Blight Fungus, Using a Rezasulin-based Spore Survival Assay 2024;30(2):131-138.
Jun 30, 2024 |
| 2024;30(2):139-147.
Jun 30, 2024 | ">Sigma S Involved in Bacterial Survival of "> 2024;30(2):148-156.
Jun 30, 2024 |
| ">Biological and Molecular Characterization of a Korean Isolate of Clover Yellow Vein Virus Infecting "> 2024;30(2):157-164.
Jun 30, 2024 | 2024;30(2):165-175.
Jun 30, 2024 |
| 2024;30(2):176-180.
Jun 30, 2024 | in Pepper Plants Using GJ6-14">Biocontrol of Southern Blight Caused by in Pepper Plants Using GJ6-14 in Pepper Plants Using GJ6-14"> in Pepper Plants Using GJ6-14 2024;30(2):181-188.
Jun 30, 2024 |
| sp. S20-465 That Are Effective in Controlling Cucumber Anthracnose">Identification of Metabolites Derived from sp. S20-465 That Are Effective in Controlling Cucumber Anthracnose sp. S20-465 That Are Effective in Controlling Cucumber Anthracnose"> sp. S20-465 That Are Effective in Controlling Cucumber Anthracnose 2024;30(2):189-193.
Jun 30, 2024 | 2024;30(2):194-198.
Jun 30, 2024 |
| Isolated from 2019 to 2023 in Korea">Changes of Sensitivity to Streptomycin in Isolated from 2019 to 2023 in Korea Isolated from 2019 to 2023 in Korea"> Isolated from 2019 to 2023 in Korea 2024;30(2):199-205.
Jun 30, 2024 |

Research in Plant Disease
Online ISSN: 2233-9191

The Plant Pathology Journal
Online ISSN: 2093-9280
> Go to The Plant Pathology Journal
| 2,291 | |
| 2,046 | |
| 2,005 | Causing Soft Rot on in Korea |
| 3,864 | Root Extract |
| 1,903 | in Korea |
| Cited By 9 | Causing Soft Rot on Graft Cactus in Korea |
| Cited By 5 | in Burial Sites of Infected Host Plants Using Sentinel Plants |
| Cited By 4 | |
| Cited By 4 | to Strobilurin Fungicides and Inhibitory Effect of Fungicides with Other Mechanisms on Resistant to Pyraclostrobin |
| Cited By 4 | Korean Strains Susceptible to the Bacteriophage phiPccP-1 |

Copyright © 2024 by The Korean Society of Plant Pathology.



Journal of Plant Diseases and Protection
Scientific Journal of the "Deutsche Phytomedizinische Gesellschaft" (DPG) - the German Society for Plant Protection and Plant Health
- It covers a wide range of interests for the global plant protection community with relevance to European plant health and protection.
- This journal bridges the gap between lab research, field application, and legislative framework.
- It is the official scientific journal of the Deutsche Phytomedizinische Gesellschaft (DPG) the German Society for Plant Protection and Plant Health.
- Offers “Topic collections” on coherent themes often arising from international conferences.
- It encourages the submission of manuscripts on virtually all aspects of plant diseases, pathogenesis, biology, and molecular biology of various pests and diseases.
- Falko Feldmann,
- Noemi Messmer
Societies and partnerships
Latest issue
Volume 131, Issue 4
Latest articles
Effect of plantain barrier plants on potyvirus-associated diseases in yam cultivation.
- José Efraín González Ramírez
- Vaniert Ventura Chávez
- Orelvis Portal

Unintended sprouts as additional resource for pathogen-free seed potato ( Solanum tuberosum ) propagation
- José Alberto Caram de Souza-Dias
- Falko Feldmann

Load of the ash dieback pathogen hymenoscyphus fraxineus differs in soil
- Jan Werner Böhm
- Christina Zübert
- Michael Kube

Efficacy assessment in crop protection: a tutorial on the use of Abbott’s formula
- Hans-Peter Piepho
- Waqas Ahmed Malik
- Ralf T. Voegele

Comparative biochemical and physiological responses to the virus-induced mosaic disease in apple ( Malus domestica )
- Subaya Manzoor
- Sajad Un Nabi
- Sheikh Mansoor

Journal updates
Reviewer acknowledgements, jpdp: a historical insight into the journal, journal information.
- CAB Abstracts
- Chemical Abstracts Service (CAS)
- Engineering Village – GEOBASE
- Google Scholar
- Japanese Science and Technology Agency (JST)
- Norwegian Register for Scientific Journals and Series
- OCLC WorldCat Discovery Service
- Pathway Studio
- Science Citation Index Expanded (SCIE)
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Editorial policies
© Deutsche Phytomedizinische Gesellschaft
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 24 February 2021
Plant diseases and pests detection based on deep learning: a review
- Jun Liu ORCID: orcid.org/0000-0001-8769-5981 1 &
- Xuewei Wang 1
Plant Methods volume 17 , Article number: 22 ( 2021 ) Cite this article
131k Accesses
388 Citations
17 Altmetric
Metrics details
Plant diseases and pests are important factors determining the yield and quality of plants. Plant diseases and pests identification can be carried out by means of digital image processing. In recent years, deep learning has made breakthroughs in the field of digital image processing, far superior to traditional methods. How to use deep learning technology to study plant diseases and pests identification has become a research issue of great concern to researchers. This review provides a definition of plant diseases and pests detection problem, puts forward a comparison with traditional plant diseases and pests detection methods. According to the difference of network structure, this study outlines the research on plant diseases and pests detection based on deep learning in recent years from three aspects of classification network, detection network and segmentation network, and the advantages and disadvantages of each method are summarized. Common datasets are introduced, and the performance of existing studies is compared. On this basis, this study discusses possible challenges in practical applications of plant diseases and pests detection based on deep learning. In addition, possible solutions and research ideas are proposed for the challenges, and several suggestions are given. Finally, this study gives the analysis and prospect of the future trend of plant diseases and pests detection based on deep learning.
Plant diseases and pests detection is a very important research content in the field of machine vision. It is a technology that uses machine vision equipment to acquire images to judge whether there are diseases and pests in the collected plant images [ 1 ]. At present, machine vision-based plant diseases and pests detection equipment has been initially applied in agriculture and has replaced the traditional naked eye identification to some extent.
For traditional machine vision-based plant diseases and pests detection method, conventional image processing algorithms or manual design of features plus classifiers are often used [ 2 ]. This kind of method usually makes use of the different properties of plant diseases and pests to design the imaging scheme and chooses appropriate light source and shooting angle, which is helpful to obtain images with uniform illumination. Although carefully constructed imaging schemes can greatly reduce the difficulty of classical algorithm design, but also increase the application cost. At the same time, under natural environment, it is often unrealistic to expect the classical algorithms designed to completely eliminate the impact of scene changes on the recognition results [ 3 ]. In real complex natural environment, plant diseases and pests detection is faced with many challenges, such as small difference between the lesion area and the background, low contrast, large variations in the scale of the lesion area and various types, and a lot of noise in the lesion image. Also, there are a lot of disturbances when collecting plant diseases and pests images under natural light conditions. At this time, the traditional classical methods often appear helpless, and it is difficult to achieve better detection results.
In recent years, with the successful application of deep learning model represented by convolutional neural network (CNN) in many fields of computer vision (CV, computer-vision), for example, traffic detection [ 4 ], medical Image Recognition [ 5 ], Scenario text detection [ 6 ], expression recognition [ 7 ], face Recognition [ 8 ], etc. Several plant diseases and pests detection methods based on deep learning are applied in real agricultural practice, and some domestic and foreign companies have developed a variety of deep learning-based plant diseases and pests detection Wechat applet and photo recognition APP software. Therefore, plant diseases and pests detection method based on deep learning not only has important academic research value, but also has a very broad market application prospect.
In view of the lack of comprehensive and detailed discussion on plant diseases and pests detection methods based on deep learning, this study summarizes and combs the relevant literatures from 2014 to 2020, aiming to help researchers quickly and systematically understand the relevant methods and technologies in this field. The content of this study is arranged as follows: “ Definition of plant diseases and pests detection problem ” section gives the definition of plant diseases and pests detection problem; “ Image recognition technology based on deep learning ” section focuses on the detailed introduction of image recognition technology based on deep learning; “ Plant diseases and pests detection methods based on deep learning ” section analyses the three kinds of plant diseases and pests detection methods based on deep learning according to network structure, including classification, detection and segmentation network; “ Dataset and performance comparison ” section introduces some datasets of plant diseases and pests detection and compares the performance of the existing studies; “ Challenges ” section puts forward the challenges of plant diseases and pests detection based on deep learning; “ Conclusions and future directions ” section prospects the possible research focus and development direction in the future.
Definition of plant diseases and pests detection problem
Definition of plant diseases and pests
Plant diseases and pests is one kind of natural disasters that affect the normal growth of plants and even cause plant death during the whole growth process of plants from seed development to seedling and to seedling growth. In machine vision tasks, plant diseases and pests tend to be the concepts of human experience rather than a purely mathematical definition.
Definition of plant diseases and pests detection
Compared with the definite classification, detection and segmentation tasks in computer vision [ 9 ], the requirements of plant diseases and pests detection is very general. In fact, its requirements can be divided into three different levels: what, where and how [ 10 ]. In the first stage, “what” corresponds to the classification task in computer vision. As shown in Fig. 1 , the label of the category to which it belongs is given. The task in this stage can be called classification and only gives the category information of the image. In the second stage, “where” corresponds to the location task in computer vision, and the positioning of this stage is the rigorous sense of detection. This stage not only acquires what types of diseases and pests exist in the image, but also gives their specific locations. As shown in Fig. 1 , the plaque area of gray mold is marked with a rectangular box. In the third stage, “how” corresponds to the segmentation task in computer vision. As shown in Fig. 1 , the lesions of gray mold are separated from the background pixel by pixel, and a series of information such as the length, area, location of the lesions of gray mold can be further obtained, which can assist the higher-level severity level evaluation of plant diseases and pests. Classification describes the image globally through feature expression, and then determines whether there is a certain kind of object in the image by means of classification operation; while object detection focuses on local description, that is, answering what object exists in what position in an image, so in addition to feature expression, object structure is the most obvious feature that object detection differs from object classification. That is, feature expression is the main research line of object classification, while structure learning is the research focus of object detection. Although the function requirements and objectives of the three stages of plant diseases and pests detection are different, yet in fact, the three stages are mutually inclusive and can be converted. For example, the “where” in the second stage contains the process of “what” in the first stage, and the “how” in the third stage can finish the task of “where” in the second stage. Also, the “what” in the first stage can achieve the goal of the second and the third stages through some methods. Therefore, the problem in this study is collectively referred to as plant diseases and pests detection as conventions in the following text, and the terminology differentiates only when different network structures and functions are adopted.
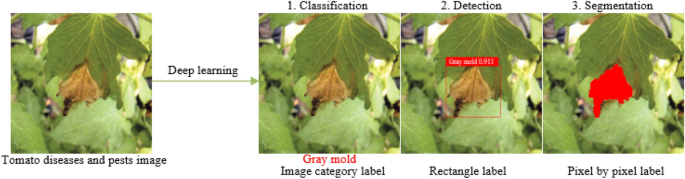
Comparison with traditional plant diseases and pests detection methods
To better illustrate the characteristics of plant diseases and pests detection methods based on deep learning, according to existing references [ 11 , 12 , 13 , 14 , 15 ], a comparison with traditional plant diseases and pests detection methods is given from four aspects including essence, method, required conditions and applicable scenarios. Detailed comparison results are shown in Table 1 .
Image recognition technology based on deep learning
Compared with other image recognition methods, the image recognition technology based on deep learning does not need to extract specific features, and only through iterative learning can find appropriate features, which can acquire global and contextual features of images, and has strong robustness and higher recognition accuracy.
Deep learning theory
The concept of Deep Learning (DL) originated from a paper published in Science by Hinton et al. [ 16 ] in 2006. The basic idea of deep learning is: using neural network for data analysis and feature learning, data features are extracted by multiple hidden layers, each hidden layer can be regarded as a perceptron, the perceptron is used to extract low-level features, and then combine low-level features to obtain abstract high-level features, which can significantly alleviate the problem of local minimum. Deep learning overcomes the disadvantage that traditional algorithms rely on artificially designed features and has attracted more and more researchers’ attention. It has now been successfully applied in computer vision, pattern recognition, speech recognition, natural language processing and recommendation systems [ 17 ].
Traditional image classification and recognition methods of manual design features can only extract the underlying features, and it is difficult to extract the deep and complex image feature information [ 18 ]. And deep learning method can solve this bottleneck. It can directly conduct unsupervised learning from the original image to obtain multi-level image feature information such as low-level features, intermediate features and high-level semantic features. Traditional plant diseases and pests detection algorithms mainly adopt the image recognition method of manual designed features, which is difficult and depends on experience and luck, and cannot automatically learn and extract features from the original image. On the contrary, deep learning can automatically learn features from large data without manual manipulation. The model is composed of multiple layers, which has good autonomous learning ability and feature expression ability, and can automatically extract image features for image classification and recognition. Therefore, deep learning can play a great role in the field of plant diseases and pests image recognition. At present, deep learning methods have developed many well-known deep neural network models, including deep belief network (DBN), deep Boltzmann machine (DBM), stack de-noising autoencoder (SDAE) and deep convolutional neural network (CNN) [ 19 ]. In the area of image recognition, the use of these deep neural network models to realize automate feature extraction from high-dimensional feature space offers significant advantages over traditional manual design feature extraction methods. In addition, as the number of training samples grows and the computational power increases, the characterization power of deep neural networks is being further improved. Nowadays, the boom of deep learning is sweeping both industry and academia, and the performance of deep neural network models are all significantly ahead of traditional models. In recent years, the most popular deep learning framework is deep convolutional neural network.
- Convolutional neural network
Convolutional Neural Networks, abbreviated as CNN, has a complex network structure and can perform convolution operations. As shown in Fig. 2 , the convolutional neural network model is composed of input layer, convolution layer, pooling layer, full connection layer and output layer. In one model, the convolution layer and the pooling layer alternate several times, and when the neurons of the convolution layer are connected to the neurons of the pooling layer, no full connection is required. CNN is a popular model in the field of deep learning. The reason lies in the huge model capacity and complex information brought about by the basic structural characteristics of CNN, which enables CNN to play an advantage in image recognition. At the same time, the successes of CNN in computer vision tasks have boosted the growing popularity of deep learning.
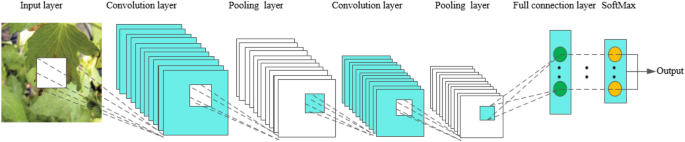
The basic structure of CNN
In the convolution layer, a convolution core is defined first. The convolution core can be considered as a local receptive field, and the local receptive field is the greatest advantage of the convolution neural network. When processing data information, the convolution core slides on the feature map to extract part of the feature information. After the feature extraction of the convolution layer, the neurons are input into the pooling layer to extract the feature again. At present, the commonly used methods of pooling include calculating the mean, maximum and random values of all values in the local receptive field [ 20 , 21 ]. After the data entering several convolution layers and pooling layers, they enter the full-connection layer, and the neurons in the full-connection layer are fully connected with the neurons in the upper layer. Finally, the data in the full-connection layer can be classified by the softmax method, and then the values are transmitted to the output layer for output results.
Open source tools for deep learning
The commonly used third-party open source tools for deep learning are Tensorflow [ 22 ], Torch/PyTorch [ 23 ], Caffe [ 24 ], Theano [ 25 ]. The different characteristics of each open source tool are shown in Table 2 .
The four commonly used deep learning third-party open source tools all support cross-platform operation, and the platforms that can be run include Linux, Windows, iOS, Android, etc. Torch/PyTorch and Tensorflow have good scalability and support a large number of third-party libraries and deep network structures, and have the fastest training speed when training large CNN networks on GPU.
Plant diseases and pests detection methods based on deep learning
This section gives a summary overview of plant diseases and pests detection methods based on deep learning. Since the goal achieved is completely consistent with the computer vision task, plant diseases and pests detection methods based on deep learning can be seen as an application of relevant classical networks in the field of agriculture. As shown in Fig. 3 , the network can be further subdivided into classification network, detection network and segmentation network according to the different network structures. As can be seen from Fig. 3 , this paper is subdivided into several different sub-methods according to the processing characteristics of each type of methods.
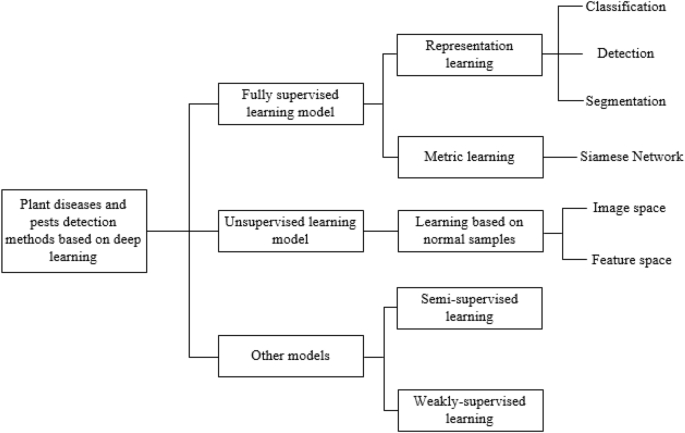
Framework of plant diseases and pests detection methods based on deep learning
Classification network
In real natural environment, the great differences in shape, size, texture, color, background, layout and imaging illumination of plant diseases and pests make the recognition a difficult task. Due to the strong feature extraction capability of CNN, the adoption of CNN-based classification network has become the most commonly used pattern in plant diseases and pests classification. Generally, the feature extraction part of CNN classification network consists of cascaded convolution layer + pooling layer, followed by full connection layer (or average pooling layer) + softmax structure for classification. Existing plant diseases and pests classification network mostly use the muture network structures in computer vision, including AlexNet [ 26 ], GoogleLeNet [ 27 ], VGGNet [ 28 ], ResNet [ 29 ], Inception V4 [ 30 ], DenseNets [ 31 ], MobileNet [ 32 ] and SqueezeNet [ 33 ]. There are also some studies which have designed network structures based on practical problems [ 34 , 35 , 36 , 37 ]. By inputting a test image into the classification network, the network analyses the input image and returns a label that classifies the image. According to the difference of tasks achieved by the classification network method, it can be subdivided into three subcategories: using the network as a feature extractor, using the network for classification directly and using the network for lesions location.
Using network as feature extractor
In the early studies on plant diseases and pests classification methods based on deep learning, many researchers took advantage of the powerful feature extraction capability of CNN, and the methods were combined with traditional classifiers [ 38 ]. First, the images are input into a pretrained CNN network to obtain image characterization features, and the acquired features are then input into a conventional machine learning classifier (e.g., SVM) for classification. Yalcin et al. [ 39 ] proposed a convolutional neural network architecture to extract the features of images while performing experiments using SVM classifiers with different kernels and feature descriptors such as LBP and GIST, the experimental results confirmed the effectiveness of the approach. Fuentes et al. [ 40 ] put forward the idea of CNN based meta architecture with different feature extractors, and the input images included healthy and infected plants, which were identified as their respective classes after going through the meta architecture. Hasan et al. [ 41 ] identified and classified nine different types of rice diseases by using the features extracted from DCNN model and input into SVM, and the accuracy achieved 97.5%.
Using network for classification directly
Directly using classification network to classify lesions is the earliest common means of CNN applied in plant diseases and pests detection. According to the characteristics of existing research work, it can be further subdivided into original image classification, classification after locating Region of Interest (ROI) and multi-category classification.
Original image classification. That is, directly put the collected complete plant diseases and pests image into the network for learning and training. Thenmozhi et al. [ 42 ] proposed an effective deep CNN model, and transfer learning is used to fine-tune the pre-training model. Insect species were classified on three public insect datasets with accuracy of 96.75%, 97.47% and 95.97%, respectively. Fang et al. [ 43 ] used ResNet50 in plant diseases and pests detection. The focus loss function was used instead of the standard cross-entropy loss function, and the Adam optimization method was used to identify the leaf disease grade, and the accuracy achieved 95.61%.
Classification after locating ROI. For the whole image acquired, we should focus on whether there is a lesion in a fixed area, so we often obtain the region of interest (ROI) in advance, and then input the ROI into the network to judge the category of diseases and pests. Nagasubramanian et al. [ 44 ] used a new three-dimensional deep convolution neural network (DCNN) and salience map visualization method to identify healthy and infected samples of soybean stem rot, and the classification accuracy achieved 95.73%.
Multi-category classification. When the number of plant diseases and pests class to be classified exceed 2 class, the conventional plant diseases and pests classification network is the same as the original image classification method, that is, the output nodes of the network are the number of plant diseases and pests class + 1 (including normal class). However, multi-category classification methods often use a basic network to classify lesions and normal samples, and then share feature extraction parts on the same network to modify or increase the classification branches of lesion categories. This approach is equivalent to preparing a pre-training weight parameter for subsequent multi-objective plant diseases and pests classification network, which is obtained by binary training between normal samples and plant diseases and pests samples. Picon et al. [ 45 ] proposed a CNN architecture to identify 17 diseases in 5 crops, which seamlessly integrates context metadata, allowing training of a single multi-crop model. The model can achieve the following goals: (a) obtains richer and more robust shared visual features than the corresponding single crop; (b) is not affected by different diseases in which different crops have similar symptoms; (c) seamlessly integrates context to perform crop conditional disease classification. Experiments show that the proposed model alleviates the problem of data imbalance, and the average balanced accuracy is 0.98, which is superior to other methods and eliminates 71% of classifier errors.
Using network for lesions location
Generally, the classification network can only complete the classification of image label level. In fact, it can also achieve the location of lesions and the pixel-by-pixel classification by combining different techniques and methods. According to the different means used, it can be further divided into three forms: sliding window, heatmap and multi-task learning network.
Sliding window. This is the simplest and intuitive method to achieve the location of lesion coarsely. The image in the sliding window is input into the classification network for plant diseases and pests detection by redundant sliding on the original image through a smaller size window. Finally, all sliding windows are connected to obtain the results of the location of lesion. Chen et al. [ 46 ] used CNN classification network based on sliding window to build a framework for characteristics automatic learning, feature fusion, recognition and location regression calculation of plant diseases and pests species, and the recognition rate of 38 common symptoms in the field was 50–90%.
Heatmap. This is an image that reflects the importance of each region in the image, the darker the color represents the more important. In the field of plant diseases and pests detection, the darker the color in the heatmap represents the greater the probability that it is the lesion. In 2017, Dechant et al. [ 47 ] trained CNN to make heatmap to show the probability of infection in each region in maize disease images, and these heatmaps were used to classify the complete images, dividing each image into containing or not containing infected leaves. At runtime, it takes about 2 min to generate a heatmap for an image (1.6 GB of memory) and less than one second to classify a set of three heatmaps (800 MB of memory). Experiments show that the accuracy is 96.7% on the test dataset. In 2019, Wiesner-Hanks et al. [ 48 ] used heatmap method to obtain accurate contour areas of maize diseases, the model can accurately depict lesions as low as millimeter scale from the images collected by UAVs, with an accuracy rate of 99.79%, which is the best scale of aerial plant disease detection achieved so far.
Multi-task learning network. If the pure classified network does not add any other skills, it could only realize the image level classification. Therefore, to accurately locate the location of plant diseases and pests, the designed network should often add an extra branch, and the two branches would share the results of the feature extracting. In this way, the network generally had the classification and segmentation output of the plant diseases and pests, forming a multi-task learning network. It takes into account the characteristics of both network. For segmentation network branches, each pixel in the image can be used as a training sample to train the network. Therefore, the multi-task learning network not only uses the segmentation branches to output the specific segmentation results of the lesions, but also greatly reduces the requirements of the classification network for samples. Ren et al. [ 49 ] constructed a Deconvolution-Guided VGNet (DGVGNet) model to identify plant leaf diseases which were easily disturbed by shadows, occlusions and light intensity. The deconvolution was used to guide the CNN classifier to focus on the real lesion sites. The test results show that the accuracy of disease class identification is 99.19%, the pixel accuracy of lesion segmentation is 94.66%, and the model has good robustness in occlusion, low light and other environments.
To sum up, the method based on classification network is widely used in practice, and many scholars have carried out application research on the classification of plant diseases and pests [ 50 , 51 , 52 , 53 ]. At the same time, different sub-methods have their own advantages and disadvantages, as shown in Table 3 .
Detection network
Object positioning is one of the most basic tasks in the field of computer vision. It is also the closest task to plant diseases and pests detections in the traditional sense. Its purpose is to obtain accurate location and category information of the object. At present, object detection methods based on deep learning emerge endlessly. Generally speaking, plant diseases and pests detection network based on deep learning can be divided into: two stage network represented by Faster R-CNN [ 54 ]; one stage network represented by SSD [ 55 ] and YOLO [ 56 , 57 , 58 ]. The main difference between the two networks is that the two-stage network needs to first generate a candidate box (proposal) that may contain the lesions, and then further execute the object detection process. In contrast, the one-stage network directly uses the features extracted in the network to predict the location and class of the lesions.
Plant diseases and pests detection based on two stages network
The basic process of two-stage detection network (Faster R-CNN) is to obtain the feature map of the input image through the backbone network first, then calculate the anchor box confidence using RPN and get the proposal. Then, input the feature map of the proposal area after ROIpooling to the network, fine-tune the initial detection results, and finally get the location and classification results of the lesions. Therefore, according to the characteristics of plant diseases and pests detection, common methods often improve on the backbone structure or its feature map, anchor ratio, ROIpooling and loss function. In 2017, Fuentes et al. [ 59 ] first used Faster R-CNN to locate tomato diseases and pests directly, combined with deep feature extractors such as VGG-Net and ResNet, the mAP value reached 85.98% in a dataset containing 5000 tomato diseases and pests of 9 categories. In 2019, Ozguven et al. [ 60 ] proposed a Faster R-CNN structure for automatic detection of beet leaf spot disease by changing the parameters of CNN model. 155 images were trained and tested. The results show that the overall correct classification rate of this method is 95.48%. Zhou et al. [ 61 ] presented a fast rice disease detection method based on the fusion of FCM-KM and Faster R-CNN. The application results of 3010 images showed that: the detection accuracy and time of rice blast, bacterial blight, and sheath blight are 96.71%/0.65 s, 97.53%/0.82 s and 98.26%/0.53 s respectively. Xie et al. [ 62 ] proposed a Faster DR-IACNN model based on the self-built grape leaf disease dataset (GLDD) and Faster R-CNN detection algorithm, the Inception-v1 module, Inception-ResNet-v2 module and SE are introduced. The proposed model achieved higher feature extraction ability, the mAP accuracy was 81.1% and the detection speed was 15.01FPS. The two-stage detection network has been devoted to improving the detection speed to improve the real-time and practicability of the detection system, but compared with the single-stage detection network, it is still not concise enough, and the inference speed is still not fast enough.
Plant diseases and pests detection based on one stage network
The one-stage object detection algorithm has eliminated the region proposal stage, but directly adds the detection head to the backbone network for classification and regression, thus greatly improving the inference speed of the detection network. The single-stage detection network is divided into two types, SSD and YOLO, both of which use the whole image as the input of the network, and directly return the position of the bounding box and the category to which it belongs at the output layer.
Compared with the traditional convolutional neural network, the SSD selects VGG16 as the trunk of the network, and adds a feature pyramid network to obtain features from different layers and make predictions. Singh et al. [ 63 ] built the PlantDoc dataset for plant disease detection. Considering that the application should predict in mobile CPU in real time, an application based on MobileNets and SSD was established to simplify the detection of model parameters. Sun et al. [ 64 ] presented an instance detection method of multi-scale feature fusion based on convolutional neural network, which is improved on the basis of SSD to detect maize leaf blight under complex background. The proposed method combined data preprocessing, feature fusion, feature sharing, disease detection and other steps. The mAP of the new model is higher (from 71.80 to 91.83%) than that of the original SSD model. The FPS of the new model has also improved (from 24 to 28.4), reaching the standard of real-time detection.
YOLO considers the detection task as a regression problem, and uses global information to directly predict the bounding box and category of the object to achieve end-to-end detection of a single CNN network. YOLO can achieve global optimization and greatly improve the detection speed while satisfying higher accuracy. Prakruti et al. [ 65 ] presented a method to detect pests and diseases on images captured under uncontrolled conditions in tea gardens. YOLOv3 was used to detect pests and diseases. While ensuring real-time availability of the system, about 86% mAP was achieved with 50% IOU. Zhang et al. [ 66 ] combined the pooling of spatial pyramids with the improved YOLOv3, deconvolution is implemented by using the combination of up-sampling and convolution operation, which enables the algorithm to effectively detect small size crop pest samples in the image and reduces the problem of relatively low recognition accuracy due to the diversity of crop pest attitudes and scales. The average recognition accuracy can reach 88.07% by testing 20 class of pests collected in real scene.
In addition, there are many studies on using detection network to identify diseases and pests [ 47 , 67 , 68 , 69 , 70 , 71 , 72 , 73 ]. With the development of object detection network in computer vision, it is believed that more and more new detection models will be applied in plant diseases and pests detection in the future. In summary, in the field of plant diseases and pests detection which emphasizes detection accuracy at this stage, more models based on two-stage are used, and in the field of plant diseases and pests detection which pursue detection speed more models based on one-stage are used.
Can detection network replace classification network? The task of detection network is to solve the location problem of plant diseases and pests. The task of classification network is to judge the class of plant diseases and pests. Visually, the hidden information of detection network includes the category information, that is, the category information of plant diseases and pests that need to be located needs to be known beforehand, and the corresponding annotation information should be given in advance to judge the location of plant diseases and pests. From this point of view, the detection network seems to include the steps of the classification network, that is, the detection network can answer “what kind of plant diseases and pests are in what place”. But there is a misconception, in which “what kind of plant diseases and pests” is given a priori, that is, what is labelled during training is not necessarily the real result. In the case of strong model differentiation, that is, when the detection network can give accurate results, the detection network can answer “what kind of plant diseases and pests are in what place” to a certain extent. However, in the real world, in many cases, it cannot uniquely reflect the uniqueness of plant diseases and pests categories, only can answer “what kind of plant diseases and pests may be in what place”, then the involvement of the classification network is necessary. Thus, the detection network cannot replace the classification network.
Segmentation network
Segmentation network converts the plant diseases and pests detection task to semantic and even instance segmentation of lesions and normal areas. It not only finely divides the lesion area, but also obtains the location, category and corresponding geometric properties (including length, width, area, outline, center, etc.). It can be roughly divided into: Fully Convolutional Networks (FCN) [ 74 ] and Mask R-CNN [ 75 ].
Full convolution neural network (FCN) is the basis of image semantics segmentation. At present, almost all semantics segmentation models are based on FCN. FCN first extracts and codes the features of the input image using convolution, then gradually restores the feature image to the size of the input image by deconvolution or up sampling. Based on the differences in FCN network structure, the plant diseases and pests segmentation methods can be divided into conventional FCN, U-net [ 76 ] and SegNet [ 77 ].
Conventional FCN. Wang et al. [ 78 ] presented a new method of maize leaf disease segmentation based on full convolution neural network to solve the problem that traditional computer vision is susceptible to different illumination and complex background, and the segmentation accuracy reached 96.26. Wang et al. [ 79 ] proposed a plant diseases and pests segmentation method based on improved FCN. In this method, a convolution layer was used to extract multi-layer feature information from the input maize leaf lesion image, and the size and resolution of the input image were restored by deconvolution operation. Compared with the original FCN method, not only the integrity of the lesion was guaranteed, but also the segmentation of small lesion area was highlighted, and the accuracy rate reached 95.87%.
U-net. U-net is not only a classical FCN structure, but also a typical encoder-decoder structure. It is characterized by introducing a layer-hopping connection, fusing the feature map in the coding stage with that in the decoding stage, which is beneficial to the recovery of segmentation details. Lin et al. [ 80 ] used U-net based convolutional neural network to segment 50 cucumber powdery mildew leaves collected in natural environment. Compared with the original U-net, a batch normalization layer was added behind each convolution layer, making the neural network insensitive to weight initialization. The experiment shows that the convolutional neural network based on U-net can accurately segment powdery mildew on cucumber leaves at the pixel level with an average pixel accuracy of 96.08%, which is superior to the existing K-means, Random-forest and GBDT methods. The U-net method can segment the lesion area in a complex background, and still has good segmentation accuracy and segmentation speed with fewer samples.
SegNet. It is also a classical encoder–decoder structure. Its feature is that the up-sampling operation in the decoder takes advantage of the index of the largest pooling operation in the encoder. Kerkech et al. [ 81 ] presented an image segmentation method for unmanned aerial vehicles. Visible and infrared images (480 samples from each range) were segmented using SegNet to identify four categories: shadows, ground, healthy and symptomatic grape vines. The detection rates of the proposed method on grape vines and leaves were 92% and 87%, respectively.
Mask R-CNN is one of the most commonly used image instance segmentation methods at present. It can be considered as a multitask learning method based on detection and segmentation network. When multiple lesions of the same type have adhesion or overlap, instance segmentation can separate individual lesions and further count the number of lesions. However, semantic segmentation often treats multiple lesions of the same type as a whole. Stewart et al. [ 82 ] trained a Mask R-CNN model to segment maize northern leaf blight (NLB) lesions in an unmanned aerial vehicle image. The trained model can accurately detect and segment a single lesion. At the IOU threshold of 0.50, the IOU between the baseline true value and the predicted lesion was 0.73, and the average accuracy was 0.96. Also, some studies combine the Mask R-CNN framework with object detection networks for plant diseases and pests detection. Wang et al. [ 83 ] used two different models, Faster R-CNN and ask R-CNN, in which Faster R-CNN was used to identify the class of tomato diseases and Mask R-CNN was used to detect and segment the location and shape of the infected area. The results showed that the proposed model can quickly and accurately identify 11 class of tomato diseases, and divide the location and shape of infected areas. Mask R-CNN reached a high detection rate of 99.64% for all class of tomato diseases.
Compared with the classification and detection network methods, the segmentation method has advantages in obtaining the lesion information. However, like the detection network, it requires a lot of annotation data, and its annotation information is pixel by pixel, which often takes a lot of effort and cost.
Dataset and performance comparison
This section first gives a brief introduction to the plant diseases and pests related datasets and the evaluation index of deep learning model, then compares and analyses the related models of plant diseases and pests detection based on deep learning in recent years.
Datasets for plant diseases and pests detection
Plant diseases and pests detection datasets are the basis for research work. Compared with ImageNet, PASCAL-VOC2007/2012 and COCO in computer vision tasks, there is not a large and unified dataset for plant diseases and pests detection. The plant diseases and pests dataset can be acquired by self-collection, network collection and use of public datasets. Among them, self-collection of image dataset is often obtained by unmanned aerial remote sensing, ground camera photography, Internet of Things monitoring video or video recording, aerial photography of unmanned aerial vehicle with camera, hyperspectral imager, near-infrared spectrometer, and so on. Public datasets typically come from PlantVillage, an existing well-known public standard library. Relatively, self-collected datasets of plant diseases and pests in real natural environment are more practical. Although more and more researchers have opened up the images collected in the field, it is difficult to compare them uniformly based on different class of diseases under different detection objects and scenarios. This section provides links to a variety of plant diseases and pests detection datasets in conjunction with existing studies. As shown in Table 4 .
Evaluation indices
Evaluation indices can vary depending on the focus of the study. Common evaluation indices include \(Precision\) , \(Recall\) , mean Average Precision (mAP) and the harmonic Mean F1 score based on \(Precision\) and \(Recall\) .
\(Precision\) and \(Recall\) are defined as:
In Formula ( 1 ) and Formula ( 2 ), TP (True Positive) is true-positive, predicted to be 1 and actually 1, indicating the number of lesions correctly identified by the algorithm. FP (False Positive) is false-positive, predicted to be 1 and actually 0, indicating the number of lesions incorrectly identified by the algorithm. FN (False Negative) is false-negative, predicted to be 0 and actually 1, indicating the number of unrecognized lesions.
Detection accuracy is usually assessed using mAP. The average accuracy of each category in the dataset needs to be calculated first:
In the above-mentioned formula, \(N\left( {class} \right)\) represents the number of all categories, \(Precision\left( j \right)\) and \(Recall\left( j \right)\) represents the precision and recall of class j respectively.
Average accuracy for each category is defined as mAP:
The greater the value of \(mAP\) , the higher the recognition accuracy of the algorithm; conversely, the lower the accuracy of the algorithm.
F1 score is also introduced to measure the accuracy of the model. F1 score takes into account both the accuracy and recall of the model. The formula is
Frames per second (FPS) is used to evaluate the recognition speed. The more frames per second, the faster the algorithm recognition speed; conversely, the slower the algorithm recognition speed.
Performance comparison of existing algorithms
At present, the research on plant diseases and pests based on deep learning involves a wide range of crops, including all kinds of vegetables, fruits and food crops. The tasks completed include not only the basic tasks of classification, detection and segmentation, but also more complex tasks such as the judgment of infection degree.
At present, most of the current deep learning-based methods for plant diseases and pests detection are applied on specific datasets, many datasets are not publicly available, there is still no single publicly available and comprehensive dataset that will allow all algorithms to be uniformly compared. With the continuous development of deep learning, the application performance of some typical algorithms on different datasets has been gradually improved, and the mAP, F1 score and FPS of the algorithms have all been increased.
The breakthroughs achieved in the existing studies are amazing, but due to the fact that there is still a certain gap between the complexity of the infectious diseases and pests images in the existing studies and the real-time field diseases and pests detection based on mobile devices. Subsequent studies will need to find breakthroughs in larger, more complex, and more realistic datasets.
Small dataset size problem
At present, deep learning methods are widely used in various computer vision tasks, plant diseases and pests detection is generally regarded as specific application in the field of agriculture. There are too few agricultural plant diseases and pests samples available. Compared with open standard libraries, self-collected data sets are small in size and laborious in labeling data. Compared with more than 14 million sample data in ImageNet datasets, the most critical problem facing plant diseases and pests detection is the problem of small samples. In practice, some plant diseases have low incidence and high cost of disease image acquisition, resulting in only a few or dozen training data collected, which limits the application of deep learning methods in the field of plant diseases and pests identification. In fact, for the problem of small samples, there are currently three different solutions.
Data amplification, synthesis and generation
Data amplification is a key component of training deep learning models. An optimized data amplification strategy can effectively improve the plant diseases and pests detection effect. The most common method of plant diseases and pests image expansion is to acquire more samples using image processing operations such as mirroring, rotating, shifting, warping, filtering, contrast adjustment, and so on for the original plant diseases and pests samples. In addition, Generative Adversarial Networks (GANs) [ 93 ] and Variational automatic encoder (VAE) [ 94 ] can generate more diverse samples to enrich limited datasets.
Transfer learning and fine-tuning classical network model
Transfer learning (TL) transfers knowledge learned from generic large datasets to specialized areas with relatively small amounts of data. When transfer learning develops a model for newly collected unlabeled samples, it can start with a training model by a similar known dataset. After fine-tuning parameters or modifying components, it can be applied to localized plant disease and pest detection, which can reduce the cost of model training and enable the convolution neural network to adapt to small sample data. Oppenheim et al. [ 95 ] collected infected potato images of different sizes, hues and shapes under natural light and classified by fine-tuning the VGG network. The results showed that, the transfer learning and training of new networks were effective. Too et al. [ 96 ] evaluated various classical networks by fine-tuning and contrast. The experimental results showed that the accuracy of Dense-Nets improved with the number of iterations. Chen et al. [ 97 ] used transfer learning and fine-tuning to identify rice disease images under complex background conditions and achieved an average accuracy of 92.00%, which proves that the performance of transfer learning is better than training from scratch.
Reasonable network structure design
By designing a reasonable network structure, the sample requirements can be greatly reduced. Zhang et al. [ 98 ] constructed a three-channel convolution neural network model for plant leaf disease recognition by combining three color components. Each channel TCCNN component is composed of three color RGB leaf disease images. Liu et al. [ 99 ] presented an improved CNN method for identifying grape leaf diseases. The model used a depth-separable convolution instead of a standard convolution to alleviate overfitting and reduce the number of parameters. For the different size of grape leaf lesions, the initial structure was applied to the model to improve the ability of multi-scale feature extraction. Compared with the standard ResNet and GoogLeNet structures, this model has faster convergence speed and higher accuracy during training. The recognition accuracy of this algorithm was 97.22%.
Fine-grained identification of small-size lesions in early identification
Small-size lesions in early identification.
Accurate early detection of plant diseases is essential to maximize the yield [ 36 ]. In the actual early identification of plant diseases and pests, due to the small size of the lesion object itself, multiple down sampling processes in the deep feature extraction network tend to cause small-scale objects to be ignored. Moreover, due to the background noise problem on the collected images, large-scale complex background may lead to more false detection, especially on low-resolution images. In view of the shortage of existing algorithms, the improvement direction of small object detection algorithm is analyzed, and several strategies such as attention mechanism are proposed to improve the performance of small target detection.
The use of attention mechanism makes resources allocated more rationally. The essence of attention mechanism is to quickly find region of interest and ignore unimportant information. By learning the characteristics of plant diseases and pests images, features can be separated using weighted sum method with weighted coefficient, and the background noise in the image can be suppressed. Specifically, the attention mechanism module can get a salient image, and seclude the object from the background, and the Softmax function can be used to manipulate the feature image, and combine it with the original feature image to obtain new fusion features for noise reduction purposes. In future studies on early recognition of plant diseases and pests, attention mechanisms can be used to effectively select information and allocate more resources to region of interest to achieve more accurate detection. Karthik et al. [ 100 ] applied attention mechanism on the residual network and experiments were carried out using the plantVillage dataset, which achieved 98% overall accuracy.
Fine-grained identification
First, there is a large difference within the class, that is, the visual characteristics of plant diseases and pests belonging to the same class are quite different. The reason is that the aforementioned external factors such as uneven illumination, dense occlusion, blurred equipment dithering and other interferences, resulting in different image samples belonging to the same kind of diseases and pests differ greatly. Plant diseases and pests detection in complex scenarios is a very challenging task of fine-grained recognition [ 101 ]. The existence of growth variations of diseases and pests results in distinct differences in the characterization of the same diseases and pests at different stages, forming the “intra-class difference” fine-grained characteristics.
Secondly, there is fuzziness between classes, that is, objects of different classes have some similarity. There are many detailed classifications of biological subspecies and subclasses of different kinds of diseases and pests, and there are some similarities of biological morphology and life habits among the subclasses, which lead to the problem of fine-grained identification of “inter-class similarity”. Barbedo believed that similar symptoms could be produced, which even phytopathologists could not correctly distinguish [ 102 ].
Thirdly, background disturbance makes it impossible for plant diseases and pests to appear in a very clean background in the real world. Background can be very complex and interfere with objects of interest, which makes plant diseases and pests detection more difficult. Some literature often ignores this issue because images are captured under controlled conditions [ 103 ].
Relying on the existing deep learning methods can not effectively identify the fine-grained characteristics of diseases and pests that exist naturally in the application of the above actual agricultural scenarios, resulting in technical difficulties such as low identification accuracy and generalization robustness, which has long restricted the performance improvement of decision-making management of diseases and pests by the Intelligent Agricultural Internet of Things [ 104 ]. The existing research is only suitable for fine-grained identification of fewer class of diseases and pests, can not solve the problem of large-scale, large-category, accurate and efficient identification of diseases and pests, and is difficult to deploy directly to the mobile terminals of smart agriculture.
Detection performance under the influence of illumination and occlusion
Lighting problems.
Previous studies have collected images of plant diseases and pests mostly in indoor light boxes [ 105 ]. Although this method can effectively eliminate the influence of external light to simplify image processing, it is quite different from the images collected under real natural light. Because natural light changes very dynamically, and the range in which the camera can accept dynamic light sources is limited, it is easy to cause image color distortion when above or below this limit. In addition, due to the difference of view angle and distance during image collection, the apparent characteristics of plant diseases and pests change greatly, which brings great difficulties to the visual recognition algorithm.
Occlusion problem
At present, most researchers intentionally avoid the recognition of plant diseases and pests in complex environments. They only focus on a single background. They use the method of directly intercepting the area of interest to the collected images, but seldom consider the occlusion problem. As a result, the recognition accuracy under occlusion is low and the practicability is greatly reduced. Occlusion problems are common in real natural environments, including blade occlusion caused by changes in blade posture, branch occlusion, light occlusion caused by external lighting, and mixed occlusion caused by different types of occlusion. The difficulties of plant diseases and pests identification under occlusion are the lack of features and noise overlap caused by occlusion. Different occlusion conditions have different degrees of impact on the recognition algorithm, resulting in false detection or even missed detection. In recent years, with the maturity of deep learning algorithms under restricted conditions, some researchers have gradually challenged the identification of plant diseases and pests under occluded conditions [ 106 , 107 ], and significant progress has been made, which lays a good foundation for the application of plant diseases and pests identification in real-world scenarios. However, occlusion is random and complex. The training of the basic framework is difficult and the dependence on the performance of hardware devices still exists, we should strengthen the innovation and optimization of the basic framework, including the design of lightweight network architecture. The exploration of GAN and other aspects should be enhanced, while ensuring the accuracy of detection, the difficulty of model training should be reduced. GAN has prominent advantages in dealing with posture changes and chaotic background, but its design is not yet mature, and it is easy to crash in learning and cause model uncontrollable problems during training. We should strengthen the exploration of network performance to make it easier to quantify the quality of the model.
Detection speed problem
Compared with traditional methods, deep learning algorithms have better results, but their computational complexity is also higher. If the detection accuracy is guaranteed, the model needs to fully learn the characteristics of the image and increase the computational load, which will inevitably lead to slow detection speed and can not meet the needs of real-time. In order to ensure the detection speed, it is usually necessary to reduce the amount of calculation. However, this will cause insufficient training and result in false or missed detection. Therefore, it is important to design an efficient algorithm with both detection accuracy and detection speed.
Plant diseases and pests detection methods based on deep learning include three main links in agricultural applications: data labeling, model training and model inference. In real-time agricultural applications, more attention is paid to model inference. Currently, most plant diseases and pests detection methods focus on the accuracy of recognition. Little attention is paid to the efficiency of model inference. In reference [ 108 ], to improve the efficiency of the model calculation process to meet the actual agricultural needs, a deep separable convolution structure model for plant leaf disease detection was introduced. Several models were trained and tested. The classification accuracy of Reduced MobileNet was 98.34%, the parameters were 29 times less than VGG, and 6 times less than MobileNet. This shows an effective compromise between delay and accuracy, which is suitable for real-time crop diseases diagnosis on resource-constrained mobile devices.
Conclusions and future directions
Compared with traditional image processing methods, which deal with plant diseases and pests detection tasks in several steps and links, plant diseases and pests detection methods based on deep learning unify them into end-to-end feature extraction, which has a broad development prospects and great potential. Although plant diseases and pests detection technology is developing rapidly, it has been moving from academic research to agricultural application, there is still a certain distance from the mature application in the real natural environment, and there are still some problems to be solved.
Plant diseases and pests detection dataset
Deep learning technology has made some achievements in the identification of plant diseases and pests. Various image recognition algorithms have also been further developed and extended, which provides a theoretical basis for the identification of specific diseases and pests. However, the collection of image samples in previous studies mostly come from the characterization of disease spots, insect appearance characteristics or the characterization of insect pests and leaves. Most of the research results are limited to the laboratory environment and are applicable only to the plant diseases and pests images obtained at the time. The main reason for this is that the growth of plants is cyclical, continuous, seasonal and regional. Similarly, the characteristics of the same disease or pest at different growing stages of crops are different. Images of different plant species vary from region to region. As a result, most of the existing research results are not universal. Even with a high recognition rate in a single trial, the validity of the data obtained at other times cannot be guaranteed.
Most of the existing studies are based on the images generated in the visible range, but the electromagnetic wave outside the visible range also contains a lot of information, so the comprehensive information such as visible light, near infrared, multi-spectral should be fused to achieve the acquisition of plant diseases and pests dataset. Future research should focus on multi-information fusion method to obtain and identify plant diseases and pests information.
In addition, image databases of different kinds of plant diseases and pests in real natural environments are still in the blank stage. Future research should make full use of the data information acquisition platform such as portable field spore auto-capture instrument, unmanned aerial vehicle aerial photography system, agricultural internet of things monitoring equipment, which performs large-area and coverage identification of farmland and makes up for the lack of randomness of image samples in previous studies. Also, it can ensures the comprehensiveness and accuracy of dataset, and improves the generality of the algorithm.
Early recognition of plant diseases and pests
In the application of plant diseases and pests identification, the manifestation symptoms are not obvious, so early diagnosis is very difficult whether it is by visual observation or computer interpretation. However, the research significance and demand of early diagnosis are greater, which is more conducive to the prevention and control of plant diseases and pests and prevent their spread and development. The best image quality can be obtained when the sunlight is sufficient, and taking pictures in cloudy weather will increase the complexity of image preprocessing and reduce the recognition effect. In addition, in the early stage of plant diseases and pests occurrence, even high-resolution images are difficult to analyze. It is necessary to combine meteorological and plant protection data such as temperature and humidity to realize the recognition and prediction of diseases and pests. By consulting the existing research literatures, there are few reports on the early diagnosis of plant diseases and pests.
Network training and learning
When plant diseases and pests are visually identified manually, it is difficult to collect samples of all plant diseases and pests types, and many times only healthy data (positive samples) are available. However, most of the current plant diseases and pests detection methods based on deep learning are supervised learning based on a large number of diseases and pests samples, so manual collection of labelled datasets requires a lot of manpower, so unsupervised learning needs to be explored. Deep learning is a black box, which requires a large number of labelled training samples for end-to-end learning and has poor interpretability. Therefore, how to use the prior knowledge of brain-inspired computing and human-like visual cognitive model to guide the training and learning of the network is also a direction worthy of studying. At the same time, deep models need a large amount of memory and are extremely time-consuming during testing, which makes them unsuitable for deployment on mobile platforms with limited resources. It is important to study how to reduce complexity and obtain fast-executing models without losing accuracy. Finally, the selection of appropriate hyper-parameters has always been a major obstacle to the application of deep learning model to new tasks, such as learning rate, filter size, step size and number, these hyper-parameters have a strong internal dependence, any small adjustment may have a greater impact on the final training results.
Interdisciplinary research
Only by more closely integrating empirical data with theories such as agronomic plant protection, can we establish a field diagnosis model that is more in line with the rules of crop growth, and will further improve the effectiveness and accuracy of plant diseases and pests identification. In the future, it is necessary to go from image analysis at the surface level to identification of the occurrence mechanism of diseases and pests, and transition from simple experimental environment to practical application research that comprehensively considers crop growth law, environmental factors, etc.
In summary, with the development of artificial intelligence technology, the research focus of plant diseases and pests detection based on machine vision has shifted from classical image processing and machine learning methods to deep learning methods, which solved the difficult problems that could not be solved by traditional methods. There is still a long distance from the popularization of practical production and application, but this technology has great development potential and application value. To fully explore the potential of this technology, the joint efforts of experts from relevant disciplines are needed to effectively integrate the experience knowledge of agriculture and plant protection with deep learning algorithms and models, so as to make plant diseases and pests detection based on deep learning mature. Also, the research results should be integrated into agricultural machinery equipment to truly land the corresponding theoretical results.
Availability of data and materials
For relevant data and codes, please contact the corresponding author of this manuscript.
Lee SH, Chan CS, Mayo SJ, Remagnino P. How deep learning extracts and learns leaf features for plant classification. Pattern Recogn. 2017;71:1–13.
Article Google Scholar
Tsaftaris SA, Minervini M, Scharr H. Machine learning for plant phenotyping needs image processing. Trends Plant Sci. 2016;21(12):989–91.
Article CAS PubMed Google Scholar
Fuentes A, Yoon S, Park DS. Deep learning-based techniques for plant diseases recognition in real-field scenarios. In: Advanced concepts for intelligent vision systems. Cham: Springer; 2020.
Google Scholar
Yang D, Li S, Peng Z, Wang P, Wang J, Yang H. MF-CNN: traffic flow prediction using convolutional neural network and multi-features fusion. IEICE Trans Inf Syst. 2019;102(8):1526–36.
Sundararajan SK, Sankaragomathi B, Priya DS. Deep belief cnn feature representation based content based image retrieval for medical images. J Med Syst. 2019;43(6):1–9.
Melnyk P, You Z, Li K. A high-performance CNN method for offline handwritten chinese character recognition and visualization. Soft Comput. 2019;24:7977–87.
Li J, Mi Y, Li G, Ju Z. CNN-based facial expression recognition from annotated rgb-d images for human–robot interaction. Int J Humanoid Robot. 2019;16(04):504–5.
Kumar S, Singh SK. Occluded thermal face recognition using bag of CNN(BoCNN). IEEE Signal Process Lett. 2020;27:975–9.
Wang X. Deep learning in object recognition, detection, and segmentation. Found Trends Signal Process. 2016;8(4):217–382.
Article CAS Google Scholar
Boulent J, Foucher S, Théau J, St-Charles PL. Convolutional neural networks for the automatic identification of plant diseases. Front Plant Sci. 2019;10:941.
Article PubMed PubMed Central Google Scholar
Kumar S, Kaur R. Plant disease detection using image processing—a review. Int J Comput Appl. 2015;124(2):6–9.
Martineau M, Conte D, Raveaux R, Arnault I, Munier D, Venturini G. A survey on image-based insect classification. Pattern Recogn. 2016;65:273–84.
Jayme GAB, Luciano VK, Bernardo HV, Rodrigo VC, Katia LN, Claudia VG, et al. Annotated plant pathology databases for image-based detection and recognition of diseases. IEEE Latin Am Trans. 2018;16(6):1749–57.
Kaur S, Pandey S, Goel S. Plants disease identification and classification through leaf images: a survey. Arch Comput Methods Eng. 2018;26(4):1–24.
CAS Google Scholar
Shekhawat RS, Sinha A. Review of image processing approaches for detecting plant diseases. IET Image Process. 2020;14(8):1427–39.
Hinton GE, Salakhutdinov R. Reducing the dimensionality of data with neural networks. Science. 2006;313(5786):504–7.
Liu W, Wang Z, Liu X, et al. A survey of deep neural network architectures and their applications. Neurocomputing. 2017;234:11–26.
Fergus R. Deep learning methods for vision. CVPR 2012 Tutorial; 2012.
Bengio Y, Courville A, Vincent P. Representation learning: a review and new perspectives. IEEE Trans Pattern Anal Mach Intell. 2013;35(8):1798–828.
Article PubMed Google Scholar
Boureau YL, Le Roux N, Bach F, Ponce J, Lecun Y. [IEEE 2011 IEEE international conference on computer vision (ICCV)—Barcelona, Spain (2011.11.6–2011.11.13)] 2011 international conference on computer vision—ask the locals: multi-way local pooling for image recognition; 2011. p. 2651–8.
Zeiler MD, Fergus R. Stochastic pooling for regularization of deep convolutional neural networks. Eprint Arxiv. arXiv:1301.3557 . 2013.
TensorFlow. https://www.tensorflow.org/ .
Torch/PyTorch. https://pytorch.org/ .
Caffe. http://caffe.berkeleyvision.org/ .
Theano. http://deeplearning.net/software/theano/ .
Krizhenvshky A, Sutskever I, Hinton G. Imagenet classification with deep convolutional networks. In: Proceedings of the conference neural information processing systems (NIPS), Lake Tahoe, NV, USA, 3–8 December; 2012. p. 1097–105.
Szegedy C, Liu W, Jia Y, Sermanet P, Reed S, Anguelov D, Erhan D, Vanhoucke V, Rabinovich A. Going deeper with convolutions. In: Proceedings of the 2015 IEEE conference on computer vision and pattern recognition, Boston, MA, USA, 7–12 June; 2015. p. 1–9.
Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. arXiv. arXiv:1409.1556 . 2014.
Xie S, Girshick R, Dollár P, Tu Z, He K. Aggregated residual transformations for deep neural networks. arXiv. arXiv:1611.05431 . 2017.
Szegedy C, Ioffe S, Vanhoucke V, et al. Inception-v4, inception-resnet and the impact of residual connections on learning. In: Proceedings of the AAAI conference on artificial intelligence. 2016.
Huang G, Lrj Z, Maaten LVD, et al. Densely connected convolutional networks. In: IEEE conference on computer vision and pattern recognition. 2017. p. 2261–9.
Howard AG, Zhu M, Chen B, Kalenichenko D, Wang W, Weyand T, Andreetto M, Adam H. MobileNets: efficient convolutional neural networks for mobile vision applications. arXiv. arXiv:1704.04861 . 2017.
Iandola FN, Han S, Moskewicz MW, Ashraf K, Dally WJ, Keutzer K. SqueezeNet: AlexNet-level accuracy with 50 × fewer parameters and < 0.5 MB model size. arXiv. arXiv:1602.07360 . 2016.
Priyadharshini RA, Arivazhagan S, Arun M, Mirnalini A. Maize leaf disease classification using deep convolutional neural networks. Neural Comput Appl. 2019;31(12):8887–95.
Wen J, Shi Y, Zhou X, Xue Y. Crop disease classification on inadequate low-resolution target images. Sensors. 2020;20(16):4601.
Article PubMed Central Google Scholar
Thangaraj R, Anandamurugan S, Kaliappan VK. Automated tomato leaf disease classification using transfer learning-based deep convolution neural network. J Plant Dis Prot. 2020. https://doi.org/10.1007/s41348-020-00403-0 .
Atila M, Uar M, Akyol K, Uar E. Plant leaf disease classification using efficientnet deep learning model. Ecol Inform. 2021;61:101182.
Sabrol H, Kumar S. Recent studies of image and soft computing techniques for plant disease recognition and classification. Int J Comput Appl. 2015;126(1):44–55.
Yalcin H, Razavi S. Plant classification using convolutional neural networks. In: 2016 5th international conference on agro-geoinformatics (agro-geoinformatics). New York: IEEE; 2016.
Fuentes A, Lee J, Lee Y, Yoon S, Park DS. Anomaly detection of plant diseases and insects using convolutional neural networks. In: ELSEVIER conference ISEM 2017—The International Society for Ecological Modelling Global Conference, 2017. 2017.
Hasan MJ, Mahbub S, Alom MS, Nasim MA. Rice disease identification and classification by integrating support vector machine with deep convolutional neural network. In: 2019 1st international conference on advances in science, engineering and robotics technology (ICASERT). 2019.
Thenmozhi K, Reddy US. Crop pest classification based on deep convolutional neural network and transfer learning. Comput Electron Agric. 2019;164:104906.
Fang T, Chen P, Zhang J, Wang B. Crop leaf disease grade identification based on an improved convolutional neural network. J Electron Imaging. 2020;29(1):1.
Nagasubramanian K, Jones S, Singh AK, Sarkar S, Singh A, Ganapathysubramanian B. Plant disease identification using explainable 3D deep learning on hyperspectral images. Plant Methods. 2019;15(1):1–10.
Picon A, Seitz M, Alvarez-Gila A, Mohnke P, Echazarra J. Crop conditional convolutional neural networks for massive multi-crop plant disease classification over cell phone acquired images taken on real field conditions. Comput Electron Agric. 2019;167:105093.
Tianjiao C, Wei D, Juan Z, Chengjun X, Rujing W, Wancai L, et al. Intelligent identification system of disease and insect pests based on deep learning. China Plant Prot Guide. 2019;039(004):26–34.
Dechant C, Wiesner-Hanks T, Chen S, Stewart EL, Yosinski J, Gore MA, et al. Automated identification of northern leaf blight-infected maize plants from field imagery using deep learning. Phytopathology. 2017;107:1426–32.
Wiesner-Hanks T, Wu H, Stewart E, Dechant C, Nelson RJ. Millimeter-level plant disease detection from aerial photographs via deep learning and crowdsourced data. Front Plant Sci. 2019;10:1550.
Shougang R, Fuwei J, Xingjian G, Peishen Y, Wei X, Huanliang X. Deconvolution-guided tomato leaf disease identification and lesion segmentation model. J Agric Eng. 2020;36(12):186–95.
Fujita E, Kawasaki Y, Uga H, Kagiwada S, Iyatomi H. Basic investigation on a robust and practical plant diagnostic system. In: IEEE international conference on machine learning & applications. New York: IEEE; 2016.
Mohanty SP, Hughes DP, Salathé M. Using deep learning for image-based plant disease detection. Front Plant Sci. 2016;7:1419. https://doi.org/10.3389/fpls.2016.01419 .
Brahimi M, Arsenovic M, Laraba S, Sladojevic S, Boukhalfa K, Moussaoui A. Deep learning for plant diseases: detection and saliency map visualisation. In: Zhou J, Chen F, editors. Human and machine learning. Cham: Springer International Publishing; 2018. p. 93–117.
Chapter Google Scholar
Barbedo JG. Plant disease identification from individual lesions and spots using deep learning. Biosyst Eng. 2019;180:96–107.
Ren S, He K, Girshick R, Sun J. Faster R-CNN: towards real-time object detection with region proposal networks. IEEE Trans Pattern Anal Mach Intell. 2017;39(6):1137–49.
Liu W, Anguelov D, Erhan D, Szegedy C, Berg AC. SSD: Single shot MultiBox detector. In: European conference on computer vision. Cham: Springer International Publishing; 2016.
Redmon J, Divvala S, Girshick R, Farhadi A. You only look once: unified, real-time object detection. In: Proceedings of the IEEE conference on computer vision and pattern recognition. 2015.
Redmon J, Farhadi A. Yolo9000: better, faster, stronger. In: Proceedings of the IEEE conference on computer vision and pattern recognition. 2017. p. 6517–25.
Redmon J, Farhadi A. Yolov3: an incremental improvement. arXiv preprint. arXiv:1804.02767 . 2018.
Fuentes A, Yoon S, Kim SC, Park DS. A robust deep-learning-based detector for real-time tomato plant diseases and pests detection. Sensors. 2017;17(9):2022.
Ozguven MM, Adem K. Automatic detection and classification of leaf spot disease in sugar beet using deep learning algorithms. Phys A Statal Mech Appl. 2019;535(2019):122537.
Zhou G, Zhang W, Chen A, He M, Ma X. Rapid detection of rice disease based on FCM-KM and faster R-CNN fusion. IEEE Access. 2019;7:143190–206. https://doi.org/10.1109/ACCESS.2019.2943454 .
Xie X, Ma Y, Liu B, He J, Wang H. A deep-learning-based real-time detector for grape leaf diseases using improved convolutional neural networks. Front Plant Sci. 2020;11:751.
Singh D, Jain N, Jain P, Kayal P, Kumawat S, Batra N. Plantdoc: a dataset for visual plant disease detection. In: Proceedings of the 7th ACM IKDD CoDS and 25th COMAD. 2019.
Sun J, Yang Y, He X, Wu X. Northern maize leaf blight detection under complex field environment based on deep learning. IEEE Access. 2020;8:33679–88. https://doi.org/10.1109/ACCESS.2020.2973658 .
Bhatt PV, Sarangi S, Pappula S. Detection of diseases and pests on images captured in uncontrolled conditions from tea plantations. In: Proc. SPIE 11008, autonomous air and ground sensing systems for agricultural optimization and phenotyping IV; 2019. p. 1100808. https://doi.org/10.1117/12.2518868 .
Zhang B, Zhang M, Chen Y. Crop pest identification based on spatial pyramid pooling and deep convolution neural network. Trans Chin Soc Agric Eng. 2019;35(19):209–15.
Ramcharan A, McCloskey P, Baranowski K, Mbilinyi N, Mrisho L, Ndalahwa M, Legg J, Hughes D. A mobile-based deep learning model for cassava disease diagnosis. Front Plant Sci. 2019;10:272. https://doi.org/10.3389/fpls.2019.00272 .
Selvaraj G, Vergara A, Ruiz H, Safari N, Elayabalan S, Ocimati W, Blomme G. AI-powered banana diseases and pest detection. Plant Methods. 2019. https://doi.org/10.1186/s13007-019-0475-z .
Tian Y, Yang G, Wang Z, Li E, Liang Z. Detection of apple lesions in orchards based on deep learning methods of CycleGAN and YOLOV3-dense. J Sens. 2019. https://doi.org/10.1155/2019/7630926 .
Zheng Y, Kong J, Jin X, Wang X, Zuo M. CropDeep: the crop vision dataset for deep-learning-based classification and detection in precision agriculture. Sensors. 2019;19:1058. https://doi.org/10.3390/s19051058 .
Arsenovic M, Karanovic M, Sladojevic S, Anderla A, Stefanović D. Solving current limitations of deep learning based approaches for plant disease detection. Symmetry. 2019;11:21. https://doi.org/10.3390/sym11070939 .
Fuentes AF, Yoon S, Lee J, Park DS. High-performance deep neural network-based tomato plant diseases and pests diagnosis system with refinement filter bank. Front Plant Sci. 2018;9:1162. https://doi.org/10.3389/fpls.2018.01162 .
Jiang P, Chen Y, Liu B, He D, Liang C. Real-time detection of apple leaf diseases using deep learning approach based on improved convolutional neural networks. IEEE Access. 2019. https://doi.org/10.1109/ACCESS.2019.2914929 .
Long J, Shelhamer E, Darrell T. Fully convolutional networks for semantic segmentation. IEEE Trans Pattern Anal Mach Intell. 2015;39(4):640–51.
He K, Gkioxari G, Dollár P, Girshick R. Mask R-CNN. In: 2017 IEEE international conference on computer vision (ICCV). New York: IEEE; 2017.
Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation. In: International conference on medical image computing and computer-assisted intervention. Berlin: Springer; 2015. p. 234–41. https://doi.org/10.1007/978-3-319-24574-4_28 .
Badrinarayanan V, Kendall A, Cipolla R. Segnet: a deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans Pattern Anal Mach Intell. 2019;39(12):2481–95.
Wang Z, Zhang S. Segmentation of corn leaf disease based on fully convolution neural network. Acad J Comput Inf Sci. 2018;1:9–18.
Wang X, Wang Z, Zhang S. Segmenting crop disease leaf image by modified fully-convolutional networks. In: Huang DS, Bevilacqua V, Premaratne P, editors. Intelligent computing theories and application. ICIC 2019, vol. 11643. Lecture Notes in Computer Science. Cham: Springer; 2019. https://doi.org/10.1007/978-3-030-26763-6_62 .
Lin K, Gong L, Huang Y, Liu C, Pan J. Deep learning-based segmentation and quantification of cucumber powdery mildew using convolutional neural network. Front Plant Sci. 2019;10:155.
Kerkech M, Hafiane A, Canals R. Vine disease detection in UAV multispectral images using optimized image registration and deep learning segmentation approach. Comput Electron Agric. 2020;174:105446.
Stewart EL, Wiesner-Hanks T, Kaczmar N, Dechant C, Gore MA. Quantitative phenotyping of northern leaf blight in UAV images using deep learning. Remote Sens. 2019;11(19):2209.
Wang Q, Qi F, Sun M, Qu J, Xue J. Identification of tomato disease types and detection of infected areas based on deep convolutional neural networks and object detection techniques. Comput Intell Neurosci. 2019. https://doi.org/10.1155/2019/9142753 .
Hughes DP, Salathe M. An open access repository of images on plant health to enable the development of mobile disease diagnostics through machine learning and crowdsourcing. Comput Sci. 2015.
Shah JP, Prajapati HB, Dabhi VK. A survey on detection and classification of rice plant diseases. In: IEEE international conference on current trends in advanced computing. New York: IEEE; 2016.
Prajapati HB, Shah JP, Dabhi VK. Detection and classification of rice plant diseases. Intell Decis Technol. 2017;11(3):1–17.
Barbedo JGA, Koenigkan LV, Halfeld-Vieira BA, Costa RV, Nechet KL, Godoy CV, Junior ML, Patricio FR, Talamini V, Chitarra LG, Oliveira SAS. Annotated plant pathology databases for image-based detection and recognition of diseases. IEEE Latin Am Trans. 2018;16(6):1749–57.
Brahimi M, Arsenovic M, Laraba S, Sladojevic S, Boukhalfa K, Moussaoui A. Deep learning for plant diseases: detection and saliency map visualisation. In: Zhou J, Chen F, editors. Human and machine learning. Human–computer interaction series. Cham: Springer; 2018. https://doi.org/10.1007/978-3-319-90403-0_6 .
Tyr WH, Stewart EL, Nicholas K, Chad DC, Harvey W, Nelson RJ, et al. Image set for deep learning: field images of maize annotated with disease symptoms. BMC Res Notes. 2018;11(1):440.
Thapa R, Snavely N, Belongie S, Khan A. The plant pathology 2020 challenge dataset to classify foliar disease of apples. arXiv preprint. arXiv:2004.11958 . 2020.
Wu X, Zhan C, Lai YK, Cheng MM, Yang J. IP102: a large-scale benchmark dataset for insect pest recognition. In: 2019 IEEE/CVF conference on computer vision and pattern recognition (CVPR). New York: IEEE; 2019.
Huang M-L, Chuang TC. A database of eight common tomato pest images. Mendeley Data. 2020. https://doi.org/10.17632/s62zm6djd2.1 .
Goodfellow I, Pouget-Abadie J, Mirza M, Xu B, Warde Farley D, Ozair S, Courville A, Bengio Y. Generative adversarial nets. In: Proceedings of the 2014 conference on advances in neural information processing systems 27. Montreal: Curran Associates, Inc.; 2014. p. 2672–80.
Pu Y, Gan Z, Henao R, et al. Variational autoencoder for deep learning of images, labels and captions [EB/OL]. 2016–09–28. arxiv:1609.08976 .
Oppenheim D, Shani G, Erlich O, Tsror L. Using deep learning for image-based potato tuber disease detection. Phytopathology. 2018;109(6):1083–7.
Too EC, Yujian L, Njuki S, Yingchun L. A comparative study of fine-tuning deep learning models for plant disease identification. Comput Electron Agric. 2018;161:272–9.
Chen J, Chen J, Zhang D, Sun Y, Nanehkaran YA. Using deep transfer learning for image-based plant disease identification. Comput Electron Agric. 2020;173:105393.
Zhang S, Huang W, Zhang C. Three-channel convolutional neural networks for vegetable leaf disease recognition. Cogn Syst Res. 2018;53:31–41. https://doi.org/10.1016/j.cogsys.2018.04.006 .
Liu B, Ding Z, Tian L, He D, Li S, Wang H. Grape leaf disease identification using improved deep convolutional neural networks. Front Plant Sci. 2020;11:1082. https://doi.org/10.3389/fpls.2020.01082 .
Karthik R, Hariharan M, Anand S, et al. Attention embedded residual CNN for disease detection in tomato leaves. Appl Soft Comput J. 2020;86:105933.
Guan W, Yu S, Jianxin W. Automatic image-based plant disease severity estimation using deep learning. Comput Intell Neurosci. 2017;2017:2917536.
Barbedo JGA. Factors influencing the use of deep learning for plant disease recognition. Biosyst Eng. 2018;172:84–91.
Barbedo JGA. Impact of dataset size and variety on the effectiveness of deep learning and transfer learning for plant disease classification. Comput Electron Agric. 2018;153:46–53.
Nawaz MA, Khan T, Mudassar R, Kausar M, Ahmad J. Plant disease detection using internet of thing (IOT). Int J Adv Comput Sci Appl. 2020. https://doi.org/10.14569/IJACSA.2020.0110162 .
Martinelli F, Scalenghe R, Davino S, Panno S, Scuderi G, Ruisi P, et al. Advanced methods of plant disease detection. A review. Agron Sustain Dev. 2015;35(1):1–25.
Liu J, Wang X. Early recognition of tomato gray leaf spot disease based on MobileNetv2-YOLOv3 model. Plant Methods. 2020;16:83.
Article CAS PubMed PubMed Central Google Scholar
Liu J, Wang X. Tomato diseases and pests detection based on improved Yolo V3 convolutional neural network. Front Plant Sci. 2020;11:898.
Kamal KC, Yin Z, Wu M, Wu Z. Depthwise separable convolution architectures for plant disease classification. Comput Electron Agric. 2019;165:104948.
Download references
Acknowledgements
Appreciations are given to the editors and reviewer of the Journal Plant Method.
This study was supported by the Facility Horticulture Laboratory of Universities in Shandong with Project Numbers 2019YY003, 2018YY016, 2018YY043 and 2018YY044; school level High-level Talents Project 2018RC002; Youth Fund Project of Philosophy and Social Sciences of Weifang College of Science and Technology with project numbers 2018WKRQZ008 and 2018WKRQZ008-3; Key research and development plan of Shandong Province with Project Number 2019RKA07012, 2019GNC106034 and 2020RKA07036; Research and Development Plan of Applied Technology in Shouguang with Project Number 2018JH12; 2018 innovation fund of Science and Technology Development centre of the China Ministry of Education with Project Number 2018A02013; 2019 basic capacity construction project of private colleges and universities in Shandong Province; and Weifang Science and Technology Development Programme with project numbers 2019GX081 and 2019GX082, Special project of Ideological and political education of Weifang University of science and technology (W19SZ70Z01).
Author information
Authors and affiliations.
Shandong Provincial University Laboratory for Protected Horticulture, Blockchain Laboratory of Agricultural Vegetables, Weifang University of Science and Technology, Weifang, 262700, Shandong, China
Jun Liu & Xuewei Wang
You can also search for this author in PubMed Google Scholar
Contributions
JL designed the research. JL and XW conducted the experiments and data analysis and wrote the manuscript. XW revised the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Correspondence to Xuewei Wang .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, J., Wang, X. Plant diseases and pests detection based on deep learning: a review. Plant Methods 17 , 22 (2021). https://doi.org/10.1186/s13007-021-00722-9
Download citation
Received : 20 September 2020
Accepted : 13 February 2021
Published : 24 February 2021
DOI : https://doi.org/10.1186/s13007-021-00722-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Deep learning
- Plant diseases and pests
- Classification
- Object detection
- Segmentation
Plant Methods
ISSN: 1746-4811
- Submission enquiries: [email protected]
METHODS article
Using deep learning for image-based plant disease detection.

- 1 Digital Epidemiology Lab, EPFL, Geneva, Switzerland
- 2 School of Life Sciences, EPFL, Lausanne, Switzerland
- 3 School of Computer and Communication Sciences, EPFL, Lausanne, Switzerland
- 4 Department of Entomology, College of Agricultural Sciences, Penn State University, State College, PA, USA
- 5 Department of Biology, Eberly College of Sciences, Penn State University, State College, PA, USA
- 6 Center for Infectious Disease Dynamics, Huck Institutes of Life Sciences, Penn State University, State College, PA, USA
Crop diseases are a major threat to food security, but their rapid identification remains difficult in many parts of the world due to the lack of the necessary infrastructure. The combination of increasing global smartphone penetration and recent advances in computer vision made possible by deep learning has paved the way for smartphone-assisted disease diagnosis. Using a public dataset of 54,306 images of diseased and healthy plant leaves collected under controlled conditions, we train a deep convolutional neural network to identify 14 crop species and 26 diseases (or absence thereof). The trained model achieves an accuracy of 99.35% on a held-out test set, demonstrating the feasibility of this approach. Overall, the approach of training deep learning models on increasingly large and publicly available image datasets presents a clear path toward smartphone-assisted crop disease diagnosis on a massive global scale.
Introduction
Modern technologies have given human society the ability to produce enough food to meet the demand of more than 7 billion people. However, food security remains threatened by a number of factors including climate change ( Tai et al., 2014 ), the decline in pollinators ( Report of the Plenary of the Intergovernmental Science-PolicyPlatform on Biodiversity Ecosystem and Services on the work of its fourth session, 2016 ), plant diseases ( Strange and Scott, 2005 ), and others. Plant diseases are not only a threat to food security at the global scale, but can also have disastrous consequences for smallholder farmers whose livelihoods depend on healthy crops. In the developing world, more than 80 percent of the agricultural production is generated by smallholder farmers ( UNEP, 2013 ), and reports of yield loss of more than 50% due to pests and diseases are common ( Harvey et al., 2014 ). Furthermore, the largest fraction of hungry people (50%) live in smallholder farming households ( Sanchez and Swaminathan, 2005 ), making smallholder farmers a group that's particularly vulnerable to pathogen-derived disruptions in food supply.
Various efforts have been developed to prevent crop loss due to diseases. Historical approaches of widespread application of pesticides have in the past decade increasingly been supplemented by integrated pest management (IPM) approaches ( Ehler, 2006 ). Independent of the approach, identifying a disease correctly when it first appears is a crucial step for efficient disease management. Historically, disease identification has been supported by agricultural extension organizations or other institutions, such as local plant clinics. In more recent times, such efforts have additionally been supported by providing information for disease diagnosis online, leveraging the increasing Internet penetration worldwide. Even more recently, tools based on mobile phones have proliferated, taking advantage of the historically unparalleled rapid uptake of mobile phone technology in all parts of the world ( ITU, 2015 ).
Smartphones in particular offer very novel approaches to help identify diseases because of their computing power, high-resolution displays, and extensive built-in sets of accessories, such as advanced HD cameras. It is widely estimated that there will be between 5 and 6 billion smartphones on the globe by 2020. At the end of 2015, already 69% of the world's population had access to mobile broadband coverage, and mobile broadband penetration reached 47% in 2015, a 12-fold increase since 2007 ( ITU, 2015 ). The combined factors of widespread smartphone penetration, HD cameras, and high performance processors in mobile devices lead to a situation where disease diagnosis based on automated image recognition, if technically feasible, can be made available at an unprecedented scale. Here, we demonstrate the technical feasibility using a deep learning approach utilizing 54,306 images of 14 crop species with 26 diseases (or healthy) made openly available through the project PlantVillage ( Hughes and Salathé, 2015 ). An example of each crop—disease pair can be seen in Figure 1 .

Figure 1. Example of leaf images from the PlantVillage dataset, representing every crop-disease pair used. (1) Apple Scab, Venturia inaequalis (2) Apple Black Rot, Botryosphaeria obtusa (3) Apple Cedar Rust, Gymnosporangium juniperi-virginianae (4) Apple healthy (5) Blueberry healthy (6) Cherry healthy (7) Cherry Powdery Mildew, Podoshaera clandestine (8) Corn Gray Leaf Spot, Cercospora zeae-maydis (9) Corn Common Rust, Puccinia sorghi (10) Corn healthy (11) Corn Northern Leaf Blight, Exserohilum turcicum (12) Grape Black Rot, Guignardia bidwellii , (13) Grape Black Measles (Esca), Phaeomoniella aleophilum, Phaeomoniella chlamydospora (14) Grape Healthy (15) Grape Leaf Blight, Pseudocercospora vitis (16) Orange Huanglongbing (Citrus Greening), Candidatus Liberibacter spp. (17) Peach Bacterial Spot, Xanthomonas campestris (18) Peach healthy (19) Bell Pepper Bacterial Spot, Xanthomonas campestris (20) Bell Pepper healthy (21) Potato Early Blight, Alternaria solani (22) Potato healthy (23) Potato Late Blight, Phytophthora infestans (24) Raspberry healthy (25) Soybean healthy (26) Squash Powdery Mildew, Erysiphe cichoracearum (27) Strawberry Healthy (28) Strawberry Leaf Scorch, Diplocarpon earlianum (29) Tomato Bacterial Spot, Xanthomonas campestris pv. vesicatoria (30) Tomato Early Blight, Alternaria solani (31) Tomato Late Blight, Phytophthora infestans (32) Tomato Leaf Mold, Passalora fulva (33) Tomato Septoria Leaf Spot, Septoria lycopersici (34) Tomato Two Spotted Spider Mite, Tetranychus urticae (35) Tomato Target Spot, Corynespora cassiicola (36) Tomato Mosaic Virus (37) Tomato Yellow Leaf Curl Virus (38) Tomato healthy.
Computer vision, and object recognition in particular, has made tremendous advances in the past few years. The PASCAL VOC Challenge ( Everingham et al., 2010 ), and more recently the Large Scale Visual Recognition Challenge (ILSVRC) ( Russakovsky et al., 2015 ) based on the ImageNet dataset ( Deng et al., 2009 ) have been widely used as benchmarks for numerous visualization-related problems in computer vision, including object classification. In 2012, a large, deep convolutional neural network achieved a top-5 error of 16.4% for the classification of images into 1000 possible categories ( Krizhevsky et al., 2012 ). In the following 3 years, various advances in deep convolutional neural networks lowered the error rate to 3.57% ( Krizhevsky et al., 2012 ; Simonyan and Zisserman, 2014 ; Zeiler and Fergus, 2014 ; He et al., 2015 ; Szegedy et al., 2015 ). While training large neural networks can be very time-consuming, the trained models can classify images very quickly, which makes them also suitable for consumer applications on smartphones.
Deep neural networks have recently been successfully applied in many diverse domains as examples of end to end learning. Neural networks provide a mapping between an input—such as an image of a diseased plant—to an output—such as a crop~disease pair. The nodes in a neural network are mathematical functions that take numerical inputs from the incoming edges, and provide a numerical output as an outgoing edge. Deep neural networks are simply mapping the input layer to the output layer over a series of stacked layers of nodes. The challenge is to create a deep network in such a way that both the structure of the network as well as the functions (nodes) and edge weights correctly map the input to the output. Deep neural networks are trained by tuning the network parameters in such a way that the mapping improves during the training process. This process is computationally challenging and has in recent times been improved dramatically by a number of both conceptual and engineering breakthroughs ( LeCun et al., 2015 ; Schmidhuber, 2015 ).
In order to develop accurate image classifiers for the purposes of plant disease diagnosis, we needed a large, verified dataset of images of diseased and healthy plants. Until very recently, such a dataset did not exist, and even smaller datasets were not freely available. To address this problem, the PlantVillage project has begun collecting tens of thousands of images of healthy and diseased crop plants ( Hughes and Salathé, 2015 ), and has made them openly and freely available. Here, we report on the classification of 26 diseases in 14 crop species using 54,306 images with a convolutional neural network approach. We measure the performance of our models based on their ability to predict the correct crop-diseases pair, given 38 possible classes. The best performing model achieves a mean F 1 score of 0.9934 (overall accuracy of 99.35%), hence demonstrating the technical feasibility of our approach. Our results are a first step toward a smartphone-assisted plant disease diagnosis system.
Dataset Description
We analyze 54,306 images of plant leaves, which have a spread of 38 class labels assigned to them. Each class label is a crop-disease pair, and we make an attempt to predict the crop-disease pair given just the image of the plant leaf. Figure 1 shows one example each from every crop-disease pair from the PlantVillage dataset. In all the approaches described in this paper, we resize the images to 256 × 256 pixels, and we perform both the model optimization and predictions on these downscaled images.
Across all our experiments, we use three different versions of the whole PlantVillage dataset. We start with the PlantVillage dataset as it is, in color; then we experiment with a gray-scaled version of the PlantVillage dataset, and finally we run all the experiments on a version of the PlantVillage dataset where the leaves were segmented, hence removing all the extra background information which might have the potential to introduce some inherent bias in the dataset due to the regularized process of data collection in case of PlantVillage dataset. Segmentation was automated by the means of a script tuned to perform well on our particular dataset. We chose a technique based on a set of masks generated by analysis of the color, lightness and saturation components of different parts of the images in several color spaces (Lab and HSB). One of the steps of that processing also allowed us to easily fix color casts, which happened to be very strong in some of the subsets of the dataset, thus removing another potential bias.
This set of experiments was designed to understand if the neural network actually learns the “notion” of plant diseases, or if it is just learning the inherent biases in the dataset. Figure 2 shows the different versions of the same leaf for a randomly selected set of leaves.
Figure 2. Sample images from the three different versions of the PlantVillage dataset used in various experimental configurations. (A) Leaf 1 color, (B) Leaf 1 grayscale, (C) Leaf 1 segmented, (D) Leaf 2 color, (E) Leaf 2 gray-scale, (F) Leaf 2 segmented.
Measurement of Performance
To get a sense of how our approaches will perform on new unseen data, and also to keep a track of if any of our approaches are overfitting, we run all our experiments across a whole range of train-test set splits, namely 80–20 (80% of the whole dataset used for training, and 20% for testing), 60–40 (60% of the whole dataset used for training, and 40% for testing), 50–50 (50% of the whole dataset used for training, and 50% for testing), 40–60 (40% of the whole dataset used for training, and 60% for testing) and finally 20–80 (20% of the whole dataset used for training, and 80% for testing). It must be noted that in many cases, the PlantVillage dataset has multiple images of the same leaf (taken from different orientations), and we have the mappings of such cases for 41,112 images out of the 54,306 images; and during all these test-train splits, we make sure all the images of the same leaf goes either in the training set or the testing set. Further, for every experiment, we compute the mean precision, mean recall, mean F 1 score, along with the overall accuracy over the whole period of training at regular intervals (at the end of every epoch). We use the final mean F 1 score for the comparison of results across all of the different experimental configurations.
We evaluate the applicability of deep convolutional neural networks for the classification problem described above. We focus on two popular architectures, namely AlexNet ( Krizhevsky et al., 2012 ), and GoogLeNet ( Szegedy et al., 2015 ), which were designed in the context of the “Large Scale Visual Recognition Challenge” (ILSVRC) ( Russakovsky et al., 2015 ) for the ImageNet dataset ( Deng et al., 2009 ).
The AlexNet architecture (see Figure S2) follows the same design pattern as the LeNet-5 ( LeCun et al., 1989 ) architecture from the 1990s. The LeNet-5 architecture variants are usually a set of stacked convolution layers followed by one or more fully connected layers. The convolution layers optionally may have a normalization layer and a pooling layer right after them, and all the layers in the network usually have ReLu non-linear activation units associated with them. AlexNet consists of 5 convolution layers, followed by 3 fully connected layers, and finally ending with a softMax layer. The first two convolution layers (conv{1, 2}) are each followed by a normalization and a pooling layer, and the last convolution layer (conv5) is followed by a single pooling layer. The final fully connected layer (fc8) has 38 outputs in our adapted version of AlexNet (equaling the total number of classes in our dataset), which feeds the softMax layer. The softMax layer finally exponentially normalizes the input that it gets from (fc8), thereby producing a distribution of values across the 38 classes that add up to 1. These values can be interpreted as the confidences of the network that a given input image is represented by the corresponding classes. All of the first 7 layers of AlexNet have a ReLu non-linearity activation unit associated with them, and the first two fully connected layers (fc{6, 7}) have a dropout layer associated with them, with a dropout ratio of 0.5.
The GoogleNet architecture on the other hand is a much deeper and wider architecture with 22 layers, while still having considerably lower number of parameters (5 million parameters) in the network than AlexNet (60 million parameters). An application of the “network in network” architecture ( Lin et al., 2013 ) in the form of the inception modules is a key feature of the GoogleNet architecture. The inception module uses parallel 1 × 1, 3 × 3, and 5 × 5 convolutions along with a max-pooling layer in parallel, hence enabling it to capture a variety of features in parallel. In terms of practicality of the implementation, the amount of associated computation needs to be kept in check, which is why 1 × 1 convolutions before the above mentioned 3 × 3, 5 × 5 convolutions (and also after the max-pooling layer) are added for dimensionality reduction. Finally, a filter concatenation layer simply concatenates the outputs of all these parallel layers. While this forms a single inception module, a total of 9 inception modules is used in the version of the GoogLeNet architecture that we use in our experiments. A more detailed overview of this architecture can be found for reference in ( Szegedy et al., 2015 ).
We analyze the performance of both these architectures on the PlantVillage dataset by training the model from scratch in one case, and then by adapting already trained models (trained on the ImageNet dataset) using transfer learning. In case of transfer learning, we re-initialize the weights of layer fc8 in case of AlexNet, and of the loss {1,2,3}/classifier layers in case of GoogLeNet. Then, when training the model, we do not limit the learning of any of the layers, as is sometimes done for transfer learning. In other words, the key difference between these two learning approaches (transfer vs. training from scratch) is in the initial state of weights of a few layers, which lets the transfer learning approach exploit the large amount of visual knowledge already learned by the pre-trained AlexNet and GoogleNet models extracted from ImageNet ( Russakovsky et al., 2015 ).
To summarize, we have a total of 60 experimental configurations, which vary on the following parameters:
1. Choice of deep learning architecture:
2. Choice of training mechanism:
Transfer Learning,
Training from Scratch.
3. Choice of dataset type:
Gray scale,
Leaf Segmented.
4. Choice of training-testing set distribution:
Train: 80%, Test: 20%,
Train: 60%, Test: 40%,
Train: 50%, Test: 50%,
Train: 40%, Test: 60%,
Train: 20%, Test: 80%.
Throughout this paper, we have used the notation of Architecture:TrainingMechanism:DatasetType:Train-Test-Set-Distribution to refer to particular experiments. For instance, to refer to the experiment using the GoogLeNet architecture, which was trained using transfer learning on the gray-scaled PlantVillage dataset on a train—test set distribution of 60–40, we will use the notation GoogLeNet:TransferLearning:GrayScale:60–40 .
Each of these 60 experiments runs for a total of 30 epochs, where one epoch is defined as the number of training iterations in which the particular neural network has completed a full pass of the whole training set. The choice of 30 epochs was made based on the empirical observation that in all of these experiments, the learning always converged well within 30 epochs (as is evident from the aggregated plots (Figure 3 ) across all the experiments).
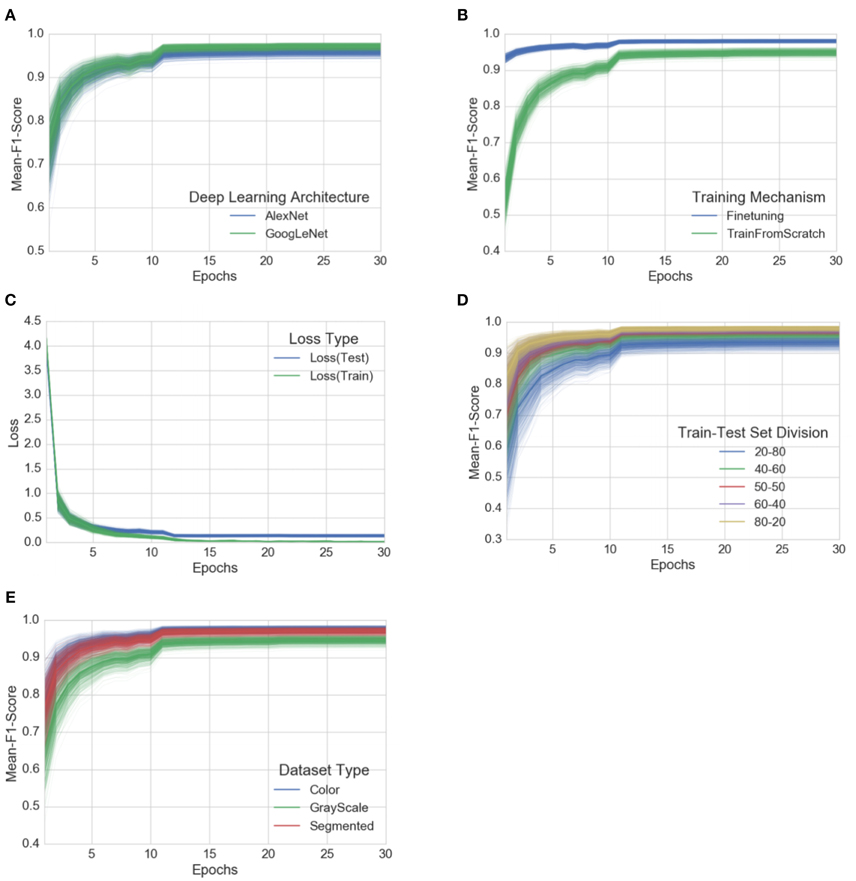
Figure 3. Progression of mean F 1 score and loss through the training period of 30 epochs across all experiments, grouped by experimental configuration parameters . The intensity of a particular class at any point is proportional to the corresponding uncertainty across all experiments with the particular configurations. (A) Comparison of progression of mean F 1 score across all experiments, grouped by deep learning architecture, (B) Comparison of progression of mean F 1 score across all experiments, grouped by training mechanism, (C) Comparison of progression of train-loss and test-loss across all experiments, (D) Comparison of progression of mean F 1 score across all experiments, grouped by train-test set splits, (E) Comparison of progression of mean F 1 score across all experiments, grouped by dataset type. A similar plot of all the observations, as it is, across all the experimental configurations can be found in the Supplementary Material.
To enable a fair comparison between the results of all the experimental configurations, we also tried to standardize the hyper-parameters across all the experiments, and we used the following hyper-parameters in all of the experiments:
• Solver type: Stochastic Gradient Descent,
• Base learning rate: 0.005,
• Learning rate policy: Step (decreases by a factor of 10 every 30/3 epochs),
• Momentum: 0.9,
• Weight decay: 0.0005,
• Gamma: 0.1,
• Batch size: 24 (in case of GoogLeNet), 100 (in case of AlexNet).
All the above experiments were conducted using our own fork of Caffe ( Jia et al., 2014 ), which is a fast, open source framework for deep learning. The basic results, such as the overall accuracy can also be replicated using a standard instance of caffe.
At the outset, we note that on a dataset with 38 class labels, random guessing will only achieve an overall accuracy of 2.63% on average. Across all our experimental configurations, which include three visual representations of the image data (see Figure 2 ), the overall accuracy we obtained on the PlantVillage dataset varied from 85.53% (in case of AlexNet::TrainingFromScratch::GrayScale::80–20 ) to 99.34% (in case of GoogLeNet::TransferLearning::Color::80–20 ), hence showing strong promise of the deep learning approach for similar prediction problems. Table 1 shows the mean F 1 score, mean precision, mean recall, and overall accuracy across all our experimental configurations. All the experimental configurations run for a total of 30 epochs each, and they almost consistently converge after the first step down in the learning rate.
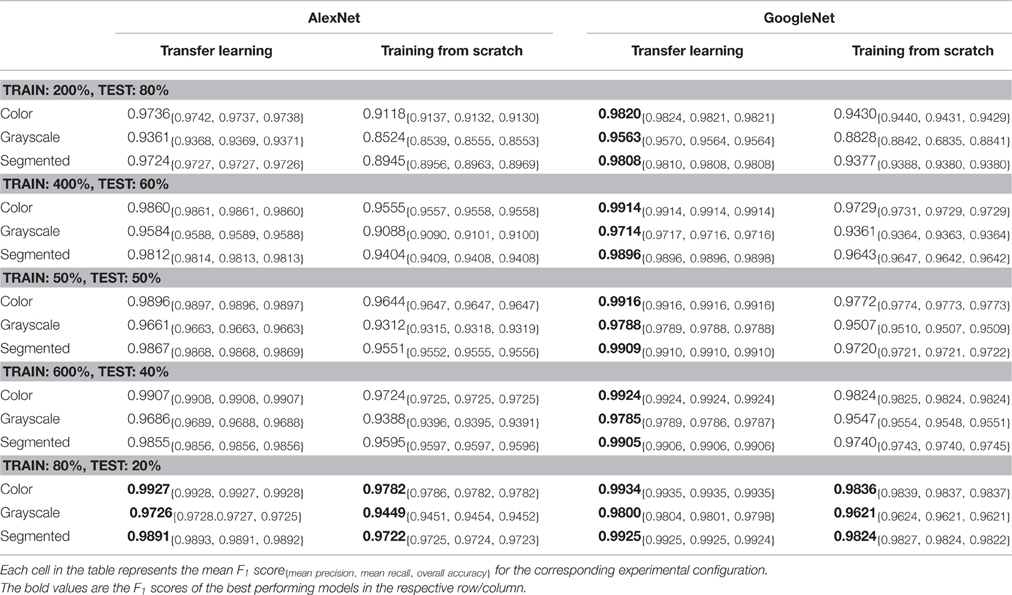
Table 1. Mean F 1 score across various experimental configurations at the end of 30 epochs .
To address the issue of over-fitting, we vary the test set to train set ratio and observe that even in the extreme case of training on only 20% of the data and testing the trained model on the rest 80% of the data, the model achieves an overall accuracy of 98.21% (mean F 1 score of 0.9820) in the case of GoogLeNet::TransferLearning::Color::20–80 . As expected, the overall performance of both AlexNet and GoogLeNet do degrade if we keep increasing the test set to train set ratio (see Figure 3D ), but the decrease in performance is not as drastic as we would expect if the model was indeed over-fitting. Figure 3C also shows that there is no divergence between the validation loss and the training loss, confirming that over-fitting is not a contributor to the results we obtain across all our experiments.
Among the AlexNet and GoogLeNet architectures, GoogLeNet consistently performs better than AlexNet (Figure 3A ), and based on the method of training, transfer learning always yields better results (Figure 3B ), both of which were expected.
The three versions of the dataset (color, gray-scale, and segmented) show a characteristic variation in performance across all the experiments when we keep the rest of the experimental configuration constant. The models perform the best in case of the colored version of the dataset. When designing the experiments, we were concerned that the neural networks might only learn to pick up the inherent biases associated with the lighting conditions, the method and apparatus of collection of the data. We therefore experimented with the gray-scaled version of the same dataset to test the model's adaptability in the absence of color information, and its ability to learn higher level structural patterns typical to particular crops and diseases. As expected, the performance did decrease when compared to the experiments on the colored version of the dataset, but even in the case of the worst performance, the observed mean F 1 score was 0.8524 (overall accuracy of 85.53%). The segmented versions of the whole dataset was also prepared to investigate the role of the background of the images in overall performance, and as shown in Figure 3E , the performance of the model using segmented images is consistently better than that of the model using gray-scaled images, but slightly lower than that of the model using the colored version of the images.
While these approaches yield excellent results on the PlantVillage dataset which was collected in a controlled environment, we also assessed the model's performance on images sampled from trusted online sources, such as academic agriculture extension services. Such images are not available in large numbers, and using a combination of automated download from Bing Image Search and IPM Images with a visual verification step, we obtained two small, verified datasets of 121 (dataset 1) and 119 images (dataset 2), respectively (see Supplementary Material for a detailed description of the process). Using the best model on these datasets, we obtained an overall accuracy of 31.40% in dataset 1, and 31.69% in dataset 2, in successfully predicting the correct class label (i.e., crop and disease information) from among 38 possible class labels. We note that a random classifier will obtain an average accuracy of only 2.63%. Across all images, the correct class was in the top-5 predictions in 52.89% of the cases in dataset 1, and in 65.61% of the cases in dataset 2. The best models for the two datasets were GoogLeNet:Segmented:TransferLearning:80–20 for dataset 1, and GoogLeNet:Color:TransferLearning:80–20 for dataset 2. An example image from theses datasets, along with its visualization of activations in the initial layers of an AlexNet architecture, can be seen in Figure 4 .
Figure 4. Visualization of activations in the initial layers of an AlexNet architecture demonstrating that the model has learnt to efficiently activate against the diseased spots on the example leaf. (A) Example image of a leaf suffering from Apple Cedar Rust, selected from the top-20 images returned by Bing Image search for the keywords “Apple Cedar Rust Leaves” on April 4th, 2016. Image Reference: Clemson University - USDA Cooperative Extension Slide Series, Bugwood. org. (B) Visualization of activations in the first convolution layer(conv1) of an AlexNet architecture trained using AlexNet:Color:TrainFromScratch:80–20 when doing a forward pass on the image in shown in panel b.
So far, all results have been reported under the assumption that the model needs to detect both the crop species and the disease status. We can limit the challenge to a more realistic scenario where the crop species is provided, as it can be expected to be known by those growing the crops. To assess this the performance of the model under this scenario, we limit ourselves to crops where we have at least n > = 2 (to avoid trivial classification) or n > = 3 classes per crop. In the n > = 2 case, dataset 1 contains 33 classes distributed among 9 crops. Random guessing in such a dataset would achieve an accuracy of 0.225, while our model has an accuracy of 0.478. In the n > = 3 case, the dataset contains 25 classes distributed among 5 crops. Random guessing in such a dataset would achieve an accuracy of 0.179, while our model has an accuracy of 0.411.
Similarly, in the n > = 2 case, dataset 2 contains 13 classes distributed among 4 crops. Random guessing in such a dataset would achieve an accuracy of 0.314, while our model has an accuracy of 0.545. In the n > = 3 case, the dataset contains 11 classes distributed among 3 crops. Random guessing in such a dataset would achieve an accuracy of 0.288, while our model has an accuracy of 0.485.
The performance of convolutional neural networks in object recognition and image classification has made tremendous progress in the past few years. ( Krizhevsky et al., 2012 ; Simonyan and Zisserman, 2014 ; Zeiler and Fergus, 2014 ; He et al., 2015 ; Szegedy et al., 2015 ). Previously, the traditional approach for image classification tasks has been based on hand-engineered features, such as SIFT ( Lowe, 2004 ), HoG ( Dalal and Triggs, 2005 ), SURF ( Bay et al., 2008 ), etc., and then to use some form of learning algorithm in these feature spaces. The performance of these approaches thus depended heavily on the underlying predefined features. Feature engineering itself is a complex and tedious process which needs to be revisited every time the problem at hand or the associated dataset changes considerably. This problem occurs in all traditional attempts to detect plant diseases using computer vision as they lean heavily on hand-engineered features, image enhancement techniques, and a host of other complex and labor-intensive methodologies.
In addition, traditional approaches to disease classification via machine learning typically focus on a small number of classes usually within a single crop. Examples include a feature extraction and classification pipeline using thermal and stereo images in order to classify tomato powdery mildew against healthy tomato leaves ( Raza et al., 2015 ); the detection of powdery mildew in uncontrolled environments using RGB images ( Hernández-Rabadán et al., 2014 ); the use of RGBD images for detection of apple scab ( Chéné et al., 2012 ) the use of fluorescence imaging spectroscopy for detection of citrus huanglongbing ( Wetterich et al., 2012 ) the detection of citrus huanglongbing using near infrared spectral patterns ( Sankaran et al., 2011 ) and aircraft-based sensors ( Garcia-Ruiz et al., 2013 ) the detection of tomato yellow leaf curl virus by using a set of classic feature extraction steps, followed by classification using a support vector machines pipeline ( Mokhtar et al., 2015 ), and many others. A very recent review on the use of machine learning on plant phenotyping ( Singh et al., 2015 ) extensively discusses the work in this domain. While neural networks have been used before in plant disease identification ( Huang, 2007 ) (for the classification and detection of Phalaenopsis seedling disease like bacterial soft rot, bacterial brown spot, and Phytophthora black rot), the approach required representing the images using a carefully selected list of texture features before the neural network could classify them.
Our approach is based on recent work Krizhevsky et al. (2012) which showed for the first time that end-to-end supervised training using a deep convolutional neural network architecture is a practical possibility even for image classification problems with a very large number of classes, beating the traditional approaches using hand-engineered features by a substantial margin in standard benchmarks. The absence of the labor-intensive phase of feature engineering and the generalizability of the solution makes them a very promising candidate for a practical and scaleable approach for computational inference of plant diseases.
Using the deep convolutional neural network architecture, we trained a model on images of plant leaves with the goal of classifying both crop species and the presence and identity of disease on images that the model had not seen before. Within the PlantVillage data set of 54,306 images containing 38 classes of 14 crop species and 26 diseases (or absence thereof), this goal has been achieved as demonstrated by the top accuracy of 99.35%. Thus, without any feature engineering, the model correctly classifies crop and disease from 38 possible classes in 993 out of 1000 images. Importantly, while the training of the model takes a lot of time (multiple hours on a high performance GPU cluster computer), the classification itself is very fast (less than a second on a CPU), and can thus easily be implemented on a smartphone. This presents a clear path toward smartphone-assisted crop disease diagnosis on a massive global scale.
However, there are a number of limitations at the current stage that need to be addressed in future work. First, when tested on a set of images taken under conditions different from the images used for training, the model's accuracy is reduced substantially, to just above 31%. It's important to note that this accuracy is much higher than the one based on random selection of 38 classes (2.6%), but nevertheless, a more diverse set of training data is needed to improve the accuracy. Our current results indicate that more (and more variable) data alone will be sufficient to substantially increase the accuracy, and corresponding data collection efforts are underway.
The second limitation is that we are currently constrained to the classification of single leaves, facing up, on a homogeneous background. While these are straightforward conditions, a real world application should be able to classify images of a disease as it presents itself directly on the plant. Indeed, many diseases don't present themselves on the upper side of leaves only (or at all), but on many different parts of the plant. Thus, new image collection efforts should try to obtain images from many different perspectives, and ideally from settings that are as realistic as possible.
At the same time, by using 38 classes that contain both crop species and disease status, we have made the challenge harder than ultimately necessary from a practical perspective, as growers are expected to know which crops they are growing. Given the very high accuracy on the PlantVillage dataset, limiting the classification challenge to the disease status won't have a measurable effect. However, on the real world datasets, we can measure noticeable improvements in accuracy. Overall, the presented approach works reasonably well with many different crop species and diseases, and is expected to improve considerably with more training data.
Finally, it's worth noting that the approach presented here is not intended to replace existing solutions for disease diagnosis, but rather to supplement them. Laboratory tests are ultimately always more reliable than diagnoses based on visual symptoms alone, and oftentimes early-stage diagnosis via visual inspection alone is challenging. Nevertheless, given the expectation of more than 5 Billion smartphones in the world by 2020—of which almost a Billion in Africa ( GSMA Intelligence, 2016 )—we do believe that the approach represents a viable additional method to help prevent yield loss. What's more, in the future, image data from a smartphone may be supplemented with location and time information for additional improvements in accuracy. Last but not least, it would be prudent to keep in mind the stunning pace at which mobile technology has developed in the past few years, and will continue to do so. With ever improving number and quality of sensors on mobiles devices, we consider it likely that highly accurate diagnoses via the smartphone are only a question of time.
Author Contributions
MS, DH, and SM conceived the study and wrote the paper. SM implemented the algorithm described.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Boris Conforty for help with the segmentation. We thank Kelsee Baranowski, Ryan Bringenberg, and Megan Wilkerson for taking the images and Kelsee Baranowski for image curation. We thank Anna Sostarecz, Kaity Gonzalez, Ashtyn Goodreau, Kalley Veit, Ethan Keller, Parand Jalili, Emma Volk, Nooeree Samdani, Kelsey Pryze for additional help with image curation. We thank EPFL, and the Huck Institutes at Penn State University for support. We are particularly grateful for access to EPFL GPU cluster computing resources.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01419
The data and the code used in this paper are available at the following locations:
Data: https://github.com/salathegroup/plantvillage_deeplearning_paper_dataset
Code: https://github.com/salathegroup/plantvillage_deeplearning_paper_analysis
More image data can be found at https://www.plantvillage.org/en/plant_images
Bay, H., Ess, A., Tuytelaars, T., and Van Gool, L. (2008). Speeded-up robust features (surf). Comput. Vis. Image Underst. 110, 346–359. doi: 10.1016/j.cviu.2007.09.014
CrossRef Full Text | Google Scholar
Chéné, Y., Rousseau, D., Lucidarme, P., Bertheloot, J., Caffier, V., Morel, P., et al. (2012). On the use of depth camera for 3d phenotyping of entire plants. Comput. Electron. Agric. 82, 122–127. doi: 10.1016/j.compag.2011.12.007
Dalal, N., and Triggs, B. (2005). “Histograms of oriented gradients for human detection,” in Computer Vision and Pattern Recognition, 2005. CVPR 2005. IEEE Computer Society Conference on. (IEEE) (Washington, DC).
Deng, J., Dong, W., Socher, R., Li, L.-J., Li, K., and Fei-Fei L. (2009). “Imagenet: A large-scale hierarchical image database,” in Computer Vision and Pattern Recognition, 2009. CVPR 2009. IEEE Conference on. (IEEE).
Google Scholar
Ehler, L. E. (2006). Integrated pest management (ipm): definition, historical development and implementation, and the other ipm. Pest Manag. Sci. 62, 787–789. doi: 10.1002/ps.1247
PubMed Abstract | CrossRef Full Text | Google Scholar
Everingham, M., Van Gool, L., Williams, C. K., Winn, J., and Zisserman, A. (2010). The pascal visual object classes (voc) challenge. Int. J. Comput. Vis. 88, 303–338. doi: 10.1007/s11263-009-0275-4
Garcia-Ruiz, F., Sankaran, S., Maja, J. M., Lee, W. S., Rasmussen, J., and Ehsani R. (2013). Comparison of two aerial imaging platforms for identification of huanglongbing-infected citrus trees. Comput. Electron. Agric. 91, 106–115. doi: 10.1016/j.compag.2012.12.002
GSMA Intelligence (2016). The Mobile Economy- Africa 2016 . London: GSMA.
Harvey, C. A., Rakotobe, Z. L., Rao, N. S., Dave, R., Razafimahatratra, H., Rabarijohn, R. H., et al. (2014). Extreme vulnerability of smallholder farmers to agricultural risks and climate change in madagascar. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130089. doi: 10.1098/rstb.2013.008
He, K., Zhang, X., Ren, S., and Sun, J. (2015). Deep residual learning for image recognition. arXiv:1512.03385.
PubMed Abstract | Google Scholar
Hernández-Rabadán, D. L., Ramos-Quintana, F., and Guerrero Juk, J. (2014). Integrating soms and a bayesian classifier for segmenting diseased plants in uncontrolled environments. Sci. World J. 2014:214674. doi: 10.1155/2014/214674
Huang, K. Y. (2007). Application of artificial neural network for detecting phalaenopsis seedling diseases using color and texture features. Comput. Electron. Agric. 57, 3–11. doi: 10.1016/j.compag.2007.01.015
Hughes, D. P., and Salathé, M. (2015). An open access repository of images on plant health to enable the development of mobile disease diagnostics. arXiv:1511.08060
ITU (2015). ICT Facts and Figures – the World in 2015. Geneva: International Telecommunication Union.
Jia, Y., Shelhamer, E., Donahue, J., Karayev, S., Long, J., Girshick, R., et al. (2014). Caffe: Convolutional architecture for fast feature embedding. arXiv:1408.5093.
Krizhevsky, A., Sutskever, I., and Hinton, G. E. (2012). “Imagenet classification with deep convolutional neural networks,” in Advances in Neural Information Processing Systems , eds F. Pereira, C. J. C. Burges, L. Bottou, and K. Q. Weinberger (Curran Associates, Inc.), 1097–1105.
LeCun, Y., Boser, B., Denker, J. S., Henderson, D., Howard, R. E., Hubbard, W., et al. (1989). Backpropagation applied to handwritten zip code recognition. Neural Comput. 1, 541–551. doi: 10.1162/neco.1989.1.4.541
LeCun, Y., Bengio, Y., and Hinton, G. (2015). Deep learning. Nature 521, 436–444. doi: 10.1038/nature14539
Lin, M., Chen, Q., and Yan, S. (2013). Network in network. arXiv:1312.4400.
Lowe, D. G. (2004). Distinctive image features from scale-invariant keypoints. Int. J. Comput. Vis. 60, 91–110. doi: 10.1023/B:VISI.0000029664.99615.94
Mokhtar, U., Ali, M. A., Hassanien, A. E., and Hefny, H. (2015). “Identifying two of tomatoes leaf viruses using support vector machine,” in Information Systems Design and Intelligent Applications , eds J. K. Mandal, S. C. Satapathy, M. K. Sanyal, P. P. Sarkar, A. Mukhopadhyay (Springer), 771–782.
Raza, S.-A., Prince, G., Clarkson, J. P., Rajpoot, N. M., et al. (2015). Automatic detection of diseased tomato plants using thermal and stereo visible light images. PLoS ONE 10:e0123262. doi: 10.1371/journal.pone.0123262. Available online at: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0123262
Report of the Plenary of the Intergovernmental Science-PolicyPlatform on Biodiversity Ecosystem Services on the work of its fourth session (2016). Plenary of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services Fourth session . Kuala Lumpur. Available online at: http://www.ipbes.net/sites/default/files/downloads/pdf/IPBES-4-4-19-Amended-Advance.pdf
Russakovsky, O., Deng, J., Su, H., Krause, J., Satheesh, S., Ma, S., et al. (2015). ImageNet large scale visual recognition challenge. Int. J. Comput. Vis. 115, 211–252. doi: 10.1007/s11263-015-0816-y
Sanchez, P. A., and Swaminathan, M. S. (2005). Cutting world hunger in half. Science 307, 357–359. doi: 10.1126/science.1109057
Sankaran, S., Mishra, A., Maja, J. M., and Ehsani, R. (2011). Visible-near infrared spectroscopy for detection of huanglongbing in citrus orchards. Comput. Electron. Agric. 77, 127–134. doi: 10.1016/j.compag.2011.03.004
Schmidhuber, J. (2015). Deep learning in neural networks: an overview. Neural Netw. 61, 85–117. doi: 10.1016/j.neunet.2014.09.003
Simonyan, K., and Zisserman, A. (2014). Very deep convolutional networks for large-scale image recognition. arXiv:1409.1556.
Singh, A., Ganapathysubramanian, B., Singh, A. K., and Sarkar, S. (2015). Machine learning for highthroughput stress phenotyping in plants. Trends Plant Sci. 21, 110–124 doi: 10.1016/j.tplants.2015.10.015
PubMed Abstract | CrossRef Full Text
Strange, R. N., and Scott, P. R. (2005). Plant disease: a threat to global food security. Phytopathology 43, 83–116. doi: 10.1146/annurev.phyto.43.113004.133839
Szegedy, C., Liu, W., Jia, Y., Sermanet, P., Reed, S., Anguelov, D., et al. (2015). “Going deeper with convolutions,” in Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition .
Tai, A. P., Martin, M. V., and Heald, C. L. (2014). Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang 4, 817–821. doi: 10.1038/nclimate2317
UNEP (2013). Smallholders, Food Security, and the Environment . Rome : International Fund for Agricultural Development (IFAD). Available online at: https://www.ifad.org/documents/10180/666cac2414b643c2876d9c2d1f01d5dd
Wetterich, C. B., Kumar, R., Sankaran, S., Junior, J. B., Ehsani, R., and Marcassa, L. G. (2012). A comparative study on application of computer vision and fluorescence imaging spectroscopy for detection of huanglongbing citrus disease in the usa and brazil. J. Spectrosc. 2013:841738. doi: 10.1155/2013/841738
Zeiler, M. D., and Fergus, R. (2014). “Visualizing and understanding convolutional networks,” in Computer Vision–ECCV 2014 , eds D. Fleet, T. Pajdla, B. Schiele, and T. Tuytelaars (Springer), 818–833.
Keywords: crop diseases, machine learning, deep learning, digital epidemiology
Citation: Mohanty SP, Hughes DP and Salathé M (2016) Using Deep Learning for Image-Based Plant Disease Detection. Front. Plant Sci. 7:1419. doi: 10.3389/fpls.2016.01419
Received: 19 June 2016; Accepted: 06 September 2016; Published: 22 September 2016.
Reviewed by:
Copyright © 2016 Mohanty, Hughes and Salathé. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcel Salathé, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents

Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Acta Naturae
- v.12(3); Jul-Sep 2020
Infectious Plant Diseases: Etiology, Current Status, Problems and Prospects in Plant Protection
P. a. nazarov.
Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119991 Russia
Moscow Institute of Physics and Technology, Dolgoprudny, Moscow region, 141701 Russia
Federal Scientific Vegetable Center, VNIISSOK, Moscow region, 143080 Russia
D. N. Baleev
All-Russian Scientific Research Institute of Medicinal and Aromatic Plants, Moscow, 117216 Russia
M. I. Ivanova
All-Russian Scientific Research Institute of Vegetable Growing, Branch of the Federal Scientific Vegetable Center, Vereya, Moscow region, 140153 Russia
L. M. Sokolova
M. v. karakozova.
Center of Life Sciences, Skolkovo Institute of Science and Technology, Moscow, 121205 Russia
In recent years, there has been an increase in the number of diseases caused by bacterial, fungal, and viral infections. Infections affect plants at different stages of agricultural production. Depending on weather conditions and the phytosanitary condition of crops, the prevalence of diseases can reach 70–80% of the total plant population, and the yield can decrease in some cases down to 80–98%. Plants have innate cellular immunity, but specific phytopathogens have an ability to evade that immunity. This article examined phytopathogens of viral, fungal, and bacterial nature and explored the concepts of modern plant protection, methods of chemical, biological, and agrotechnical control, as well as modern methods used for identifying phytopathogens.
INTRODUCTION
A plant is considered to be susceptible to infection if environmental factors alter its physiological processes thus resulting in a disrupted structure, growth, functions, or other parameters. Plant diseases are classified as infectious and non-infectious depending on the nature of a causative agent. The symptoms of the disease may depend on its cause, nature, and the location of the impact site. The factors causing plant diseases can be of biotic and abiotic nature. Non-infectious diseases are caused by unfavorable growth conditions; they are not transmitted from a diseased plant to a healthy one. Infectious diseases, on the contrary, can spread from one susceptible host to another, since the infectious agent can reproduce in the plant or on its surface.
The signs of plant diseases include wilting, spotting (necrosis), mold, pustules, rot, hypertrophy and hyperplasia (overgrowth), deformation, mummification, discoloration, and destruction of the affected tissue. Wilting results from the loss of turgor pressure in the cells and tissues. It is caused by both abiotic and biotic factors. Spotting is mostly associated with the partial death of plant tissues due to biotic factors. Mold and pustules occur as a result of fungal damage to a plant. Rot leads to both the death of intracellular contents (bacterial wet or fungal dry rot) and destruction of the intercellular substance and cell membrane (fungal dry rot). Hypertrophy and hyperplasia represent an excessive growth and proliferation of the affected tissue caused by pathogens. Deformations (leaf wrinkling, twisting, and curling; threadlike leaves, fruit ugliness, and double-floweredness) can be caused by various biotic and abiotic factors due to an outflow of the products of photosynthesis, uneven intake of nutrients by the plant, or uneven growth of various tissue elements. In mummification, plant organs are damaged by the fungal mycelium, which leads to plant shrinkage, darkening, or compaction. Color changes usually occur due to chloroplast dysfunction and low content of chlorophyll in the leaves, which manifests itself in the light color of some leaf areas (mosaic discoloration) or the entire leaf (chlorosis) [ 1 , 2 ].
Infectious agents can spread through the air, with water, be transmitted by animals, humans, and remain infectious for many months or years. The natural reservoirs of infectious agents are soil, water, and animals: especially insects.
Infectious plant diseases are mainly caused by pathogenic organisms such as fungi, bacteria, viruses, protozoa, as well as insects and parasitic plants [ 1 ]. With the development of agriculture, infectious plant diseases have become an increasingly significant factor affecting crop yield and economic efficiency. In the field environment, each plant cultivated as a monoculture has uniform conditions and requirements for planting, care, and harvesting, which leads to higher yields and lower production costs than in polyculture [ 3 ]. Over the past half century, the use of modern technologies, including cultivation of monocultures, has allowed us to reduce the amount of additional land needed for food production. However, growing the same crop in the same location year after year depletes the soil and renders it unable to ensure healthy plant growth. Another crucial issue is the susceptibility of monocultures to infectious diseases. Losses can amount to up to 30% even at the stage of storage, transportation, and distribution to the consumer ( Fig. 1 ) [ 4 , 5 ]. Therefore, it is necessary to arrest or prevent the development of infectious diseases at all stages of crop production: starting from seed handling technologies and ending with the delivery and storage of the product on store shelves and in consumers’ homes. This review summarizes existing data on the causes and pathogenetic mechanisms of infectious plant diseases caused by viruses, bacteria, and fungi that affect major agricultural crops, including cereals, vegetables, and industrial crops. The article considers the current status, as well as the problems and prospects of plant protection.

A - crop losses in industrialized countries (medium and high per capita income) at each stage of the production process, starting from cultivation and ending with consumption by households. The results present data for three regions: 1 – Europe (including Russia), 2 – North America and Oceania (USA, Canada, Australia, and New Zealand) and 3 – Industrial Asia (Japan, China, South Korea). Losses are calculated by weight as a percentage of the total mass of the product at the production stage [ 4 ]. B - top 10 most grown crops in the world (by import). C - the most grown plant crops in Russia. D - the main exported plant products from Russia [ 5 ]
PLANT IMMUNITY AND MECHANISMS FOR ITS EVASION
Plants typically are resistant to non-specific pathogens thanks to the presence of a waxy cuticle covering the epidermal cell layer and the constant synthesis of various antimicrobial compounds. Specific pathogens use a variety of strategies to penetrate plants, which often render such protection ineffective. Fungi can penetrate directly into epidermal cells or form hyphae over plant cells and between them, which does not require special structures or conditions. Meanwhile, bacterial and viral infections often require either damaged tissues, specialized structures (e.g., stomata) for entering the cell, or a specific carrier (vector). The latter is usually an insect, a fungus, or protozoa. How does plant infection with phytopathogens occur? In order to understand this, it is important to keep in mind that, unlike animals, plants rely on the innate immunity of each cell and systemic signals emanating from the sites of the infection and not on mobile defense cells and the somatic adaptive immune system. Moreover, an infection by pathogenic microorganisms is not always successful because of the structural changes in the cell wall or programmed cell death.
Plants have so-called trichomes: outgrowths of the epidermis that prevent pathogen growth and penetration. Trichomes may contain antimicrobial compounds or exert an inhibitory effect on the microbial hydrolytic enzymes involved in cell wall damage. The role of the cell wall cannot be overestimated: it is the first obstacle that pathogenic microorganisms must evade; successful protection at this line of defense is most effective against non-specific pathogens. The cell wall consists of cellulose microfibrils and hemicellulose; it is reinforced with lignin and contains a significant amount of proteins that perform structural and enzymatic functions [ 6 ]. The heterogeneity of the structure of the plant cell wall forces pathogens to use various strategies to penetrate it.
Antimicrobial plant compounds, which contain low-molecular-weight non-protein substances, are divided into two groups: phytoanticipins and phytoalexins. Phytoanticipins, such as saponins, phenylpropanoids, alkaloids, cyanogenic glycosides, and glucosinolates, are antimicrobial compounds pre-synthesized by plants. Phytoalexins are formed in response to a pathogenic attack and include various phenylpropanoids, alkaloids, and terpenes. An overlap between these groups of antimicrobial agents is explained by the fact that the phytoalexins of some plants can act as phytoanticipins in others [ 7 ]. In addition, small RNAs regulate the expression of a wide range of genes in plants and comprise natural immunity against viruses [ 8 ]. Plants can also absorb and process exogenous hairpin double-stranded RNAs (dsRNAs) to suppress the genes responsible for the life maintenance and virulence of viruses pathogenic to plants, fungi, and insects [ 9 ]. Aspartate-specific apoptotic proteases (phytaspases), which induce apoptosis, the process of programmed cell death, play an important role in plant defense [ 10 ].
Plants have two types of immune system. The first one uses transmembrane pattern recognition receptors that respond to slowly evolving microbial or pathogen-associated molecular patterns, while the second one acts mainly inside the cell using the polymorphic protein products encoded by most disease resistance (R) genes [ 11 ].
Plant R genes interact with the avr (avirulence) gene products of the corresponding pathogens. In the presence of the corresponding R gene encoding a receptor that triggers the defense response cascade, the receptor recognizes the avr gene product and the plant exhibits a resistance phenotype. For protection against bacterial, viral, and fungal infections, as well as against insects, plants encode only eight classes of the R gene products [ 12 ] that trigger the downstream reaction cascade, which indicates degeneracy of the plant immune system. The number of R genes in the genome can amount to about 100, which is clearly not enough to recognize all possible pathogens. Apparently, recognition of pathogens by the plant immune system is also of a degenerative nature [ 13 ].
The general mechanism of protection against pathogens is, apparently, as follows: during the first phase of an infection, receptors recognize pathogen-associated molecular structures (for instance, flagellin) and trigger an immune response to prevent colonization, which leads to the elimination of a non-specific infection. A specific pathogen produces effector molecules that interfere with the molecules of the immune response, which triggers the so-called effector-mediated susceptibility in susceptible plants. In resistant plants, the R gene products recognize effectors, with further formation of effector-mediated resistance, which can trigger a hypersensitivity (programmed cell death) response in the pathogen-infected area [ 13 ]. During the course of evolution, pathogens have developed several strategies to suppress plant defense responses, such as altering the programmed cell death pathway, inhibiting protective compounds in the cell wall, as well as changing the hormonal status of plants and the expression pattern of defense genes [ 14 ]. However, the products of R defense genes against a viral infection can trigger a series of responses at once. For instance, the defense against potato virus X first starts with the inhibition of viral replication in the absence of a hypersensitivity reaction, while overexpression of the avr gene induces a hypersensitivity reaction, which renders the plant extremely resistant to this virus [ 15 ].
Plants can develop the so-called acquired resistance if the infection that causes resistance in one part of the plant spreads to other parts. This fact indicates that the signaling molecules can move from the affected area to other cells and enhance immunity to the previously encountered pathogen. It should be noted that acquired resistance is not a de novo acquired resistance but an activation of the existing resistance genes in response to a pathogenic attack. The cells accumulate salicylic acid and the various proteins associated with pathogenesis (e.g., chitinase). Such acquired resistance is of a temporary nature and can be both systemic and local [ 16 ].
Symbiotic bacteria colonizing the rhizosphere antagonize soil pathogens through various mechanisms: siderophores suppress plant pathogens by competing for iron; antibiotics suppress competing microorganisms, while chitinases and glucanases lyse microbial cells. Moreover, as a result of symbiosis with bacteria, plants can develop another, extremely peculiar type of resistance: induced systemic resistance, which is also mediated by salicylic acid, ethylene, jasmonic acid, and lipopolysaccharides. In contrast to acquired systemic resistance, induced systemic resistance provides non-specific protection, has no dose-dependent correlation with the effect, does not affect the pathogen directly, and does not depend on the proteins associated with pathogenesis [ 16 ]. Instead, it is determined by the plant genotype and can cause changes in plant metabolism, leading to a general increase in resistance [ 16 ].
Thus, understanding the mechanisms of plant defense and the pathways utilized by phytopathogens to overcome that defense allows one to devise a systematic approach to plant protection.
THE MOST SIGNIFICANT PHYTOPATHOGENS
Viruses and viroids
Viruses are non-cellular infectious agents that can only replicate in living cells. Viruses infect all types of organisms, from plants and animals to bacteria and archaea [ 17 ]. They can be integrated into the host’s genome and remain there as an inactive provirus or actively replicate and regulate the host’s biosynthesis processes. The suppression of viral gene transcription can lead to a latent infection [ 18 ]. Plant viruses mainly come in the form of single-stranded (ss) and double-stranded (ds) RNA viruses, as well as single-stranded and DNA-containing retroviruses [ 17 ]. Due to a wide diversity of their genetic material, the reproductive cycle and life pattern often vary from virus to virus ( Fig. 2A ). Viruses are composed of a nucleic acid molecule and a protective protein coat (capsid). Capsid can sometimes contain a combination of proteins and lipids, which form a lipoprotein membrane. The typical size of a plant virus is 30 nm [ 19 ].

A – plant viruses (and viroids): replication and translation strategies. Tr – transcription, R – replication, RCR – rolling-circle replication, T – translation, RT – reverse transcription, E – encapsulation, ds – double-stranded, ss – single-stranded, “–”– minus-strand, “+”– plus-strand. B - schematic representation of infection of neighboring cells by a virus (viroid) via plasmodesmata. C - symmetric and asymmetric mechanisms of viroid replication
The virion enters the cytoplasm of the plant cell via passive transport through wounds caused by mechanical damage to the cuticle and cell wall, since it is unable to pass through these structures on its own. Upon entering the cell, the virus uncoats. DNA-containing viruses also need to penetrate the nucleus in order to start transcription and mRNA synthesis. All viruses encode at least two types of proteins: replication proteins, which are required for the synthesis of nucleic acid, and structural proteins, which form the capsid. In some cases, there are also proteins that are responsible for virion motility; they ensure transport of virus particles between the plant cells. Viral replication proteins bind to cellular proteins to form a complex that produces multiple copies of the viral genome which interact with structural proteins to form new virions, which are then released from the cell. This is the standard viral life cycle.
Plant viruses can be transmitted vertically (from parents to offspring) and horizontally (from diseased plants to healthy ones). Viruses utilize small intercellular channels called plasmodesmata to penetrate neighboring cells ( Fig. 2B ). Viruses often express the proteins that ensure virion motility by modifying channels to facilitate the transmission of the infection to a neighboring cell [ 20 ]. This is how a local infection of a plant takes place. In order to infect an entire plant, a virus must enter its vascular system, where it then moves passively through the sieve tubes of the phloem with the flow of substances: this is how it can infect cells distant from the primary site of the infection [ 19 , 20 ].
Some viruses are very stable and resistant to heat, can remain viable for a long time in plant cells and the products derived from them [ 21 , 22 ], and can spread through passive mechanical transport from one plant to another [ 23 ]. However, most plant viruses actively spread from infected plants to healthy ones using a carrier organism (vector). Carriers are divided into a mechanical vector, in which the agent does not propagate, and a biological one, in which part of the viral life cycle takes place [ 24 ]. The main vectors of plant viruses are arthropods, nematodes, and fungi that feed on plants [ 25 ].
Plant viruses pose a serious threat to a wide range of crops, while the economic losses caused by viruses are second only to the losses caused by other pathogens [ 26 ]. Moreover, some viruses can infect more than 1,000 different plant species comprising more than 85 families [ 27 ]. In the majority of subtropical and tropical regions, a viral infection can lead to a loss of up to 98% of the crop [ 28 ]. Viruses manifest themselves in a different way depending on the stage of crop production: they can inflict colossal damage at the stage of crop growth, while at the stage of harvesting, storage, and transportation, the damage from a viral infection is minimal. It should be also noted that, in some cases, plants are found infected with viruses in the absence of any obvious symptoms [ 29 ].
The symptoms of viral diseases can be divided into five main types: growth suppression (reduced growth of the entire plant or its leading shoots); discoloration (mosaic, chlorotic rings, leaf chlorosis, variegation); deformations (leaf wrinkling, corrugation, threadlike leaves); necrosis; and impaired reproduction (flower sterility, parthenocarpy, shedding of flowers and ovaries) [ 2 ].
There is another type of infectious agents: viroids, which are circular RNAs that cause various diseases in plants and animals. Taxonomically, they belong to viruses (families Pospiviroidae and Avsunviroidae ). In contrast to viruses, viroids lack a protein envelope (capsid) and present covalently linked ssRNA molecules 200–500 nucleotides long, which is 50-80 times shorter than the viral genome. Viroids do not encode proteins and cannot replicate autonomously. It is considered that the viroid can employ the DNA-dependent RNA polymerase, endoribonuclease, and DNA ligase 1 (which is usually silent) of the host cell for its replication [ 30 ]. Viroids replicate via a rolling-circle mechanism, with members of the families Pospiviroidae and Avsunviroidae replicating through an asymmetric and symmetric pathway, respectively ( Fig. 2C ). The molecular mechanism of the pathogenic action of viroids is not fully understood. It is believed that viroids can alter the phosphorylation state of gene products via binding to cellular kinases [ 31 ], affect the expression of the genes associated with growth, stress, development, and protection [ 32 ], induce the proteins associated with pathogenesis during an infection [ 33 ], cause post-transcriptional suppression of gene expression by RNA interference, impair splicing [ 34 ], and induce demethylation of rRNA genes. It is surprising that the substitution of one nucleotide at a certain position alters the pathogenicity of the viroid significantly [ 35 ]. The RNA molecule of Pospiviroidae family members has five domains: a central domain (C) containing the central, conserved region, which plays an important role in viroid replication; a pathogenicity domain (P) implicated in the manifestation of disease symptoms; a variable domain (V), which is, apparently, responsible for viroid adaptation; and the transport domains T1 and T2 (in cases of co-infection with two viroids, they can exchange with these domains, which can contribute to their evolution). Viroids of the family Avsunviroidae lack the central conserved region but contain the sequences involved in the formation of the ribozyme structures necessary for self-cleavage of RNA strands [ 36 ].
The main symptoms of viroid diseases are reduced growth of the entire plant or its parts, discoloration (chlorosis, anthocyanosis), and deformation of various organs [ 2 ].
Thus, viruses and viroids represent a rather large group of pathogens that cause plant diseases and can result in serious damage to crops in the absence of management and preventive measures, especially when infected at early stages of plant growth.
Bacteria and phytoplasmas
Bacteria are found almost everywhere and can be pathogenic to animals, plants, and fungi [ 37 ]. Bacterial genetic information is encoded in the DNA in the form of a chromosome; more than one chromosome can be found in a cell. A bacterial cell can contain extrachromosomal mobile genetic elements: plasmids that can carry important virulence factors or, on the contrary, biological control factors. Bacteria can also contain a prophage, which represents bacteriophage DNA integrated into the genome. Most bacteria divide by binary fission, usually with simultaneous duplication of both chromosomal DNA and extrachromosomal elements. Division of a bacterial cell requires the presence of the membrane potential [ 38 ]. Bacteria can contain more than one plasmid, since some of them can be lost during division. For instance, Pantoea stewartii can harbor up to 13 different plasmids [ 39 ]. Although bacteria usually transfer plasmids within their population [ 40 ], horizontal transfer of genetic information remains quite common in the prokaryotic world.
Bacteria have a cell membrane which separates the cytoplasm from the external environment. Bacteria are divided into Gram-positive and Gram-negative organisms depending on the cell wall structure [ 41 ]. The cell wall of Gram-positive bacteria consists of a membrane and a thick peptidoglycan layer. The main component of the latter is multilayered murein. Peptidoglycan also contains proteins, lipids, and teichoic and teichuronic acids. The cell wall of Gram-negative bacteria has two membranes with a peptidoglycan layer between them. The outer membrane contains lipopolysaccharides and porins but lacks teichoic and lipoteichoic acids.
Due to the presence of a cell wall, bacteria need secretion systems to pump out xenobiotics, as well as release various proteins and virulence factors ( Fig. 3A ). The secretion systems are divided into several groups based on their structure. There are at least six different types of secretion systems typical of Gram-negative bacteria, four types found in Gram-positive bacteria, and two types present in both groups [ 42 ]. The secretion systems also play a key role in the virulence of phytopathogenic bacteria. It should be noted that, during the division of a bacterial cell, an asymmetry between mother and daughter cells can be observed, where the mother cell retains most of the secretion system transporters, while the daughter cell receives a smaller part of transporters and is forced to synthesize them de novo [ 43 ].

A – bacterial secretory systems that are used to infect plant cells and tissues. B - development of bacterial infection: 1 – penetration through the stomata due to phytotoxins, 2 – secretion of phytotoxins to modify the physiology, immune system, and metabolism of plants, 3 – secretion of phytotoxins for degradation of the cell wall and cytotoxic effect on plant cells, 4 – surface colonization and formation of biofilms, 5 – damage to plant cells due to ice nucleation and formation of crystals. C - development of fungal infection: 1 – penetration into an intact cell at the site of appressorium attachment through the combined effect of mechanical force and enzymes that destroy the plant cell wall, 2 – penetration of the fungus through stomata, 3 – secretion of phytotoxins to modify plant physiology, immune system, and metabolism in biotrophic fungi 4 – penetration of the fungus through the wound; 5 – secretion of phytotoxins for degradation of the cell wall and cytotoxic effect on plant cells
As a rule, phytopathogenic bacteria grow more slowly than non-pathogenic ones isolated from plants and have a temperature optimum of 20–30°C.
Bacterial pathogens contain several types of genes: virulence genes, which play a major role in infection and contribution to virulence, and disease-specific genes, which are important for disease manifestation ( Fig. 3B ). There are a series of genes that are required for host recognition, pathogen attachment to the plant surface, formation of infectious structures, as well as penetration and colonization of the host tissue. Pathogenic factors may either remain attached to the bacterial surface or can be released to the external environment. Pathogenic bacteria cause many serious plant diseases around the world, although not as many as fungi or viruses; however, the economic damage from bacterial diseases is relatively less severe than that from fungi and viruses [ 44 ]. Bacteria wreak havoc at all stages of crop production. Furthermore, due to the increase in the average annual temperature, there is reason to believe that the damage from bacterial spot and economic loses will only continue to grow in the coming years [ 45 ]. With an annual increase in the average daily temperature in summer of 3–4°C, the prevalence of bacterial diseases increases twofold, while the prevalence of plant infection grows by 30–50% [ 45 ].
There are two types of bacterial diseases: systemic bacterial blight (penetration of the pathogen in the plant’s vascular system, its further spread through the conductive bundles and adjacent tissues with disruption of the normal process of water consumption) and local bacterial blight (damage to the parenchymal tissues of individual plant organs). The main symptoms of bacterial diseases are wilting, necrosis, chlorosis, rot, overgrowth (galls), and scab.
Phytoplasmas and spiroplasmas are two groups of very small (about 1 μm in diameter) bacteria without a cell wall (they are separated from the external environment by a cytoplasmic membrane). They cause phytoplasmosis and growth retardation. Like mycoplasmas, a related genus of bacteria, phytoplasmas are apparently one of the most primitive and autonomously reproducing living organisms [ 46 ]. The genome of phytoplasmas is 0.5–1.3 million bp [ 47 ], while the genome of Mycoplasma genitalium , a model organism for studying the minimal genome, comprises 0.58 million bp [ 48 ]. Phytoplasmas exhibit gliding motility [ 49 ], while representatives of the genus Spiroplasma have a spiral shape and move in a twisting motion [ 50 ]. Cultivation of phytoplasmas in axenic cultures is quite difficult, which indicates their greater dependence on the host metabolism [ 51 ].
Phytoplasmosis significantly decreases both crop yield and its quality. Crop losses reach 40% for eggplants, 60% for tomatoes, 93% for pepper, 30–80% for potatoes, and 100% for cucumbers [ 52 ]. Plants with phytoplasmosis are characterized by such disorders of generative organs as virescence (greening of flowers and loss of normal pigmentation), phyllodia (transformation of part of a flower into a leaf-like formation), and proliferation (appearance of several “pseudo” flowers instead of one). In addition, phytoplasmosis can lead to the witches’ broom symptom (increased bushiness), dwarfism and wilting of plants, as well as leaf deformations. There is only one known case of positive phytoplasmosis, which leads to an economically useful effect: it is phytoplasmosis of poinsettia, a popular seasonal ornamental plant.
Fungi are characteristic representatives of the domain Eukaryota. Unlike bacteria, they have a complex cell structure with a distinct nucleus and mitochondria. Fungal genome is much smaller than that of most eukaryotes but much larger than prokaryotic. Fungi have a cell wall, which usually consists of chitin, mannan, and chitosan, and also includes various proteins, lipids, and polyphosphates. Fungi form a mycelium: a system of thin branching hyphae, which sometimes lacks intercellular septa and forms a syncytium. Fungi are found in all ecological niches and can cause significant harm. Fungi appear to be evolutionarily much older than plants; the duration of their coexistence can be compared to the evolutionary age of higher plants [ 53 ]. About 80% of the plants present on our planet to date are symbiotic with fungi [ 54 ]. However, fungi sometimes disrupt the delicate balance of the mutually beneficial cooperation by turning into plant pathogens classified as biotrophs, hemibiotrophs, and necrotrophs. As a rule, pathogenic fungi enter plants through damaged leaves and stomata. However, in many cases, fungi secrete specific infectious structures and enzymes that destroy a plant’s cell wall ( Fig. 3C ). In the case of necrotrophs, which have a wide range of hosts, the host cells die quickly from the combined action of enzymes destroying the plant’s cell wall, reactive oxygen species, and/or toxins [ 55 , 56 ]. Biotrophs, whose life cycle is associated with a living host cell, secrete effector molecules that suppress the plant’s immune system. These fungi exhibit specificity and interact with the host via special biotrophic hyphae in the interphase region where biomolecules synthesized by the plant are absorbed [ 57 ]. Fungi can develop specific outgrowths of hyphae, so-called apressoria, which provide attachment of the fungus to the substrate, thus allowing the pathogen to penetrate the cell wall using a combination of mechanical force and enzymes that degrade the plant’s cell wall. Haustoria move from the base of the appressorium through the destroyed areas and penetrate the lumen. As a rule, haustoria contain a large number of mitochondria and ribosomes with a well-developed endoplasmic reticulum; haurtorium is usually separated from the plant cell by invagination of the host plasmalemma [ 58 ]. At the same time, one can assume that an increased pressure of plant defense can cause a transition from biotrophy to necrotrophy [ 53 ].
Phytopathogenic fungi are the most dangerous plant pathogens to cause harm at all stages of crop production. The most common way to fight fungi is considered to be treatment with fungicides. The use of fungicides is associated with serious environmental and medical risks, namely the emergence of resistance and horizontal transfer of resistance genes, with the occurrence of species with multiple resistance [ 59 ]. At least 150 chemical compounds with different mechanisms of action are used as fungicides in world agriculture; however, there have been cases of resistance among various types of phytopathogens against almost all major classes of fungicides recorded to date [ 60 ].
The main symptoms of fungal diseases include wilting, spotting, mold (mycelium and sporulation of the fungus on the surface of affected organs), pustules (accumulation of fungal spores), overgrowth, deformations, mummification (shrinkage, darkening, and compaction of the infected tissue), and rot [ 2 ].
To date, more than 10,000 fungal species associated with plants have been discovered, and it is not surprising that fungal infections cause more harm than the diseases caused by other pathogenic microorganisms [ 61 ].
Complex diseases
Although it is believed that a plant disease is caused by one pathogen species or strain, microbes occur in nature mainly as part of complex multi-species consortia/communities. Most laboratory studies focus on individual strains grown in a pure culture. However, they cannot explain the complex course of certain plant diseases. Therefore, the diseases where more than one pathogen is involved are usually termed “complex” due to their complicated diagnosis and subsequent control [ 62 ]. Synergistic interactions can occur between viruses, bacteria, fungi, and different groups of pathogens. For instance, the synergism of virus–virus type is observed when cowpea is co-infected with cowpea mosaic virus and cucumber mosaic virus, with the severity of the disease and the degree of growth retardation being greater than in the case of infection with individual viruses [ 63 ]. Synergism of the type bacterium–bacterium, which exacerbates the disease severity, can be observed when tomato is co-infected with the bacteria Pseudomonas corrugata and P. mediterranea , which cause tomato pith necrosis [ 64 ]. Synergism of the type fungus–fungus occurs quite often; it causes complex diseases such as ascochyta blight complex of pea [ 65 ], mango malformation disease [ 66 ], etc. Brown apical necrosis of walnut resulting from the interaction of numerous pathogenic fungi and bacterium Xanthomonas arboricola represents an example of a synergistic interaction between different groups of pathogens [ 67 ]. Synergism between different pathogens resulting in more severe disease symptoms is more common than expected and may be crucial in understanding microbial pathogenesis and evolution, as well as further developing effective strategies of disease management [ 62 ].
Thus, phytopathogens are ubiquitous and cause various plant diseases ( Fig. 4 ).

Infectious plant diseases. From left to right, top row: tomato mosaic virus, downy mildew of lettuce, bacterial blight of cauliflower, rye ergot, middle row: potato spindle tuber viroid (William M. Brown Jr, amended), lettuce bacterial blight, mixed viral infection on the ramson (cucumber mosaic virus, tobacco rattle virus, tobacco mosaic virus), Septoria blight of celery; bottom row: Fusarium blight of dill, onion rust, black rot (alternariosis) of carrots, and tomato leaf curl virus
Identification of phytopathogens
Early diagnosis of plant diseases is a key factor that determines the timely use of protective measures and, as a result, determines the yield and quality of crop products. To date, in addition to conventional visual examination and the method of indicator plants, serological methods and methods based on DNA and RNA technologies are required in order to accurately identify plant diseases. The most common methods of serological diagnosis include enzyme immunoassay, immunoblotting, dot-blot hybridization, immunochromatography [ 68 ], and serologically specific electron microscopy [ 69 ]. Methods based on DNA detection include fluorescence in situ hybridization [ 70 ], various polymerase chain reaction (PCR) techniques, including nested PCR, cooperative PCR, multiplex PCR, real-time PCR, and DNA fingerprinting. There are also RNA-based approaches: isothermal amplification of nucleic acids [ 71 ], the AmpliDet RNA real-time diagnostic system [ 72 ], and reverse-transcription PCR. These methods allow for quick and accurate detection of the pathogen and identification of its taxonomic rank. Novel approaches for a more accurate and sensitive detection are now being developed. These are the next-generation sequencing and metagenomic analysis, two-hybrid analysis, phage display, as well as biosensor technologies based on electrochemistry and biophotonics [ 73 ]. Thus, modern methods allow for accurate identification of a phytopathogen even in the absence of infection symptoms.
Integrated pest management (IPM)
The system of managing the phytosanitary state of ecosystems using integrated methods of pest management to ensure the phytosanitary prosperity of the territory is effectively used in many countries [ 74 ].
IPM is based on the assessment of an acceptable level of pests for determining the pest threshold. A prerequisite for this is the constant monitoring of pests, quarantine measures and seed purity, as well as the selection of resistant varieties cultivated in the area. If the level of harmfulness is reached, then methods of mechanical and biological control are mostly applied; however, if necessary, chemical-control methods can be used in a responsible and targeted manner.
The costs of IPM and chemical management are practically comparable, while IPM provides longer duration of the effect, increases yields by 10–30%, improves product quality, reduces climate risks, and has a pronounced environmental upside [ 75 ].
Seed reserves
In the IPM paradigm, healthy planting material is a prerequisite for the effective use of the system. Unfortunately, the seeds of most plants often serve as reservoirs for various phytopathogens, and the infection can be located both on the surface of the seed and inside of it. There are several strategies for regulating the seed transmission of a pathogen existing to date: the use of pathogen-free seeds and the search for methods of pre-sowing seed treatment. The most effective way to combat fungi is considered to be treatment of seeds with fungicides. Contact fungicides are used to destroy pathogens on the seed surface, while translaminar fungicides can penetrate into the seed and destroy the pathogen inside of it. These agents must act delicately to avoid damaging the fetus [ 76 ]. In recent years, there have been various strategies developed to control the pathogens on seeds, including physical treatment (mechanical and thermal treatment, ultrasonic and ultraviolet light exposure), treatment with natural compounds and biological control agents, as well as substances inducing resistance [ 77 ].
About 11 million tons of agricultural seeds are sown in Russia annually. The volume rate of domestic seeds in the world’s cereal crops is 90%; it is 46% for corn, 43% for vegetables, 42% for soybeans, 32% for spring rape, and 26% for sunflower [ 78 ]. On the contrary, the volume rate of foreign seeds used in Russia varies from 30 to 90% depending on the culture, with the cost reaching 681,000 US dollars. The share of the seed business in the total sales of large agrochemical companies such as Syngenta, Bayer, DuPont, Dow, and Monsanto, is on the increase; they have acquired seed companies and comprehensively expanded their research on crop protection by developing and creating resistant varieties and hybrids using modern high-end and high-performance technologies, including genome editing [ 79 ].
Plant breeding and bioengineering
Modern plant breeding for resistance to pathogens utilizes approaches and methods of conventional and cell selection. The emergence of the complete genomic sequences of some economically important crops now makes it possible to effectively search for resistance genes, as well as the corresponding DNA markers. Today, genetic markers based on DNA polymorphism (RFLP, RAPD, AFLP, CAPS) and short tandem repeats (STRs, or SSRs), as well as DNA microarray technology Diversity Arrays Technology (DArT) [ 80 , 81 ], are actively used. A long-term increase in plant resistance can be achieved by using gene pyramiding [ 82 ]; namely through the development of genetically engineered varieties and distant hybridization technology.
Modern biotechnology approaches are becoming increasingly important for the production of virus-resistant plant varieties and hybrids. Introduction of an antisense gene in the plant for its modification allows one to disrupt viral reproduction [ 83 ]. The gene encoding the protein that has an affinity for viral RNA and inhibits its replication is also inserted into the plant’s genome [ 84 ] to cause a delay in the expression of the transport protein or a modification of plasmodesma [ 85 ]. Constant expression of chitinase or lysozyme of bacteriophage T4 results in enhanced plant resistance to fungal and bacterial infections [ 86 , 87 ]. Transgenic potato plants transcribing an RNA ribozyme that cleaves the RNA minus-strand of the spindle tuber viroid have been obtained [ 88 ].
New breeding methods to select varieties resistant to plant pathogens include powerful molecular tools for precise genetic modification, including the CRISPR/ Cas9 system, which allows for more accurate genome editing than the use of Agrobacterium -mediated transformation [ 89 ].
Agrotechnical control is a mandatory component of the IPM system. Adequate agricultural technology provides enhanced plant resistance to diseases and prevents massive infection by creating optimal conditions for plant growth and development. At the same time, crop rotation and selection of predecessors, the system of soil cultivation, fertilizers, dates of sowing and harvesting, as well as the destruction of weeds and post-harvest plant residues are of primary importance [ 90 ]. Placement of neighboring crops in the crop rotation and soil tillage are also essential [ 91 ]. Destroying post-harvest residues and weeds, which retain a large number of pathogens, while many weeds serve as reservoirs for them, is also of prime importance.
Chemical control
Chemical control plays a crucial role in preventing losses associated with plant diseases, especially with the advent of numerous fungicides with selective toxicity, which expands possibilities for using them in targeted fashion.
The total cost of research, development, and registration of a new crop protection product rose from USD 152 million in 1995 to USD 286 million in 2014. Worldwide sales have been increasing by about 6.5% annually since 1999 [ 92 ]. There are more than 600 different chemical control agents on the market to date (fungicides, pesticides, herbicides, nematicides, molluscicides, rodenticides, and antibiotics), and the economic sector is now valued at more than USD 50 billion [ 93 ]. There are now strict regulations on the use of chemical pesticides; and many products have been taken off the market, banned or have failed to pass re-registration. For instance, six out of the ten major chemical control products used in 1968 are currently banned as household and agricultural pesticides in the United States.
Biological control and alternative to antibiotics
Modern agriculture is becoming an increasingly high-end and multidisciplinary industry with each passing year [ 94 ]. The uncontrolled use of herbicides leads to the appearance of populations of weeds that are resistant to them [ 95 ]. Although success in disease management mainly depends on crop resistance and the agricultural methods used, antibiotics such as gentamicin, oxolinic acid, oxytetracycline, and streptomycin are widely used in crop production [ 96 ]. The use of antibiotics in crop production is about 0.12%. However, in recent years, due to the widespread antibiotic resistance, more emphasis has been placed on alternative forms of combating phytopathogens. One such approach is the use of various methods of biological control [ 97 ]. Examples of biological control include the use of antagonist strains and antibiotic producers, bacteriophages, insects for weed control, and parasitic insects for controlling insect pests. For plant disease management, substances that are not themselves representatives of the groups of antibiotics or antimycotics, such as photosensitizers, bacteriophages, phagolysins, antimicrobial peptides, and antibiofilm agents [ 98 ], are used. They are especially useful if, in addition to antibacterial activity, they have other properties, e.g., the ability to reduce the level of reactive oxygen species or inhibit bacterial multidrug efflux pumps [ 99 ].
The most significant plant pathogens
Several years ago, Molecular Plant Pathology conducted a series of surveys among specialists in the field of molecular plant pathology, which allowed the journal to select the ten most significant phytopathogenic fungi [ 100 ], viruses [ 101 ], and bacteria [ 102 ] ( Table ).
The most significant phytopathogens
| Viruses | Bacteria | Fungi |
|---|---|---|
| The world’s most significant phytopathogens | ||
| Tobacco mosaic virus | Pseudomonas syringae | Magnaporthe oryzae |
| Tomato spotted wilt virus | Ralstonia solanacearum | Botrytis cinerea |
| Tomato yellow leaf curl virus | Agrobacterium tumefaciens | Puccinia spp. |
| Cucumber mosaic virus | Xanthomonas oryzae | Fusarium graminearum |
| Potato virus Y | Xanthomonas campestris | Fusarium oxysporum |
| Cauliflower mosaic virus | Xanthomonas axonopodis | Blumeria graminis |
| African cassava mosaic virus | Erwinia amylovora | Mycosphaerella graminicola |
| Plum pox virus | Xylella fastidiosa | Colletotrichum spp. |
| Brome mosaic virus | Dickeya dadantii | Ustilago maydis |
| Potato virus X | Dickeya solani | Melampsora lini |
| Citrus tristeza virus | Pectobacterium carotovorum | Phakopsora pachyrhizi |
| Barley yellow dwarf virus | Pectobacterium atrosepticum | Rhizoctonia solani |
| Potato leafroll virus | Clavibacter michiganensis | |
| Tomato bushy stunt virus | ||
| The most significant phytopathogens in Russia | ||
| Barley stripe mosaic virus | Candidatus Phytoplasma spp. | Alternaria solani |
| Wheat streak mosaic virus | Xanthomonas translucens | Fusarium avenaceum |
| Winter wheat Russian mosaic virus | Pseudomonas cichorii | Plasmopara halstedii |
| Oat Siberian mosaic virus | Rathayibacter tritici | Phytophthora infestans |
| Beet necrotic yellow vein virus | Pseudomonas fuscovaginae | Tilletia caries |
| Lettuce mosaic virus | Acidovorax citrulli | |
One cannot but agree with such a choice. However, the structure of agricultural products and crops grown in Russia differs from global ones and is predominantly comprised of wheat, sugar beet, potatoes, barley, oats, sunflower, and corn and, thus, requires adjustments to the list of pathogens specific to these cultures [ 2 , 65 , 79 , 103 , 104 ] .
With the advent of modern diagnostic approaches, genome editing and sequencing technologies, as well as microbiome and proteomic analysis methods, the study of the mechanisms and effect of phytopathogens on plants has moved to a multidisciplinary level. In this review, we have attempted to provide a comprehensive picture of the current state of pest management. However, to our deep regret, we could not consider many aspects of the interaction between plants and phytopathogens, such as damage by ice nucleation proteins, which cause the formation of ice crystals in plant cells [ 105 ] or the conserved nature of the sequences of effector molecules in bacteria: pathogens of humans, animals, and plants [ 106 ].
Acknowledgments
Acknowledgments: The reported study was funded by the Russian Foundation for Basic Research (project number 19-116-50156).
Abbreviations
| IPM | integrated pest management |
| RNA | ribonucleic acid, |
| DNA | deoxyribonucleic acid |
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Open access
- Published: 05 August 2024
Coming of age for Microbiome gene breeding in plants
- Tomislav Cernava ORCID: orcid.org/0000-0001-7772-4080 1
Nature Communications volume 15 , Article number: 6623 ( 2024 ) Cite this article
22 Altmetric
Metrics details
- Agricultural genetics
- Plant breeding
The plant microbiota can complement host functioning, leading to improved growth and health under unfavorable conditions. Microbiome engineering could therefore become a transformative technique for crop production. Microbiome genes, abbreviated as M genes, provide valuable targets for shaping plant-associated microbial communities.
New strategies are needed to secure global crop production
Agriculture currently relies on high inputs of agrochemicals to maintain crop production. This has not only led to polluted environments and health issues, but also contributed to the crossing of planetary boundaries 1 . Various global initiatives, such as the European Green Deal, aim at substantially reducing agrochemical inputs during the next years. The implementation of such initiatives is currently mainly hindered by the fact that we lack viable strategies that would allow us to maintain the required crop production without chemical plant protection. Plant breeding focusing on resistance genes ( R genes) and susceptibility genes ( S genes) has emerged as a promising solution to develop crop plants that are less susceptible to pathogens. However, such approaches, especially those based on R genes, can only provide short-term solutions as diverse and rapidly evolving pathogens can overcome them 2 .
Microbiome studies, which have been carried out intensively over the last 15 years, have contributed to a better understanding of plant health and disease. They have also provided support for the holobiont theory. This theory is based on the assumption that microbial communities together with multicellular hosts can result in various phenotypes, offering extended possibilities for adaptation in various environments. In rice, it was demonstrated that the presence of a specific seed-endophytic bacterium can shape an entirely disease-resistant plant phenotype 3 . A high number of studies conducted during the last decades has provided evidence that various microorganisms have plant-beneficial traits when naturally present or artificially introduced. Introduced biological agents often result in significant plant growth promotion and disease protection. However, there are substantial limitations in terms of their applicability due to frequently occurring inefficacy or low efficacy under certain field conditions 4 . Competition with the local microbiota, which can vary between hosts and environments, is one of the unpredictable factors that must be accounted for when living organisms are used for plant protection.
Significant public and private investments are currently being made to support the development of technologies that manipulate the host microbiota as a whole, also known as ‘microbiome engineering’. Such technologies hold more promise than the currently available ones based on the application single strains or defined microbial consortia. One general strategy to engineer microbiomes is to alter traits of the host. A recent review article highlights the potential of plant microbiome engineering, but the authors also point out that we currently lack detailed knowledge related to specific host genes that are involved in directed assembly of microbial communities with desired functions 5 . The identification of such genes will play a pivotal role in enabling targeted modifications of the plant microbiome.
Engineering crop microbiomes via host genetics
Studies conducted during the last years have shown that plants can recruit specific microbes via their metabolites 6 , 7 , 8 . The mechanisms that were discovered so far are needed for targeted recruitment of bacteria as well as fungi 8 , 9 . Such findings provide new options to harness specific host genetic traits to shape and maintain a microbiota with desirable functioning. Knowledge about microbiome-regulating host genes could be harnessed for the generation of genetically modified plants and more importantly to specifically screen for natural variation in these genes. The latter will likely result in targeted breeding approaches that will face less restrictions in agricultural applications as compared to genetically modified plants. The overall approach was introduced as M gene breeding by Su and colleagues 8 . Observations made in this study indicate that specific M gene haplotypes in rice plants can significantly enrich specific microbiome components resulting in increased protection against pathogens. This could be further exploited in targeted breeding approaches. Implementation of M genes in plant breeding has the potential to provide long-term protection against phytopathogens. This is mainly supported by microbial diversity being accompanied by chemical diversity due to a wide range of metabolites that can be produced by different members of the microbiota. Pathogens would therefore have to develop resistances against a diverse set of bioactive compounds that are synthesized by M gene-enriched microbes. This is in contrast to the direct effect of certain plant metabolites against pathogens, which can more readily result in resistance development. In addition, knowledge about M gene activity can likely be harnessed to bidirectionally optimize plant-microbe interactions that result in improved plant growth or productivity 10 , 11 . In all the examples described, it will be important to take potential adaptations of plants to local environments and their microbiomes into consideration 10 . It is also noteworthy to mention that it is currently unclear if certain plant pathogens also respond to specific M genes, which would allow them to potentially hijack certain mechanisms for their own benefit.
Various genes involved in the biosynthesis or regulation of plant metabolites known to shape the microbiota are promising targets for M gene breeding. Distinct lignin precursors, and especially p-coumaric acid, regulate bacterial community structures in the phyllosphere and endosphere of plants 8 , 12 . Scopoletin, a coumarin that branches out of lignin biosynthesis, was shown to selectively enrich plant-beneficial microorganisms in the rhizosphere 13 . Several other plant coumarins are also known for their effects on the plant microbiome 7 . Targeted plant breeding to optimize the production of microbiome-shaping compounds could result in less susceptibility to phytopathogens and increased plant productivity. This has the potential to substantially lower the global use of chemical pesticides and fertilizers. A growing knowledge base about plant genes that are in control of the microbiome will pave the way for such approaches 14 , 15 . Obstacles that will be likely encountered are related to multiple different M genes and exometabolites that are sometimes shaping the microbiome in a concerted way 16 , 17 . This will increase the complexity related to the implementation of M gene breeding approaches in such cases but is not an impassable hurdle if the connected genes in plants are known.
Further considerations related to M gene breeding
The fundamental basis for M gene breeding is readily available, meaning that the first attempts can already be made. Screening for M gene activity can be implemented as part of pre-breeding approaches where available germplasm material is utilized. Targeted approaches can also be implemented to further improve elite plant varieties by fine-tuning their M genes into a desired direction. When designing studies to identify novel M genes, distinct particularities of plant microbiomes should be taken into account. Intra-species genetic diversity provides a highly suitable basis for the identification of novel M genes by allowing to search for microbiome associations at single-nucleotide polymorphism (SNP) level (Fig. 1 ) 8 . Future identification of M genes will be facilitated if plants subjected to microbiome profiling are obtained from the same location and growth stage, as environmental and host-specific factors could obscure their implications in shaping microbial communities. In addition, upcoming genome-wide association studies (GWAS) targeting microbiomes would generally benefit from relying on shotgun-sequenced metagenomes; currently they are mostly based on marker gene amplicons that are more prone to bias 15 . Underutilized crops, also known as orphan crops, could be a valuable resource to study co-evolved associations between plants and their microbiota. This is especially due to their generally better adaptation to local environments, where also specialized microbial communities are more likely to be found. To enable such approaches, suitable genetic resources must be generated 18 . Such resources are currently scarce, however, the importance of intensifying research efforts to generate them was recognized 19 .

Specific host genes are associated with the structure and composition of the microbiome. Single-nucleotide polymorphisms (SNPs) in these genes can lead to alterations of the microbiome. Knowledge about SNPs associated with the whole microbiota, or distinct components of it, provides the basis for targeted M gene breeding approaches. Screening for specific M gene haplotypes connected with desirable microbiome structures can be implemented as part of pre-breeding strategies with germplasm collections. If successfully implemented, crop plants with an optimized microbiome will be less reliant on chemical pesticides and fertilizers.
The more associations are identified between plant genes and microbiome components, the more likely it is that these findings will lead to practical applications. This can be accelerated if targeted approaches are implemented in plant microbiome research. If it proves viable, M gene breeding not only can increase sustainability in agriculture but also contribute to global food security.
Richardson, K. et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 9 , eadh2458 (2023).
Article PubMed PubMed Central Google Scholar
Stam, R. & McDonald, B. A. When resistance gene pyramids are not durable—the role of pathogen diversity. Mol. Plant Pathol. 19 , 521 (2018).
Matsumoto, H. et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 7 , 60–72 (2021).
Article CAS PubMed Google Scholar
Jurburg, S. D. et al. Potential of microbiome-based solutions for agrifood systems. Nat. Food 3 , 557–560 (2022).
Article PubMed Google Scholar
Zhan, C., Matsumoto, H., Liu, Y. & Wang, M. Pathways to engineering the phyllosphere microbiome for sustainable crop production. Nat. Food 3 , 997–1004 (2022).
O’Banion, B. S. et al. Plant inositol transport influences bacterial colonization phenotypes. Curr. Biol. 33 , 3111–3124 (2023).
Stringlis, I. A., De Jonge, R. & Pieterse, C. M. The age of coumarins in plant–microbe interactions. Plant Cell Physiol. 60 , 1405–1419 (2019).
Article CAS PubMed PubMed Central Google Scholar
Su, P. et al. Microbiome homeostasis on rice leaves is regulated by a precursor molecule of lignin biosynthesis. Nat. Commun. 15 , 23 (2024).
Article ADS CAS PubMed PubMed Central Google Scholar
Xu, P. et al. Temporal metabolite responsiveness of microbiota in the tea plant phyllosphere promotes continuous suppression of fungal pathogens. J. Adv. Res. 39 , 49–60 (2022).
He, X. et al. Heritable microbiome variation is correlated with source environment in locally adapted maize varieties. Nat. Plants 10 , 598–617 (2024).
Wintermans, P. C., Bakker, P. A. & Pieterse, C. M. Natural genetic variation in Arabidopsis for responsiveness to plant growth-promoting rhizobacteria. Plant Mol. Biol. 90 , 623–634 (2016).
Beckers, B. et al. Lignin engineering in field-grown poplar trees affects the endosphere bacterial microbiome. Proc. Natl Acad. Sci. USA 113 , 2312–2317 (2016).
Stringlis, I. A. et al. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl Acad. Sci.USA 115 , E5213–E5222 (2018).
Escudero-Martinez, C. et al. Identifying plant genes shaping microbiota composition in the barley rhizosphere. Nat. Commun. 13 , 3443 (2022).
Oyserman, B. O. et al. Disentangling the genetic basis of rhizosphere microbiome assembly in tomato. Nat. Commun. 13 , 3228 (2022).
Escudero-Martinez, C. & Bulgarelli, D. Engineering the crop microbiota through host genetics. Annu. Rev. Phytopathol. 61 , 257–277 (2023).
Sasse, J., Martinoia, E. & Northen, T. Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 23 , 25–41 (2018).
Njaci, I. et al. Chromosome-level genome assembly and population genomic resource to accelerate orphan crop lablab breeding. Nat. Commun. 14 , 1915 (2023).
Shorinola, O. et al. Integrative and inclusive genomics to promote the use of underutilised crops. Nat. Commun. 15 , 320 (2024).
Download references
Acknowledgements
The help of Martin Stonitsch (Graz, Austria) in preparing figure elements with the assistance of AI-generated rendering by Midjourney and Adobe Firefly is highly appreciated. The author also wants to thank Mark Chapman (University of Southampton) for discussions related to underutilized crops.
Author information
Authors and affiliations.
School of Biological Sciences, Faculty of Environmental and Life Sciences, University of Southampton, Southampton, UK
Tomislav Cernava
You can also search for this author in PubMed Google Scholar
Contributions
T.C. conceptualized and wrote the manuscript.
Corresponding author
Correspondence to Tomislav Cernava .
Ethics declarations
Competing interests.
The author declares no competing interests.
Peer review
Peer review information.
Nature Communications thanks Jos Raaijmakers, Joelle Sasse and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cernava, T. Coming of age for Microbiome gene breeding in plants. Nat Commun 15 , 6623 (2024). https://doi.org/10.1038/s41467-024-50700-7
Download citation
Received : 29 January 2024
Accepted : 18 July 2024
Published : 05 August 2024
DOI : https://doi.org/10.1038/s41467-024-50700-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Plant leaves
Plant Leaf Disease Prediction
- 9(VII):1295-1305

- Chaitanya Bharathi Institute of Technology
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- G. Shyamsundar Reddy
- K. Venkata Rao
- P. Anil Kumar
- Sai Sharvesh R
- Suresh Kumar K
- C. J. Raman
- Rasendram Muralitharan
- Nilani Inuvaisubramaniam

- Gramma Niladhari

- P.Rohit Reddy
- M. Vinay Kumar
- K. H Vijaya Kumari
- Sugamya Katta
- R. Thamilselvan

- M Sowndharya

- Ede Venkatesh
- Karnam Balaji

- DISCRETE DYN NAT SOC

- Chengxin Yin

- Aniirudh Ramesh

- Pappula Srinivasu
- Sharada Mohanty
- David P. Hughes
- Marcel Salathé
- Vijeta Shrivastava
- Samreen Pushpanjali
- Indrajit Fatima
- Konstantinos Ferentinos
- P Alagumariappan
- G N Muthukrishnan
- Adrian Rosebrock
- Mk Gurucharan
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
share this!
July 30, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
Tea plant's genetic guardians: lncRNA-protein pairs bolster disease resistance
by Anhui Agricultural University
Long non-coding RNAs (lncRNAs) are vital in plant growth and stress resistance but are not well understood in tea plants. Previous research in model plants has shown that lncRNAs play crucial roles in various biological processes. However, the unique characteristics of tea plants present challenges in studying these RNAs.
Due to these issues, further in-depth research on trans-lncRNAs in tea plants was necessary. This study addresses these challenges by exploring the role of lncRNAs in enhancing disease resistance in tea plants through the jasmonic acid signaling pathway.
A new study, conducted by researchers from Anhui Agricultural University and published on May 6, 2024, in Horticulture Research , explores the role of evolutionarily conserved trans-lncRNA pairs in disease resistance in tea plants. The study identified and analyzed 24 trans-lncRNA pairs, focusing on their interaction with the 12-oxophytodienoate reductase gene and its impact on the jasmonic acid signaling pathway.
Researchers developed an innovative method to identify evolutionarily conserved trans-lncRNA pairs, uncovering 24 such pairs across various plant species. In tea plants, the CsOPRL gene cluster was found to regulate disease resistance by interacting with CsOPR genes. Experiments revealed that knocking out the CsOPRL gene in potato plants led to increased resistance to fungal infections , underscoring its crucial role in plant defense.
The study further demonstrated that CsOPRL forms RNA-DNA triplexes with target genes , inhibiting their expression and affecting the synthesis of jasmonic acid , a key compound in plant defense mechanisms.
These findings highlight the significant role of CsOPRL in modulating CsOPR gene expression and enhancing disease resistance. This research provides valuable insights into the genetic mechanisms underlying plant resilience and opens up new possibilities for improving crop resistance through genetic manipulation.
Dr. Tao Xia, one of the corresponding authors, stated, "Our findings provide new insights into the regulatory mechanisms of plant disease resistance. The identification of these trans-lncRNA pairs opens up new avenues for enhancing crop resilience through genetic manipulation."
This research lays the groundwork for developing new strategies to improve disease resistance in crops, particularly in non-model plants like tea. By leveraging the regulatory mechanisms of trans-lncRNAs, scientists can potentially enhance the resilience of various crops, leading to more sustainable agricultural practices and improved food security.
Journal information: Horticulture Research
Provided by Anhui Agricultural University
Explore further
Feedback to editors
Advanced chelators offer efficient and eco-friendly rare earth element recovery
11 minutes ago

'Baby talk:' Decoding how children's vocal and cognitive cues sway adults
23 minutes ago

Calculating faster: Coupling AI with fundamental physics
25 minutes ago

Hunt for herbicide solution in snap bean reveals master switch for stress resistance
36 minutes ago
Understanding the forces that regulate crystallization by particle attachment
37 minutes ago

Fossil hunter discovers new species of 210-million-year-old lungfish
45 minutes ago
The shape of molecules to come: A Q&A on designing DNA nanostructures for biomedical applications
48 minutes ago

Researchers identify gene responsible for marsupial fur color
50 minutes ago

Heating for fusion: Why toast plasma when you can microwave it
52 minutes ago

Antarctic survey of plant life to aid conservation efforts
Relevant physicsforums posts, contradictory statements made by two different professors about iq scores.
Aug 2, 2024
New and Interesting Publications Relevant to the Origin of Life
The cass report (uk).
Jul 30, 2024
The predictive brain (Stimulus-Specific Error Prediction Neurons)
Jul 21, 2024
Understanding COVID Quarantine Guidance
Jul 19, 2024
Innovative ideas and technologies to help folks with disabilities
Jul 18, 2024
More from Biology and Medical
Related Stories
Study reveals how wounding boosts anthocyanin in grapes
Jul 2, 2024
Pear-derived discovery: A genetic mechanism to fortify crops against drought
Jul 5, 2024
Grape white rot resistance and the role of VvWRKY5 in enhancing pathogen defense through the jasmonic acid pathway
Feb 5, 2024

Plants' hidden allies: Root microbiota fight back against leaf-mining flies
Jul 29, 2024
Heat and disease: The genetic tug-of-war in pepper immunity
Jun 25, 2024

The hidden drivers of evolution: Transposable elements in Rosaceae genomes
Recommended for you.

Male poison frogs may use finger placement to channel pheromones to females while mating
How friendly fungi is helping rice thrive

Researchers find book scorpion venom effective against hospital germs
2 hours ago
Researchers identify over 2,000 potential toxins using machine learning
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Chitinase gene fochi20 in fusarium oxysporum reduces its pathogenicity and improves disease resistance in cotton.

1. Introduction
2.1. identification of disease resistance in cotton and restoration culture, 2.2. hub-genes with pathogenicity of f. ox, 2.3. fochi genes in f. ox were involved in resistant and susceptible cotton root secretions, 2.4. silenced fochi20 gene attenuated the pathogenicity of f. ox and enhanced disease resistance in cotton, 3. discussion, 3.1. root secretions could be widely used to inhibit f. ox growth, 3.2. pathogenicity-related genes in f. ox were predicated, 3.3. chitinase genes influenced the pathogenicity of pathogenic bacteria, 3.4. the value of higs technology in plant disease resistance research, 4. materials and methods, 4.1. sample collection of cotton and fusarium oxysporum f. sp. vasinfectum, 4.2. f. ox morphological and molecular biological characterization, 4.3. rt-qpcr experiment, 4.4. identification of chitinase genes in f. ox, 4.5. evolutionary tree and location of the chitinase genes, 4.6. host-induced fochi20 gene silencing assay, 5. conclusions, supplementary materials, author contributions, data availability statement, acknowledgments, conflicts of interest.
- Chávez Montes, R.A.; Ulloa, M.; Biniashvili, T.; Zackay, A.; Kfir, N.; Lopez-Arredondo, D.; Herrera-Estrella, L. Assembly and annotation of the Gossypium barbadense L.‘Pima-S6’ genome raise questions about the chromosome structure and gene content of Gossypium barbadense genomes. BMC Genom. 2023 , 24 , 11. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, S.; Kong, L.; Xiao, X.; Li, P.; Liu, A.; Li, J.; Gong, J.; Gong, W.; Ge, Q.; Shang, H. Genome-wide artificial introgressions of Gossypium barbadense into G. hirsutum reveal superior loci for simultaneous improvement of cotton fiber quality and yield traits. J. Adv. Res. 2023 , 53 , 1–16. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, S.T.; Chen, H.; Hou, Z.; Li, Y.; Yang, C.L.; Wang, D.J.; Song, C.P. Screening of abiotic stress-responsive cotton genes using a cotton full-length cDNA overexpressing Arabidopsis library. J. Integr. Plant Biol. 2020 , 62 , 998–1016. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Xiao, X.; Liu, R.; Gong, J.; Li, P.; Li, Z.; Gong, W.; Liu, A.; Ge, Q.; Deng, X.; Li, S. Fine mapping and candidate gene analysis of qFL-A12-5 : A fiber length-related QTL introgressed from Gossypium barbadense into Gossypium hirsutum . Theor. Appl. Genet. 2023 , 136 , 48. [ Google Scholar ] [ CrossRef ]
- Xiong, X.; Sun, C.; Chen, B.; Sun, J.; Fei, C.; Xue, F. Transcriptomic datasets of Verticillium wilt resistant and non-resistant Gossypium barbadense varieties during pathogen inoculation. Sci. Data 2024 , 11 , 11. [ Google Scholar ] [ CrossRef ]
- Man, M.W.; Zhu, Y.Q.; Liu, L.L.; Luo, L.; Han, X.P.; Qiu, L.; Li, F.G.; Ren, M.Z.; Xing, Y.D. Defense Mechanisms of Cotton Fusarium and Verticillium Wilt and Comparison of Pathogenic Response in Cotton and Humans. Int. J. Mol. Sci. 2022 , 23 , 12217. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Qin, G.L.; Zhao, N.; Wang, W.R.; Wang, M.; Zhu, J.H.; Yang, J.; Lin, F.; Huang, X.L.; Zhang, Y.H.; Min, L.; et al. Glyphosate-Induced Abscisic Acid Accumulation Causes Male Sterility in Sea Island Cotton. Plants 2023 , 12 , 1058. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Su, X.J.; Zhu, G.Z.; Song, X.H.; Xu, H.J.; Li, W.X.; Ning, X.Z.; Chen, Q.J.; Guo, W.Z. Genome-wide association analysis reveals loci and candidate genes involved in fiber quality traits in sea island cotton ( Gossypium barbadense ). BMC Plant Biol. 2020 , 20 , 289. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, Y.; Yu, Y.H.; Wan, H.A.; Tang, J.; Ni, Z.Y. The sea-island cotton GbTCP4 transcription factor positively regulates drought and salt stress responses. Plant Sci. 2022 , 322 , 111329. [ Google Scholar ] [ CrossRef ]
- Wang, Y.; Yu, Y.H.; Wan, H.N.; Ni, Z.Y. Sea-island cotton ( Gossypium barbadense L.) GbTCP5 improves plant adaptation to drought and salt stress by directly activating GbERD7 , GbUBC19 , and GbGOLS2 expression. Ind. Crops Prod. 2023 , 203 , 117209. [ Google Scholar ] [ CrossRef ]
- Abdelraheem, A.; Zhu, Y.; Zeng, L.; Stetina, S.; Zhang, J. A genome-wide association study for resistance to Fusarium wilt ( Fusarium oxysporum f. sp. vasinfectum ) race 4 in diploid cotton ( Gossypium arboreum ) and resistance transfer to tetraploid Gossypium hirsutum . Mol. Genet. Genom. 2024 , 299 , 30. [ Google Scholar ] [ CrossRef ]
- Bustamante, M.I.; Todd, C.; Elfar, K.; Hamid, M.I.; Garcia, J.F.; Cantu, D.; Rolshausen, P.E.; Eskalen, A. Identification and Pathogenicity of Fusarium Species Associated with Young Vine Decline in California. Plant Dis. 2024 , 108 , 1053–1061. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zu, Q.; Deng, X.; Qu, Y.; Chen, X.; Cai, Y.; Wang, C.; Li, Y.; Chen, Q.; Zheng, K.; Liu, X. Genetic Channelization Mechanism of Four Chalcone Isomerase Homologous Genes for Synergistic Resistance to Fusarium wilt in Gossypium barbadense L. Int. J. Mol. Sci. 2023 , 24 , 14775. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Babilonia, K.; Wang, P.; Liu, Z.Y.; Jamieson, P.; Mormile, B.; Rodrigues, O.; Zhang, L.; Lin, W.W.; Clement, C.D.; de Moura, S.M.; et al. A nonproteinaceous Fusarium cell wall extract triggers receptor-like protein-dependent immune responses in Arabidopsis and cotton. New Phytol. 2021 , 230 , 275–289. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, W.; Guo, Y.; Xu, J.; Zhang, H.; Ma, Z.; Wu, H. Isolation of anthraquinone derivatives from Rubia cordifolia (Rubiaceae) and their bioactivities against plant pathogenic microorganisms. Pest Manag. Sci. 2024 . [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhu, Y.; Elkins-Arce, H.; Wheeler, T.A.; Dever, J.; Whitelock, D.; Hake, K.; Wedegaertner, T.; Zhang, J.F. Effect of growth stage, cultivar, and root wounding on disease development in cotton caused by Fusarium wilt race 4 ( Fusarium oxysporum f. sp. vasinfectum ). Crop Sci. 2023 , 63 , 101–114. [ Google Scholar ] [ CrossRef ]
- Zhu, Y.; Lujan, P.A.; Wedegaertner, T.; Nichols, R.; Abdelraheem, A.; Zhang, J.F.; Sanogo, S. First Report of Fusarium oxysporum f. sp. vasinfectum Race 4 Causing Fusarium Wilt of Cotton in New Mexico, USA. Plant Dis. 2020 , 104 , 588. [ Google Scholar ] [ CrossRef ]
- Sakane, K.; Akiyama, M.; Ando, A.; Shigyo, M.; Ito, S.-i.; Sasaki, K.J.P.; Pathology, M.P. Identification of a novel effector gene and its functional tradeoff in Fusarium oxysporum f. sp. cepae that infects Welsh onion. Physiol. Mol. Plant Pathol. 2023 , 123 , 101939. [ Google Scholar ] [ CrossRef ]
- Srivastava, V.; Patra, K.; Pai, H.; Aguilar-Pontes, M.V.; Berasategui, A.; Kamble, A.; Di Pietro, A.; Redkar, A. Molecular Dialogue During Host Manipulation by the Vascular Wilt Fungus Fusarium oxysporum . Annu. Rev. Phytopathol. 2024 , 62 . [ Google Scholar ] [ CrossRef ]
- Yang, D.; Zhang, X.; Ming, Y.; Liu, C.; Zhang, X.; Liu, S.; Zhu, L. Characterization of the High-Quality Genome Sequence and Virulence Factors of Fusarium oxysporum f. sp. vasinfectum Race 7. J. Fungi 2024 , 10 , 242. [ Google Scholar ] [ CrossRef ]
- Zhu, Y.; Abdelraheem, A.; Cooke, P.; Wheeler, T.; Dever, J.K.; Hake, K.; Bissonnette, K.; Zhang, J. Comparative analysis of infection process in Upland cottons differing in resistance to Fusarium wilt caused by Fusarium oxysporum f. sp. vasinfectum race 4. Crop Sci. 2023 , 63 , 1330–1343. [ Google Scholar ] [ CrossRef ]
- Maqsood, A.; Aslam, M.N.; Khaliq, H.; Shakeel, M.T.; Wu, H.; Fahad, S. Endophytic Bacillus spp. Mediated Plant Growth Promotion of Tomato Seedlings and Suppression of Meloidogyne incognita and Fusarium oxysporum Disease Complex. J. Plant Growth Regul. 2024 , 43 , 2454–2469. [ Google Scholar ] [ CrossRef ]
- Mokhtari, S.; Chavez, M.; Ali, A. First Report of Fusarium oxysporum f. sp. vasinfectum Causing Fusarium Wilt of Cotton in Kansas, USA. Plant Dis. 2023 , 107 , 1239. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cen, Z.X.; Hu, B.J.; Yang, S.Y.; Ma, G.L.; Zheng, Y.R.; Dong, Y. Mechanism of benzoxazinoids affecting the growth and development of Fusarium oxysporum f. sp. fabae . Plant Mol. Biol. 2024 , 114 , 42. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wen, T.; Ding, Z.X.; Thomashow, L.S.; Hale, L.; Yang, S.D.; Xie, P.H.; Liu, X.Y.; Wang, H.Q.; Shen, Q.R.; Yuan, J. Deciphering the mechanism of fungal pathogen-induced disease-suppressive soil. New Phytol. 2023 , 238 , 2634–2650. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yang, S.; Zhuo, Y.H.; Lin, Y.Q.; Huang, M.M.; Tang, W.; Zheng, W.H.; Lu, G.D.; Wang, Z.H.; Yun, Y.Z. The Signal Peptidase FoSpc2 Is Required for Normal Growth, Conidiation, Virulence, Stress Response, and Regulation of Light Sensitivity in Fusarium odoratissimum. Microbiol. Spectr. 2023 , 11 , e04403-22. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, N.; Lv, F.J.; Qiu, F.H.; Han, D.H.; Xu, Y.; Liang, W.X. Pathogenic fungi neutralize plant-derived ROS via Srpk1 deacetylation. EMBO J. 2023 , 42 , e112634. [ Google Scholar ] [ CrossRef ]
- Bani, M.; Cimmino, A.; Evidente, A.; Rubiales, D.; Rispail, N. Pisatin involvement in the variation of inhibition of Fusarium oxysporum f. sp pisi spore germination by root exudates of Pisum spp. germplasm. Plant Pathol. 2018 , 67 , 1046–1054. [ Google Scholar ] [ CrossRef ]
- Xu, W.H.; Wang, Z.G.; Wu, F.Z. Companion cropping with wheat increases resistance to Fusarium wilt in watermelon and the roles of root exudates in watermelon root growth. Physiol. Mol. Plant Pathol. 2015 , 90 , 12–20. [ Google Scholar ] [ CrossRef ]
- Wu, F.Z.; Liu, B.; Zhou, X.G. Effects of root exudates of watermelon cultivars differing in resistance to Fusarium wilt on the growth and development of Fusarium oxysporum f. sp. niveum . Allelopath. J. 2010 , 25 , 403–413. [ Google Scholar ]
- Yang, S.Y.; Zheng, Y.R.; Guo, Y.T.; Yang, Z.X.; Dong, Y. Faba bean-wheat intercropping and appropriate nitrogen supply control fusarium wilt in faba bean via altering specific amino acids in the root exudate of faba bean. Physiol. Mol. Plant Pathol. 2023 , 124 , 101961. [ Google Scholar ] [ CrossRef ]
- Yang, Y.; Wu, F.Z.; Liu, S.W. Allelopathic effects of root exudates of Chinese onion accessions on cucumber yield and Fusarium oxysporum f. sp. cucumerinum . Allelopath. J. 2011 , 27 , 75–85. [ Google Scholar ]
- Aro, N.; Pakula, T.; Penttilä, M. Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol. Rev. 2005 , 29 , 719–739. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chevalier, L.; Pinar, M.; Le Borgne, R.; Durieu, C.; Penalva, M.A.; Boudaoud, A.; Minc, N. Cell wall dynamics stabilize tip growth in a filamentous fungus. PLoS Biol. 2023 , 21 , e3001981. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pate, P.K.; Free, S.J. The Genetics and Biochemistry of Cell Wall Structure and Synthesis in Neurospora crassa , a Model Filamentous Fungus. Front. Microbiol. 2019 , 10 , 2294. [ Google Scholar ]
- Yoshimi, A.; Miyazawa, K.; Kawauchi, M.; Abe, K. Cell Wall Integrity and Its Industrial Applications in Filamentous Fungi. J. Fungi 2022 , 8 , 435. [ Google Scholar ] [ CrossRef ]
- Z hao, S.; Zhang, T.; Hasunuma, T.; Kondo, A.; Zhao, X.Q.; Feng, J.X. Every road leads to Rome: Diverse biosynthetic regulation of plant cell wall-degrading enzymes in filamentous fungi Penicillium oxalicum and Trichoderma reesei . Crit. Rev. Biotechnol. 2023 , 1–21. [ Google Scholar ] [ CrossRef ]
- Zhou, J.S.; Kang, L.Q.; Liu, C.C.; Niu, X.; Wang, X.J.; Liu, H.L.; Zhang, W.M.; Liu, Z.H.; Latge, J.P.; Yuan, S. Chitinases Play a Key Role in Stipe Cell Wall Extension in the Mushroom Coprinopsis cinerea . Appl. Environ. Microbiol. 2019 , 85 , e00532-19. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- El-Katatny, M.H.; Somitsch, W.; Robra, K.H.; El-Katatny, M.S.; Gubitz, G.M. Production of chitinase and beta-1,3-glucanase by Trichoderma harzianum for control of the phytopathogenic fungus Sclerotium rolfsii . Food Technol. Biotechnol. 2000 , 38 , 173–180. [ Google Scholar ]
- Muñoz, W.R.; Ramírez, M.I.; Molina, F.R.; Crespo, S.G.; Rodríguez-Medina, J.R. Myosin II is important for maintaining regulated secretion and asymmetric localization of chitinase 1 in the budding yeast Saccharomyces cerevisiae . Arch. Biochem. Biophys. 2003 , 409 , 411–413. [ Google Scholar ] [ CrossRef ]
- Pocsi, I.; Leiter, E.; Kwon, N.J.; Shin, K.S.; Kwon, G.S.; Pusztahelyi, T.; Emri, T.; Abuknesha, R.A.; Price, R.G.; Yu, J.H. Asexual sporulation signalling regulates autolysis of Aspergillus nidulans via modulating the chitinase ChiB production. J. Appl. Microbiol. 2009 , 107 , 514–523. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guo, J.; Mou, Y.; Li, Y.X.; Yang, Q.; Wang, X.; Lin, H.C.; Kang, Z.S.; Guo, J. Silencing a Chitinase Gene, PstChia1, Reduces Virulence of Puccinia striiformis f. sp. tritici . Int. J. Mol. Sci. 2023 , 24 , 8215. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Han, Y.J.; Song, L.L.; Peng, C.L.; Liu, X.; Liu, L.H.; Zhang, Y.H.; Wang, W.Z.; Zhou, J.; Wang, S.H.; Ebbole, D.; et al. A Magnaporthe Chitinase Interacts with a Rice Jacalin-Related Lectin to Promote Host Colonization. Plant Physiol. 2019 , 179 , 1416–1430. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhao, Y.L.; Li, C.; Chen, X.F.; Cheng, J.S.; Xie, J.T.; Lin, Y.; Fu, Y.P.; Jiang, D.H.; Chen, T. Overexpression of chitinase PbChia1 from Plasmodiophora brassicae improves broad-spectrum disease resistance of Arabidopsis. Virulence 2023 , 14 , 2233147. [ Google Scholar ] [ CrossRef ]
- Li, Y.; Liu, X.Y.; Liu, M.X.; Wang, Y.; Zou, Y.B.; You, Y.M.; Yang, L.N.; Hu, J.X.; Zhang, H.F.; Zheng, X.B.; et al. Magnaporthe oryzae Auxiliary Activity Protein MoAa91 Functions as Chitin-Binding Protein to Induce Appressorium Formation on Artificial Inductive Surfaces and Suppress Plant Immunity. Mbio 2020 , 11 , e03304-19. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lou, H.; Zhu, J.C.; Yang, Y.; Zhang, W. Effects of root secretions of resistant and susceptible varieties of cotton on the growth and gene expression of Fusarium spinosum . Biotechnol. Bull. 2023 , 39 , 156–167. [ Google Scholar ]
- Sun, J.; Yang, J.; Zhao, S.Y.; Yu, Q.; Weng, L.L.; Xiao, C.P. Root exudates influence rhizosphere fungi and thereby synergistically regulate Panax ginseng yield and quality. Front. Microbiol. 2023 , 14 , 1194224. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, X.G.; Wei, Q.; Liu, B.; Alam, M.S.; Wang, X.X.; Shen, W.J.; Han, Z.M. Root exudates of transgenic cotton and their effects on Fusarium oxysporum . Front. Biosci. Landmark 2013 , 18 , 725–733. [ Google Scholar ] [ CrossRef ]
- Were, E.; Schone, J.; Viljoen, A.; Rasche, F. Phenolics mediate suppression of Fusarium oxysporum f. sp. cubense TR4 by legume root exudates. Rhizosphere 2022 , 21 , 100459. [ Google Scholar ] [ CrossRef ]
- Tang, W.X.; Wang, G.S.; Chen, R.; Liu, X.; Chen, X.S.; Shen, X.; Yin, C.M.; Mao, Z.Q. Allium fistulosum L. Alleviates Apple Replant Disease by Suppressing Fusarium solani . J. Fungi 2022 , 8 , 1071. [ Google Scholar ] [ CrossRef ]
- Wu, F.Z.; Han, X.; Wang, X.Z. Allelopathic effect of root exudates of cucumber cultivars on Fusarium oxysporum . Allelopath. J. 2006 , 18 , 163–172. [ Google Scholar ]
- Wang, R.Q.; Wang, Y.T.; Yao, W.J.; Ge, W.G.; Jiang, T.B.; Zhou, B.R. Transcriptome Sequencing and WGCNA Reveal Key Genes in Response to Leaf Blight in Poplar. Int. J. Mol. Sci. 2023 , 24 , 10047. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yao, M.M.; Wang, X.H.; Long, J.H.; Bai, S.Y.; Cui, Y.Y.; Wang, Z.Y.; Liu, C.X.; Liu, F.L.; Wang, Z.J.; Li, Q.F. Identification of Key Modules and Candidate Genes for Powdery Mildew Resistance of Wheat-Agropyron cristatum Translocation Line WAT-2020-17-6 by WGCNA. Plants 2023 , 12 , 335. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cui, M.J.; Han, S.Y.; Wang, D.; Haider, M.S.; Guo, J.J.; Zhao, Q.; Du, P.; Sun, Z.Q.; Qi, F.Y.; Zheng, Z.; et al. Gene Co-expression Network Analysis of the Comparative Transcriptome Identifies Hub Genes Associated with Resistance to Aspergillus flavus L. in Cultivated Peanut ( Arachis hypogaea L.). Front. Plant Sci. 2022 , 13 , 899177. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, Q.B.; Pan, Y.B.; Su, Y.C.; Zou, W.H.; Xu, F.; Sun, T.T.; Grisham, M.P.; Yang, S.L.; Xu, L.P.; Que, Y.X. WGCNA Identifies a Comprehensive and Dynamic Gene Co-Expression Network That Associates with Smut Resistance in Sugarcane. Int. J. Mol. Sci. 2022 , 23 , 10770. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yang, Y.; Sossah, F.L.; Li, Z.; Hyde, K.D.; Li, D.; Xiao, S.J.; Fu, Y.P.; Yuan, X.H.; Li, Y. Genome-Wide Identification and Analysis of Chitinase GH18 Gene Family in Mycogone perniciosa . Front. Microbiol. 2021 , 11 , 596719. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McCreath, K.J.; Specht, C.A.; Liu, Y.L.; Robbins, P.W. Molecular cloning of a third chitinase gene ( CHT1 ) from Candida albicans . Yeast 1996 , 12 , 501–504. [ Google Scholar ] [ CrossRef ]
- Yang, C.; Yu, Y.Q.; Huang, J.K.; Meng, F.W.; Pang, J.H.; Zhao, Q.Q.; Islam, M.A.; Xu, N.; Tian, Y.; Liu, J. Binding of the Magnaporthe oryzae Chitinase MoChia1 by a Rice Tetratricopeptide Repeat Protein Allows Free Chitin to Trigger Immune Responses. Plant Cell 2019 , 31 , 172–188. [ Google Scholar ] [ CrossRef ]
- Tzelepis, G.D.; Melin, P.; Jensen, D.F.; Stenlid, J.; Karlsson, M. Functional analysis of glycoside hydrolase family 18 and 20 genes in Neurospora crassa . Fungal Genet. Biol. 2012 , 49 , 717–730. [ Google Scholar ] [ CrossRef ]
- Koch, A.; Wassenegger, M. Host-induced gene silencing—Mechanisms and applications. New Phytol. 2021 , 231 , 54–59. [ Google Scholar ] [ CrossRef ]
- Wang, Z.W.; Gao, X.; Zhong, S.; Li, Y.; Shi, M.R.; Zhang, B.R.; Zhang, S.C.; Shen, H.L.; Liu, X.L. Host-induced gene silencing of PcCesA3 and PcOSBP1 confers resistance to Phytophthora capsici in Nicotiana benthamiana through NbDCL3 and NbDCL4 processed small interfering RNAs. Int. J. Biol. Macromol. 2022 , 222 , 1665–1675. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ghag, S.B.; Shekhawat, U.K.S.; Ganapathi, T.R. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol. J. 2014 , 12 , 541–553. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, B.; Tian, J.; Feng, Z.D.; Wang, H.; Sun, J.; Kong, Z.S. Chitin synthases containing myosin motor-like domain are required for cell wall integrity and virulence of vascular wilt pathogen Verticillium dahliae . Phytopathol. Res. 2023 , 5 , 21. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Lou, H.; Zhu, J.; Zhao, Z.; Han, Z.; Zhang, W. Chitinase Gene FoChi20 in Fusarium oxysporum Reduces Its Pathogenicity and Improves Disease Resistance in Cotton. Int. J. Mol. Sci. 2024 , 25 , 8517. https://doi.org/10.3390/ijms25158517
Lou H, Zhu J, Zhao Z, Han Z, Zhang W. Chitinase Gene FoChi20 in Fusarium oxysporum Reduces Its Pathogenicity and Improves Disease Resistance in Cotton. International Journal of Molecular Sciences . 2024; 25(15):8517. https://doi.org/10.3390/ijms25158517
Lou, Hui, Jincheng Zhu, Zengqiang Zhao, Zegang Han, and Wei Zhang. 2024. "Chitinase Gene FoChi20 in Fusarium oxysporum Reduces Its Pathogenicity and Improves Disease Resistance in Cotton" International Journal of Molecular Sciences 25, no. 15: 8517. https://doi.org/10.3390/ijms25158517
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 26 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

How Alzheimer’s disease could be reversed through lifestyle changes: new research
- A plant-based diet, strength training exercise and meditation can help reverse the disease’s symptoms, study says

It is not often you hear a hopeful story or read an encouraging study about dementia, given the sobering statistics about the disease.
Someone in the world develops dementia every three seconds, according to the non-profit Alzheimer’s Disease International. More than 55 million people worldwide lived with dementia in 2020, a number that is expected to double every 20 years, reaching 78 million in 2030 and 139 million in 2050.
Dr Dean Ornish, a professor of medicine at the University of California San Francisco and founder and president of the non-profit Preventive Medicine Research Institute, also in California, paints a brighter picture in a paper published in June in the journal Alzheimer’s Research & Therapy.

He suggests that radical lifestyle changes might not only slow the progression of dementia, but could even reverse it.
It sounds too good to be true given the less encouraging stories of a cure for the disease. But Ornish has reason to be optimistic.
“People talk about Alzheimer’s disease today just like they used to talk about heart disease,” he said in a recent podcast from the non-profit Us Against Alzheimer’s. “Forty-five years ago, the best that was hoped for was that the progression of heart disease might be slowed, not reversed altogether.”
It has since been proven to be reversible.

Ornish’s rules for health – and a healthy brain – as we age are simple: “Eat well, move more, stress less and love more.”
Ornish’s study – admittedly a small study sample – reviewed 51 adults in their 70s who all had signs of mild cognitive impairment or early Alzheimer’s.
At the end of the five-month study period, 70 per cent of the control group had worse cognitive function. Of the group who engaged in healthy interventions, 70 per cent were either stable or markedly improved.
The changes in some individuals were significant. Several participants whose symptoms had seen them stop reading began to read again; a musician remembered his music; a businessman who had not been able to manage his affairs was able to again; and a number of people who had struggled to follow complicated movie plots were able to enjoy films again.
The positive changes among participants who undertook interventions were astonishing given the short length of the study, the authors said.
Cici Zerbe, a Californian now in her mid-80s, was one of the patients in the control group. Her dementia appeared to worsen during the study but by the end, she committed to changing her lifestyle radically.
Five years later, in the documentary The Last Alzheimer’s Patient made for CNN by Dr Sanjay Gupta, Zerbe opens the door to Gupta and greets him by name.
In the documentary, Zerbe says her symptoms have been reversed after changes she made following the end of the study. She now walks every day and admits to a big change in her diet. “I haven’t had a breaded veal cutlet in years,” she laughs.
Gupta, who has Alzheimer’s in his family, is acutely aware of his own risk and had his profile analysed for the documentary.

In the course of assessing his risk, he spoke to neurologist and researcher Dr Richard Isaacson, director of the Florida Atlantic University Centre for Brain Health.
Isaacson, who also has the disease in his family, describes the recent case of Simon Nicholls. Nicholls lost his mother to Alzheimer’s and, at 55, was concerned about his memory. He spent a year under Isaacson’s care to manage his risk.
“His brain grew and his belly size got smaller,” Isaacson notes in the documentary.
Gupta’s tests were all normal. Isaacson calls him a “walking modifiable risk factor for Alzheimer’s” – in other words, he can keep managing his lifestyle to minimise his chance of developing the disease.
There were no signs or amyloid plaques or tau tangles in his brain, but as Gupta notes, he still has to watch for them.
Isaacson’s tips for best brain health in old age include eating a mostly plant-based diet; exercising regularly; taking brisk walks, particularly while wearing a weighted belt; and wearing a glucose monitor to keep an eye on blood sugar fluctuations.

Ornish – who designed a diet that experts recognised as being among the top healthy diets in the world in 2024 – says the problem with Alzheimer’s disease at the moment is that it can isolate a person, which further hastens the development of the disease.
Changing the way you live now – whatever your age – for better brain health later is a message of hope, he says, not despair.

IMAGES
VIDEO
COMMENTS
a wider plantation size, thus reducing crop production. Therefore, many smart agricultural practices are. deployed to control plant diseases and pests. Most of these approaches, for example, use ...
Plant Disease is the leading international journal for rapid reporting of research on new, emerging, and established plant diseases. The journal publishes papers that describe translational and applied research focusing on practical aspects of disease diagnosis, development, and management in agricultural and horticultural crops.
There are some research papers previously presented to summarize the research based on agriculture (including plant disease recognition) by DL [43,54], but they lacked some of the recent developments in terms of visualization techniques implemented along with the DL and modified/cascaded version of famous DL models, which were used for plant ...
Res. Plant Dis. 2024;30 (2):199-205. Research in Plant Disease is an international journal for papers related to fundamental research that advances understanding of the nature of plant diseases and rapid reporting of research on new diseases, epidemics and methods for disease control.
The research papers for this study were selected using various keywords from peer-reviewed publications from various databases published between 2010 and 2022. A total of 182 papers were identified and reviewed for their direct relevance to plant disease detection and classification, of which 75 papers were selected for this review after ...
Journal of Plant Diseases and Protection is an international scientific journal dedicated to applied scientific aspects of plant pathology, plant health, and plant protection. It covers a wide range of interests for the global plant protection community with relevance to European plant health and protection. This journal bridges the gap between ...
This review provides the research progress of deep learning technology in the field of crop leaf disease identification in recent years. In this paper, we present the current trends and challenges for the detection of plant leaf disease using deep learning and advanced imaging techniques.
The National Academy of Sciences recently published an ambitious agricultural research agenda that emphasized the need for breakthrough technology for the early and rapid detection and prevention of plant diseases ().Emerging plant diseases are diseases that 1) have increased in either incidence, geographical, or host range; 2) have changed pathogenesis; 3) have newly evolved; or 4) have been ...
Among the 57 papers, 16 papers were selected for specific analysis based on the research object (plant disease) and research method (CNN). On this basis, the most recent research in 2022 is ...
Plant Disease is the leading international journal for rapid reporting of research on new, emerging, and established plant diseases. The journal publishes papers that describe translational and applied research focusing on practical aspects of disease diagnosis, development, and management in agricultural and horticultural crops.
The present shortcomings and limitations of offered detection of plant disease models are discussed and presented. In the year of 2020, the authors "MonuBhagat et al.", proposed a paper related to plant leaf disease detection with respect to classical Support Vector Classification methodology [4]. In this paper the authors illustrated such ...
Plant diseases and pests detection is a very important research content in the field of machine vision. It is a technology that uses machine vision equipment to acquire images to judge whether there are diseases and pests in the collected plant images [].At present, machine vision-based plant diseases and pests detection equipment has been initially applied in agriculture and has replaced the ...
At the same time, an early and deep understanding of plant disease epidemiology is needed to tackle future challenges ahead and to relate directly with the disease control strategies. Comprehensively, the Special Issue collected 13 original contributions (1 review, 1 perspective, and 11 research papers).
Here, we demonstrate the technical feasibility using a deep learning approach utilizing 54,306 images of 14 crop species with 26 diseases (or healthy) made openly available through the project PlantVillage ( Hughes and Salathé, 2015 ). An example of each crop—disease pair can be seen in Figure 1. Figure 1.
Plant Disease is the leading international journal for rapid reporting of research on new, emerging, and established plant diseases. The journal publishes papers that describe translational and applied research focusing on practical aspects of disease diagnosis, development, and management in agricultural and horticultural crops.
Plant Disease Detection using CNN. Emma Harte. School of Computing. National College of Ireland. Mayor Street, IFSC, Dublin 1. Dublin, Ireland. [email protected]. Abstract — Plant ...
In this paper, we are using ML to give a solution to Plant Diseases. In this method, we have divided the process into three stages Identity, Analyse and Verify with the Available database [9]. The key issues and challenges [10, 11] are identified by the researchers and the scientists, while analysing the leaf diseases of plant. Some of them are ...
The plant disease identification and classification method based on the FL-EfficientNet network proposed in this paper solves the problem of imbalance in the number of samples of different kinds of plant diseases by introducing the Focal loss function in the task of multi-class plant disease classification, and effectively improves the accuracy ...
The detection of the disease includes methods including. image segregation, pre-processing data, fragmentation of the image, detection, and recognition of characteristics. This paper also examines ...
Fusarium head blight (FHB) is mainly caused by Fusarium graminearum (Fg) and is a very widespread disease throughout the world, leading to severe damage to wheat with losses in both grain yield and quality. FHB also leads to mycotoxin contamination in the infected grains, being toxic to humans and animals. In spite of the continuous advancements to elucidate more and more aspects of FHB host ...
Identification of the plant diseases is the key to preventing the losses in the yield and quantity of the agricultural product. The studies of the plant diseases mean the studies of visually observable patterns seen on the plant. Health monitoring and disease detection on plant is very critical for sustainable agriculture. It is very difficult to monitor the plant diseases manually. It ...
Plant diseases are classified as infectious and non-infectious depending on the nature of a causative agent. The symptoms of the disease may depend on its cause, nature, and the location of the impact site. The factors causing plant diseases can be of biotic and abiotic nature. Non-infectious diseases are caused by unfavorable growth conditions ...
In rice, it was demonstrated that the presence of a specific seed-endophytic bacterium can shape an entirely disease-resistant plant phenotype 3. A high number of studies conducted during the last ...
We then reconstructed the evolution of macrofibril size in seed plants to study the transition from large to small macrofibrils (Fig. 2a). Amborella trichopoda is the earliest diverged extant angiosperm species (Amborella Genome Project, 2013), and its tracheids have a macrofibril diameter (average 28.3 nm) similar to coniferous gymnosperms meaning the transition to the smaller eudicot ...
A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the ...
Moreover, nanoparticles may stimulate plant defensive responses such as the manufacture of defense-related chemicals and the activation of defense signaling pathways, hence increasing the plant's resistance to diseases or pests [15 - 18]. It is vital to highlight the presence of accompanying issues and limits that demand careful study and ...
A public dataset of 54,306 images o f healthy and diseased plant leaves has be en used to train a deep convolutional neu ral network to. identify 14 crops and 26 diseases. An accuracy of 99.35% ...
This research lays the groundwork for developing new strategies to improve disease resistance in crops, particularly in non-model plants like tea. By leveraging the regulatory mechanisms of trans ...
WGCNA was first applied to medical research and has begun to be increasingly applied to agricultural research in recent years, making important contributions to the cultivation of disease-resistant germplasm resources by mining disease-causing-related genes of plant pathogenic bacteria [52,53,54]. The researchers used WGCNA to analyze the ...
New Alzheimer's disease research finds that a plant-based diet, strength training exercise and meditation can help reverse the symptoms of the most common type of dementia.