An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Soc Cogn Affect Neurosci
- v.17(5); 2022 May

Social cognitive network neuroscience
Anne c krendl.
Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN 47405, USA
Richard F Betzel
Over the past three decades, research from the field of social neuroscience has identified a constellation of brain regions that relate to social cognition. Although these studies have provided important insights into the specific neural regions underlying social behavior, they may overlook the broader neural context in which those regions and the interactions between them are embedded. Network neuroscience is an emerging discipline that focuses on modeling and analyzing brain networks—collections of interacting neural elements. Because human cognition requires integrating information across multiple brain regions and systems, we argue that a novel social cognitive network neuroscience approach—which leverages methods from the field of network neuroscience and graph theory—can advance our understanding of how brain systems give rise to social behavior. This review provides an overview of the field of network neuroscience, discusses studies that have leveraged this approach to advance social neuroscience research, highlights the potential contributions of social cognitive network neuroscience to understanding social behavior and provides suggested tools and resources for conducting network neuroscience research.
Over the past three decades, research from the field of social neuroscience has identified a myriad of brain regions that support social cognition—the process by which people understand, store and apply information about others (e.g. Mitchell, 2008 ; Adolphs, 2009 ; Kliemann and Adolphs, 2018 ). This research has provided fundamental insights into mapping discrete brain regions to specific social cognitive functions (e.g. theory of mind, face processing, stereotyping and prejudice). Recently, social neuroscience research has begun to leverage complex computational approaches, such as multivariate pattern analysis and psychophysiological interactions (PPIs), to better characterize functionality within brain regions ( Weaverdyck et al. , 2020 ; Parkinson, 2021 ) or between two brain regions (e.g. O’Reilly et al. , 2012 ; Cassidy et al. , 2016 ). However, these approaches may overlook the broader neural context in which individual brain regions are embedded.
The human brain is fundamentally a multiscale network. The average human brain contains 86 billion neurons with trillions of connections ( Azevedo et al. , 2009 ). These interconnections represent the anatomical scaffolding along which information is transferred throughout the brain and are therefore strongly related to the way the brain functions ( McIntosh, 2000 ; Park and Friston, 2013 ; Smith et al. , 2013 ). Human cognition requires integrating information across multiple brain regions (e.g. McIntosh, 2000 ), forming a distributed network composed of systems that support specialized brain function ( Bassett and Sporns, 2017 ). Thus, in addition to identifying the individual brain regions underlying social cognition, understanding their collective interactions and organization into systems may provide deeper insight into social behavior. The field of network neuroscience provides the mathematical framework for doing so, leveraging sophisticated tools for mapping the interactions within and among brain systems, modeling them as a network and understanding how those networks help to organize, segregate and integrate information ( Bassett et al. , 2018 ).
Network neuroscience has the potential to make important contributions to social neuroscience. Specifically, recent research suggests that examining brain function at a network level instead of at a region level may provide more comprehensive insight into how the brain gives rise to socially relevant behavior (see Tompson et al. , 2018 ). Consistent with this assertion, although studies examining age deficits in social cognitive function (e.g. theory of mind and deception detection) have identified age differences in the extent to which specific brain regions are engaged during social cognition, these patterns of activation are not necessarily linked to behavior ( Castle et al. , 2012 ; Moran et al. , 2012 ; Cassidy et al. , 2016 ). For example, one study examining older adults’ deficits in theory of mind found that they had weaker activation than young adults in the medial prefrontal cortex when performing theory of mind tasks, but it was unclear whether this related to their behavioral deficits ( Moran et al. , 2012 ). However, a recent study using a network neuroscience approach found that age-related deficits in connectivity mediated age deficits in theory of mind ( Hughes et al. , 2019 ). Together, these studies suggest that a network neuroscience approach may provide novel insights into understanding social behavior. In this review, our goal is to provide an overview of the field of network neuroscience and suggest several strategies by which social neuroscience can capitalize on these approaches to better characterize the social brain.
What is network neuroscience?
Network neuroscience is the study of brain networks—mathematical abstractions of the brain in which cells, populations or regions are modeled as nodes and their pairwise interactions as connections or edges ( Bullmore and Sporns, 2009 , 2012 ; Sporns and Betzel, 2016 ). (See Table 1 for glossary). This definition of a network is consistent with historical definitions of networks (e.g. Friston, 1994 ), even those preceding functional magnetic resonance imaging (fMRI) research (e.g. Mesulam, 1998 ). However, it is distinct from its other uses in recent neuroimaging and cognitive neuroscience research, where the term ‘network’ has been used to refer to a collection of voxels whose activities increase from baseline during a task, spatial components obtained from independent component analysis or functionally defined systems (e.g. the default-mode network). In this review, however, we use the term ‘network’ to refer to examining the brain as a collection of nodes linked to one another by edges (for discussion, see Uddin et al. , 2019 ).
Table 1.
Glossary of terms
| Term | Definition |
|---|---|
| Node | The smallest unit of interest in an analysis; may be a voxel (or grayordinate) or collection of spatially contiguous voxels grouped into a parcel |
| Edge | A measure of whether two nodes interact with one another or are connected, usually operationalized as a correlation. In general, can be weighted/binary or directed/undirected |
| Network | A collection of nodes interconnected to one another by edges. Also referred to as a graph |
| Module or community | Sub-networks of densely interconnected nodes embedded within a larger network |
| System | A special class of sub-network usually defined based on neuroscientific knowledge, e.g. groups of nodes known to be co-active across conditions. Often referred to by names that reference the cognitive functions, the system is supposed to subtend, e.g. somatomotor, visual and attention systems |
| Grayordinates | Gray matter vertices on a surface projection |
| Multilayer network | The connectivity between the same set of nodes may be across connectivity modality, time and individuals. Multilayer networks are a way of representing differential patterns of connectivity among those nodes using a single model |
In network models of the brain, connections typically come in two different ‘flavors’: structural or functional. Structural connections represent the physical and material pathways between brain regions. At the macroscale (measurable with MRI), they correspond to interregional white-matter pathways. Functional connections, on the other hand, represent statistical associations between the activity recorded from pairs of voxels, grayordinates or regions. Historically, they have been defined as ‘temporal correlations between spatially remote neurophysiological events’ ( Friston, 1994 , p. 57). In practice, functional connections are measured as a correlation, but could be estimated using a wide range of other measures ( Friston, 1994 ; Honey et al. , 2007 ; Smith et al. , 2011 ).
The functional connections that comprise brain networks are typically measured during ‘resting-state’—a period typified by the absence of explicit task instruction in which participants are engaged in undirected thought ( Greicius et al. , 2003 ; Meindl et al. , 2010 ). There are several important benefits to defining brain networks in this manner. First, resting-state connectivity is broadly related to the brain’s anatomical connections of white-matter fascicles ( Hagmann et al. , 2008 ; Honey et al. , 2009 ), suggesting that there is an overlap between structural and functional connectivity (see also Suárez et al. , 2020 ; Tovar and Chavez, 2021 ). However, dynamic changes in functional connectivity throughout resting state suggest that, although functional connectivity may be constrained by structural connectivity, the two are dissociable (e.g. Buckner et al. , 2013 ). Second, although the brain consumes as much as 25% of the body’s metabolic energy ( Herculano-Houzel, 2012 ), 60–80% of that energy is consumed during resting state, whereas only 0.5–1% of its energy is task-specific ( Raichle and Mintun, 2006 ). Finally, task-based state connectivity patterns are highly correlated with resting-state connectivity ( Smith et al. , 2009 ; Cole et al. , 2014 ; Hughes et al. , 2020 ), suggesting that resting-state connectivity may serve as a functional backbone, constraining task-evoked connectivity and offering more comprehensive insight into network reconfiguration (e.g. Damoiseaux et al. , 2006 ; see also Hughes et al. , 2019 ).
An important challenge to resting-state functional connectivity is that resting state, by definition, is an unconstrained period in which individuals’ minds are allowed to wander and are not constrained to a specific type, or even domain, of thought. As such, individual differences in functional connectivity patterns during resting state likely relate to differences in participants’ mental states during this task ( Buckner et al. , 2013 ; Gonzalez-Castillo et al. , 2021 ). Indeed, a recent study found that functional connectivity measured during naturalistic viewing (e.g. movie-watching) yielded more accurate predictions of individuals’ cognition and emotion (as measured in separate tasks) than did their resting-state functional connectivity patterns ( Finn and Bandettini, 2021 ). Moreover, this study found that although cognition was better predicted than emotion from either functional connectivity source, watching movies with social content gave the most accurate predictions for both cognition and emotion. The findings from this study raise important questions for future research about the suitability of resting-state vs passive movie-watching tasks for extracting functional connectivity patterns to relate to social behavior. In order to ascertain the most suitable approach for social cognitive network neuroscience research, future work should compare functional connectivity patterns from resting-state vs passive movie-watching and determine which best relates to explicit measures of targeted social behaviors.
In the following sections, we introduce some of the canonical findings from network neuroscience and discuss some of the technical challenges associated with the construction of brain networks from MRI data. Next, we review some of the ways that network neuroscience is being used to probe brain–behavior associations and for extending our understanding of ‘the social brain’, Finally, we conclude by discussing some of the latest methodological advances in network neuroscience and explore how they might be used within the context of social neuroscience.
Constructing brain networks from neuroimaging data
An important consideration in network neuroscience is how to construct brain networks from neuroimaging data. Although brain networks can be constructed using data collected from virtually any recording modality (see Box 1 ), here we will focus on fMRI data. Two of the greatest sources of variability across network studies using fMRI are (i) how to define regions of interest or parcels that later become the nodes in the network and (ii) how to measure the presence/absence of a functional connection between two regions and its weight (the edges between the nodes) (e.g. Smith et al. , 2011 ; Eickhoff et al. , 2015 ; Arslan et al. , 2018 ; Pervaiz et al. , 2020 ).
| Although our discussion of network analysis focuses primarily on fMRI approaches, networks can be defined using multiple other neuroimaging tools [e.g. diffusion tensor imaging, structural data, EEG, MEG and functional near-infrared spectroscopy (fNIRS)]. Diffusion imaging measures the microscopic motion of water molecules to detect the presence of white-matter fascicles using ‘tractography’ algorithms. These algorithms provide maps of the gray matter starting and end points of myelinated fiber bundles. The result is a (usually sparse) network of interregional white-matter connectivity ( , 2007; , 2008; , 2013). There exist a number of strategies for weighting edges. On one hand, one could simply weight edges based on the number of streamlines between two regions. This number can be inflated based on region (parcel) volume and surface area, so typically a correction is necessary. On the other hand, one could weight white-matter edges using biophysical measures, e.g. mean fractional anisotropy or mean diffusivity, which are related to fiber integrity. Additionally, networks can be constructed from structural data (T1 or T2 images). One popular strategy for doing so is to generate ‘structural covariance matrices’ ( , 2012; ). The weight of the edge between regions and is usually defined as the population-level covariance of and ’s cortical thickness (or some other structural measure). Accordingly, structural covariance matrices are typically defined at the group level. However, recent studies have extended this approach to the level of individual subjects by computing the covariance (or correlation) between ensembles of morphological metrics defined regionally (e.g. , 2018). |
| Apart from MRI data, networks can also be constructed from scalp and intracranial electroencephalography (sEEG and iEEG; e.g. , 2019; , 2019; , 2021), MEG (e.g. ., 2011; , 2021) and fNIRS (e.g. , 2013; , 2018) data. These methods record brain activity using electrical, magnetic and spectral properties. While some clinical conditions require placing recording electrodes directly onto the exposed cortical surface, more commonly EEG, MEG and fNIRS record signals on the scalp and generally offer poorer spatial resolution compared to fMRI. However, they acquire data at a frequency that is orders of magnitude faster than that of fMRI, making it possible (in principle) to detect and characterize changes in network structure with sub-second precision. Network nodes can be defined either as the sensors themselves, or, following source reconstruction, anatomically, which makes it possible to use familiar parcellation-based approaches for defining network nodes. The improved temporal resolution along with the oscillatory basis of EEG and MEG signals have contributed to the widespread use of phase-based measures of synchrony to define edge weights, e.g. phase-locking values. The spectral content of EEG and MEG is much broader than that of fMRI; it is common to define connectivity within specific canonical frequency ranges. |
Defining nodes
In principle, one could define nodes as the smallest possible unit of interest. In neuroimaging, this corresponds to voxels or surface vertices (grayordinates). However, voxel-wise networks are large (on the order of 10 5 number of nodes) and can present computational challenges. Additionally, many voxels and vertices connect similarly to the rest of the brain, suggesting that those voxels could be merged together without a loss of much information. There are numerous approaches to take to defining nodes, including functional specificity, topographic organization and connectivity ( Felleman and Van Essen, 1991 ; Van Essen and Glasser, 2018 ). However, in practice, most studies elect to parcellate the cerebral cortex into, roughly, hundreds of non-overlapping parcels (e.g. regions of interest) by assigning every voxel/vertex to one parcel.
There are, of course, many strategies for generating these parcellations. Early studies took advantage of existing divisions of the brain into regions based on anatomical (e.g. Automated anatomical labeling; Rolls et al. , 2020 ) or cytoarchitectonic information (e.g. Brodmann areas; Sporns, 2011 ). While these parcellations continue to be used, they have been largely supplanted by data-driven approaches in which parcels are defined using functional connectivity data ( Power et al. , 2011 ; Shen et al. , 2013 ; Gordon et al. , 2016 ; Schaefer et al. , 2018 ) and sometimes other microstructural properties (see Glasser et al. , 2016 ). The aim of these approaches is to generate functionally homogeneous parcels such that the voxels assigned to any given parcel exhibit similar patterns of connectivity with respect to the rest of the brain. Additionally, parcels are usually defined to be spatially contiguous and should be generalizable, so that when they are imposed on new brains, the resulting parcels are still functionally homogeneous. Homogeneity, as it is discussed here, does not refer to the blood-oxygen-level-dependent (BOLD) signal, but rather is assessed using similarity or distance-based metrics on measures such as functional or anatomical connectivity, cytoarchitectural properties or topography.
Recently, it has become clear that parcels generated from pooled, group-averaged functional connectivity may systematically distort individual features ( Braga and Buckner, 2017 ; Gordon et al. , 2017 ; Gratton et al. , 2018 ), igniting new efforts to generate flexible parcellations that can adapt group-level parcels to individual brains ( Chong et al. , 2017 ; Bijsterbosch et al. , 2018 ; Kong et al. , 2019 ; Mejia et al. , 2020 ) by leveraging data collection tools such as multi-echo fMRI ( Lynch et al. , 2020 ). Using individual parcellations may be particularly beneficial for social cognitive network neuroscience research, given that its goal is to estimate individual differences in social behavior from brain networks (see Mwilambwe-Tshilobo et al. , 2019 for a relevant example).
In network analyses, parcels are treated as nodes. In general, the choice of parcellation will impact the properties of a network such that two different parcellations of the same brain can exhibit contradictory properties or provide misleading summaries of interregional connectivity ( Wang et al. , 2009 ; Zalesky et al. , 2010 ). Since there is, generally, no ground truth by which to assess the validity of any parcellation, it is difficult to unambiguously and objectively determine which parcellation is ‘best’. Even measures of parcellation quality, e.g. the average homogeneity of parcels, can be biased by the number and size of parcels, with finer parcellations exhibiting greater levels of homogeneity ( Gordon et al. , 2016 ). Further complicating this process is the fact that parcel boundaries vary across conditions ( Salehi et al. , 2020 ) and time ( Iraji et al. , 2019 ), thereby resulting in there not being a universally optimal parcellation.
To increase the reliability and replicability of their parcellations, some of the more widely cited parcellations are those that were generated (and validated) using large datasets (e.g. Power et al. , 2011 ; Yeo et al. , 2011 ; Schaefer et al. , 2018 ). For example, Yeo and colleagues (2011) used resting-state data collected from 1000 brains to identify network parcellations. They generated their initial network structure from a subset of 500 brains and replicated the structure across the second set of 500. Using this approach, they ultimately identified (and cross-validated) 17 putative systems that largely divide seven core cognitive domains—visual, somatomotor, default mode, limbic, dorsal attention, ventral attention and frontoparietal ( Yeo et al. , 2011 ). At present, one of the most widely used atlases was developed by Schaefer and colleagues (2018) using a multi-modal approach and data from nearly 1500 participants. Similar to the approach by Yeo and colleagues, the data were divided in half to create a discovery and replication sample. Rather than a fixed number of nodes, this study resulted in a multiresolution network parcellation comprising between 100 and 1000 parcels (in increments of 100), each of which could be mapped to one of 17 validated brain systems, analogous to those in the Yeo atlas ( Yeo et al. , 2011 ). Importantly, the parcels generated by Schaefer et al. were more functionally homogeneous compared to a set of comparable parcellations (for discussion, see Schaefer et al. , 2018 ), including the well-known Gordon atlas ( Gordon et al. , 2016 ). Although these differences emphasize the importance of standardizing approaches, they also demonstrate that using a parcellation that was defined in a rigorous manner may reduce the likelihood of spurious findings (for discussion, see Arslan et al. , 2018 ).
Since nodes vary across conditions ( Salehi et al. , 2020 ), another approach is to define the nodes of interest through a task-based localizer ( Chai et al. , 2016 ; Schmälzle et al. , 2017 ; Hughes et al. , 2019 ). Although such approaches are less common, they may provide a more targeted and hypothesis-driven method for modeling resting-state and especially task-based functional connectivity. For example, one study found that global vs specific task-defined parcellations had dissociable effects in predicting task performance on language tasks ( Bansal et al. , 2018 ). Two recent social neuroscience studies have also used this approach. One compared functional connectivity within the default-mode system to connectivity derived from a task-based localizer (e.g. Hughes et al. , 2019 ) and found that only the latter predicted social behavior (theory of mind performance). Another study found that connectivity between nodes in a theory-defined ‘mentalizing system’ during a social exclusion task predicted aspects of adolescent males’ friendship networks ( Schmälzle et al. , 2017 ). An important future direction for social cognitive network neuroscience research will be to evaluate the effectiveness of using a data-driven vs theory-driven approach in predicting social behavior.
Defining edges
A second, but oftentimes less explored, source of variation in network organization is how one determines the presence or absence of a functional connection and its weight (the edges between pairs of nodes). In general, functional connectivity is defined as a measure of statistical dependence between activities recorded from distinct neural sources ( Friston, 1994 ; Sporns, 2010 ; Figure 1A–C ). In practice, however, resting-state functional connectivity is almost always calculated as a temporal correlation of activity, i.e. a Pearson correlation ( Smith et al. , 2009 ). Note that in studies of task-evoked connectivity, additional preprocessing steps need to be performed prior to computing the correlation. Specifically, activations driven by presentation should be regressed out so that they do not artifactually drive correlations ( Cole et al. , 2019 ).

Functional network construction and canonical properties of brain networks. Functional networks are constructed by extracting fMRI BOLD time courses from voxels, grayordinates or parcels (A) and computing their temporal correlation (B). Note that, in principle, other measures of statistical dependence can be substituted, e.g. that account for nonlinearities. This process is repeated for all pairs of neural elements, usually defined to be parcels. The result is a correlation matrix that is referred to as a ‘functional network’ (C). The network can be viewed in anatomical space by drawing a circle (node) at the spatial center of each parcel and linking connected nodes by lines (edges). The resulting network can be analyzed with tools from network neuroscience. These tools can (D) track the flow of information through the network by studying paths, (E) identify influential nodes based on the number of connections (a node’s degree), (F) assess the contribution of the brain’s spatial embedding on its network architecture (in general, brain networks prefer to form low-cost, short-range connections rather than costly long-distance connections) and (G) identify functionally specialized sub-networks known as modules or communities.
Other studies have used temporal precedence measures to establish pseudo-causal relationships between time series, e.g. Granger causality and transfer entropy ( Smith et al. , 2011 ; Holper et al. , 2012 ; Maki-Marttunen et al. , 2013 ; Ribeiro et al. , 2021 ). Broadly, these measures test whether the past activity of one region helps predict the future of another region’s activity above and beyond what its own activity can predict. While temporal precedence measures like Granger causality and transfer entropy hold tremendous promise in revealing directed interactions between brain regions, their accurate estimation requires large amounts of data and, in the case of transfer entropy, necessitate the binarization of time series or force the user to make assumptions about the underlying distribution of brain activity. Finally, care must be taken in interpreting these measures. While their application to spike trains and cellular levels lead to straightforward interpretations, the slow and serially correlated fMRI BOLD signal (itself an indirect measure of population-level activity) may obscure true causal relationships.
In general, any bivariate measure of statistical dependence can be used to assess the presence, absence and/or weight of an edge. However, the decision to use a particular measure should be motivated by the experimental paradigm. For example, PPI, which assesses the effect of tasks influence the coupling strength between two regions ( Friston et al. , 1997 ; O’Reilly et al. , 2012 ), should only be used for tasks, and for maintaining continuity with extant literature. Introducing a novel measure when an existing measure is capable of capturing the desired effect makes it challenging to compare the present results against those from previous studies and may have the unwanted effect of confusing readers.
As with node definition, how one defines a functional connection has implications for the organization of the inferred network. Different measures also have the capacity to summarize different modes of coupling between network nodes. For instance, the commonly used Pearson correlation assesses the linear relationship between the activities recorded from two nodes. However, if that relationship is nonlinear, the Pearson correlation might be misleading. In contrast, measures like mutual information may be better suited for capturing generic, nonlinear relationships between nodes ( Smith et al. , 2011 ; Pervaiz et al. , 2020 ).
Figure 2 provides an overview of the steps discussed in this section. In summary, social cognitive network neuroscience should carefully consider how to reduce variability in node selection and in how functional connectivity is calculated. One potential way to do this would be to build on existing models from the field of network neuroscience, such as using well-studied network parcellations (e.g. Schaefer et al. , 2018 ). In addition to providing a clearer foundation upon which the field can evolve, such an approach would provide more domain-general insights into social neuroscience.

Constructing brain networks from neuroimaging data. Graphical depiction of the suggested pipeline for constructing brain networks. Each step is associated with multiple user-defined parameters and options, whose strengths and weaknesses are discussed in detail in the review. Several choices (e.g. selecting the dataset to use in the first step) will largely be dictated by the research question. The steps are presented in sequential order but are not intended to be prescriptive. Rather, they are intended to offer a summary of the key steps discussed in this review and are presented for convenience and clarity. Constructing a network begins with data selection (Step 1). Traditionally this meant collecting data under different conditions (while performing a task, while passively listening/viewing audiovisual stimuli or in the absence of explicit task instruction). Recent initiatives, however, have made high-quality data publicly available, organized according to field-defined standards and processed via distributed computing. After data selection, data undergo basic preprocessing steps (Step 2; alignment, intensity normalization, nuisance regression, frame censoring, etc.). This procedure results in ‘cleaned’ voxel/vertex time series. In most network analyses, the dimensionality of these data is reduced via a parcellation step (Step 3), in which voxels/vertices are aggregated into parcels. Parcels may be determined a priori based on existing atlases, meta-analytic activation maps or using localizers to co-locate similar functional territories across individuals. The final step (Step 4) is to establish whether a connection exists between pairs of parcels (nodes). In human neuroimaging (especially of the resting brain), the convention is to estimate connection strength based on the extent to which two regions’ activity time courses are correlated with one another. Other measures (e.g. mutual information, spectral coherence and Granger causality) are discussed in the review.
Organizing principles of human brain networks
Brain network analyses necessarily shift focus away from measuring activation in specific brain regions and instead emphasize on how different parts of the brain interact with one another as components of distributed networks. This increasingly global view allows for network neuroscientists to identify the overarching principles by which brains are organized and operate. Inspired by advances in other scientific disciplines ( Watts and Strogatz, 1998 ), early work in network neuroscience focused on ‘small-worldness’ ( Sporns and Zwi, 2004 ; Bassett and Bullmore, 2006 ), which refers to the propensity for nervous systems to simultaneously exhibit locally dense (interconnected) clusters and shorter-than-expected path length. These two characteristics are thought to support specialized information processing and rapid transmission of information, respectively ( Figure 1D ). Small-world organization has been observed in brain networks across phylogeny and at virtually every spatial scale, from synaptic contacts among single cells ( Latora and Marchiori, 2001 ; Varshney et al. , 2011 ) to large-scale brain networks ( Iturria-Medina et al. , 2008 ; Muldoon et al. , 2016 ).
More recent studies have provided evidence that brain networks are organized around an exclusive set of hub regions—highly connected and highly central regions that occupy positions of influence within the brain ( Hagmann et al. , 2008 ; Power et al. , 2013 ). Because of high levels of connectivity, these regions are capable of both delivering and receiving information to and from large portions of the brain, respectively ( Figure 1E ). Moreover, these putative hubs tend to be connected to one another, forming an integrative structure known as a ‘rich club’, which serves as a backbone for efficient information transfer ( Zamora-López et al. , 2010 ; van den Heuvel and Sporns, 2011 ). Once again, hubs and rich clubs are conserved across phylogeny ( Harriger et al. , 2012 ; de Reus and van den Heuvel, 2013 ; Towlson et al. , 2013 ; Shih et al. , 2015 ).
Other studies have suggested that embedding the brain in three-dimensional space serves as an overarching organizing principle ( Stiso and Bassett, 2018 ; Figure 1F ). All things equal, long-distance connections require proportionally more material and energy, of which the brain has limited amounts, than short-range connections. Consequently, brains need to balance the formation of functionally adaptive features like efficient processing paths, hubs and rich clubs with the material and metabolic cost of forming and supporting those features ( Kaiser and Hilgetag, 2006 ; Samu et al. , 2014 ). This trade-off restricts the types of features that brain networks can support simultaneously and gives rise to a heavy-tailed distribution of connection lengths that favors short (low-cost) connections ( Ercsey-Ravasz et al. , 2013 ; Betzel and Bassett, 2018 ).
Studying brain networks as sub-networks, modules and systems
One of the hallmark features of biological neural networks—and one that is increasingly becoming the focus of network science applications in neuroimaging and cognitive neuroscience—is their decomposability into cohesive sub-networks known as ‘modules’ or ‘communities’ ( Power et al. , 2011 ; Sporns and Betzel, 2016 ; Yeo et al. , 2011 ; Figure 1G ). Modular structure is evident at all spatial scales ( Jarrell et al. , 2012 ; Betzel and Bassett, 2018 ), but it has been investigated in depth at the macroscale using human fMRI data. At rest, modules correspond closely to patterns of task-evoked activity and delineate well-known functional systems ( Smith et al. , 2009 ; Crossley et al. , 2013 ) and at multiple resolutions ( Gordon et al. , 2020 ). The correspondence of module boundaries with well-established functional systems suggests that the brain’s modular structure helps support functionally specialized processing ( Stevens and Spreng, 2014 ).
For example, partitions of functional brain networks identify modules corresponding to frontoparietal and both dorsal and ventral attention systems, which include collections of brain regions known to play central roles in control (for review, see Scolari et al. , 2015 ), top-down guided attention and processing of sensory or perceptual information, respectively ( Corbetta and Shulman, 2002 ; Vossel et al. , 2014 ). The default-mode system, one of the most widely studied collections of regions in the brain, has the unique property of being more active during resting than task states (see Raichle, 2015 ). Numerous animal studies have also identified a homologous default-mode system in non-human primates, rats and mice ( Mantini et al. , 2011 ; Lu et al. , 2012 ; Stafford et al. , 2014 ). Within humans, the default-mode system has been implicated in a myriad of functions, including receiving and conveying sensory information from the external world (for review, see Raichle, 2015 ), mind wandering ( Andrews-Hanna et al. , 2010 ) and, directly relevant in the current review, social cognition (e.g. Mars et al. , 2012 ; Meyer, 2019 ).
Modules represent collections of densely connected brain regions that, on their own, are thought to support specific cognitive functions. Complex cognition, then, is thought to arise from interactions between these modules. Accordingly, relating functional connectivity strength within and between modules during resting and/or task states to behavior has been one of the most common applications of network neuroscience, with applications in personality neuroscience ( Markett et al. , 2018 ), cognitive neuroscience ( Medaglia et al. , 2015 ) and even social neuroscience ( Schmälzle et al. , 2017 ; Wasylyshyn et al. , 2018 ; Hughes et al. , 2019 ). This approach will be discussed in more detail in a later section.
There are several key takeaways from these sections. First, the field of network neuroscience studies brain networks, which models brain regions as nodes and their pairwise functional interactions as connections. Second, small-world organization, hubs and short path length are key organizing principles of networks. Third, sub-networks or modules support specific cognitive functions. These features are expressed to some extent in virtually all brains and are thought to be critical ‘ingredients’ for healthy brain function. An important future direction for social cognitive network neuroscience research will be to apply these principles to social behavior, specifically. This includes, but is not limited to, identifying how modules and their interactions give rise to social cognition and better understanding of the roles of hubs and rich clubs in social cognition, by mediating the flow of information between modules. In the next section, we explore how network neuroscience techniques have been applied to advance our understanding of social behavior.
Social cognitive network neuroscience and the default mode
The limited work applying a network neuroscience approach to understanding social behavior has focused primarily on within- and/or between-module functional connectivity patterns. One of the primary modules of interest in this research has been the default mode, which is generally viewed as comprising a core aspect of the social brain ( Mars et al. , 2012 ; Meyer, 2019 ). The default mode is typically defined as a set of brain regions that are more active when the brain is at rest than during a task ( Raichle et al. , 2001 ). An emerging body of research applying network neuroscience techniques to social behavior has shown that default-mode connectivity relates to numerous aspects of social behavior, including perceived social isolation ( Spreng et al. , 2020 ), theory of mind ( Hughes et al. , 2019 ), social rejection ( Schmälzle et al. , 2017 ; Wasylyshyn et al. , 2018 ), conformity ( Wasylyshyn et al. , 2018 ), creativity ( Beaty et al. , 2019 ) and even real-world social outcomes (e.g. Falk and Bassett, 2017 ; Joo et al. , 2017 ; Pillemer et al. , 2017 ; Schmälzle et al. , 2017 ; Hyon et al. , 2020 ; Tompson et al. , 2020 ).
A recent study examined whether functional connectivity differed as a function of being socially included or excluded ( Schmälzle et al. , 2017 ). The study had adolescent males perform the Cyberball task ( Williams and Jarvis, 2006 ), a widely used manipulation of social exclusion, and evaluated whether being socially excluded during the task was associated with increased within-network connectivity in networks related to mentalizing or social pain. Mentalizing and social pain were targets of interest in this study because prior work has shown that being socially excluded is painful ( Rotge et al. , 2015 ) and elicits greater activity from brain regions associated with mentalizing (inferring other people’s mental states; Powers et al. , 2013 ). The parcellations in this study were operationalized in two ways: first, by using theory-driven approach that identified nodes associated with mentalizing and social pain using meta-analytic data from NeuroSynth ( Yarkoni et al. , 2011 ); and second, by using a data-driven whole-brain network parcellation approach. Both approaches used largely overlapping nodes: mentalizing consisted primarily of nodes within the default-mode system, whereas social pain comprised nodes from portions of the salience and cingulo-opercular systems. The results were consistent using both the theory- and data-driven approaches: when participants were socially excluded, they showed higher within-system connectivity in the mentalizing system than when they were included. No significant changes in connectivity were observed within the social pain system nor were there changes in between-system connectivity. Another study found that increased within-system connectivity in the mentalizing and social pain systems during social exclusion predicted adolescent males’ increased likelihood to subsequently comply with perceived social norms (e.g. be more or less risky while in a driving simulator; Wasylyshyn et al. , 2018 ).
An interesting aspect of the finding by Wasylyshyn and colleagues (2018) is that it demonstrated that connectivity during the social exclusion task predicted behavior outside of the scanner. Similarly, a recent study found that resting-state functional connectivity was associated with theory of mind performance ( Hughes et al. , 2019 ). Specifically, Hughes and colleagues (2019) examined resting-state connectivity within a localizer-defined theory of mind system in the default mode for young adults (individuals between the ages of 18 and 25) and older adults (individuals over the age of 65). They found that age differences in resting-state connectivity within this system predicted older adults’ theory of mind deficits on a separate task. Importantly, overall age deficits in global default-mode connectivity did not predict older adults’ theory of mind deficits, suggesting that theory-driven approaches (e.g. focusing on a sub-system or a localizer-defined system) may provide more insight into social behavior in some cases than a strictly data-driven approach. An important implication of this study is that it suggests that resting-state functional connectivity may constrain social behavior. Related to this finding, Christov-Moore and colleagues (2020) examined whether resting-state connectivity predicted empathic concern, which is essential for everyday communication and survival in the social environment ( Eisenberg and Strayer, 1987 ). The authors found that greater resting-state connectivity within the somatomotor system predicted greater empathic concern.
A burgeoning area of interest has been to use network neuroscience techniques to examine real-world social outcomes, including loneliness ( Spreng et al. , 2020 ), and the number and structure of individuals’ social relationships (e.g. Falk and Bassett, 2017 ; Schmälzle et al. , 2017 ; Hyon et al. , 2020 ). These studies have examined both resting-state (e.g. Spreng et al. , 2020 ) and task-based connectivity (e.g. Schmälzle et al. , 2017 ). With respect to the former, individuals with greater within-system resting-state default-mode connectivity had higher levels of loneliness (perceived social isolation; Spreng et al. , 2020 ). This finding has important implications for social neuroscience research because longitudinal and cross-sectional studies have found that, even when controlling for other risk factors (e.g. socioeconomic status and cognitive and physical health), loneliness is associated with poorer mental, physical and cognitive health and higher mortality rates ( Cornwell and Waite, 2009 ; Cacioppo et al. , 2010 ; Luo et al. , 2012 ; Perissinotto et al. , 2012 ; Kuiper et al. , 2015 ).
Several recent studies have also examined whether functional connectivity predicts aspects of individuals’ personal social networks—the group of people with whom an individual is socially embedded ( Joo et al. , 2017 ; Pillemer et al. , 2017 ; Schmälzle et al. , 2017 ; Tompson et al. , 2020 ). These studies primarily have examined connectivity as it relates to unique individual’s social connections ( Schmälzle et al ., 2017 ; Pillemer et al. , 2017 ; Hyon et al. , 2020 ; Tompson et al. , 2020 ; but see, Joo et al. , 2017 ). In some cases, these studies have been more qualitative, focused on identifying patterns of resting-state connectivity that predict features of an individual’s social network ( Joo et al. , 2017 ; Pillemer et al. , 2017 ). For example, one study with older adults found that their resting-state connectivity in a subcomponent of the default-mode system was positively related to the number of individuals in their network, whereas connectivity within the frontoparietal system was positively related to the number of network members with whom the older adult was close (e.g. was in contact with at least biweekly; Pillemer et al. , 2017 ).
Other studies, however, have used task-based connectivity to explore potential mechanisms underlying the relationship between functional connectivity and individuals’ social networks ( Schmälzle et al. , 2017 ; Tompson et al. , 2020 ). For example, Schmälzle and colleagues (2017) explored the possibility that changes in adolescents’ functional connectivity in response to being socially excluded might relate to the structure of their social network. They found that having greater within-system connectivity in a subcomponent of the default-mode system during social exclusion predicted having less dense (e.g. fewer interconnections among friendships) social networks.
Another potential application of network neuroscience methods to understanding social behavior is to determine whether social intelligence may facilitate non-social cognitive performance. To do this, Tompson and colleagues (2020) examined whether greater engagement of ‘social brain’ networks offsets adolescent males’ underdeveloped or underutilized inhibitory abilities to improve their cognitive performance. The two social brain systems of interest in this study were theory-defined self-referential and mentalizing brain systems. They also examined a theory-defined inhibition system. They found that adolescent males who performed better on a measure of inhibition (a go/no-go task) had stronger connectivity between the self-referential and response inhibition systems and weaker within-system connectivity in the self-referential system. Moreover, they found that the relationship between task performance and greater between-system connectivity was most pronounced for adolescents with less dense social networks. Together, these findings suggest that some aspects of social functioning may facilitate non-social cognitive function.
Although this section has focused primarily on the default-mode system, it is important to note that there are multiple other systems that play a key role in social cognition (e.g. Lamm et al. , 2011 ; Alcalá-López et al. , 2018 ; Redcay and Schilbach, 2019 ). A recent meta-analysis that classified regions comprising the “social brain connectome” ( Alcalá-López et al. , 2018 ) provides a potential overview of different “social brain systems”. Specifically, the authors identified four main functional systems, including a visual-sensory system, composed of the fusiform gyrus and superior temporal sulcus, a limbic system, composed of the amygdala, hippocampus and nucleus accumbens, and two cognitive systems: one that included the anterior insula, middle cingulate cortex and inferior frontal gyrus, and a second that included the dorsal medial prefrontal cortex, posterior cingulate cortex, precuneus and temporoparietal junction. Future work should align the social brain systems with those commonly defined by network neuroscience work to bring parsimony between the two fields.
Together, these studies provide important insights into a myriad of potential applications of a social cognitive network neuroscience approach. Identifying the brain systems and interactions among systems that give rise to social behavior is an important future direction for social neuroscience research. A social cognitive network neuroscience approach that builds on extant research from the field of network neuroscience thus may provide a more comprehensive and accurate map of the ‘social brain’ (e.g. Charpentier and O’Doherty, 2018 ; Kliemann and Adolphs, 2018 ). We next review research from the fields of personality and cognitive neuroscience that uses network neuroscience techniques to study individual differences and cognition. Our goal is to highlight potential techniques or approaches that social cognitive network neuroscience research may leverage to study social behavior.
Using functional connections to characterize individual differences and behavior
To date, one of the most common approaches to applying network neuroscience techniques to personality neuroscience, cognitive neuroscience and social neuroscience research has been to measure functional connectivity strength within or between modules during resting and/or task states and relate it to behavior or performance ( Medaglia et al. , 2015 ; Schmälzle et al. , 2017 ; Markett et al. , 2018 ; Wasylyshyn et al. , 2018 ; Hughes et al. , 2019 ). A basic premise of this approach is that weaker connectivity within a module (sometimes referred to as dysregulation) during resting state is considered a measure of relative dysfunction in that module (for review, see Ferreira and Busatto, 2013 ). However, increased between-module connectivity during task performance is generally considered to facilitate performance because it is thought to relate to the exchange of task-relevant information between systems (e.g. Cohen and D’Esposito, 2016 ; Rosenberg et al. , 2016 ; Bassett and Mattar, 2017 ). Studies examining fixed behaviors (e.g. personality traits) have tended to relate the behavior of interest to resting-state connectivity, whereas studies examining transient behaviors (e.g. attention) have tended to relate the behavior of interest to task-based connectivity.
Personality neuroscience research has frequently used measures of within-module resting-state functional connectivity to predict a myriad of individual differences in real-life function ( Vaidya and Gordon, 2013 ; Dubois and Adolphs, 2016 ; Bassett and Sporns, 2017 ; Shen et al. , 2017 ; Markett et al. , 2018 ; Christov-Moore et al. , 2020 ), including intelligence (e.g. Song et al. , 2008 ; Cole et al. , 2012 ; Smith et al. , 2015 ), attention ( Finn et al. , 2015 ), cognitive control ( Marek et al. , 2015 ; Spielberg et al. , 2015 ) and working memory ( Cohen and D’Esposito, 2016 ). Within-module resting-state functional connectivity patterns also predict other individual differences, including mind wandering ( Wang et al. , 2018 ), lifestyle factors, including education, income and life satisfaction ( Smith et al. , 2015 ), and socially relevant traits, such as empathic concern ( Christov-Moore et al. , 2020 ) and creativity ( Beaty et al. , 2019 ). Challenges to interpreting relationships between resting-state functional connectivity and these myriad of individual differences arise due to the noteworthy heterogeneity in participants’ mental states during resting state (e.g. Buckner et al. , 2013 ; Gonzalez-Castillo et al. , 2021 ). Indeed, a recent study found that functional connectivity patterns associated with passive movie-watching better predicted participants’ cognition and emotion than did their functional connectivity patterns during resting state ( Finn and Bandettini, 2021 ). Future work is thus needed to identify the optimal states in which to measure functional connectivity patterns.
Cognitive neuroscience research, however, has explored the extent to which task-based between-network connectivity facilitates performance ( Medaglia et al. , 2015 ). The premise of this work is that greater between-network connectivity reflects the exchange of task-relevant information between systems (e.g. Cohen and D’Esposito, 2016 ; Rosenberg et al. , 2016 ; Bassett and Mattar, 2017 ). For example, Rosenberg and colleagues (2016) found that stronger functional connectivity between motor and visual systems during a sustained attention task predicted better performance than having stronger connectivity between temporal and parietal regions. Another study compared connectivity during resting state to connectivity during a motor and working memory task ( Cohen and D’Esposito, 2016 ). They predicted that since the motor task likely engaged one system (e.g. somatomotor), but the working memory likely engaged multiple systems (e.g. visual, frontoparietal and somatomotor), greater between-system connectivity would facilitate working memory, but not motor, performance. Indeed, this is what the authors found.
Another approach to using functional connectivity strength as a measure of behavior or performance is to examine the extent to which connectivity flexibly reconfigures within and between modules during both rest ( Betzel et al. , 2017 ) and task states ( Bassett et al. , 2011 ; Cole et al. , 2013 ; Vatansever et al. , 2015 ; Shine et al. , 2016 ). For example, greater variations in flexible reconfiguration of connectivity during resting state predicts individual differences in positive affect ( Betzel et al. , 2017 ). Moreover, although motor learning is accompanied by increased autonomy of visual and somatomotor systems ( Bassett et al. , 2015 ), greater flexibility during a motor learning task predicts improved performance ( Bassett et al. , 2011 ). Other work has shown that the frontoparietal system, which is involved in cognitive control (for review, see Scolari et al. , 2015 ), flexibly reconfigures connectivity to other systems to support ongoing task demands ( Cole et al. , 2013 ). These studies suggest that although there is a great deal of overlap in system configuration between resting and task states (e.g. Cole et al. , 2014 ; Krienen et al. , 2014 ; Hughes et al. , 2020 ), there are important task-specific differences. Indeed, a recent study comparing functional connectivity patterns among a group of 18 healthy individuals during resting state and also during movie-watching found that connectivity patterns became more consistent across individuals when they were watching a movie ( van der Meer et al. , 2020 ).
An important benefit of relating patterns of functional connectivity to behavior is that they have clear and measurable individual differences. Finn and colleagues (2015) examined whether functional connectivity patterns serve as ‘fingerprints’ to identify individuals (see also Miranda-Dominguez et al. , 2014 ). To do this, they examined whether individuals (among a pool of 126) could be correctly identified across scan sessions based solely on their functional connectivity patterns. In addition to finding that functional connectivity patterns were uniquely characteristic to each individual, the authors found that resting-state connectivity in the medial frontal and frontoparietal systems were the most accurate in individual subject identification, with nearly 100% accuracy. In a related study by Miranda-Dominguez and colleagues (2014) , the researchers identified unique ‘fingerprints’ (which they refer to as connectotypes) for humans and non-primates. An important contribution of these findings is that they suggest that functional connectivity ‘fingerprints’ may provide a gateway for studying individual differences. However, it is important to note that functional connectivity patterns may be conflated by inter-subject differences in node location (e.g. due to warping and distortion during preprocessing). Future work should examine this possibility.
Finally, a widely used application of functional connectivity patterns has been to identify potential biomarkers that may have clinical relevance, including for Alzheimer’s disease ( Wang et al. , 2006 ; Supekar et al. , 2008 ; Damoiseaux et al. , 2012 ), ASD ( Hull et al. , 2017 ), schizophrenia ( Garrity et al. , 2007 ; Lynall et al. , 2010 ; Venkataraman et al. , 2012 ) and depression ( Fox et al. , 2013 ; Drysdale et al. , 2017 ). A widely targeted population for this work has been with cognitively normal older adults ( Betzel et al. , 2014 ; Chan et al. , 2014 ; Wig, 2017 ; Spreng and Turner, 2019 ). In both healthy and pathological aging, older adults have weaker within-module connectivity coupled with stronger between-module connectivity during resting state ( Wang et al. , 2006 ; Betzel et al. , 2014 ; Chan et al. , 2014 ; Spreng and Turner, 2019 ; Hughes et al. , 2020 ), which have been suggested to reflect decreased functional specialization of the systems over the lifespan (e.g. Betzel et al. , 2014 ; Wig, 2017 ; Spreng and Turner, 2019 ; Koen et al. , 2020 ).
A key module of interest in this work has been the default mode (for review see Broyd et al. , 2009 ; also, Badhwar et al. , 2017 ; Garrity et al. , 2007 ), which consists of medial and lateral parietal cortex, medial prefrontal cortex, and the medial and lateral temporal cortices ( Raichle, 2015 ). Research on autism spectrum disorder (ASD), a development disorder widely associated with disrupted social function, has shown that ASD is associated with weaker resting-state default-mode system connectivity ( Assaf et al. , 2010 ; Weng et al. , 2010 ; Gotts et al. , 2012 ; Hull et al. , 2017 ), the magnitude of which relates to the magnitude of individuals’ social and communication impairments (e.g. Assaf et al. , 2010 ; Gotts et al. , 2012 ). Recent work in network neuroscience has defined three functionally distinct subdivisions of the default-mode system: two in the prefrontal cortex (the ventral medial and the dorsal medial prefrontal cortices) and one in the posterior cortex that is composed of the posterior cingulate cortex, precuneus and lateral parietal cortex ( Figure 3 ; Raichle, 2015 ). Although Alzheimer’s disease has been widely associated with disruptions in resting-state functional connectivity throughout the default-mode system ( Greicius et al. , 2004 ; Koch et al. , 2012 ; Badhwar et al. , 2017 ), some evidence suggests that connectivity within the default-mode system subcomponents differs over the course of Alzheimer’s disease ( Damoiseaux et al. , 2012 ). Thus, although the preponderance of research on the default-mode system focuses on it as a whole system, future work may benefit from disentangling its unique subcomponents.

Cortical components of the default-mode network. The activation map was obtained using NeuroSynth ( Yarkoni et al. , 2011 ) and the term ‘default mode’.
Given its unique role in social cognition ( Mars et al. , 2012 ; Meyer, 2019 ), the default-mode system has already emerged as an important target for social cognitive network neuroscience research. In the next section, we will explore some key limitations in network neuroscience research that should be considered in the context of studying social behavior. We then consider future directions for the field of social cognitive network neuroscience.
Limitations
The goal of the current review was to highlight the potential impact of using a social cognitive network neuroscience approach to advance our understanding of social cognition. However, there are several limitations to this approach that should be considered. First, network neuroscience research lacks consistency in how nodes are defined in brain parcellations (e.g. Power et al. , 2011 ; Yeo et al. , 2011 ; Gordon et al. , 2016 ; Schaefer et al. , 2018 ). Variability among parcellations may contribute to spurious findings (for discussion, see Arslan et al. , 2018 ). Further complicating node selection is the fact that nodes vary across conditions, even for the same individuals ( Salehi et al. , 2020 ). To increase reliability and replicability, social cognitive network neuroscience research should consider building on existing models from the field of network neuroscience. This could be accomplished by using well-studied network parcellations (e.g. Schaefer et al. , 2018 ) that were generated (and validated) from large datasets (see also, Power et al. , 2011 ; Yeo et al. , 2011 ).
Recent concerns have also emerged regarding reliability of functional connectivity across tasks and sessions. A recent meta-analysis examining test–retest reliability of functional connectivity found relatively poor reliability across scanning sessions ( Noble et al. , 2019 ). However, an important caveat to these findings is that because reliability was measured using mean edge-level intraclass correlation coefficients, the meta-analysis was based on a small pool (about 12%) of studies examining test–retest reliability. Thus, these results should be interpreted with caution.
Finally, an important consideration in all neuroimaging research is the magnitude of the effects associated with the different analytical approaches. The effect sizes associated with functional connectivity and behavior remain largely unexplored (but see Seguin et al. , 2020 ), particularly as they might compare to region-specific activation and other measures of brain activity (e.g. Tompson et al. , 2018 ). Although some research suggests that neuroimaging doubles the amount of variance explained in some behavior (e.g. health) relative to self-report measures alone ( Falk et al. , 2011 ), meta-analyses have demonstrated that the effect sizes in cognitive neuroscience research are relatively low ( Button et al. , 2013 ; Szucs and Ioannidis, 2017 ). This limitation is confounded by relatively small sample sizes in neuroimaging work (e.g. N = 20–30; Cremers et al. , 2017 ), which have been consistently subject to criticism ( Button et al. , 2013 ; David et al. , 2013 ; Szucs and Ioannidis, 2017 ; Clayson et al. , 2019 ). Although some research suggests that at least 50 subjects may be necessary to detect reliable effects in targeted (e.g. not whole-brain) analyses ( Yarkoni, 2009 ), other work points to even higher sample sizes (e.g. N = 80) to detect reliable effects on social tasks (e.g. face processing; Bossier et al. , 2020 ). Thus, more work is needed to identify ideal sample sizes for social cognitive network neuroscience research.
A potential benefit of a social cognitive network neuroscience approach is that the availability of large datasets that measure a variety of domains of cognition, including social cognition (e.g. the Human Connectome Project; Van Essen et al. , 2013 ), provide opportunities to replicate findings within datasets (e.g. Bossier et al. , 2020 ). Moreover, they also facilitate replicability by other researchers, given the established infrastructure for accessing these data.
Future directions
Network neuroscience offers a set of tools for representing brains as networks of nodes and edges. This abstraction necessarily results in a loss of detail, but allows researchers to interrogate network data at different spatiotemporal scales using a rich and ever-growing suite of quantitative methods. To date, most social network neuroscience studies have focused on brain systems and modules, with particular emphasis being placed on the default mode and its interactions with other systems and the rest of the brain. However, network neuroscience offers a much more diverse and comprehensive set of tools to interrogate networks. In this section, we highlight several tools from network neuroscience that are ( Table 2 provides links to code for these measures), at present, underutilized within the social neuroscience community. We further speculate on how these tools could be used to enhance our understanding of the role played by networks in shaping social cognition.
Table 2.
A summary of measures discussed in this review that concisely enumerate and articulate how to interpret different graph measures. This is not intended to be an exhaustive list, nor does it include mathematical descriptions of measures (for a more comprehensive list, see Rubinov and Sporns, 2010 ). We also direct the reader to the corresponding functions in the Brain Connectivity Toolbox (BCT; https://sites.google.com/site/bctnet/ ) that make these measurements. If that function does not exist in the BCT, we include links to an alternative source. We note that there may be other implementations of these same functions through other software packages and scientific programming languages (e.g. NetworkX in Python; https://networkx.org/ )
| Name | What it measures | Where can I find code? |
|---|---|---|
| Density | density_und.m (BCT) | |
| Community (module) | Community_louvain.m (BCT) | |
| Participation coefficient | Participation_coef.m, Participation_coef_sign.m (BCT) | |
| Modularity maximization | community_louvain.m (BCT) | |
| Infomap | infomap ( ) | |
| Degree | Degrees_und.m, degrees_dir.m, strengths_und.m, strengths_dir.m, strengths_und_sign.m | |
| Centrality | Betweenness_bin.m, betweenness_wei.m | |
| Rich club | Rich_club_bd.m, rich_club_bu.m, rich_club_wd.m, rich_club_wu.m | |
| Sliding-window time-varying connectivity | ||
| Multilayer network | Genlouvain.m, Community_louvain.m ( ) | |
| Flexibility |
Modules and hubs
In the previous sections, we discussed brain systems—groups of brain regions that are cohesively connected internally but sparsely connected between one another. Oftentimes, the identities of these systems are treated as ‘given’. For instance, one might define the default-mode system based on the parcel labels that accompany the Schaefer atlas ( Schaefer et al. , 2018 ). This approach is appropriate and reasonable, but only under the assumption that systems are identical across individuals. However, recent work has shown that the boundaries of brain systems vary systematically and reliably across individuals ( Gordon et al. , 2017 ), suggesting that to study brain systems meaningfully, we need to estimate them at the subject level. Such an approach could be particularly useful for social cognitive research targeting focal brain regions, such as the fusiform ‘face’ area ( Kanwisher et al. , 1997 ).
How does one go about doing this? One solution is to algorithmically discover a network’s systems using ‘community detection’ methods ( Fortunato, 2010 ). In network neuroscience parlance, a ‘community’ or ‘module’ refers to a collection of nodes that exhibit similar connectivity profiles, usually such that nodes belonging to the same community are strongly connected to one another, a property called community assortativity ( Sporns and Betzel, 2016 ; Betzel and Bassett, 2018 ). Community detection, then, refers to data-driven methods that attempt to identify the optimal partition of nodes into communities for a given individual. In network neuroscience, the two most popular methods are Infomap ( Rosvall and Bergstrom, 2008 ), which identifies communities as groups of nodes that ‘trap’ the probabilistic flow of a random walker over the network, and modularity maximization ( Newman and Girvan, 2004 ), which defines communities as groups of nodes whose internal density of connections is maximally greater than what would be expected by chance. With either of these simple heuristics, one can identify putative communities in a network without any prior knowledge—the community boundaries are informed by the network itself. Knowing a network’s community structure is useful—it allows for ‘coarse graining’, the discovery of functionally related regions, and can be applied meaningfully to both structural and functional networks.
Another way to leverage modules and communities is to use them to determine nodal roles, e.g. identifying hubs whose connections span module boundaries and therefore may play outsized roles in mediating inter-modular communication and information transfer ( Figure 4A ; Guimera and Amaral, 2005 ). Hub regions can be detected quantitatively using the participation coefficient measure, which has a value close to unity when a node’s connections are uniformly distributed across multiple modules and zero when a node’s connections are concentrated within a single module. Interestingly, previous studies have found that hubs tend to be situated within transmodal cortex in higher-order cognitive networks ( Power et al. , 2013 ; Bertolero et al. , 2015 ) and that damage to hub regions as a result of focal lesions corresponds to widespread cognitive deficits ( Warren et al. , 2014 ). Notably, however, there are other methods for identifying and defining hubs, including their more common definitions as highly connected and highly central regions ( Figure 4A ). Collectively, these dissimilar hub definitions offer a suite of measurements for classifying and categorizing brain regions based on their connectivity patterns.

Frontiers in social cognitive network neuroscience. Network neuroscience offers a suite of computational tools, many of which are not currently widely used in social neuroscience. Here, we identify several approaches that could be used to better understand the neural bases of social cognition. (A) The definition of a ‘hub’ in network neuroscience is imprecise. In practice, hubs could be defined in a number of ways. For instance, hubs could correspond to nodes that make many connections and occupy positions of influence. They can also be defined as nodes that are central or important to a process taking place on the network, e.g. the transmission of information over a network’s shortest paths. Hubs can also be defined as nodes whose connections straddle the boundary (e.g. are bridges) between communities. Exploring the variety of alternative hub definitions has the potential to enrich social neuroscience studies. (B) Another area of interest for future studies is the decomposition of modules into hierarchies and multiple scales. Large high-level modules correspond to groups of brain regions that share a broad set of functions (e.g. are domain general). Lower-level and smaller modules reflect increasing functional specialization (e.g. may be more domain specific). (C) A final topic that could be explored in future studies is the study of changes in network structure over short timescales. Many cognitive processes unfold over timescales of seconds. In contrast, fMRI resting-state scan sessions can last as long as 30 minutes. This incongruity of timescales makes it difficult to track fast changes in network structure associated with rapid fluctuations in cognitive state. Time-varying or ‘dynamic’ connectivity studies segment time points into windows and separately estimate connectivity for each window, resulting in a time series of dissimilar connectivity matrices.
One particularly intriguing hub that should be targeted in social cognitive network neuroscience is the insula. In social neuroscience research, the insula has been implicated in a variety of social cognitive functions, including several affective states (e.g. disgust and empathy), social decision-making (e.g. Singer et al. , 2009 ) and even loneliness ( Eisenberger and Cole, 2012 ). Network neuroscience has identified the insula as a hub that plays an important role in saliency, task switching, attention and control ( Menon and Uddin, 2010 ). Focusing on the insula as a hub may provide novel insight into its broader role in social cognition.
Multiscale and hierarchical modules
Many brain systems exhibit known hierarchies and subdivisions, such that they are composed of systems within systems within systems, etc. ( Figure 4B ; Betzel and Bassett, 2018 ). Consider, for instance, the organization of the somatomotor system. At a coarse scale, it can be viewed as a singular system associated with representing sensory information and executing movements. However, its territories can be meaningfully subdivided based on the type of information that a given patch of cortex represents, with distinct subsystems associated differentially with one’s hands, feet, mouth, etc. These subsystems, in turn, can be even further subdivided according to individual digits. Clearly, the coarse grouping of these areas as a singular ‘somatomotor system’ label fails to resolve these fine-scale features. Even at rest there are questions surrounding the correct number of brain systems. For instance, some studies have characterized the brain in terms of a bipartition into large ‘task-positive’ and ‘task-negative’ communities ( Golland et al. , 2008 ), while other studies have focused on finer subdivisions of canonical systems ( Gordon et al. , 2020 ). But how might one access these details using data-driven and network science approaches? Is there a way to resolve different sized communities and modules and, from these different estimates, arrange them into a hierarchy of communities?
Fortunately, the data-driven methods described in the previous section are well-suited for addressing these questions. Both Infomap and modularity maximization include tunable parameters that effectively vary the size and number of detected communities ( Reichardt and Bornholdt, 2006 ). These parameters can be fixed ahead of time to uncover either smaller or larger communities but can also be varied systematically as part of a ‘parameter sweep’ to discover communities across a range of sizes. At the coarsest level, this type of multiscale analysis yields a bipartition of the network into two communities. A parameter sweep will reveal communities of different sizes but will not, unfortunately, determine whether those communities are hierarchically related to one another. However, new methods like multiresolution consensus clustering ( Jeub et al. , 2018 ) use a statistical criterion to arrange a multiscale ensemble of communities into a coherent hierarchy of communities within communities within communities.
Together, these two approaches offer a framework for flexibly examining brain network modules at different scales. Not only does this allow a user to carry out an analysis at one scale or another, but the hierarchy itself can be characterized in the form of different summary statistics, e.g. number of levels, which may vary with cognitive state or clinical condition. This approach may be an interesting complement or alternative to multivariate pattern analyses, which have been widely used in social neuroscience research in recent years (e.g. Weaverdyck et al. , 2020 ). That is, rather than focusing on patterns of activations within specific regions, this approach would allow researchers to explore patterns of connectivity within specific modules.
Dynamic and time-varying network analyses
Functional and structural connectivity represent static network maps of the brain. That is, their connections represent interaction weights between pairs of brain regions either at a specific moment in time or averaged over a longer period. However, brain networks are constantly in flux. Functional connections fluctuate over timescales of seconds and minutes ( Hutchison et al. , 2013 ), possibly reflecting instantaneous changes in cognitive state or performing homeostatic function ( Laumann and Snyder, 2021 ). Similarly, anatomical connections wax and wane over longer timescales with learning, development and aging. Clearly, then, the view of networks as static and temporally invariant objects cannot capture this rich temporal variation. Social neuroscience research has recently embraced a similar viewpoint, integrating techniques such as hyperscanning to measure neural synchrony between two individuals during social interactions ( Misaki et al. , 2021 ).
To better characterize how a network changes over time, a growing number of studies have begun modeling time-varying or dynamic networks, usually in the context of functional connectivity ( Lurie et al. , 2020 ). Estimating time-varying networks is usually carried out using a sliding-window analysis, in which a functional network is estimated using a small subset of time points (those that fall within a temporally contiguous window of fixed length; Shakil et al. , 2016 ; Hindriks et al. , 2016 ; Leonardi and Van De Ville, 2015 ; see Figure 4C ). The window is then advanced by some number of frames, and a new network is generated. This procedure is repeated until the window can be advanced no further, yielding a time series of networks, each corresponding to a different window in time. These networks can be analyzed to track time-varying changes in individual connections or even network properties, like modularity ( Betzel et al. , 2016 ; Fukushima et al. , 2018 ), distribution of hubs and the segregation/integration of brain systems ( Shine et al. , 2016 ).
Time-varying networks can be treated like static networks and analyzed independently of one another. However, they can also be analyzed collectively as part of a multilayer network ( Vaiana and Muldoon, 2020 ). This allows researchers to take advantage of multilayer network analyses tools, including analogs of community detection methods, like modularity maximization ( Mucha et al. , 2010 ). When applied to a multilayer network, this approach yields temporally resolved estimates of communities, allowing users to trivially track changes in community assignments and estimate the network measure of ‘flexibility’—how frequently a node changes its community assignment from one time point to the next ( Bassett et al. , 2011 , 2013 ). In previous studies, flexibility has been linked with learning rate ( Bassett et al. , 2011 ), affective state ( Betzel et al. , 2017 ) and clinical status ( Braun et al. , 2016 ), among others, suggesting that it serves as a powerful marker of behavior.
Time-varying network analysis, however, is not without limitations. Sliding-window analyses require that the user specify the window duration and the amount of overlap between successive windows. Care must be taken in selecting these parameters; short windows can exhibit aliasing effects, and overlap between windows means that the resulting networks are no long independent from one another, which can have implications for their subsequent analysis. To circumvent these issues, several studies have developed ‘point-wise’ estimates of functional connectivity, thus obviating the need for a sliding window while still generating temporally resolved estimates of functional connectivity ( Liu and Duyn, 2013 ; Shine et al. , 2015 ; Esfahlani et al. , 2020 ). These newer methods are relatively untested, but in principle allow users to address some of the issues associated with sliding-window analyses.
In this review, we have provided an overview of the field of network neuroscience with the goal of demonstrating how a social cognitive network neuroscience approach may advance our understanding of the social brain. In addition to providing more comprehensive insights into how the brain gives rise to social behavior, another potential contribution of social cognitive network neuroscience is that it may help address recent critiques arguing that social neuroscience research is too domain specific and overly simplifies our understanding of how the brain gives rise to social behavior ( Barrett and Satpute, 2013 ; Spunt and Adolphs, 2017 ; Ramsey and Ward, 2020 ). These critiques build on prior work highlighting the overlap between seemingly unique cognitive processes (e.g. working memory and intelligence), noting, for instance, that working memory accounts for 40% of the variance in global fluid intelligence ( Fukuda et al. , 2010 ). Extending these observations to the field of social neuroscience, the critiques argue against the notion of a ‘social brain’, suggesting that social and non-social cognitive processes are likely largely overlapping (e.g. Ramsey and Ward, 2020 ). However, an important caveat to these critiques is that although specific brain regions may share social and non-social functions (e.g. Spunt and Adolphs, 2017 ; Ramsey and Ward, 2020 ), the manner in which brain regions communicate information may differ for social and non-social information. Thus, a potential contribution of social cognitive network neuroscience could be to determine whether the manner in which brain systems communicate information differs for social vs non-social information.
Finally, a social cognitive network neuroscience approach has several key benefits. First, it could provide novel insights into how, if at all, brain regions work together to give rise to social behavior. Second, it provides potential resources by which to minimize concerns about power and sample sizes in neuroscience research (e.g. Button et al. , 2013 ; David et al. , 2013 ; Szucs and Ioannidis, 2017 ; Clayson et al. , 2019 ). Specifically, large, publicly available datasets (e.g. the Human Connectome Project; Van Essen et al. , 2013 ) contain resting-state and task-based neuroimaging data from more than 1000 participants, as well as extensive behavioral measures. Large datasets provide opportunities to conceptually replicate findings (e.g. across different subsamples of the dataset; e.g. Bossier et al. , 2020 ), as well as ease for replicability by other researchers, given the established infrastructure for accessing these data. Finally, it could allow us to determine whether a social cognitive network neuroscience approach potentially accounts for unique, or even more, variance in behavior than traditional social neuroscience approaches.
Contributor Information
Anne C Krendl, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN 47405, USA.
Richard F Betzel, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN 47405, USA.
This project was supported in part by a R01 AG070931 from the National Institute on Aging (PI: Krendl).
Conflict of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
- Adolphs R. (2009). The social brain: neural basis of social knowledge . Annual Review of Psychology , 60 , 693–716. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alcalá-López D., Smallwood J., Jefferies E., et al. (2018). Computing the social brain connectome across systems and states . Cerebral Cortex , 28 ( 7 ), 2207–32. [ PubMed ] [ Google Scholar ]
- Andrews-Hanna J.R., Reidler J.S., Huang C., Buckner R.L. (2010). Evidence for the default network’s role in spontaneous cognition . Journal of Neurophysiology , 104 ( 1 ), 322–35. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Arslan S., Ktena S.I., Makropoulos A., Robinson E.C., Rueckert D., Parisot S. (2018). Human brain mapping: a systematic comparison of parcellation methods for the human cerebral cortex . Neuroimage , 170 , 5–30. [ PubMed ] [ Google Scholar ]
- Assaf M., Jagannathan K., Calhoun V.D., et al. (2010). Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients . Neuroimage , 53 ( 1 ), 247–56. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Azevedo F.A., Carvalho L.R., Grinberg L.T., et al. (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled‐up primate brain . Journal of Comparative Neurology , 513 ( 5 ), 532–41. [ PubMed ] [ Google Scholar ]
- Badhwar A., Tam A., Dansereau C., Orban P., Hoffstaedter F., Bellec P. (2017). Resting-state network dysfunction in Alzheimer’s disease: a systematic review and meta-analysis . Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring , 8 , 73–85. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bansal K., Medaglia J.D., Bassett D.S., Vettel J.M., Muldoon S.F. (2018). Data-driven brain network models differentiate variability across language tasks . PLoS Computational Biology , 14 ( 10 ), e1006487. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barrett L.F., Satpute A.B. (2013). Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain . Current Opinion in Neurobiology , 23 ( 3 ), 361–72. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bassett D.S., Wymbs N.F., Porter M.A., Mucha P.J., Carlson J.M., Grafton S.T. (2011). Dynamic reconfiguration of human brain networks during learning . Proceedings of the National Academy of Sciences , 108 ( 18 ), 7641–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bassett D.S., Wymbs N.F., Rombach M.P., Porter M.A., Mucha P.J., Grafton S.T. (2013). Task-based core-periphery organization of human brain dynamics . PLoS Computational Biology , 9 ( 9 ), e1003171. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bassett D.S., Yang M., Wymbs N.F., Grafton S.T. (2015). Learning-induced autonomy of sensorimotor systems . Nature Neuroscience , 18 ( 5 ), 744–51. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bassett D.S., Zurn P., Gold J.I. (2018). On the nature and use of models in network neuroscience . Nature Reviews Neuroscience , 19 ( 9 ), 566–78. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bassett D.S., Bullmore E.D. (2006). Small-world brain networks . The Neuroscientist , 12 ( 6 ), 512–23. [ PubMed ] [ Google Scholar ]
- Bassett D.S., Mattar M.G. (2017). A network neuroscience of human learning: potential to inform quantitative theories of brain and behavior . Trends in Cognitive Sciences , 21 ( 4 ), 250–64. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bassett D.S., Sporns O. (2017). Network neuroscience . Nature Neuroscience , 20 ( 3 ), 353–64. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Beaty R.E., Seli P., Schacter D.L. (2019). Network neuroscience of creative cognition: mapping cognitive mechanisms and individual differences in the creative brain . Current Opinion in Behavioral Sciences , 27 , 22–30. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bertolero M.A., Yeo B.T., D’Esposito M. (2015). The modular and integrative functional architecture of the human brain . Proceedings of the National Academy of Sciences , 112 ( 49 ), E6798–807. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Betzel R.F., Byrge L., He Y., Goñi J., Zuo X.N., Sporns O. (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan . Neuroimage , 102 , 345–57. [ PubMed ] [ Google Scholar ]
- Betzel R.F., Fukushima M., He Y., Zuo X.N., Sporns O. (2016). Dynamic fluctuations coincide with periods of high and low modularity in resting-state functional brain networks . Neuroimage , 127 , 287–97. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Betzel R.F., Satterthwaite T.D., Gold J.I., Bassett D.S. (2017). Positive affect, surprise, and fatigue are correlates of network flexibility . Scientific Reports , 7 ( 1 ), 1–10. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Betzel R.F., Medaglia J.D., Kahn A.E., Soffer J., Schonhaut D.R., Bassett D.S. (2019). Structural, geometric and genetic factors predict interregional brain connectivity patterns probed by electrocorticography . Nature Biomedical Engineering , 3 ( 11 ), 902–16. [ PubMed ] [ Google Scholar ]
- Betzel R.F., Bassett D.S. (2018). Specificity and robustness of long-distance connections in weighted, interareal connectomes . Proceedings of the National Academy of Sciences , 115 ( 21 ), E4880–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bijsterbosch J.D., Woolrich M.W., Glasser M.F., et al. (2018). The relationship between spatial configuration and functional connectivity of brain regions . Elife , 7 , e32992. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bossier H., Roels S.P., Seurinck R., Banaschewski T., Barker G.J., Bokde A.L. IMAGEN Consortium . (2020). The empirical replicability of task-based fMRI as a function of sample size . Neuroimage , 212 , 116601. [ PubMed ] [ Google Scholar ]
- Braga R.M., Buckner R.L. (2017). Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity . Neuron , 95 ( 2 ), 457–71. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Braun U., Schäfer A., Bassett D.S., et al. (2016). Dynamic brain network reconfiguration as a potential schizophrenia genetic risk mechanism modulated by NMDA receptor function . Proceedings of the National Academy of Sciences , 113 ( 44 ), 12568–73. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Broyd S.J., Demanuele C., Debener S., Helps S.K., James C.J., Sonuga-Barke E.J. (2009). Default-mode brain dysfunction in mental disorders: a systematic review . Neuroscience and Biobehavioral Reviews , 33 ( 3 ), 279–96. [ PubMed ] [ Google Scholar ]
- Buckner R.L., Krienen F.M., Yeo B.T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI . Nature Neuroscience , 16 ( 7 ), 832–7. [ PubMed ] [ Google Scholar ]
- Bullmore E., Sporns O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems . Nature Reviews Neuroscience , 10 ( 3 ), 186–98. [ PubMed ] [ Google Scholar ]
- Bullmore E., Sporns O. (2012). The economy of brain network organization . Nature Reviews Neuroscience , 13 ( 5 ), 336–49. [ PubMed ] [ Google Scholar ]
- Button K.S., Ioannidis J.P., Mokrysz C., et al. (2013). Power failure: why small sample size undermines the reliability of neuroscience . Nature Reviews Neuroscience , 14 ( 5 ), 365–76. [ PubMed ] [ Google Scholar ]
- Cacioppo J.T., Hawkley L.C., Thisted R.A. (2010). Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study . Psychology and Aging , 25 ( 2 ), 453. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cai L., Dong Q., Niu H. (2018). The development of functional network organization in early childhood and early adolescence: a resting-state fNIRS study . Developmental Cognitive Neuroscience , 30 , 223–35. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cassidy B.S., Lee E.J., Krendl A.C. (2016). Age and executive ability impact the neural correlates of race perception . Social Cognitive and Affective Neuroscience , 11 ( 11 ), 1752–61. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Castle E., Eisenberger N.I., Seeman T.E., et al. (2012). Neural and behavioral bases of age differences in perceptions of trust . Proceedings of the National Academy of Sciences , 109 ( 51 ), 20848–52. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chai L.R., Mattar M.G., Blank I.A., Fedorenko E., Bassett D.S. (2016). Functional network dynamics of the language system . Cerebral Cortex , 26 ( 11 ), 4148–59. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chan M.Y., Park D.C., Savalia N.K., Petersen S.E., Wig G.S. (2014). Decreased segregation of brain systems across the healthy adult lifespan . Proceedings of the National Academy of Sciences , 111 ( 46 ), E4997–5006. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Charpentier C.J., O’Doherty J.P. (2018). The application of computational models to social neuroscience: promises and pitfalls . Social Neuroscience , 13 ( 6 ), 637–47. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chong M., Bhushan C., Joshi A.A., et al. (2017). Individual parcellation of resting fMRI with a group functional connectivity prior . Neuroimage , 156 , 87–100. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Christov-Moore L., Reggente N., Douglas P.K., Feusner J.D., Iacoboni M. (2020). Predicting empathy from resting state brain connectivity: a multivariate approach . Frontiers in Integrative Neuroscience , 14 , 3. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Clayson P.E., Carbine K.A., Baldwin S.A., Larson M.J. (2019). Methodological reporting behavior, sample sizes, and statistical power in studies of event‐related potentials: barriers to reproducibility and replicability . Psychophysiology , 56 ( 11 ), e13437. [ PubMed ] [ Google Scholar ]
- Cohen J.R., D’Esposito M. (2016). The segregation and integration of distinct brain networks and their relationship to cognition . Journal of Neuroscience , 36 ( 48 ), 12083–94. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cole M.W., Yarkoni T., Repovš G., Anticevic A., Braver T.S. (2012). Global connectivity of prefrontal cortex predicts cognitive control and intelligence . Journal of Neuroscience , 32 ( 26 ), 8988–99. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cole M.W., Laurent P., Stocco A. (2013). Rapid instructed task learning: a new window into the human brain’s unique capacity for flexible cognitive control . Cognitive, Affective & Behavioral Neuroscience , 13 ( 1 ), 1–22. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cole M.W., Bassett D.S., Power J.D., Braver T.S., Petersen S.E. (2014). Intrinsic and task-evoked network architectures of the human brain . Neuron , 83 ( 1 ), 238–51. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cole M.W., Ito T., Schultz D., Mill R., Chen R., Cocuzza C. (2019). Task activations produce spurious but systematic inflation of task functional connectivity estimates . Neuroimage , 189 , 1–18. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cole M.W., Ito T., Cocuzza C., Sanchez-Romero R. (2021). The functional relevance of task-state functional connectivity . Journal of Neuroscience , 41 ( 12 ), 2684–702. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain . Nature Reviews Neuroscience , 3 ( 3 ), 201–15. [ PubMed ] [ Google Scholar ]
- Cornwell E.Y., Waite L.J. (2009). Social disconnectedness, perceived isolation, and health among older adults . Journal of Health and Social Behavior , 50 ( 1 ), 31–48. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cremers H.R., Wager T.D., Yarkoni T. (2017). The relation between statistical power and inference in fMRI . PLoS One , 12 ( 11 ), e0184923. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Crossley N.A., Mechelli A., Vértes P.E., et al. (2013). Cognitive relevance of the community structure of the human brain functional coactivation network . Proceedings of the National Academy of Sciences , 110 ( 28 ), 11583–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., et al. (2006). Consistent resting-state networks across healthy subjects . Proceedings of the National Academy of Sciences , 103 ( 37 ), 13848–53. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Damoiseaux J.S., Prater K.E., Miller B.L., Greicius M.D. (2012). Functional connectivity tracks clinical deterioration in Alzheimer’s disease . Neurobiology of Aging , 33 ( 4 ), 828–e19. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- David S.P., Ware J.J., Chu I.M., et al. (2013). Potential reporting bias in fMRI studies of the brain . PLoS One , 8 ( 7 ), e70104. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- de Reus M.A., van den Heuvel M.P. (2013). Rich club organization and intermodule communication in the cat connectome . Journal of Neuroscience , 33 ( 32 ), 12929–39. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Drysdale A.T., Grosenick L., Downar J., et al. (2017). Resting-state connectivity biomarkers define neurophysiological subtypes of depression . Nature Medicine , 23 ( 1 ), 28–38. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dubois J., Adolphs R. (2016). Building a science of individual differences from fMRI . Trends in Cognitive Sciences , 20 ( 6 ), 425–43. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eickhoff S.B., Thirion B., Varoquaux G., Bzdok D. (2015). Connectivity‐based parcellation: critique and implications . Human Brain Mapping , 36 ( 12 ), 4771–92. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eisenberg N., Strayer J. (1987). Critical issues in the study of empathy. In: Eisenberg, N., Strayer, J., editors. Empathy and Its Development . New York, NY: Cambridge University Press, 3–13. [ Google Scholar ]
- Eisenberger N.I., Cole S.W. (2012). Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health . Nature Neuroscience , 15 ( 5 ), 669. [ PubMed ] [ Google Scholar ]
- Ercsey-Ravasz M., Markov N.T., Lamy C., et al. (2013). A predictive network model of cerebral cortical connectivity based on a distance rule . Neuron , 80 ( 1 ), 184–97. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Esfahlani F.Z., Jo Y., Faskowitz J., et al. (2020). High-amplitude cofluctuations in cortical activity drive functional connectivity . Proceedings of the National Academy of Sciences , 117 ( 45 ), 28393–401. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans A.C. (2013). Networks of anatomical covariance . Neuroimage , 80 , 489–504. [ PubMed ] [ Google Scholar ]
- Falk E.B., Berkman E.T., Whalen D., Lieberman M.D. (2011). Neural activity during health messaging predicts reductions in smoking above and beyond self-report . Health Psychology , 30 ( 2 ), 177. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Falk E.B., Bassett D.S. (2017). Brain and social networks: fundamental building blocks of human experience . Trends in Cognitive Sciences , 21 ( 9 ), 674–90. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Felleman D.J., Van Essen D.C. (1991). Distributed hierarchical processing in the primate cerebral cortex . Cerebral Cortex (New York, NY: 1991) , 1 ( 1 ), 1–47. [ PubMed ] [ Google Scholar ]
- Ferreira L.K., Busatto G.F. (2013). Resting-state functional connectivity in normal brain aging . Neuroscience and Biobehavioral Reviews , 37 ( 3 ), 384–400. [ PubMed ] [ Google Scholar ]
- Finn E.S., Shen X., Scheinost D., et al. (2015). Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity . Nature Neuroscience , 18 ( 11 ), 1664–71. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Finn E.S., Bandettini P.A. (2021). Movie-watching outperforms rest for functional connectivity-based prediction of behavior . Neuroimage , 235 , 117963. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fortunato S. (2010). Community detection in graphs . Physics Reports , 486 ( 3–5 ), 75–174. [ Google Scholar ]
- Fox M.D., Liu H., Pascual-Leone A. (2013). Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity . Neuroimage , 66 , 151–60. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Friston K.J. (1994). Functional and effective connectivity in neuroimaging: a synthesis . Human Brain Mapping , 2 ( 1‐2 ), 56–78. [ Google Scholar ]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging . Neuroimage , 6 ( 3 ), 218–29. [ PubMed ] [ Google Scholar ]
- Fukuda K., Vogel E., Mayr U., Awh E. (2010). Quantity, not quality: the relationship between fluid intelligence and working memory capacity . Psychonomic Bulletin & Review , 17 ( 5 ), 673–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fukushima M., Betzel R.F., He Y., et al. (2018). Fluctuations between high- and low-modularity topology in time-resolved functional connectivity . Neuroimage , 180 , 406–16. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Garofalo M., Nieus T., Massobrio P., Martinoia S. (2009). Evaluation of the performance of information theory-based methods and cross-correlation to estimate the functional connectivity in cortical networks . PLoS One , 4 ( 8 ), e6482. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Garrity A.G., Pearlson G.D., McKiernan K., Lloyd D., Kiehl K.A., Calhoun V.D. (2007). Aberrant ‘default mode’ functional connectivity in schizophrenia . American Journal of Psychiatry , 164 ( 3 ), 450–7. [ PubMed ] [ Google Scholar ]
- Glasser M.F., Coalson T.S., Robinson E.C., et al. (2016). A multi-modal parcellation of human cerebral cortex . Nature , 536 ( 7615 ), 171–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Golland Y., Golland P., Bentin S., Malach R. (2008). Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems . Neuropsychologia , 46 ( 2 ), 540–53. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gong G., He Y., Chen Z.J., Evans A.C. (2012). Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex . Neuroimage , 59 ( 2 ), 1239–48. [ PubMed ] [ Google Scholar ]
- Gonzalez-Castillo J., Kam J.W., Hoy C.W., Bandettini P.A. (2021). How to interpret resting-state fMRI: ask your participants . Journal of Neuroscience , 41 ( 6 ), 1130–41. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., Petersen S.E. (2016). Generation and evaluation of a cortical area parcellation from resting-state correlations . Cerebral Cortex , 26 ( 1 ), 288–303. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gordon E.M., Laumann T.O., Gilmore A.W., et al. (2017). Precision functional mapping of individual human brains . Neuron , 95 ( 4 ), 791–807. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gordon E.M., Laumann T.O., Marek S., et al. (2020). Default-mode network streams for coupling to language and control systems . Proceedings of the National Academy of Sciences , 117 ( 29 ), 17308–19. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gotts S.J., Simmons W.K., Milbury L.A., Wallace G.L., Cox R.W., Martin A. (2012). Fractionation of social brain circuits in autism spectrum disorders . Brain , 135 ( 9 ), 2711–25. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gratton C., Laumann T.O., Nielsen A.N., et al. (2018). Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation . Neuron , 98 ( 2 ), 439–52. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis . Proceedings of the National Academy of Sciences , 100 ( 1 ), 253–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI . Proceedings of the National Academy of Sciences , 101 ( 13 ), 4637–42. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Guimera R., Amaral L.A.N. (2005). Cartography of complex networks: modules and universal roles . Journal of Statistical Mechanics: Theory and Experiment , 2005 ( 02 ), P02001. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hagmann P., Cammoun L., Gigandet X., et al. (2008). Mapping the structural core of human cerebral cortex . PLoS Biology , 6 ( 7 ), e159. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Harriger L., Van Den Heuvel M.P., Sporns O. (2012). Rich club organization of macaque cerebral cortex and its role in network communication . PLoS One , 7 ( 9 ), e46497. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Herculano-Houzel S. (2012). The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost . Proceedings of the National Academy of Sciences , 109 ( Supplement 1 ), 10661–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hermundstad A.M., Bassett D.S., Brown K.S., et al. (2013). Structural foundations of resting-state and task-based functional connectivity in the human brain . Proceedings of the National Academy of Sciences , 110 ( 15 ), 6169–74. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hindriks R., Adhikari M.H., Murayama Y., et al. (2016). Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage , 127 , 242–56. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Holper L., Scholkmann F., Wolf M. (2012). Between-brain connectivity during imitation measured by fNIRS . Neuroimage , 63 ( 1 ), 212–22. [ PubMed ] [ Google Scholar ]
- Honey C.J., Kötter R., Breakspear M., Sporns O. (2007). Network structure of cerebral cortex shapes functional connectivity on multiple time scales . Proceedings of the National Academy of Sciences , 104 ( 24 ), 10240–5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Honey C.J., Sporns O., Cammoun L., et al. (2009). Predicting human resting-state functional connectivity from structural connectivity . Proceedings of the National Academy of Sciences , 106 ( 6 ), 2035–40. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hughes C., Cassidy B.S., Faskowitz J., Avena-Koenigsberger A., Sporns O., Krendl A.C. (2019). Age differences in specific neural connections within the default mode network underlie theory of mind . Neuroimage , 191 , 269–77. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hughes C., Faskowitz J., Cassidy B.S., Sporns O., Krendl A.C. (2020). Aging relates to a disproportionately weaker functional architecture of brain networks during rest and task states . Neuroimage , 209 , 116521. [ PubMed ] [ Google Scholar ]
- Hull J.V., Dokovna L.B., Jacokes Z.J., Torgerson C.M., Irimia A., Van Horn J.D. (2017). Resting-state functional connectivity in autism spectrum disorders: a review . Frontiers in Psychiatry , 7 , 205. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hutchison R.M., Womelsdorf T., Allen E.A., et al. (2013). Dynamic functional connectivity: promise, issues, and interpretations . Neuroimage , 80 , 360–78. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hyon R., Youm Y., Kim J., Chey J., Kwak S., Parkinson C. (2020). Similarity in functional brain connectivity at rest predicts interpersonal closeness in the social network of an entire village . Proceedings of the National Academy of Sciences , 117 ( 52 ), 33149–60. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Iraji A., Deramus T.P., Lewis N., et al. (2019). The spatial chronnectome reveals a dynamic interplay between functional segregation and integration . Human Brain Mapping , 40 ( 10 ), 3058–77. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Iturria-Medina Y., Canales-Rodríguez E.J., Melie-García L., et al. (2007). Characterizing brain anatomical connections using diffusion weighted MRI and graph theory . Neuroimage , 36 ( 3 ), 645–60. [ PubMed ] [ Google Scholar ]
- Iturria-Medina Y., Sotero R.C., Canales-Rodríguez E.J., Alemán-Gómez Y., Melie-García L. (2008). Studying the human brain anatomical network via diffusion-weighted MRI and graph theory . Neuroimage , 40 ( 3 ), 1064–76. [ PubMed ] [ Google Scholar ]
- Jarrell T.A., Wang Y., Bloniarz A.E., et al. (2012). The connectome of a decision-making neural network . Science , 337 ( 6093 ), 437–44. [ PubMed ] [ Google Scholar ]
- Jeub L.G., Sporns O., Fortunato S. (2018). Multiresolution consensus clustering in networks . Scientific Reports , 8 ( 1 ), 1–16. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jin S.H., Seol J., Kim J.S., Chung C.K. (2011). How reliable are the functional connectivity networks of MEG in resting states? Journal of neurophysiology , 106 ( 6 ), 2888–95. [ PubMed ] [ Google Scholar ]
- Joo W.T., Kwak S., Youm Y., Chey J. (2017). Brain functional connectivity difference in the complete network of an entire village: the role of social network size and embeddedness . Scientific Reports , 7 ( 1 ), 1–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kaiser M., Hilgetag C.C. (2006). Nonoptimal component placement, but short processing paths, due to long-distance projections in neural systems . PLoS Computational Biology , 2 ( 7 ), e95. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kanwisher N., McDermott J., Chun M.M. (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception . Journal of Neuroscience , 17 ( 11 ), 4302–11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kliemann D., Adolphs R. (2018). The social neuroscience of mentalizing: challenges and recommendations . Current Opinion in Psychology , 24 , 1–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koch W., Teipel S., Mueller S., et al. (2012). Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease . Neurobiology of Aging , 33 ( 3 ), 466–78. [ PubMed ] [ Google Scholar ]
- Koen J.D., Srokova S., Rugg M.D. (2020). Age-related neural dedifferentiation and cognition . Current Opinion in Behavioral Sciences , 32 , 7–14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kong R., Li J., Orban C., et al. (2019). Spatial topography of individual-specific cortical networks predicts human cognition, personality, and emotion . Cerebral Cortex , 29 ( 6 ), 2533–51. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Krienen F.M., Yeo B.T., Buckner R.L. (2014). Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture . Philosophical Transactions of the Royal Society B: Biological Sciences , 369 ( 1653 ), 20130526. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kuiper J.S., Zuidersma M., Voshaar R.C.O., et al. (2015). Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies . Ageing Research Reviews , 22 , 39–57. [ PubMed ] [ Google Scholar ]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain . Neuroimage , 54 ( 3 ), 2492–502. [ PubMed ] [ Google Scholar ]
- Latora V., Marchiori M. (2001). Efficient behavior of small-world networks . Physical Review Letters , 87 ( 19 ), 198701. [ PubMed ] [ Google Scholar ]
- Laumann T.O., Snyder A.Z. (2021). Brain activity is not only for thinking . Current Opinion in Behavioral Sciences , 40 , 130–6. [ Google Scholar ]
- Leonardi N., Van De Ville D. (2015). On spurious and real fluctuations of dynamic functional connectivity during rest . Neuroimage , 104 , 430–6. [ PubMed ] [ Google Scholar ]
- Liu X., Duyn J.H. (2013). Time-varying functional network information extracted from brief instances of spontaneous brain activity . Proceedings of the National Academy of Sciences , 110 ( 11 ), 4392–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lu H., Zou Q., Gu H., Raichle M.E., Stein E.A., Yang Y. (2012). Rat brains also have a default mode network . Proceedings of the National Academy of Sciences , 109 ( 10 ), 3979–84. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Luo Y., Hawkley L.C., Waite L.J., Cacioppo J.T. (2012). Loneliness, health, and mortality in old age: a national longitudinal study . Social Science & Medicine , 74 ( 6 ), 907–14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lurie D.J., Kessler D., Bassett D.S., et al. (2020). Questions and controversies in the study of time-varying functional connectivity in resting fMRI . Network Neuroscience , 4 ( 1 ), 30–69. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lynall M.E., Bassett D.S., Kerwin R., et al. (2010). Functional connectivity and brain networks in schizophrenia . Journal of Neuroscience , 30 ( 28 ), 9477–87. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lynch C.J., Power J.D., Scult M.A., Dubin M., Gunning F.M., Liston C. (2020). Rapid precision functional mapping of individuals using multi-echo fMRI . Cell Reports , 33 ( 12 ), 108540. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maki-Marttunen V., Diez I., Cortes J.M., Chialvo D.R., Villarreal M. (2013). Disruption of transfer entropy and inter-hemispheric brain functional connectivity in patients with disorder of consciousness . Frontiers in Neuroinformatics , 7 , 24. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mantini D., Gerits A., Nelissen K., et al. (2011). Default mode of brain function in monkeys . Journal of Neuroscience , 31 ( 36 ), 12954–62. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. (2015). The contribution of network organization and integration to the development of cognitive control . PLoS Biology , 13 ( 12 ), e1002328. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Markett S., Montag C., Reuter M. (2018). Network neuroscience and personality . Personality Neuroscience , 1 , 1–14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mars R.B., Neubert F.X., Noonan M.P., Sallet J., Toni I., Rushworth M.F. (2012). On the relationship between the ‘default mode network’ and the ‘social brain’ . Frontiers in Human Neuroscience , 6 , 18. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McIntosh A.R. (2000). Towards a network theory of cognition . Neural Networks , 13 ( 8–9 ), 861–70. [ PubMed ] [ Google Scholar ]
- Medaglia J.D., Lynall M.E., Bassett D.S. (2015). Cognitive network neuroscience . Journal of Cognitive Neuroscience , 27 ( 8 ), 1471–91. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meindl T., Teipel S., Elmouden R., et al. (2010). Test-retest reproducibility of the default-mode network in healthy individuals . Human Brain Mapping , 31 ( 2 ), 237–46. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mejia A.F., Nebel M.B., Wang Y., Caffo B.S., Guo Y. (2020). Template independent component analysis: targeted and reliable estimation of subject-level brain networks using big data population priors . Journal of the American Statistical Association , 115 ( 531 ), 1151–77. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function . Brain Structure & Function , 214 ( 5–6 ), 655–67. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Messaritaki E., Foley S., Schiavi S., et al. (2021). Predicting MEG resting-state functional connectivity from microstructural information . Network Neuroscience , 5 ( 2 ), 477–504. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mesulam M.M. (1998). From sensation to cognition . Brain: A Journal of Neurology , 121 ( 6 ), 1013–52. [ PubMed ] [ Google Scholar ]
- Meyer M.L. (2019). Social by default: characterizing the social functions of the resting brain . Current Directions in Psychological Science , 28 ( 4 ), 380–6. [ Google Scholar ]
- Miranda-Dominguez O., Mills B.D., Carpenter S.D., et al. (2014). Connectotyping: model based fingerprinting of the functional connectome . PLoS One , 9 ( 11 ), e111048. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Misaki M., Kerr K.L., Ratliff E.L., et al. (2021). Beyond synchrony: the capacity of fMRI hyperscanning for the study of human social interaction . Social Cognitive and Affective Neuroscience , 16 ( 1–2 ), 84–92. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mitchell J.P. (2008). Contributions of functional neuroimaging to the study of social cognition . Current Directions in Psychological Science , 17 ( 2 ), 142–6. [ Google Scholar ]
- Moran J.M., Jolly E., Mitchell J.P. (2012). Social-cognitive deficits in normal aging . Journal of Neuroscience , 32 ( 16 ), 5553–61. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mucha P.J., Richardson T., Macon K., Porter M.A., Onnela J.P. (2010). Community structure in time-dependent, multiscale, and multiplex networks . Science , 328 ( 5980 ), 876–8. [ PubMed ] [ Google Scholar ]
- Muldoon S.F., Bridgeford E.W., Bassett D.S. (2016). Small-world propensity and weighted brain networks . Scientific Reports , 6 ( 1 ), 1–13. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mwilambwe-Tshilobo L., Ge T., Chong M., et al. (2019). Loneliness and meaning in life are reflected in the intrinsic network architecture of the brain . Social Cognitive and Affective Neuroscience , 14 ( 4 ), 423–33. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Newman M.E., Girvan M. (2004). Finding and evaluating community structure in networks . Physical Review E , 69 ( 2 ), 026113. [ PubMed ] [ Google Scholar ]
- Niu H., Li Z., Liao X., et al. (2013). Test-retest reliability of graph metrics in functional brain networks: a resting-state fNIRS study . PLoS One , 8 ( 9 ), e72425. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Noble S., Scheinost D., Constable R.T. (2019). A decade of test-retest reliability of functional connectivity: a systematic review and meta-analysis . Neuroimage , 203 , 116157. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- O’Reilly J.X., Woolrich M.W., Behrens T.E., Smith S.M., Johansen-Berg H. (2012). Tools of the trade: psychophysiological interactions and functional connectivity . Social Cognitive and Affective Neuroscience , 7 ( 5 ), 604–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paban V., Modolo J., Mheich A., Hassan M. (2019). Psychological resilience correlates with EEG source-space brain network flexibility . Network Neuroscience , 3 ( 2 ), 539–50. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Park H., Friston K. (2013). Structural and functional brain networks: from connections to cognition . Science , 342 ( 6158 ), 1238411. [ PubMed ] [ Google Scholar ]
- Parkinson C. (2021). Computational methods in social neuroscience: recent advances, new tools, and future directions . Social Cognitive and Affective Neuroscience , 16 ( 8 ), 739–44. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Perissinotto C.M., Cenzer I.S., Covinsky K.E. (2012). Loneliness in older persons: a predictor of functional decline and death . Archives of Internal Medicine , 172 ( 14 ), 1078–84. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pervaiz U., Vidaurre D., Woolrich M.W., Smith S.M. (2020). Optimising network modelling methods for fMRI . Neuroimage , 211 , 116604. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pillemer S., Holtzer R., Blumen H.M. (2017). Functional connectivity associated with social networks in older adults: a resting-state fMRI study . Social Neuroscience , 12 ( 3 ), 242–52. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Power J.D., Cohen A.L., Nelson S.M., et al. (2011). Functional network organization of the human brain . Neuron , 72 ( 4 ), 665–78. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Power J.D., Schlaggar B.L., Lessov-Schlaggar C.N., Petersen S.E. (2013). Evidence for hubs in human functional brain networks . Neuron , 79 ( 4 ), 798–813. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Powers K.E., Wagner D.D., Norris C.J., Heatherton T.F. (2013). Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes . Social Cognitive and Affective Neuroscience , 8 ( 2 ), 151–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function . Proceedings of the National Academy of Sciences , 98 ( 2 ), 676–82. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Raichle M.E. (2015). The brain’s default mode network . Annual Review of Neuroscience , 38 , 433–47. [ PubMed ] [ Google Scholar ]
- Raichle M.E., Mintun M.A. (2006). Brain work and brain imaging . Annual Review of Neuroscience , 29 , 449–76. [ PubMed ] [ Google Scholar ]
- Ramsey R., Ward R. (2020). Putting the nonsocial into social neuroscience: a role for domain-general priority maps during social interactions . Perspectives on Psychological Science , 15 ( 4 ), 1076–94. [ PubMed ] [ Google Scholar ]
- Redcay E., Schilbach L. (2019). Using second-person neuroscience to elucidate the mechanisms of social interaction . Nature Reviews Neuroscience , 20 ( 8 ), 495–505. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Reichardt J., Bornholdt S. (2006). Statistical mechanics of community detection . Physical Review E , 74 ( 1 ), 016110. [ PubMed ] [ Google Scholar ]
- Ribeiro A.H., Vidal M.C., Sato J.R., Fujita A. (2021). Granger causality among graphs and application to functional brain connectivity in autism spectrum disorder . Entropy , 23 ( 9 ), 1204. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rolls E.T., Huang C.C., Lin C.P., Feng J., Joliot M. (2020). Automated anatomical labelling atlas 3 . Neuroimage , 206 , 116189. [ PubMed ] [ Google Scholar ]
- Rosenberg M.D., Finn E.S., Scheinost D., et al. (2016). A neuromarker of sustained attention from whole-brain functional connectivity . Nature Neuroscience , 19 ( 1 ), 165–71. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosvall M., Bergstrom C.T. (2008). Maps of random walks on complex networks reveal community structure . Proceedings of the National Academy of Sciences , 105 ( 4 ), 1118–23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rotge J.Y., Lemogne C., Hinfray S., et al. (2015). A meta-analysis of the anterior cingulate contribution to social pain . Social Cognitive and Affective Neuroscience , 10 ( 1 ), 19–27. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rubinov M., Sporns O. (2010). Complex network measures of brain connectivity: uses and interpretations . Neuroimage , 52 ( 3 ), 1059–69. [ PubMed ] [ Google Scholar ]
- Salehi M., Greene A.S., Karbasi A., Shen X., Scheinost D., Constable R.T. (2020). There is no single functional atlas even for a single individual: functional parcel definitions change with task . NeuroImage , 208 , 116366. [ PubMed ] [ Google Scholar ]
- Samu D., Seth A.K., Nowotny T. (2014). Influence of wiring cost on the large-scale architecture of human cortical connectivity . PLoS Computational Biology , 10 ( 4 ), e1003557. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schaefer A., Kong R., Gordon E.M., et al. (2018). Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI . Cerebral Cortex , 28 ( 9 ), 3095–114. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scheid B.H., Ashourvan A., Stiso J., et al. (2021). Time-evolving controllability of effective connectivity networks during seizure progression . Proceedings of the National Academy of Sciences , 118 ( 5 ), 1–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schmälzle R., O’Donnell M.B., Garcia J.O., et al. (2017). Brain connectivity dynamics during social interaction reflect social network structure . Proceedings of the National Academy of Sciences , 114 ( 20 ), 5153–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scolari M., Seidl-Rathkopf K.N., Kastner S. (2015). Functions of the human frontoparietal attention network: evidence from neuroimaging . Current Opinion in Behavioral Sciences , 1 , 32–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Seguin C., Tian Y., Zalesky A. (2020). Network communication models improve the behavioral and functional predictive utility of the human structural connectome . Network Neuroscience , 4 ( 4 ), 980–1006. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Seidlitz J., Váša F., Shinn M., Romero-Garcia R., Whitaker K.J., Vértes P.E. NSPN Consortium . (2018). Morphometric similarity networks detect microscale cortical organization and predict inter-individual cognitive variation . Neuron , 97 ( 1 ), 231–47. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shakil S., Lee C.H., Keilholz S.D. (2016). Evaluation of sliding window correlation performance for characterizing dynamic functional connectivity and brain states . Neuroimage , 133 , 111–28. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shen X., Tokoglu F., Papademetris X., Constable R.T. (2013). Groupwise whole-brain parcellation from resting-state fMRI data for network node identification . Neuroimage , 82 , 403–15. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shen X., Finn E.S., Scheinost D., et al. (2017). Using connectome-based predictive modeling to predict individual behavior from brain connectivity . Nature Protocols , 12 ( 3 ), 506–18. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shih C.T., Sporns O., Yuan S.L., et al. (2015). Connectomics-based analysis of information flow in the Drosophila brain . Current Biology , 25 ( 10 ), 1249–58. [ PubMed ] [ Google Scholar ]
- Shine J.M., Koyejo O., Bell P.T., Gorgolewski K.J., Gilat M., Poldrack R.A. (2015). Estimation of dynamic functional connectivity using multiplication of temporal derivatives . Neuroimage , 122 , 399–407. [ PubMed ] [ Google Scholar ]
- Shine J.M., Bissett P.G., Bell P.T., et al. (2016). The dynamics of functional brain networks: integrated network states during cognitive task performance . Neuron , 92 ( 2 ), 544–54. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Singer T., Critchley H.D., Preuschoff K. (2009). A common role of insula in feelings, empathy and uncertainty . Trends in Cognitive Sciences , 13 ( 8 ), 334–40. [ PubMed ] [ Google Scholar ]
- Smith S.M., Fox P.T., Miller K.L., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest . Proceedings of the National Academy of Sciences , 106 ( 31 ), 13040–5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smith S.M., Miller K.L., Salimi-Khorshidi G., et al. (2011). Network modelling methods for FMRI . Neuroimage , 54 ( 2 ), 875–91. [ PubMed ] [ Google Scholar ]
- Smith S.M., Vidaurre D., Beckmann C.F., et al. (2013). Functional connectomics from resting-state fMRI . Trends in Cognitive Sciences , 17 ( 12 ), 666–82. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smith S.M., Nichols T.E., Vidaurre D., et al. (2015). A positive-negative mode of population covariation links brain connectivity, demographics and behavior . Nature Neuroscience , 18 ( 11 ), 1565–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Song M., Zhou Y., Li J., et al. (2008). Brain spontaneous functional connectivity and intelligence . Neuroimage , 41 ( 3 ), 1168–76. [ PubMed ] [ Google Scholar ]
- Spielberg J.M., Miller G.A., Heller W., Banich M.T. (2015). Flexible brain network reconfiguration supporting inhibitory control . Proceedings of the National Academy of Sciences , 112 ( 32 ), 10020–5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sporns O. (2010). Networks of the Brain . Cambridge, MA: MIT Press. [ Google Scholar ]
- Sporns O. (2011). The human connectome: a complex network . Annals of the New York Academy of Sciences , 1224 ( 1 ), 109–25. [ PubMed ] [ Google Scholar ]
- Sporns O., Betzel R.F. (2016). Modular brain networks . Annual Review of Psychology , 67 , 613–40. [ Google Scholar ]
- Sporns O., Zwi J.D. (2004). The small world of the cerebral cortex . Neuroinformatics , 2 ( 2 ), 145–62. [ PubMed ] [ Google Scholar ]
- Spreng R.N., Dimas E., Mwilambwe-Tshilobo L., et al. (2020). The default network of the human brain is associated with perceived social isolation . Nature Communications , 11 ( 1 ), 1–11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Spreng R.N., Turner G.R. (2019). The shifting architecture of cognition and brain function in older adulthood . Perspectives on Psychological Science , 14 ( 4 ), 523–42. [ PubMed ] [ Google Scholar ]
- Spunt R.P., Adolphs R. (2017). A new look at domain specificity: insights from social neuroscience . Nature Reviews Neuroscience , 18 ( 9 ), 559–67. [ PubMed ] [ Google Scholar ]
- Stafford J.M., Jarrett B.R., Miranda-Dominguez O., et al. (2014). Large-scale topology and the default mode network in the mouse connectome . Proceedings of the National Academy of Sciences , 111 ( 52 ), 18745–50. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stevens W.D., Spreng R.N. (2014). Resting-state functional connectivity MRI reveals active processes central to cognition . Wiley Interdisciplinary Reviews: Cognitive Science , 5 ( 2 ), 233–45. [ PubMed ] [ Google Scholar ]
- Stiso J., Bassett D.S. (2018). Spatial embedding imposes constraints on neuronal network architectures . Trends in Cognitive Sciences , 22 ( 12 ), 1127–42. [ PubMed ] [ Google Scholar ]
- Suárez L.E., Markello R.D., Betzel R.F., Misic B. (2020). Linking structure and function in macroscale brain networks . Trends in Cognitive Sciences , 24 ( 4 ), 302–15. [ PubMed ] [ Google Scholar ]
- Supekar K., Menon V., Rubin D., Musen M., Greicius M.D. (2008). Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease . PLoS Computational Biology , 4 ( 6 ), e1000100. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Szucs D., Ioannidis J.P. (2017). Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature . PLoS Biology , 15 ( 3 ), e2000797. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tompson S.H., Falk E.B., Vettel J.M., Bassett D.S. (2018). Network approaches to understand individual differences in brain connectivity: opportunities for personality neuroscience . Personality Neuroscience , 1 , 1–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tompson S.H., Falk E.B., O’Donnell M.B., et al. (2020). Response inhibition in adolescents is moderated by brain connectivity and social network structure . Social Cognitive and Affective Neuroscience , 15 ( 8 ), 827–37. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tovar D.T., Chavez R.S. (2021). Large-scale functional coactivation patterns reflect the structural connectivity of the medial prefrontal cortex . Social Cognitive and Affective Neuroscience , 16 ( 8 ), 875–82. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Towlson E.K., Vértes P.E., Ahnert S.E., Schafer W.R., Bullmore E.T. (2013). The rich club of the C. elegans neuronal connectome . Journal of Neuroscience , 33 ( 15 ), 6380–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Uddin L.Q., Yeo B.T., Spreng R.N. (2019). Towards a universal taxonomy of macro-scale functional human brain networks . Brain Topography , 32 ( 6 ), 926–42. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vaiana M., Muldoon S.F. (2020). Multilayer brain networks . Journal of Nonlinear Science , 30 ( 5 ), 2147–69. [ Google Scholar ]
- Vaidya C.J., Gordon E.M. (2013). Phenotypic variability in resting-state functional connectivity: current status . Brain Connectivity , 3 ( 2 ), 99–120. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van den Heuvel M.P., Sporns O. (2011). Rich-club organization of the human connectome . Journal of Neuroscience , 31 ( 44 ), 15775–86. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van der Meer J.N., Breakspear M., Chang L.J., Sonkusare S., Cocchi L. (2020). Movie viewing elicits rich and reliable brain state dynamics . Nature Communications , 11 ( 1 ), 1–14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van Essen D.C., Smith S.M., Barch D.M., Behrens T.E., Yacoub E., Ugurbil K. Wu-Minn HCP Consortium . (2013). The WU-Minn human connectome project: an overview . Neuroimage , 80 , 62–79. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van Essen D.C., Glasser M.F. (2018). Parcellating cerebral cortex: how invasive animal studies inform noninvasive mapmaking in humans . Neuron , 99 ( 4 ), 640–63. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Varshney L.R., Chen B.L., Paniagua E., Hall D.H., Chklovskii D.B. (2011). Structural properties of the Caenorhabditis elegans neuronal network . PLoS Computational Biology , 7 ( 2 ), e1001066. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vatansever D., Menon D.K., Manktelow A.E., Sahakian B.J., Stamatakis E.A. (2015). Default mode network connectivity during task execution . Neuroimage , 122 , 96–104. [ PubMed ] [ Google Scholar ]
- Venkataraman A., Whitford T.J., Westin C.F., Golland P., Kubicki M. (2012). Whole brain resting state functional connectivity abnormalities in schizophrenia . Schizophrenia Research , 139 ( 1–3 ), 7–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vossel S., Geng J.J., Fink G.R. (2014). Dorsal and ventral attention systems: distinct neural circuits but collaborative roles . The Neuroscientist , 20 ( 2 ), 150–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang H.T., Poerio G., Murphy C., Bzdok D., Jefferies E., Smallwood J. (2018). Dimensions of experience: exploring the heterogeneity of the wandering mind . Psychological Science , 29 ( 1 ), 56–71. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang J., Wang L., Zang Y., et al. (2009). Parcellation‐dependent small‐world brain functional networks: a resting‐state fMRI study . Human Brain Mapping , 30 ( 5 ), 1511–23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang L., Zang Y., He Y., et al. (2006). Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI . Neuroimage , 31 ( 2 ), 496–504. [ PubMed ] [ Google Scholar ]
- Warren D.E., Power J.D., Bruss J., et al. (2014). Network measures predict neuropsychological outcome after brain injury . Proceedings of the National Academy of Sciences , 111 ( 39 ), 14247–52. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wasylyshyn N., Hemenway Falk B., Garcia J.O., et al. (2018). Global brain dynamics during social exclusion predict subsequent behavioral conformity . Social Cognitive and Affective Neuroscience , 13 ( 2 ), 182–91. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Watts D.J., Strogatz S.H. (1998). Collective dynamics of ‘small-world’ networks . Nature , 393 ( 6684 ), 440–2. [ PubMed ] [ Google Scholar ]
- Weaverdyck M.E., Lieberman M.D., Parkinson C. (2020). Tools of the trade multivoxel pattern analysis in fMRI: a practical introduction for social and affective neuroscientists . Social Cognitive and Affective Neuroscience , 15 ( 4 ), 487–509. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weng S.J., Wiggins J.L., Peltier S.J., et al. (2010). Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders . Brain Research , 1313 , 202–14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wig G.S. (2017). Segregated systems of human brain networks . Trends in Cognitive Sciences , 21 ( 12 ), 981–96. [ PubMed ] [ Google Scholar ]
- Williams K.D., Jarvis B. (2006). Cyberball: a program for use in research on interpersonal ostracism and acceptance . Behavior Research Methods , 38 ( 1 ), 174–80. [ PubMed ] [ Google Scholar ]
- Yarkoni T. (2009). Big correlations in little studies: inflated fMRI correlations reflect low statistical power—commentary on Vul et al. (2009) . Perspectives on Psychological Science , 4 ( 3 ), 294–8. [ PubMed ] [ Google Scholar ]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data . Nature Methods , 8 ( 8 ), 665–70. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yeo B.T., Krienen F.M., Sepulcre J., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity . Journal of Neurophysiology , 106 ( 3 ), 1125–65. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zalesky A., Fornito A., Harding I.H., et al. (2010). Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage , 50 ( 3 ), 970–83. [ PubMed ] [ Google Scholar ]
- Zamora-López G., Zhou C., Kurths J. (2010). Cortical hubs form a module for multisensory integration on top of the hierarchy of cortical networks . Frontiers in Neuroinformatics , 4 , 1. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- A-Z Publications
Annual Review of Psychology
Volume 71, 2020, review article, implicit social cognition.
- Anthony G. Greenwald 1 , and Calvin K. Lai 2
- View Affiliations Hide Affiliations Affiliations: 1 Department of Psychology, University of Washington, Seattle, Washington 98195, USA; email: [email protected] 2 Department of Psychological and Brain Sciences, Washington University in St. Louis, St. Louis, Missouri 63130, USA
- Vol. 71:419-445 (Volume publication date January 2020) https://doi.org/10.1146/annurev-psych-010419-050837
- First published as a Review in Advance on October 22, 2019
- Copyright © 2020 by Annual Reviews. All rights reserved
In the last 20 years, research on implicit social cognition has established that social judgments and behavior are guided by attitudes and stereotypes of which the actor may lack awareness. Research using the methods of implicit social cognition has produced the concept of implicit bias, which has generated wide attention not only in social, clinical, and developmental psychology, but also in disciplines outside of psychology, including business, law, criminal justice, medicine, education, and political science. Although this rapidly growing body of research offers prospects of useful societal applications, the theory needed to confidently guide those applications remains insufficiently developed. This article describes the methods that have been developed, the findings that have been obtained, and the theoretical questions that remain to be answered.
Article metrics loading...
Full text loading...
Literature Cited
- Agosta S , Sartori G. 2013 . The autobiographical IAT: a review. Front. Psychol. 4 : 519 [Google Scholar]
- Ajzen I , Fishbein M. 1977 . Attitude-behavior relations: a theoretical analysis and review of empirical research. Psychol. Bull. 84 : 5 888– 918 [Google Scholar]
- Banaji MR. 2001 . Implicit attitudes can be measured. The Nature of Remembering: Essays in Honor of Robert G. Crowder HL Roediger, JS Nairne 117– 50 Washington, DC: Am. Psychol. Assoc. [Google Scholar]
- Banse R , Seise J , Zerbes N 2001 . Implicit attitudes towards homosexuality: reliability, validity, and controllability of the IAT. Z. Exp. Psychol. 48 : 2 145– 60 [Google Scholar]
- Bar-Anan Y , Nosek BA. 2014 . A comparative investigation of seven indirect attitude measures. Behav. Res. Methods 46 : 668– 88 [Google Scholar]
- Bargh JA. 1994 . The four horsemen of automaticity: awareness, intention, efficiency, and control in social cognition. Handbook of Social Cognition , Vol. 1: Basic Processes RS Wyer Jr., TK Srull 1– 40 Hillsdale, NJ: Lawrence Erlbaum Assoc. [Google Scholar]
- Bargh JA. 1999 . The cognitive monster: the case against the controllability of automatic stereotype effects. Dual-Process Theories in Social Psychology S Chaiken, Y Trope 361– 82 New York: Guilford Press [Google Scholar]
- Bargh JA , Chen M , Burrows L 1996 . Automaticity of social behavior: direct effects of trait construct and stereotype activation on action. J. Pers. Soc. Psychol. 71 : 2 230– 44 [Google Scholar]
- Barnes-Holmes D , Barnes-Holmes Y , Power P , Hayden E , Milne R , Stewart I 2006 . Do you really know what you believe? Developing the Implicit Relational Assessment Procedure (IRAP) as a direct measure of implicit beliefs. Irish Psychol 32 : 7 169– 77 [Google Scholar]
- Bezrukova K , Spell CS , Perry JL , Jehn KA 2016 . A meta-analytical integration of over 40 years of research on diversity training evaluation. Psychol. Bull. 142 : 11 1227– 74 [Google Scholar]
- Blair IV. 2002 . The malleability of automatic stereotypes and prejudice. Pers. Soc. Psychol. Rev. 6 : 3 242– 61 [Google Scholar]
- Blair IV , Banaji MR. 1996 . Automatic and controlled processes in stereotype priming. J. Pers. Soc. Psychol. 70 : 6 1142– 63 [Google Scholar]
- Bluemke M , Friese M. 2008 . Reliability and validity of the Single-Target IAT (ST-IAT): assessing automatic affect towards multiple attitude objects. Eur. J. Soc. Psychol. 38 : 6 977– 97 [Google Scholar]
- Cai H , Sriram N , Greenwald AG , McFarland SG 2004 . The Implicit Association Test's D measure can minimize a cognitive skill confound: comment on McFarland and Crouch 2002. Soc. Cogn. 22 : 6 673– 84 [Google Scholar]
- Cameron CD , Brown-Iannuzzi JL , Payne BK 2012 . Sequential priming measures of implicit social cognition: a meta-analysis of associations with behavior and explicit attitudes. Pers. Soc. Psychol. Rev. 16 : 4 330– 50 [Google Scholar]
- Campion MA , Pursell ED , Brown BK 1988 . Structured interviewing: raising the psychometric properties of the employment interview. Pers. Psychol. 41 : 1 25– 42 [Google Scholar]
- Carnes M , Devine PG , Manwell LB , Byars-Winston A , Fine E et al. 2015 . The effect of an intervention to break the gender bias habit for faculty at one institution: a cluster randomized, controlled trial. Acad. Med. 90 : 2 221– 30 [Google Scholar]
- Chen M , Bargh JA. 1999 . Consequences of automatic evaluation: immediate behavioral predispositions to approach or avoid the stimulus. Pers. Soc. Psychol. Bull. 25 : 215– 24 [Google Scholar]
- Conrey FR , Sherman JW , Gawronski B , Hugenberg K , Groom CJ 2005 . Separating multiple processes in implicit social cognition: the Quad model of implicit task performance. J. Pers. Soc. Psychol. 89 : 469– 87 [Google Scholar]
- Cronbach LJ , Meehl PE. 1955 . Construct validity in psychological tests. Psychol. Bull. 52 : 4 281– 302 [Google Scholar]
- Cunningham WA , Preacher KJ , Banaji MR 2001 . Implicit attitude measures: consistency, stability, and convergent validity. Psychol. Sci. 12 : 163– 70 [Google Scholar]
- Cvencek D , Greenwald AG , Brown AS , Gray NS , Snowden RJ 2010 . Faking of the Implicit Association Test is statistically detectable and partly correctable. Basic Appl. Soc. Psychol. 32 : 302– 14 [Google Scholar]
- Cvencek D , Greenwald AG , Meltzoff AN 2016 . Implicit measures for preschool children confirm self-esteem's role in maintaining a balanced identity. J. Exp. Soc. Psychol. 62 : 50– 57 [Google Scholar]
- De Houwer J. 2003 . The extrinsic affective Simon task. Exp. Psychol. 50 : 2 77– 85 [Google Scholar]
- De Houwer J , Teige-Mocigemba S , Spruyt A , Moors A 2009 . Implicit measures: a normative analysis and review. Psychol. Bull. 135 : 347– 68 [Google Scholar]
- Devine PG. 1989 . Stereotypes and prejudice: their automatic and controlled components. J. Pers. Soc. Psychol. 56 : 1 5– 18 [Google Scholar]
- Devine PG , Forscher PS , Cox WTL , Kaatz A , Sheridan J , Carnes M 2017 . A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J. Exp. Soc. Psychol. 73 : 211– 15 [Google Scholar]
- Dobbin F , Schrage D , Kalev A 2015 . Rage against the iron cage: the varied effects of bureaucratic personnel reforms on diversity. Am. Sociol. Rev. 80 : 5 1014– 44 [Google Scholar]
- Donders FC. 1969 . 1868 . Over de snelheid van psychische processen [On the speed of mental processes], transl. WG Koster. Attention and Performance II WG Koster 412– 31 Amsterdam, Neth: North Holland [Google Scholar]
- Fazio RH. 1990 . Multiple processes by which attitudes guide behavior: the MODE model as an integrative framework. Advances in Experimental Social Psychology MP Zanna 265– 343 San Diego, CA: Academic [Google Scholar]
- Fazio RH , Olson MA. 2003 . Implicit measures in social cognition research: their meaning and uses. Annu. Rev. Psychol. 54 : 297– 327 [Google Scholar]
- Fazio RH , Sanbonmatsu DM , Powell MC , Kardes FR 1986 . On the automatic activation of attitudes. J. Pers. Soc. Psychol. 50 : 229– 38 [Google Scholar]
- Freeman JB , Ambady N. 2010 . MouseTracker: software for studying real-time mental processing using a computer mouse-tracking method. Behav. Res. Methods 42 : 1 226– 41 [Google Scholar]
- Gawronski B , Bodenhausen GV. 2006 . Associative and propositional processes in evaluation: an integrative review of implicit and explicit attitude change. Psychol. Bull. 132 : 692– 731 [Google Scholar]
- Gawronski B , De Houwer J 2014 . Implicit measures in social and personality psychology. Handbook of Research Methods in Social and Personality Psychology HT Reis, CM Judd 283– 310 Cambridge, UK: Cambridge Univ. Press [Google Scholar]
- Gawronski B , Payne BK , eds. 2010 . Handbook of Implicit Social Cognition: Measurement, Theory, and Applications New York: Guilford Press Comprehensive edited volume reviewing findings and theories across the domain of implicit social cognition. [Google Scholar]
- Gawronski B , Sritharan R. 2010 . Formation, change, and contextualization of mental associations. See Gawronski & Payne 2010 216– 40
- Goldin C , Rouse C. 2000 . Orchestrating impartiality: the impact of “blind” auditions on female musicians. Am. Econ. Rev. 90 : 715– 41 [Google Scholar]
- Graf P , Schacter DL. 1985 . Implicit and explicit memory for new associations in normal and amnesic subjects. J. Exp. Psychol. Learn. Mem. Cogn. 11 : 501– 18 [Google Scholar]
- Greenberg J , Solomon S , Pyszczynski T , Rosenblatt A , Burling J et al. 1992 . Why do people need self-esteem? Converging evidence that self-esteem serves an anxiety-buffering function. J. Pers. Soc. Psychol. 63 : 913– 22 [Google Scholar]
- Greenwald AG , Banaji MR. 1995 . Implicit social cognition: attitudes, self-esteem, and stereotypes. Psychol. Rev. 102 : 4– 27 [Google Scholar]
- Greenwald AG , Banaji MR. 2017 . The implicit revolution: reconceiving the relation between conscious and unconscious. Am. Psychol. 72 : 861– 71 Describes the changing understanding of the relation between conscious and unconscious cognition in the past half century. [Google Scholar]
- Greenwald AG , Banaji MR , Rudman LA , Farnham SD , Nosek BA , Mellott DS 2002 . A unified theory of implicit attitudes, stereotypes, self-esteem, and self-concept. Psychol. Rev. 109 : 3– 25 Theory that predicts observed affective-cognitive consistency among attitudes, stereotypes, self-esteem, and identities. [Google Scholar]
- Greenwald AG , McGhee DE , Schwartz JLK 1998 . Measuring individual differences in implicit cognition: the Implicit Association Test. J. Pers. Soc. Psychol. 74 : 1464– 80 [Google Scholar]
- Greenwald AG , Nosek BA , Banaji MR 2003 . Understanding and using the implicit association test: I. An improved scoring algorithm. J. Pers. Soc. Psychol. 85 : 197– 216 [Google Scholar]
- Greenwald AG , Nosek BA , Banaji MR , Klauer KC 2005 . Validity of the salience asymmetry interpretation of the IAT: comment on Rothermund and Wentura 2004. J. Exp. Psychol. Gen. 134 : 420– 25 [Google Scholar]
- Greenwald AG , Poehlman TA , Uhlmann EL , Banaji MR 2009 . Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J. Pers. Soc. Psychol. 97 : 17– 41 [Google Scholar]
- Hahn A , Gawronski B. 2018 . Implicit social cognition. In The Stevens' Handbook of Experimental Psychology and Cognitive Neuroscience , Vol. 4 JT Wixted 395– 427 New York: Wiley. , 4th ed.. [Google Scholar]
- Hahn A , Gawronski B. 2019 . Facing one's implicit biases: from awareness to acknowledgment. J. Pers. Soc. Psychol. 116 : 769– 94 [Google Scholar]
- Hahn A , Judd CM , Hirsh HK , Blair IV 2014 . Awareness of implicit attitudes. J. Exp. Psychol. Gen. 143 : 3 1369– 92 [Google Scholar]
- Hart M. 2005 . Subjective decisionmaking and unconscious discrimination. Ala. Law Rev. 56 : 741– 91 [Google Scholar]
- Hedges LV , Tipton E , Johnson MC 2010 . Robust variance estimation in meta‐regression with dependent effect size estimates. Res. Synth. Methods 1 : 1 39– 65 [Google Scholar]
- Heider F. 1946 . Attitudes and cognitive organization. J. Psychol. 21 : 107– 12 [Google Scholar]
- Heider F. 1958 . The Psychology of Interpersonal Relations New York: Wiley [Google Scholar]
- Heilman ME , Haynes MC. 2008 . Subjectivity in the appraisal process: a facilitator of gender bias in work settings. Beyond Common Sense: Psychological Science in the Courtroom E Borgida, ST Fiske 127– 56 Oxford, UK: Blackwell Publ. [Google Scholar]
- Hofmann W , Gawronski B , Gschwendner T , Le H , Schmitt M 2005a . A meta-analysis on the correlation between the Implicit Association Test and explicit self-report measures. Pers. Soc. Psychol. Bull. 31 : 1369– 85 [Google Scholar]
- Ito TA , Friedman NP , Bartholow BD , Correll J , Loersch C et al. 2015 . Toward a comprehensive understanding of executive cognitive function in implicit racial bias. J. Pers. Soc. Psychol. 108 : 2 187– 218 [Google Scholar]
- Jacoby LL. 1991 . A process dissociation framework: separating automatic from intentional uses of memory. J. Mem. Lang. 30 : 513– 41 [Google Scholar]
- James W. 1890 . The Principles of Psychology Vol. 2 New York: Henry Holt & Co. [Google Scholar]
- Jordan CH , Spencer SJ , Zanna MP , Hoshino-Browne E , Correll J 2003 . Secure and defensive high self-esteem. J. Pers. Soc. Psychol. 85 : 969– 78 [Google Scholar]
- Jung CG. 1910 . The association method. Am. J. Psychol. 21 : 219– 69 [Google Scholar]
- Kalev A , Dobbin F , Kelly E 2006 . Best practices or best guesses? Assessing the efficacy of corporate affirmative action and diversity policies. Am. Sociol. Rev. 71 : 589– 617 Review using Equal Employment Opportunity Commission data to appraise the effectiveness of corporate strategies for improving diversity in hiring. [Google Scholar]
- Karpinski A , Steinman RB. 2006 . The single category implicit association test as a measure of implicit social cognition. J. Pers. Soc. Psychol. 91 : 1 16– 32 [Google Scholar]
- Kim D-Y. 2003 . Voluntary controllability of the Implicit Association Test (IAT). Soc. Psychol. Q. 66 : 83– 96 [Google Scholar]
- Klauer KC , Voss A , Schmitz F , Teige-Mocigemba S 2007 . Process components of the Implicit Association Test: a diffusion-model analysis. J. Pers. Soc. Psychol. 93 : 3 353– 68 [Google Scholar]
- Kurdi B , Seitchik AE , Axt JR , Carroll TJ , Karapetyan A et al. 2018 . Relationship between the Implicit Association Test and intergroup behavior: a meta-analysis. Am. Psychol. 74 : 5 569– 86 [Google Scholar]
- Lai CK , Hoffman KM , Nosek BA 2013 . Reducing implicit prejudice. Soc. Pers. Psychol. Compass 7 : 5 315– 30 [Google Scholar]
- Lai CK , Marini M , Lehr SA , Cerruti C , Shin J-EL et al. 2014 . Reducing implicit racial preferences: I. A comparative investigation of 17 interventions. J. Exp. Psychol. Gen. 143 : 1765– 85 Series of large-scale experiments comparing 17 interventions to reduce implicit racial preferences. [Google Scholar]
- Lai CK , Skinner AL , Cooley E , Murrar S , Brauer M et al. 2016 . Reducing implicit racial preferences: II. Intervention effectiveness across time. J. Exp. Psychol. Gen. 145 : 8 1001– 16 [Google Scholar]
- Maass A , Salvi D , Arcuri L , Semin GR 1989 . Language use in intergroup contexts: the linguistic intergroup bias. J. Pers. Soc. Psychol. 57 : 6 981– 93 [Google Scholar]
- MacCallum RC , Austin JT. 2000 . Applications of structural equation modeling in psychological research. Annu. Rev. Psychol. 51 : 201– 26 [Google Scholar]
- Macrae CN , Bodenhausen GV , Milne AB , Jetten J 1994 . Out of mind but back in sight: stereotypes on the rebound. J. Pers. Soc. Psychol. 67 : 5 808– 17 [Google Scholar]
- McNulty JK , Olson MA , Jones RE , Acosta LM 2017 . Automatic associations between one's partner and one's affect as the proximal mechanism of change in relationship satisfaction: evidence from evaluative conditioning. Psychol. Sci. 28 : 8 1031– 40 [Google Scholar]
- Meissner F , Rothermund K. 2013 . Estimating the contributions of associations and recoding in the Implicit Association Test: the ReAL model for the IAT. J. Pers. Soc. Psychol. 104 : 45– 69 [Google Scholar]
- Meyer DE , Schvaneveldt RW. 1971 . Facilitation in recognizing pairs of words: evidence of a dependence between retrieval operations. J. Exp. Psychol. 90 : 2 227– 34 [Google Scholar]
- Mierke J , Klauer KC. 2001 . Implicit association measurement with the IAT: evidence for effects of executive control processes. Z. Exp. Psychol. 48 : 2 107– 22 [Google Scholar]
- Mierke J , Klauer KC. 2003 . Method-specific variance in the Implicit Association Test. J. Pers. Soc. Psychol. 85 : 6 1180– 92 [Google Scholar]
- Mogg K , Bradley BP , Field M , De Houwer J 2003 . Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction 98 : 825– 36 [Google Scholar]
- Moors A , De Houwer J 2006 . Automaticity: a theoretical and conceptual analysis. Psychol. Bull. 132 : 2 297– 326 [Google Scholar]
- Morgan CD , Murray HA. 1935 . A method for investigating fantasies: the thematic apperception test. Arch. Neurol. Psychiatry 34 : 289– 306 [Google Scholar]
- Nock MK , Park JM , Finn CT , Deliberto TL , Dour HJ , Banaji MR 2010 . Measuring the suicidal mind: Implicit cognition predicts suicidal behavior. Psychol. Sci. 21 : 4 511– 17 Validation of an indirect (IAT) measure in predicting the likelihood of a repeat suicide attempt. [Google Scholar]
- Nosek BA. 2005 . Moderators of the relationship between implicit and explicit evaluation. J. Exp. Psychol. Gen. 134 : 4 565– 84 [Google Scholar]
- Nosek BA , Banaji MR. 2001 . The go/no-go association task. Soc. Cogn. 19 : 6 625– 66 [Google Scholar]
- Nosek BA , Banaji MR , Greenwald AG 2002 . Math = male, me = female, therefore math ≠ me. J. Pers. Soc. Psychol. 83 : 44– 59 Demonstration of math–gender stereotypes’ role in reducing women's association of math with self. [Google Scholar]
- Nosek BA , Bar-Anan Y , Sriram N , Axt J , Greenwald AG 2014 . Understanding and using the Brief Implicit Association Test: recommended scoring procedures. PLOS ONE 9 : 12 e110938 [Google Scholar]
- Nosek BA , Hansen JJ. 2008 . The associations in our heads belong to us: searching for attitudes and knowledge in implicit evaluation. Cogn. Emot. 22 : 553– 94 [Google Scholar]
- Nosek BA , Hawkins CB , Frazier RS 2011 . Implicit social cognition: from measures to mechanisms. Trends Cogn. Sci. 15 : 4 152– 59 [Google Scholar]
- Nosek BA , Hawkins CB , Frazier RS 2012 . Implicit social cognition. Handbook of Social Cognition S Fiske, CN Macrae 31– 53 New York: Sage [Google Scholar]
- Nosek BA , Smyth FL , Hansen JJ , Devos T , Lindner NM et al. 2007 . Pervasiveness and correlates of implicit attitudes and stereotypes. Eur. Rev. Soc. Psychol. 18 : 36– 88 Study that summarizes data from 2.5 million completed IATs involving 17 topics. [Google Scholar]
- Nunnally J , Bernstein I. 1994 . Psychometric Theory New York: McGraw-Hill. , 3rd ed.. [Google Scholar]
- Nuttin JM Jr 1985 . Narcissism beyond Gestalt and awareness: the name letter effect. Eur. J. Soc. Psychol. 15 : 3 353– 61 [Google Scholar]
- Olson MA , Fazio RH. 2004 . Reducing the influence of extrapersonal associations on the Implicit Association Test: personalizing the IAT. J. Pers. Soc. Psychol. 86 : 653– 67 [Google Scholar]
- Oswald FL , Mitchell G , Blanton H , Jaccard J , Tetlock PE 2013 . Predicting ethnic and racial discrimination: a meta-analysis of IAT criterion studies. J. Pers. Soc. Psychol. 105 : 2 171– 92 [Google Scholar]
- Paluck EL , Green DP. 2009 . Prejudice reduction: What works? A review and assessment of research and practice. Annu. Rev. Psychol. 60 : 339– 67 [Google Scholar]
- Payne BK , Cheng CM , Govorun O , Stewart BD 2005 . An inkblot for attitudes: affect misattribution as implicit measurement. J. Pers. Soc. Psychol. 89 : 3 277– 93 [Google Scholar]
- Payne BK , Gawronski B. 2010 . A history of implicit social cognition. See Gawronski & Payne 2010 1– 15
- Payne BK , Vuletich HA , Lundberg KB 2017 . The bias of crowds: how implicit bias bridges personal and systemic prejudice. Psychol. Inq. 28 : 4 233– 48 [Google Scholar]
- Ratcliff R , Gomez P , McKoon G 2004 . A diffusion model account of the lexical decision task. Psychol. Rev. 111 : 1 159– 82 [Google Scholar]
- Reingold EM , Merikle PM. 1988 . Using direct and indirect measures to study perception without awareness. Percept. Psychophys. 44 : 563– 75 [Google Scholar]
- Rinck M , Becker ES. 2007 . Approach and avoidance in fear of spiders. J. Behav. Ther. Exp. Psychiatry 38 : 2 105– 20 [Google Scholar]
- Rogers CR. 1959 . A theory of therapy, personality, and interpersonal relationships, as developed in the client-centered framework. In Psychology: A Study of a Science , Vol. 3 S Koch 184– 256 New York: McGraw-Hill [Google Scholar]
- Rothermund K , Teige-Mocigemba S , Gast A , Wentura D 2009 . Minimizing the influence of recoding in the implicit association test: the Recoding-Free Implicit Association Test (IAT-RF). Q. J. Exp. Psychol. 62 : 84– 98 [Google Scholar]
- Rothermund K , Wentura D. 2004 . Underlying processes in the Implicit Association Test: dissociating salience from associations. J. Exp. Psychol. Gen. 133 : 139– 65 [Google Scholar]
- Rudman LA , Greenwald AG , McGhee DE 2001 . Implicit self-concept and evaluative implicit gender stereotypes: Self and ingroup share desirable traits. Pers. Soc. Psychol. Bull. 27 : 1164– 78 [Google Scholar]
- Schacter DL. 1987 . Implicit memory: history and current status. J. Exp. Psychol. Learn. Mem. Cogn. 13 : 501– 18 [Google Scholar]
- Schröder-Abé M , Rudolph A , Schütz A 2007 . High implicit self-esteem is not necessarily advantageous: discrepancies between explicit and implicit self-esteem and their relationship with anger expression and psychological health. Eur. J. Pers. 21 : 319– 39 [Google Scholar]
- Sekaquaptewa D , Espinoza P , Thompson M , Vargas P , von Hippel W 2003 . Stereotypic explanatory bias: implicit stereotyping as a predictor of discrimination. J. Exp. Soc. Psychol. 39 : 1 75– 82 [Google Scholar]
- Shook NJ , Fazio RH. 2008 . Interracial roommate relationships: an experimental field test of the contact hypothesis. Psychol. Sci. 19 : 7 717– 23 [Google Scholar]
- Sloman SA. 1996 . The empirical case for two systems of reasoning. Psychol. Bull. 119 : 1 3– 22 [Google Scholar]
- Smeijers D , Vrijsen JN , van Oostrom I , Isaac L , Speckens A et al. 2017 . Implicit and explicit self-esteem in remitted depressed patients. J. Behav. Ther. Exp. Psychiatry 54 : 301– 6 [Google Scholar]
- Smith ER , DeCoster J. 2000 . Dual-process models in social and cognitive psychology: conceptual integration and links to underlying memory systems. Pers. Soc. Psychol. Rev. 4 : 2 108– 31 [Google Scholar]
- Spencer SJ , Steele CM , Quinn DM 1999 . Stereotype threat and women's math performance. J. Exp. Soc. Psychol. 35 : 1 4– 28 [Google Scholar]
- Sriram N , Greenwald AG. 2009 . The brief implicit association test. Exp. Psychol. 56 : 4 283– 94 [Google Scholar]
- Stanovich KE , West RF , Toplak ME 2014 . Rationality, intelligence, and the defining features of Type 1 and Type 2 processing. Dual-Process Theories of the Social Mind JW Sherman, B Gawronski, Y Trope 80– 91 New York: Guilford Press Review of the wide variety of dual-construct theories in social and cognitive psychology. [Google Scholar]
- Strack F , Deutsch R. 2004 . Reflective and impulsive determinants of social behavior. Pers. Soc. Psychol. Rev. 8 : 3 220– 47 [Google Scholar]
- Stroop JR. 1935 . Studies of interference in serial verbal reactions. J. Exp. Psychol. 18 : 6 643– 62 [Google Scholar]
- Tajfel H , Billig MG , Bundy RF , Flament C 1971 . Social categorization and intergroup behaviour. Eur. J. Psychol. 1 : 149– 77 [Google Scholar]
- Teige-Mocigemba S , Klauer KC , Rothermund K 2008 . Minimizing method-specific variance in the IAT: a Single Block IAT. Eur. J. Psychol. Assess. 24 : 4 237– 45 [Google Scholar]
- Turner JC , Hogg MA , Oakes PJ , Reicher SD , Wetherell MS 1987 . Rediscovering the Social Group: A Self-Categorization Theory Cambridge, MA: Basil Blackwell [Google Scholar]
- Uhlmann EL , Cohen GL. 2005 . Constructed criteria: redefining merit to justify discrimination. Psychol. Sci. 16 : 474– 80 [Google Scholar]
- Wilson TD , Lindsey S , Schooler TY 2000 . A model of dual attitudes. Psychol. Rev. 107 : 1 101– 26 [Google Scholar]
- Wittenbrink B , Judd CM , Park B 1997 . Evidence for racial prejudice at the implicit level and its relationship with questionnaire measures. J. Pers. Soc. Psychol. 72 : 2 262– 74 [Google Scholar]
Data & Media loading...
Supplementary Data
Download Supplemental Text (PDF).
- Article Type: Review Article
Most Read This Month
Most cited most cited rss feed, job burnout, executive functions, social cognitive theory: an agentic perspective, on happiness and human potentials: a review of research on hedonic and eudaimonic well-being, sources of method bias in social science research and recommendations on how to control it, mediation analysis, missing data analysis: making it work in the real world, grounded cognition, personality structure: emergence of the five-factor model, motivational beliefs, values, and goals.
Current research topics in embodied social cognition
- Published: 20 July 2014
- Volume 15 , pages 235–236, ( 2014 )
Cite this article

- Fernando Marmolejo-Ramos 1 &
- Amedeo D’Angiulli 2
2756 Accesses
5 Citations
Explore all metrics
Avoid common mistakes on your manuscript.
Although a great deal of research has focused on the study of the embodiment of cognition, only recently has the association between the embodiment of cognition and social cognition been acknowledged. Current behavioural and neuroscientific research in cognitive psychology has started to provide empirical evidence, demonstrating the clear link between embodied cognition and cognitive processes dependent on social situations (see Leung et al. 2011 ). That is, sensorimotor experience with the environment can be shown to shape the acquisition and use of knowledge where this environment is embedded in social situations. Hence, cognition is both embodied and social and is referred to as embodied social cognition (EmSoCo). The five empirical articles composing this special section provide some examples of the cutting edge research being carried out in relation to EmSoCo.
As gestures are kinesic components essential to communication (see Cevasco and Marmolejo-Ramos 2013 ), their role in cognition is, therefore, straightforward. Parzuchowski et al. demonstrate that gestures of honesty, e.g. putting a hand on one’s heart, can influence the judgment of others and one’s actions. This supports the idea that abstract concepts are associated with concrete actions and that non-verbal behaviour serves to convey social signals (see also Tagai et al. 2013 ).
That bodily actions portray and communicate social clues, requires also that emotional correlates be appraised. As shown by Stins et al., body sway is influenced by emotionally valenced facial expressions only when an explicit emotional assessment is required; when emotional assessment is irrelevant the effect vanishes. This finding demonstrates that people tend to approach positive and avoid negative situations (see Eerland et al. 2012 ), but adds the cautionary note that such affect only manifests when an explicit emotion is task relevant.
Marmolejo-Ramos et al. have also demonstrated that only when explicit evaluation of the emotionality of stimuli is required, does an association between emotions and concrete concepts emerge. Specifically, these researchers found that emotion concepts can be mapped on vertical spatial locations such that positive concepts are mapped onto upper locations and negative concepts onto lower locations. Although current research has already demonstrated such an association (e.g. Ansorge and Bohner 2013 ), Marmolejo-Ramos et al. extend those results by showing that the effect also holds for linguistic units larger than words, but only when the emotional evaluation is task relevant.
Abstract concepts, e.g. happiness and honesty, can be understood as being mediated by knowledge of concrete entities that can be linked to the abstract referents by conceptual metaphors or metaphoric mapping (see Landau et al. 2010 ). Metaphoric mapping occurs when, for instance, emotions are mapped onto spatial locations (as described above). But less is known about how other abstract concepts, such as time, are mapped onto space. Xue et al. use event-related potentials from Chinese–English bilinguals to demonstrate that sensorimotor systems activate during the processing of temporal markers embedded in sentences presented in these two languages. Although the authors refer to embodied theories of cognition, they present interesting ideas concerning how metaphoric mappings can explain the results that were found.
Behavioural and neuropsychological evidence indicates that language is grounded in the brain’s motor system (see current evidence by Cardona et al. 2014 ). Although most studies in the embodiment framework have used emotion, action and abstract words, very few studies have focused on concepts that refer to schematic representations of embodied experiences. Prieto Velasco and Tercedor Sánchez investigate how concepts referring to image schemas underlie verbal and visual representations of the human body in medical texts. The authors focus particularly on the concept of ‘pain’. Their work provides insights as to how the concept of ‘pain’ is verbally and graphically represented from a medical standpoint. Their findings are in line with current research in cognitive linguistics suggesting that conceptual knowledge, via words and concepts, is closely linked to perceptual properties (see Milin and Zdravković 2013 ).
Embodied cognition has evolved from the biological notion of autopoiesis (see Maturana 2002 ) to the psychological notion of embodiment which is now enriched through its application in accounting for social cognition. Such evolution from biological to psychological notions is evidenced by recent research demonstrating that from early developmental stages, humans display bodily interactions that can be seen as precursors of social skills (see interesting work by Castiello et al. 2010 ). Thus, EmSoCo is a new field of research that extends the broad field of cognitive science. It is expected that future work will not only address new and compelling questions but will also develop new methodologies to help answer these questions. We hope the articles presented in the current special section will provide novel insights on the ways in which social processes reflect the embodiment of cognition. While EmSoCo is still in its infancy, the present studies form a basis for future studies that will either corroborate, extend or even refute the present findings.
Ansorge U, Bohner G (2013) Investigating the association between valence and elevation with an implicit association task that requires upward and downward responding. Univ Psychol 12(5):1453–1471
Google Scholar
Cardona JF, Kargieman L, Sinay V, Gershanik O, Gelormini C, Amoruso L, Roca M, Pineda D, Trujillo N, Michon M, García A, Szenkman D, Bekinschtein T, Manes F, Ibáñez A (2014) How embodied is action language? Neurological evidence from motor diseases. Cognition 131(2):311–322
Article PubMed Google Scholar
Castiello U, Becchio C, Zoia S, Nelini C, Sartori L, Blason L, D’Ottavio G, Bulgheroni M, Gallese V (2010) Wired to be social: the ontogeny of human interaction. PLoS One 5(10):e13199. doi: 10.1371/journal.pone.0013199
Article PubMed Central PubMed Google Scholar
Cevasco J, Marmolejo-Ramos F (2013) The importance of studying the role of prosody in the comprehension of spontaneous spoken discourse. Rev Latinoam Psicol 45(1):21–33
Eerland A, Guadalupe T, Zwaan RA (2012) Posture as index for approach-avoidance behavior. PLoS One 7(2):e31291. doi: 10.1371/journal.pone.0031291
Article CAS PubMed Central PubMed Google Scholar
Landau MJ, Keefer LA, Meier BP (2010) A metaphor-enriched social cognition. Psychol Bull 136(6):1046–1067
Article Google Scholar
Leung A, Qiu L, Ong L, Tam K-P (2011) Embodied cultural cognition: situating the study of embodied cognition in socio-cultural contexts. Soc Pers Psychol Compass 5(9):591–608
Maturana H (2002) Autopoiesis, structural coupling and cognition: a history of these and other notions in the biology of cognition. Cybern Hum Knowing 9(3–4):5–34
Milin P, Zdravković S (2013) Bi-dimensional semantic scales: the embodied maps of meanings. Univ Psychol 12(5):1543–1558
Tagai K, Takata S, Nagai M, Watanabe K, Kumada T (2013) A near-infrared spectroscopy study of differential brain responses to one or two-handed handing actions: an implication for cultural difference in perceived politeness. Univ Psychol 12(5):1567–1581
Download references
Author information
Authors and affiliations.
School of Psychology, The University of Adelaide, Adelaide, Australia
Fernando Marmolejo-Ramos
Department of Neuroscience, Carleton University, Ottawa, Canada
Amedeo D’Angiulli
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Fernando Marmolejo-Ramos .
Additional information
This editorial is part of the Special Section on ‘Embodied Social Cognition’, guest-edited by Fernando Marmolejo-Ramos and Amedeo D’Angiulli.
Rights and permissions
Reprints and permissions
About this article
Marmolejo-Ramos, F., D’Angiulli, A. Current research topics in embodied social cognition. Cogn Process 15 , 235–236 (2014). https://doi.org/10.1007/s10339-014-0624-2
Download citation
Received : 10 July 2014
Accepted : 11 July 2014
Published : 20 July 2014
Issue Date : August 2014
DOI : https://doi.org/10.1007/s10339-014-0624-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
- Find a journal
- Publish with us
- Track your research
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Social Cognition
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Last »
- Embodied Cognition Follow Following
- Cognitive Science Follow Following
- Philosophy of Cognitive Science Follow Following
- Theory of Mind Follow Following
- Philosophy of Mind Follow Following
- Embodied Mind and Cognition Follow Following
- Social Neuroscience Follow Following
- Cognitive Neuroscience Follow Following
- Embodiment Follow Following
- Cognitive Psychology Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Journals
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Skip to main content
- Skip to primary sidebar
IResearchNet
Social Psychology Topics
Social psychology is a dynamic and multidisciplinary field that delves into the intricate interplay between individuals and their social environments. It seeks to unravel the mysteries of human behavior by exploring how our thoughts, feelings, and actions are shaped by the presence and influence of others. In this introduction, we will define social psychology, emphasize its profound significance, and provide an overview of the diverse range of topics it encompasses. Furthermore, we will present a thesis statement that underscores the paramount importance of comprehending social psychology topics for fostering a deeper understanding of human nature and facilitating positive social change.
Definition of Social Psychology and Its Significance
Social psychology can be defined as the scientific study of how people’s thoughts, feelings, and behaviors are influenced by the actual, imagined, or implied presence of others. It scrutinizes the intricate web of social interactions, exploring how individuals perceive themselves and others, form attitudes, make decisions, and navigate the complexities of human relationships. At its core, social psychology seeks to unravel the underlying mechanisms that govern our social world, shedding light on the often subtle and nuanced factors that drive human behavior.
The significance of social psychology lies in its ability to provide profound insights into the fundamental aspects of human existence. By examining the ways in which individuals are influenced by their social surroundings, it offers a lens through which we can comprehend societal phenomena, from prejudice and discrimination to cooperation and altruism. Social psychology equips us with the tools to decipher the mysteries of interpersonal dynamics, group behavior, and the intricacies of communication. As we explore the diverse topics within this field, we gain a deeper understanding of the human experience and the profound impact of social influences on our lives.
Overview of the Diversity of Topics within Social Psychology
Social psychology is a vast and multifaceted discipline, encompassing an array of topics that illuminate the complexities of human behavior. These topics span the breadth of human interactions, attitudes, and group dynamics, providing valuable insights into the intricacies of our social world. Some of the key areas of focus within social psychology include:
- Attitudes and Attitude Change: Exploring the formation, modification, and influence of attitudes on behavior.
- Social Influence and Conformity: Investigating how individuals are shaped by the pressures of conformity and obedience to authority figures.
- Prejudice and Discrimination: Analyzing the origins and consequences of prejudice, stereotyping, and discriminatory behavior.
- Group Dynamics and Teamwork: Examining the dynamics of group behavior, cohesion, leadership, and decision-making processes.
- Social Cognition and Perception: Uncovering the cognitive processes that underlie social judgments, biases, and heuristics.
- Interpersonal Relationships: Delving into the complexities of attraction, love, relationship maintenance, and dissolution.
- Aggression and Prosocial Behavior: Studying the roots of aggressive behavior, as well as factors promoting empathy, cooperation, and altruism.
- Social Psychology in the Digital Age: Exploring the impact of technology and social media on social behavior, identity, and communication.
As we navigate the rich tapestry of social psychology topics, we gain valuable insights into the intricate mechanisms that govern our social interactions. These insights not only enhance our comprehension of human behavior but also empower us to address pressing societal challenges, foster positive relationships, and promote greater social harmony.
In an increasingly interconnected world, a comprehensive understanding of social psychology topics is indispensable for unraveling the mysteries of human behavior, promoting empathy and tolerance, and fostering positive social change. By delving into the depths of social psychology, we equip ourselves with the knowledge and insights necessary to navigate the complexities of our social world, challenge ingrained prejudices, and build more compassionate and inclusive societies. As we embark on this exploration of social psychology topics, we will uncover the profound ways in which our thoughts, feelings, and actions are intertwined with the social tapestry that surrounds us, ultimately enhancing our capacity for empathy and transforming the world we inhabit.
Social Psychology Research Topics List
This list of social psychology topics performs two functions. One, the headings alone describe, at a broad level, the kinds of topics covered in the field of social psychology. Looking at the overarching categories, one can see that social psychology studies cognition (thought) and action, helpful and hurtful behaviors, emotions and decisions, culture and evolution, the self and social relationships, as well as health and problematic behaviors. That’s quite a range of topics! The second purpose of the list of social psychology research topics is related to the first in that it helps readers who are already interested in a topic find new topics that may be of interest. In this way, the list provides links among topics.
- Antisocial Behavior Topics
- Attitudes Topics
- Control Topics
- Decision Making Topics
- Emotions Topics
- Groups Topics
- Interpersonal Relationships Topics
- Personality Topics
- Prejudice Topics
- Prosocial Behavior Topics
- Self Topics
- Social Cognition Topics
- Social Influence Topics

Social psychology, as a scientific discipline, is dedicated to the exploration of how individuals think about, influence, and interact with one another. In this pursuit, social psychologists delve into various dimensions of human behavior and cognition. They scrutinize the intricacies of social thinking to unravel how we perceive ourselves and those around us. Their examination of social influence delves into the subtle forces at play in conformity, persuasion, and group dynamics. Additionally, social psychologists investigate the complex realm of social relations, seeking to understand the origins of both animosity and empathy among individuals.
Positioned at the intersection of personality psychology and sociology, social psychology occupies a unique niche. To draw a metaphor, while personality psychologists focus on the attributes of individual “boats,” and sociologists navigate the broader “ocean,” social psychologists are primarily concerned with comprehending how these “boats” maneuver within their environment. They investigate the impact of situational factors and social forces on individual behavior and cognition. When an individual, symbolized as a “boat,” encounters a particular environment, analogous to an “ocean,” social psychologists endeavor to discern how external factors, akin to winds and currents, shape their actions and decisions.
While social psychology shares some common inquiries with sociology, it leans toward answers that illuminate the roles of individual actors and their perceptions within social contexts. Rather than concentrating solely on group-level phenomena like poverty or family cohesion, social psychology hones in on the intricacies of individual responses to social situations. Although distinct from personality psychology, which primarily concerns itself with individual differences, social psychology does consider how such differences interact with situational factors. For instance, it might explore how a person with high self-esteem reacts to a threat in their relationship by developing a stronger affinity for their partner.
The practical applications of social psychological research are wide-ranging and have found utility in numerous real-world domains. Researchers have harnessed social psychology to gain insights into health behaviors, such as smoking and condom use, resulting in valuable advancements. For instance, they have played a pivotal role in implementing graphic warnings, like decayed teeth and lungs, on cigarette packaging in Canada to dissuade smoking. In the realm of political psychology, scholars have examined models of persuasion and attitude formation, contributing to our understanding of political behavior. Furthermore, organizational psychologists have applied social psychological theories concerning group dynamics, job satisfaction, and workplace engagement to enhance the functioning of work environments.
The legal arena has also seen the extensive application of social psychological research. In the domain of law, social psychology has exposed the fallibility of eyewitness identification, a crucial element of legal evidence. This research has unveiled the challenges individuals face in accurately identifying those they have witnessed, even after a prolonged observation. Consequently, efforts have been made to refine identification lineup procedures to minimize false positives. For instance, witnesses are now informed that the suspect may or may not be present in the lineup, reducing the likelihood of misidentifications.
Moreover, social psychologists have actively participated in contentious debates surrounding the accuracy of “recovered memories”—recollections of past trauma that individuals believe they have rediscovered later in life. While some of these cases may indeed be genuine, research has demonstrated that false memories can be implanted in individuals. This underscores the need for rigorous scrutiny when evaluating the validity of such memories and highlights the pivotal role of social psychology in shaping the discourse on this topic.
Social Psychology in the Digital Age
The advent of the digital age has ushered in a transformative era in which technology and social media have become integral aspects of our daily lives. This paradigm shift has not only redefined how we communicate and connect but has also had a profound impact on the field of social psychology. In this discussion, we will explore the multifaceted implications of the digital age on social psychology, encompassing online behavior, identity, self-presentation, the role of social media in information dissemination and social movements, and the ethical considerations that underpin research and interventions in this rapidly evolving landscape.
Online Behavior, Identity, and Self-Presentation
The digital age has given rise to new platforms and mediums through which individuals engage in online behavior. Social networking sites, virtual communities, and online forums have become virtual arenas for social interaction, where individuals communicate, form relationships, and express themselves. Within this context, social psychology examines how online behavior mirrors or deviates from offline behavior, exploring concepts such as online disinhibition, digital self-presentation, and the influence of anonymity.
The creation of online identities, often distinct from one’s offline persona, poses intriguing questions about the construction of self in the digital realm. Social psychologists investigate the factors that influence the portrayal of self online, from the selection of profile pictures to the crafting of digital narratives. Additionally, the digital age has given rise to novel aspects of self-presentation, including the cultivation of curated online personas that may not align with an individual’s authentic self.
The Role of Social Media in Information Dissemination and Social Movements
Social media platforms have revolutionized the way information is disseminated, shared, and consumed. These platforms serve as powerful amplifiers of information, enabling the rapid spread of news, opinions, and ideas. Social psychology explores the dynamics of information flow on social media, examining how content goes viral, the role of algorithms in shaping content exposure, and the formation of online echo chambers where individuals are exposed to information congruent with their existing beliefs.
Furthermore, the digital age has witnessed the emergence of social media as a catalyst for social movements and activism. Movements like #BlackLivesMatter and #MeToo have harnessed the power of social media to mobilize support, raise awareness, and effect social change. Social psychologists investigate the psychological processes underlying online activism, including the role of moral outrage, collective identity, and social support in shaping participation in digital social movements.
Ethical Considerations in Online Research and Interventions
The digital landscape presents unique ethical challenges for researchers and practitioners in social psychology. Online research methodologies, such as studying online communities or analyzing social media data, raise questions about informed consent, privacy, and data security. Researchers must grapple with issues related to the use of publicly available online information versus intrusive data collection.
Moreover, ethical considerations extend to the realm of online interventions and behavior change efforts. The use of persuasive techniques, such as nudges and digital interventions, raises questions about the manipulation of online behavior and the potential for unintended consequences. Social psychologists are tasked with navigating the ethical boundaries of online research and interventions, ensuring that their work respects the autonomy and well-being of individuals in the digital space.
In conclusion, the digital age has ushered in a new frontier for social psychology, one in which the study of online behavior, identity, and social media dynamics is of paramount importance. As technology continues to evolve, social psychologists must grapple with the ethical complexities of this digital landscape while unraveling the intricate ways in which technology and social media shape our perceptions, interactions, and understanding of the social world.
In the realm of social psychology, we have embarked on a captivating journey through the intricate landscape of human behavior and social interactions. Our exploration has unveiled a diverse array of topics, each offering valuable insights into the complex web of influences that shape our thoughts, feelings, and actions. As we conclude our discussion, let us recap the key social psychology topics we have encountered and emphasize the profound significance of this interdisciplinary field. Furthermore, we issue a resounding call to action for the ongoing pursuit of research and understanding of social behavior.
Recap of Key Social Psychology Topics and Their Significance
Throughout our exploration, we have encountered a rich tapestry of social psychology topics, each shedding light on a different facet of human nature and social dynamics. We have delved into the formation and change of attitudes, grappled with the complexities of social influence and conformity, confronted the challenges of prejudice and discrimination, and examined the intricate dynamics of groups and teams. Our journey has taken us through the realms of social cognition and perception, interpersonal relationships, aggression, prosocial behavior, and the transformative impact of technology on social behavior.
These topics are not merely academic pursuits but hold profound significance in our lives and societies. They offer us the tools to comprehend the mechanisms behind our behaviors, beliefs, and interactions with others. They equip us with the knowledge to challenge stereotypes, biases, and discriminatory practices, fostering greater empathy, tolerance, and inclusivity. Moreover, they empower us to navigate the complexities of group dynamics, relationships, and digital interactions in an ever-evolving world.
Emphasis on the Interdisciplinary Nature of Social Psychology
Social psychology is a field that bridges disciplines, drawing insights from psychology, sociology, anthropology, and neuroscience, among others. It underscores the interconnectedness of these disciplines and highlights the fundamental role of social factors in shaping individual and collective behavior. As we have seen, social psychology thrives on collaboration and the integration of diverse perspectives to provide a holistic understanding of human behavior in its social context.
Call to Action for Continued Research and Understanding of Social Behavior
Our exploration of social psychology has only scratched the surface of this vast and ever-evolving field. It is imperative that we recognize the ongoing relevance and necessity of research in this domain. The challenges and opportunities presented by our interconnected world demand a deeper understanding of social behavior, both online and offline. We must continue to explore the intricacies of attitudes, influence, prejudice, relationships, and the impact of technology with unwavering curiosity and dedication.
As individuals, scholars, and global citizens, we are called to action. We must engage in ongoing research that deepens our understanding of the human experience and promotes positive social change. We must challenge stereotypes and biases, foster empathy, and build inclusive communities. In an era of rapid technological advancement and global interconnectedness, the lessons of social psychology are more relevant than ever.
In conclusion, social psychology is not merely an academic pursuit but a lens through which we can better comprehend ourselves and the world around us. It offers the potential for transformative change, a bridge between disciplines, and a roadmap to a more compassionate and harmonious society. Let us heed this call to action, embrace the interdisciplinary nature of social psychology, and continue our quest for a deeper understanding of social behavior—one that brings us closer to a more inclusive, empathetic, and interconnected world.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Sweepstakes
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
Social Cognition in Psychology
The Way We Think About Others
- How It Develops
- Cultural Differences
Research and Challenges
Social cognition refers to the different psychological processes that influence how people process, interpret, and respond to social signals. These processes allow people to understand social behavior and respond in ways that are appropriate and beneficial.
Social cognition is a sub-topic of social psychology that focuses on how people process, store, and apply information about others and social situations. It focuses on the role that cognitive processes play in our social interactions. How we think about others plays a major role in how we think, feel, and interact with the world around us.
This article explores the processes involved in social cognition and how this ability forms. It also explores how psychologists study the processes involved in social cognition.
What Is Social Cognition?
Social cognition encompasses a range of processes. Some common factors that many experts have identified as being important include:
- The processes involved in perceiving other people and how we learn about the people in the world around us.
- The study of the mental processes involved in perceiving, remembering, thinking about, and attending to the other people in our social world.
- The reasons we attend to certain information about the social world, how it is stored in memory, and how it is used to interact with other people.
Another important topic in social cognition is the concept of social schemas. Social schemas refer to people's mental representations of social patterns and norms. These representations can include information about societal roles and the expectations of different individuals within a group.
Social cognition is not simply a topic within social psychology —it is an approach to studying any subject with social psychology. Using a social-cognitive perspective, researchers can study a wide range of topics, including:
- Person perception
- Stereotypes
- Self-concept
- Discrimination
- Decision-making
Examples of Social Cognition
Imagine that you are getting ready to go on a blind date. Not only do you worry about the impression and signals that you are sending to the other person, but you are also concerned with interpreting the signals given by your date.
Questions you might ask include:
- How do you form an impression of this person?
- What meaning do you read into the other person's behavior?
- How do you attribute their actions?
This is just one example of how social cognition influences a single social interaction, but you can probably think of many more examples from your daily life. We spend a considerable portion of every day interacting with others, which is why this branch of psychology formed to help understand how we feel, think, and behave in social situations.
Development of Social Cognition
Social cognition develops in childhood and adolescence. As children grow, they become more aware not only of their own feelings, thoughts, and motives but also of the emotions and mental states of others.
Children become more adept at understanding how others feel, learning how to respond in social situations, engaging in prosocial behaviors , and taking the perspective of others.
While many different theories look at how social cognition develops, one of the most popular focuses on the work of the psychologist Jean Piaget. According to Piaget, a child's cognitive development goes through several stages .
- During the earliest stages of development, children are very egocentric . They see the world from their own perspective and struggle to think about how other people may view the world.
- As children grow older, children become increasingly adept at perspective-taking and have an increased ability to think about how and why people act the way they do in social situations.
More recently, research has provided evidence that children develop the ability to think about other people's perspectives at an earlier age than Piaget believed. Even young preschoolers exhibit some ability to think about how other people might view a situation.
One of the most important developments in the early emergence of social cognition is the growth of a theory of mind. A theory of mind refers to a person's ability to understand and think about the mental states of other people.
It is the emergence of a theory of mind that is critical to being able to consider the thoughts, motives, desires, needs, feelings, and experiences that other people may have. Being able to think about how these mental states can influence how people act is critical to forming social impressions and explaining how and why people do the things that they do.
Disorders That Impact Social Cognition
Certain mental health conditions are characterized by disruptions in social cognition. Examples include:
- Bipolar disorder
- Borderline personality disorder (BPD)
- Post-traumatic stress disorder (PTSD)
- Schizophrenia
- Traumatic brain injury
- Williams syndrome
Cultural Differences in Social Cognition
Social psychologists have also found that there are often important cultural differences in social cognition. When looking at a social situation, any two people may have wildly different interpretations. Each person brings a unique background of experiences, knowledge, social influences, feelings, and cultural variations.
Collective cultural influences can also affect how people interpret social situations. The same social behavior in one cultural setting might have a very different meaning and interpretation if it were to occur or be observed in another culture.
As people interpret behavior, extract meaning from the interaction, and then act based upon their beliefs about the situation, they are then further reinforcing and reproducing the cultural norms that influence their social cognitions.
Research into social cognition is ongoing. But there are also challenges to some established theories.
Future Areas of Study
So what are some of the different questions related to social cognition that researchers are interested in understanding? Our perceptions of others play such an important role in how we forge relationships, how we interact with others, how we treat others, and how others treat us.
Some of the topics that psychologists are interested in when it comes to social cognition include:
- How do we develop attitudes ? What role do these attitudes play in our social lives?
- How do we interpret other people's feelings and emotions? How do we figure out what they are thinking or feeling? What cues or indicators do we use to make these assumptions?
- How is self-concept formed and how does it influence our relationships with others?
- What influence do our thoughts have on our feelings?
- What mental processes influence person perception , or how we form impressions of other people?
One criticism of some of the research on social cognition suggests that it is too focused on individual behavior. Because the topic is so social, some suggest that many information-processing models traditionally used to understand the cognitive processes behind social cognition are too limited.
Focusing on the collective and interactive aspects of human thought may provide a better understanding of how people think about and understand social behavior.
Other critics have noted that the field often focuses too heavily on the reasons for a behavior and not on the underlying causes.
A Word From Verywell
Social cognition is the cognitive processes that influence social behavior. Learning more about this perspective offers insights into how other people shape our behaviors and choices. It also plays a role in understanding how individual cognitions affect how we perceive and respond to others.
Arioli M, Crespi C, Canessa N. Social cognition through the lens of cognitive and clinical neuroscience . Biomed Res Int . 2018;2018:4283427. doi:10.1155/2018/4283427
Kaneko A, Asaoka Y, Lee YA, Goto Y. Cognitive and affective processes associated with social biases . Int J Neuropsychopharmacol . 2021;24(8):645-655. doi:10.1093/ijnp/pyab022
Moll H, Meltzoff AN. How does it look? Level 2 perspective-taking at 36 months of age . Child Dev. 2011;82(2):661-73. doi:10.1111/j.1467-8624.2010.01571.x
Schaafsma SM, Pfaff DW, Spunt RP, Adolphs R. Deconstructing and reconstructing theory of mind . Trends Cogn Sci (Regul Ed). 2015;19(2):65-72. doi:10.1016/j.tics.2014.11.007
Dickerson BC. Dysfunction of social cognition and behavior . Continuum (Minneap Minn) . 2015;21(3 Behavioral Neurology and Neuropsychiatry):660-677. doi:10.1212/01.CON.0000466659.05156.1d
Legare CH. The development of cumulative cultural learning . Annu Rev Dev Psychol . 2019;1(1):119-147. doi:10.1146/annurev-devpsych-121318-084848
Kim D, Hommel B. Social cognition 2.0: Toward mechanistic theorizing [published correction appears in Front Psychol. 2020 Feb 05;11:41]. Front Psychol . 2019;10:2643. doi:10.3389/fpsyg.2019.02643
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
ORIGINAL RESEARCH article
Human reasoning on social interactions in ecological contexts: insights from the theory of mind brain circuits.

- 1 IRCCS Fondazione Don Carlo Gnocchi Onlus, Milan, Italy
- 2 Department of Electronics, Information, and Bioengineering, Politecnico di Milano, Milan, Italy
- 3 Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, United Kingdom
- 4 South London and Maudsley NHS Foundation Trust, London, United Kingdom
- 5 The LonDownS Consortium, London, United Kingdom
- 6 Department of Psychology, School of Philosophy, Psychology and Language Sciences, University of Edinburgh, Edinburgh, United Kingdom
Introduction: The relationship between neural social cognition patterns and performance on social cognition tasks in daily life is a topic of debate, with key consideration given to the extent to which theory of mind (ToM) brain circuits share properties reflecting everyday social functioning. To test the efficacy of ecological stimuli in eliciting brain activation within the ToM brain circuits, we adapted the Edinburgh Social Cognition test social scenarios, consisting of dynamic ecological contextually embedded social stimuli, to a fMRI paradigm.
Methods: Forty-two adults (21 men, mean age ± SD = 34.19 years ±12.57) were enrolled and underwent an fMRI assessment which consisted of a ToM task using the Edinburgh Social Cognition test scenarios. We used the same stimuli to prompt implicit ( movie viewing ) and explicit ( silent and two-choice answers ) reasoning on cognitive and affective mental states. The fMRI analysis was based on the classical random effect analysis. Group inferences were complemented with supplemental analyses using overlap maps to assess inter-subject variability.
Results: We found that explicit mentalizing reasoning yielded wide neural activations when two-choice answers were used. We also observed that the nature of ToM reasoning, that is, affective or cognitive, played a significant role in activating different neural circuits.
Discussion: The ESCoT stimuli were particularly effective in evoking ToM core neural underpinnings and elicited executive frontal loops. Future work may employ the task in a clinical setting to investigate ToM network reorganization and plasticity.
1 Introduction
While navigating in the social world, individuals’ proficiency in interpreting and reacting to social cues is indispensable to allow them to behave appropriately and participate in successful social interactions ( Dziobek et al., 2006 ; Frith, 2008 ; Adolphs, 2009 ; Henry et al., 2015 ; Love et al., 2015 ; Baez et al., 2017 ). This social proficiency relies on a complex set of processes known as social cognition abilities, which are acquired in infancy and continuously developed during the lifespan. Among these abilities, theory of mind (ToM) ( Adolphs, 2009 ; Henry et al., 2013 ; Happé et al., 2017 ), the capacity to infer and respond to others’ mental states driving their behavior, assuring the prediction and adequate response to others’ social conduct ( Baglio and Marchetti, 2016 ), is considered a key multidimensional social cognitive process, constituted by two main components, namely, affective and cognitive ToM. Specifically, reasoning on emotions refers to affective ToM, and reasoning on thoughts and beliefs refers to the cognitive ToM component.
Existing tests of ToM, however, have been criticized for their unnaturalistic and artificial nature, and therefore, the ecological validity of ToM measures to reflect social processing in daily life has gained attention ( Mathersul et al., 2013 ). The adoption of ecological stimuli resembling the richness and complexity of daily life scenarios may reliably stimulate everyday social processing ( Redcay and Moraczewski, 2020 ). In particular, movies depicting social interactions, rather than static images or written text, may be capable of eliciting social cognition operations in daily life. Real-world social cognitive processes rely on the online processing of dynamic multimodal, contextually embedded, temporally extended social events ( Redcay and Moraczewski, 2020 {Msika, 2024, Dynamic and/or multimodal assessments for social cognition in neuropsychology: Results from a systematic literature review}). Such processes are scarcely resembled by static and simplistic stimuli.
Some movie-based social cognition tests exist in the literature including the Movie for the Assessment of Social Cognition (MASC; Dziobek et al., 2006 ), the Awareness of Social Inference Test (TASIT; McDonald et al., 2003 ), the Awkward Moments Test ( Heavey et al., 2000 ), and the Empathic Accuracy Paradigm ( Roeyers et al., 2001 ). However, these tests are not without their limitations such as lacking important contextual information, offering exaggerated interactions, or being dubbed from other languages. Interestingly, virtual reality is starting to be adopted in neuropsychological assessment ( Krohn et al., 2020 ), and the Virtual Assessment of Mentalizing Abilities (VAMA) has been proposed as an ecologically valid tool allowing evaluation of mental state reasoning in an interactive virtual environment ( Canty et al., 2017 ). However, virtual reality systems may be not easily accessible due to the high cost related to technologies. In addition, interindividual differences in computer experience and in adaptation to the virtual environment may affect performance ( Parsey and Schmitter-Edgecombe, 2013 ).
The Edinburgh Social Cognition test (ESCoT; Baksh et al., 2018 , 2020 , 2021 ; Poveda et al., 2022 ) is a measure of ToM and social norm understanding, and it has been implemented and validated in the United Kingdom and recently adapted for the Italian culture ( Isernia et al., 2022a ). The ESCoT assesses social abilities through everyday scenarios presented by dynamic cartoons showing a social interaction in which a character adheres to or violates a social rule in the face of a contextual request. The ESCoT provides several advantages: each cartoon measures both affective and cognitive ToM separately; its stimuli resemble the complexity of everyday social interactions; and it enables a multidimensional assessment of ToM. In fact, both social rules and contextual events are crucial to understand and infer the characters’ mental states during the social interactions in the ESCoT scenarios. Each movie depicts an expected or unexpected behavior of one character toward another based on social norms and contextual events (e.g., helping an older woman when her shopping bag breaks; not giving a pregnant woman a seat on the bus). The ESCoT scenarios show ten disparate social situations: helping the elderly, disobeying parking regulations, being considerate on the bus, cleaning up after own pet, assisting a neighbor, smoking in a prohibited area, talking in the cinema, serving a customer, skipping a bus queue, and assisting a stranger. By reasoning about the mental states of characters in those social scenarios, people are prompted to contextually embed social inferences, which are tightly dependent on how social capabilities adapt to the complexity of real-life situations.
Previous research has revealed that the ESCoT is not influenced by intellectual abilities or executive functions in healthy participants ( Baksh et al., 2018 ; Isernia et al., 2022a ), distinguishing itself from other social cognition tests ( Charlton et al., 2009 ; Aboulafia-Brakha et al., 2011 ). In addition, the ESCoT has shown good diagnostic validity in discriminating between autistic and non-autistic participants ( Baksh et al., 2021 ), individuals with and without acquired brain injuries ( Poveda et al., 2022 ), and people with dementia with and without behavior change, unlike established tests of social cognition ( Baksh et al., 2023 ). Given these advantages of the ESCoT, its stimuli may be a suitable way to study the neural mechanisms of mentalizing in real-life scenarios, where contextual events and social rule understanding are involved in the processing of social information.
The ToM neural-network model of Abu-Akel and Shamay-Tsoory (2011) introduces a highly complex map of interconnected neuroanatomical hubs devoted to mental state representation, attribution, and application. Based on this paradigm, mental state representations involve the temporoparietal junction (TPJ), the precuneus, the posterior cingulate cortex (PCC), and superior temporal sulcus (STS), that is, the core ToM network. In this model, partially dissociated mechanisms underlying cognitive and affective ToM have been identified in the limbic and paralimbic areas (the limbic–paralimbic ToM network): the amygdala and the ventral portion of the anterior cingulate cortex, temporal pole, and striatum are especially devoted to affective mental state understanding (affective ToM), whereas the dorsal part of the anterior cingulate cortex, temporal pole, and striatum are involved in cognitive mental state comprehension (cognitive ToM). The dissociation between affective and cognitive ToM neural hubs also involves frontal portions: the dorsomedial and dorsolateral prefrontal cortex (cognitive ToM), the ventromedial prefrontal cortex, and inferolateral and orbitofrontal cortex (affective ToM). In particular, the communication between the limbic–paralimbic ToM areas and key frontal regions enables behavior predictions based on affective and cognitive mental states.
Ecologically valid stimuli in fMRI may reveal neural regions engaged while mentalizing in a naturalistic context ( Wolf et al., 2010 ; Redcay and Moraczewski, 2020 ; Hildebrandt et al., 2021 ). Jacoby et al. (2016) demonstrated that a non-verbal animated movie with segments eliciting emotions and beliefs was able to efficiently localize ToM functional networks, such as the bilateral STS, TPJ, precuneus, ventromedial prefrontal and dorsomedial prefrontal cortices. In addition, Pantelis et al. (2015) administered movies from a TV series in fMRI to healthy participants and autistic people to identify neural areas involved in the perception of socially awkward moments. They found activation in the right TPJ, which was selectively engaged in healthy controls and decreased in autistic people. Wolf et al. (2010) explored the neural mechanisms for both implicit and explicit mental state reasoning by adapting the MASC test into an fMRI task; they reported correspondence with typical mentalizing network areas recruited in implicit ToM reasoning during the passive movie viewing, such as the left TPJ, left precuneus, bilateral occipitotemporal cortex, and left precentral gyrus, and explicit ToM reasoning, left TPJ, left precuneus, left dorsomedial prefrontal cortex, left superior prefrontal gyrus, bilateral STS, bilateral temporal pole, and bilateral inferior frontal gyrus.
We believe that the ESCoT scenarios could be used as fMRI ecological stimuli able to elicit ToM reasoning neural patterns and differential networks for ToM affective and cognitive components.
The aim of the current study was to test the capacity of dynamic, contextually embedded, social scenarios, such as the ESCoT stimuli, in eliciting neural circuits involved in human mentalizing, and, then, propose the ESCoT scenarios as an effective and comprehensive fMRI paradigm for the assessment of cognitive and affective ToM networks.
We adapted the ESCoT into an fMRI task and administered it to a group of healthy adults. Our approach focused on verifying the engagement of ToM neural networks considering a well-recognized ToM neural-network model ( Abu-Akel and Shamay-Tsoory, 2011 ). Specifically, when investigating the overall mentalizing reasoning compared to physical inference (control condition), we expect to find a prevalent involvement of the core ToM network, according to Wolf et al. (2010) . Moreover, when the two ToM components are separately prompted, we expect to observe an extended neural pattern also involving specific cognitive and affective ToM limbic–paralimbic and executive frontal areas as detailed in the ToM neural-network model.
This is a prospective cross-sectional study conducted at the IRCCS Don Gnocchi Foundation of Milan between May 2022 and April 2023. The study protocol was approved by the “Fondazione Don Gnocchi-Milan” Ethics Committee: protocol number 08_23/02/2022. The research conformed to the ethical principles of the Helsinki Declaration revised.
2.1 Participants
Healthy adults were enrolled in the IRCCS Don Gnocchi Foundation clinic (Milan). They were researchers, volunteers, administrative staff, interns, and students attending the clinic. All participants enrolled agreed to take part in the research without receiving monetary compensation. They received a magnetic resonance report at the end of the study.
Before accepting eligible participants for the study, a brief clinical interview was performed to ensure they complied with the research study’s inclusion/exclusion criteria: (i) age ≥ 18; (ii) absence of neurologic and/or major psychiatric conditions; (iii) absence of pharmacological treatment with antipsychotics, antidepressants, and/or antiepileptic that may interfere with the fMRI acquisition; (iv) absence of non-corrected visual impairment able to impact the fMRI acquisition (i.e., inability to wear contact lenses for myopia); (v) absence of hearing impairment able to impact the behavioral assessment; (vi) absence of MRI contraindications (i.e., pacemaker, metal implants, and crystalline surgery in the last month). All participants read and signed the written informed consent.
We enrolled a total of 42 healthy adults (21 men, mean age ± SD = 34.19 years ±12.57, mean full-time years of education ± SD = 16.27 years ±3.08). Among these, seven participants were excluded from the analyses: one due to a brain lesion and six due to low-quality MRI data due to movement artifacts (head motion above 2 mm/2°).
2.2 Procedure
After recruitment into the research study, participants were involved in an individual session in the clinic to perform: (i) an MRI acquisition lasting approximately 40 min in total and (ii) a neuropsychological assessment lasting approximately 1 h. The MRI examination included brain structural MRI sequences to study brain morphometry and exclude gross brain abnormalities, and the ESCoT fMRI ToM task, preceded by 10 min of familiarization with the task instructions and stimuli outside the MRI scanner (see Section 2.3.2). The neuropsychological assessment comprised a test battery to evaluate both non-social cognitive level and social cognitive abilities (see Section 2.3.3).
2.3 Materials
2.3.1 fmri tom task implementation.
A new fMRI ToM task was derived from the Edinburgh Social Cognition Test (ESCoT, Baksh et al., 2018 ), originally developed in the United Kingdom ( Baksh et al., 2018 , 2020 , 2021 ; Poveda et al., 2022 ) and then adapted for the Italian language ( Isernia et al., 2022a ), assessing affective and cognitive ToM and social norm understanding with an ecological and multidimensional approach. The test consists of 11 cartoon-style silent animations (30 s each) depicting real-life social interactions. The animations show social interactions complying with or violating social norms in a daily life context (e.g., assisting a stranger, skipping a bus queue, and smoking in a prohibited area). Each animation evaluates separately cognitive and affective components of ToM, and interpersonal and intrapersonal comprehension of social norms (i.e., social knowledge). In its original version, participants are invited to watch animations and then answer open-ended questions about what happened in the videos (animation comprehension), what a character is thinking (cognitive ToM), how a character is feeling (affective ToM), whether a character behaves as other people should behave (interpersonal comprehension of the social norm), and whether the participant would have acted the same as the character (intrapersonal comprehension of the social norm). Each answer is scored from 0 to 3 points, with 3 points indicating an optimal interpretation of the social dynamics, explicitly extracting and integrating relevant social information and contextual factors influencing characters’ behavior. A score of 2 indicates a response that explicitly extracts the relevant social information but does not integrate it into the context related to the interaction or refers to the extraction of low-level social information related to the context. A score of 1 recognizes a non-social information response, even with the mention of contextual requests. A score of 0 indicates an “I do not know” answer. For a detailed description of the scoring procedure, please refer to Isernia et al. (2022a) and Baksh et al. (2018) (see Figure 1 for an example).
Figure 1 . Storyboard of an ESCoT movie (“Helping the elderly”) and the description of the social scenario of the movie.
For the fMRI adaptation, the ESCoT animation stimuli were adapted to have the same duration (23 s), specifically the vignettes have been shortened by cutting only the start or the end frames when the social interaction has not yet occurred/was already concluded. In addition, instructions for both open- (silent) and closed-ended questions were implemented for each animation assessing social cognition. Then, before implementing the definitive version of the task, pilot versions were administered inside the MRI scanner. The first pilot version of the task included both questions on ToM and comprehension of social norms. However, after preliminary analyses reporting inconsistent neural activations related to social norm comprehension among participants, the task was modified to assess only ToM (affective and cognitive components).
In its final version, the ESCoT fMRI task has been implemented in a way that the same stimuli (video clips) were used to investigate neural correlates elicited by ToM reasoning (experimental condition) and physical inference (control condition). To this purpose, the ESCoT fMRI task consisted of two blocks (A–B) modeling two different conditions: the ToM experimental condition (A) and the physical inference (PI) control condition (B). Moreover, in each block, the same stimuli were used to test the neural activations of implicit reasoning , namely, the reasoning spontaneously elicited by the stimulus itself, not resulting from a specific question, and the explicit reasoning , which instead consists of reasoning elicited by questions that purposely direct attention toward mental states or physical elements (see Figure 2 ).
Figure 2 . Overview of the block design of the task-fMRI experiment. The items of the two blocks (namely, Block A—Theory of Mind Experimental Condition, and Block B—Physical Inference Control Condition) are shown, together with the stimuli duration, according to the order of presentation.
Specifically, the ToM experimental block (A) included the following items:
1. Social cognition instructions : written instructions for the movie scene ( “Focus on characters’ interactions” ) lasting on the screen for 4 s;
2. Implicit ToM reasoning : cartoon-style animation movie watching, lasting 23 s;
3. Explicit affective ToM reasoning – Silent answer : silent answer to affective ToM question ( “How is the woman feeling?” ), lasting on the screen for 9.5 s;
4. Explicit affective ToM reasoning – Closed-ended answer : two -choice answer to affective ToM question ( “Is the woman disappointed by the man behavior?” ), lasting on the screen for 3.5 s;
5. Explicit cognitive ToM reasoning – Silent answer : Silent answer to cognitive ToM question ( “What is the woman thinking?” ), lasting on the screen for 9.5 s;
6. Explicit cognitive ToM reasoning – Closed-ended answer : two-choice answer to cognitive ToM question ( “Did the man act as the woman would expect?” ), lasting on the screen for 3.5 s.
The PI control block (B) included the following items:
1. Physical instructions: written instruction for the movie scene (“ Focus on the elements in the scene ”) lasting 4 s;
2. Implicit physical inference: cartoon-style animation movie watching, lasting 23 s;
3. Explicit physical inference – Silent answer: silent answer to PI question (“ What color is the man’s hair? ”), lasting on the screen for 9.5 s;
4. Explicit physical inference – Closed-ended answer: two-choice answer to PI question (“ Is the man’s hair black? ”), lasting on the screen for 3.5 s.
All items presented in each block were interleaved by a white fixation cross on a black background of variable duration (ranging between 2 to 3 s, and 6 s between blocks) (see Figure 2 ).
The fMRI task included a total of 10 animation movies (the remaining animation was used for familiarization of the task outside the MRI scanner), each of which was administered twice: once during the ToM experimental condition and once for the PI control condition, according to a randomized order. The task administration was split into two separate, but sequential runs (5 movies each) during the same scanning session. Both the task sessions lasted approximately 10 min for a total fMRI task duration of 20 min. The closed-ended two-choice answer was recorded using a dedicated device, namely, an Evoke Response Pad System (Resonance Technology Inc.), and consisted of pressing a button with the index or middle finger to indicate positive or negative answers, respectively. The task was implemented and successively administered using E-Prime 3.0 (Psychology software tools) 1 .
2.3.2 MRI data acquisition
The data were acquired on a 3 T Siemens Prisma scanner (Erlangen, Germany) equipped with a 64-channel head/neck coil. The acquisition protocol included the following: (1) a T1-3D magnetization prepared rapid acquisition with gradient-echo (MPRAGE) sequence with a repetition time (TR) = 2,300 ms, echo time (TE) = 3.1 ms, isotropic resolution = 0.8 × 0.8 × 0.8 mm 3 , 224 slices, which was used as an anatomical reference; (2) a sagittal fluid-attenuated inversion recovery (FLAIR) sequence was also acquired TR = 5,000 ms, TE = 394 ms, resolution = 0.8 × 0.8 × 1 mm 3 , acquisition matrix = 288 × 320, 176 slices, to exclude gross brain abnormalities; (3) an accelerated GE sequence with TR = 2000 ms, TE = 30 ms, resolution 3 × 3 × 3 mm 3 , multi-slice acceleration factor = 2, 52 slices, 333 measurements, 2 runs for fMRI.
The ESCoT visual stimuli were delivered using E-Prime 3.0 [Psychology software tools (see Footnote 1)] by means of a NordicNeuroLab system 2 comprising an “in-room viewing device.” Specifically, an MR-compatible display was located at the end of the gantry, and a mirror was placed on the head coil to allow the participant to see the monitor. The stimuli administration was synchronized with the MR acquisition by means of a dedicated device (SyncBox). The participants were trained before entering the MRI scanner and performed a trial mimicking the fMRI experiment structure involving an ESCoT test video.
2.3.3 Neuropsychological assessment
The neuropsychological test battery included both conventional non-social cognitive measures and social cognition tools.
Non-social cognitive measures comprised the following:
Montreal Cognitive Assessment (MoCA; Conti et al., 2015 ; Santangelo et al., 2015 ) to assess global cognition. The total score ranging from 0 to 30 (greater cognitive level) was adjusted for age and years of education based on the instructions of Santangelo et al. (2015) .
Trail Making Test (TMT; Giovagnoli et al., 1996 ) to evaluate shifting. Performance time was recorded both for TMT parts A and B, and total scores were adjusted for age and years of education according to Giovagnoli et al. (1996) .
Stroop Color-Word Test ( Strauss et al., 2006 ) to assess inhibition. Performance time and errors were registered, and total time and total errors were computed and adjusted for age and years of education according to Caffarra et al. (2002) .
Digit Span Test ( Monaco et al., 2013 ) to assess short-term and working memory. Forward and backward total scores were computed and adjusted for sex, age, and years of education based on the instructions of Monaco et al. (2013) .
Symbol Digit Modality Test (SDMT; Sheridan et al., 2006 ) to evaluate processing speed and attention. The total score was computed and adjusted according to Rao (1990) .
Social cognition measures included the following:
Yoni Task ( Shamay-Tsoory and Aharon-Peretz, 2007 ; Isernia et al., 2022c ) to evaluate cognitive and affective, first-order and second-order ToM with visual cartoon-like stimuli. The 48-item version was administered, and total accuracy and total response time indexes (range 0–1) were computed and adjusted for sex, age, and education according to Isernia et al. (2022c) .
Reading the Mind in the Eyes test (RME, Baron-Cohen et al., 2001 ) to evaluate ToM through photographs of the eye region expressing complex mental states. The total score (range 0–36) was computed and adjusted according to Maddaluno et al. (2022) .
2.4 MRI data analysis
2.4.1 fmri data preprocessing.
The EPI functional data were preprocessed, according to a standard pipeline, using the Statistical Parametric Mapping toolbox (SPM12) 3 running on MATLAB (MathWorks) 4 . The first 10 acquired volumes were considered as ‘dummy scans’ and were discarded to account for magnetization to reach the steady state. The two runs were preprocessed together by setting two different sessions. The first step of the preprocessing involved motion correction and realignment of functional volumes to an average reference volume. The degree of head motion was assessed, and participants with movements exceeding the threshold set at 2 mm/2° were excluded from further analysis. Then, the co-registration with individuals’ anatomical volumes (MPRAGE) was performed. Specifically, the MPRAGE anatomical volumes were preprocessed using the FMRIB Software Library v6.0 (FSL) 5 according to the following steps: bias field correction ( Tustison et al., 2010 ) and brain extraction ( Smith, 2002 ; Jenkinson et al., 2005 ). The individual volumes were successively used as anatomical references for the registration of functional volumes, performed in SPM, at the subject level. The last steps involved segmentation and normalization to the standard MNI template and smoothing (8 mm full width at half-maximum isotropic Gaussian). The preprocessed volumes served as input to the following first-level statistical analyses.
2.4.2 fMRI statistics
A priori sample size calculation: in accordance with the recommendations reported in Szucs and Ioannidis (2020) , we used G*Power (version 3.1.9.7) to a priori estimate the sample size for the study. A total of 31 participants resulted as necessary to achieve a power equal to 0.85 for one-sample t -test analysis (d = 0.5) and α threshold = 0.05. We considered enrolling an additional 15% of subjects to account for eventual exclusions from fMRI analysis due to low-quality data or due to non-compliance with the MRI scan.
2.4.2.1 First-level analyses
The general linear model (GLM) was used to construct and fit the statistical model on the BOLD response to perform the first-level analysis at the subject level. Every item was modeled as a single event inside each block (ToM “experimental” block and PI “control” block), namely, movie viewing, silent answering, and two-choice question answering, and represented the regressors of interest. The six motion parameters were instead inserted in the model as nuisance regressors. Seven different contrasts were considered comparing the different items between the two conditions (i.e., ToM Experimental condition and PI Control condition), specifically, (1) implicit ToM reasoning vs. implicit PI (movie viewing following “ToM instruction” vs. movie viewing following “PI instruction”), (2) explicit ToM reasoning vs. explicit PI silent answer, (3) explicit ToM reasoning vs. explicit PI closed-ended answer, (4) explicit affective ToM (aToM) reasoning vs. explicit PI silent answer, (5) explicit aToM reasoning vs. explicit PI closed-ended answer, (6) explicit cognitive ToM (cToM) reasoning vs. explicit PI silent answer, and (7) explicit cToM reasoning vs. explicit PI closed-ended answer.
To directly test the selective activation of the affective and cognitive ToM dimension, four additional contrasts, directly testing the differences between ToM components during both silent and closed-ended explicit reasoning, have been computed: (8) explicit aToM reasoning vs. explicit cToM reasoning silent answer, (9) explicit cToM reasoning vs. explicit aToM reasoning silent answer, (10) explicit aToM reasoning vs. explicit cToM reasoning closed-ended answer, and (11) explicit cToM reasoning vs. explicit aToM reasoning closed-ended answer.
Moreover, to complement the classical GLM factorial analysis, subjects’ interindividual variability was performed. Specifically, subject-specific activation maps derived for all the above-mentioned contrasts have been used to compute threshold-dependent overlap maps representing the proportion of subject activation in a given region of interest (ROI) ( Seghier and Price, 2016 ). The ROIs were defined according to an in-house developed atlas-derived inclusive mask previously described in Isernia et al. (2022b) , comprising the cerebral areas relevant to ToM reasoning according to Abu-Akel and Shamay-Tsoory’s model ( Abu-Akel and Shamay-Tsoory, 2011 ). The subject-specific activation maps were derived according to the following thresholds: p unc < 0.001 and cluster size ≥30.
2.4.2.2 Second-level analyses
The resulting subject-level contrasts were used to perform second-level group analysis, modeled in the GLM as one-sample t -tests. The statistics were restricted using an in-house developed atlas-derived inclusive mask previously described in Isernia et al. (2022b) , to the cerebral areas relevant to ToM processing according to the Abu-Akel and Shamay-Tsoory’s model ( Abu-Akel and Shamay-Tsoory, 2011 ). In brief, the mask was composed of 11 bilateral non-overlapping ROIs, constituting the four ToM circuits depicted by the model: the ‘ Core ToM Network’ , composed of TPJ, precuneus, and PCC and the anterior division of the STS; the ‘ Limbic–Paralimbic ToM Network’ , composed by anterior cingulate cortex (ACC), TP, dorsal striatum, ventral striatum, and amygdala; the ‘ Cognitive Execution Loop’ , composed by dorsal medial and dorsal lateral PFC; the ‘ Affective Execution Loop’ , composed by the orbitofrontal cortex and ventromedial PFC, and the inferolateral PFC. The functional activation maps were considered statistically significant for p FWE < 0.05 considering the family-wise error (FWE) correction for multiple comparisons to account for false positives. A threshold on cluster size was also set to consider clusters larger than 30 voxels.
3.1 Participants
Thirty-five participants (18 men, mean age ± SD = 34.23 years ±12.72, mean years of education ± SD = 16.46 years ±2.70) were included in the analyses. Table 1 reports the cognitive and psychosocial characteristics of the participants included in the analyses. Participants showed high performance on all the neuropsychological tests.
Table 1 . Neurocognitive profile of the sample.
3.2 fMRI task performance
The task-fMRI performance for the closed-ended questions, for both the ToM experimental and PI control conditions, is reported in Table 2 . Specifically, the reaction times (RT), namely, the elapsed time between the question presentation and the participants pressing the button, the number of missing answers, and the number of wrong answers were assessed for the affective ToM (aToM), cognitive ToM (cToM), and PI closed-ended questions. All participants correctly answered at least 75% of overall questions with a low rate of missing/wrong answers and average RT below the 3,000-ms time limit.
Table 2 . Performance indexes of closed-ended questions recorded during MRI examination.
3.3 fMRI GLM results
The results of the fMRI analysis are reported in detail in Table 3 and Figure 1 for the ToM performance vs. PI and separately for affective ToM and cognitive ToM vs. PI in Table 4 and Figure 2 . Specifically, for the first contrast ( implicit ToM reasoning vs. implicit PI ), investigating the implicit ToM reasoning, no significant neural activation was retrieved. For the second contrast ( explicit ToM reasoning vs. explicit PI silent answer ), the functional activations were located in the left TP, both superior and middle (BA 21, 22, 38) ( Figure 3 ). The third contrast ( explicit ToM reasoning vs. explicit PI closed-ended answer ) yielded significant activation in the bilateral temporal cortex, specifically superior and middle temporal gyri (BA 21, 22, 39), and precuneus (BA 7), left temporal pole (BA 38), inferior frontal cortex (pars orbitalis and pars triangularis), and insula ( Figure 3 ). Furthermore, significant clusters were retrieved in the left middle frontal cortex and precentral gyrus.
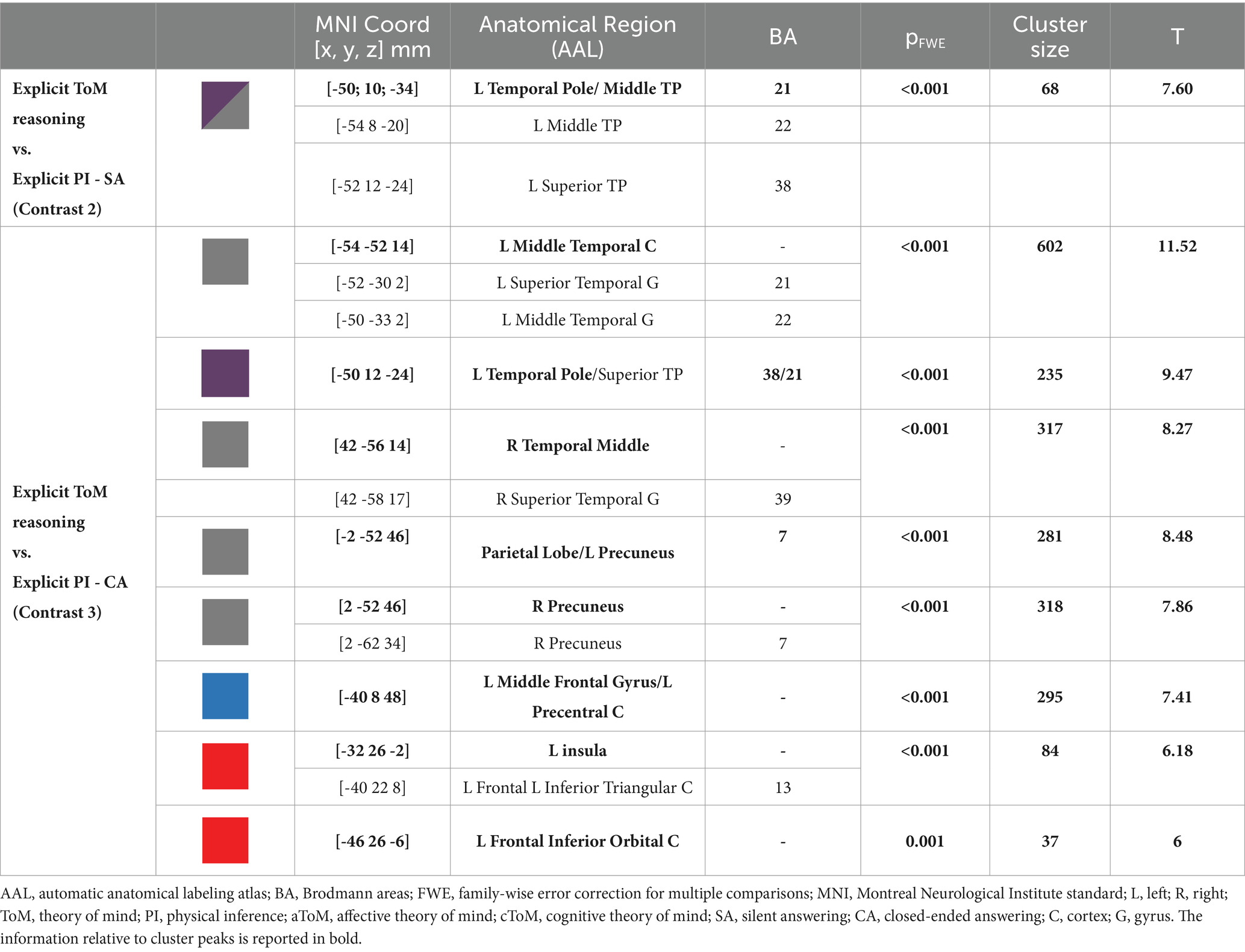
Table 3 . MRI GLM Results.
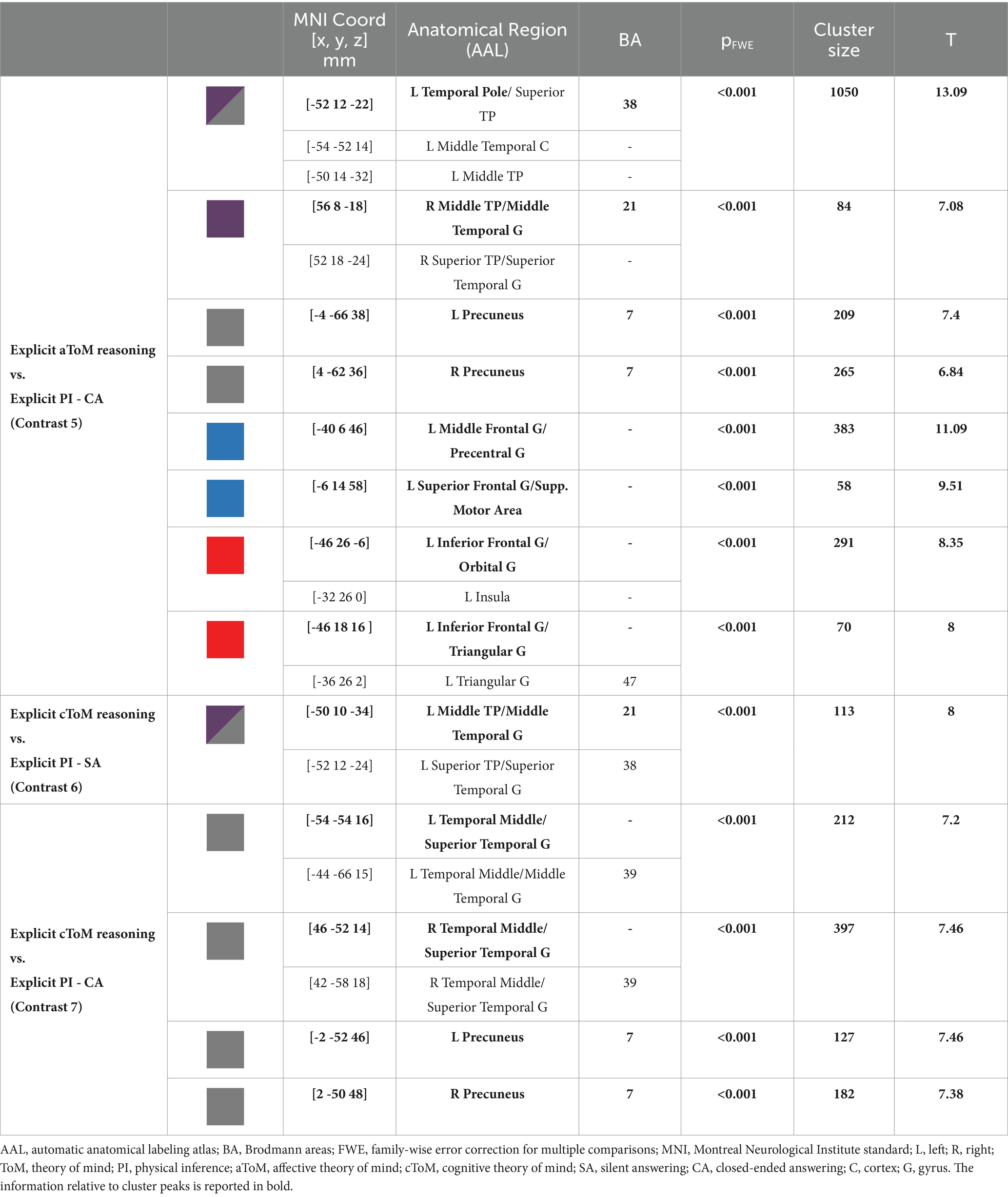
Table 4 . MRI GLM Results.
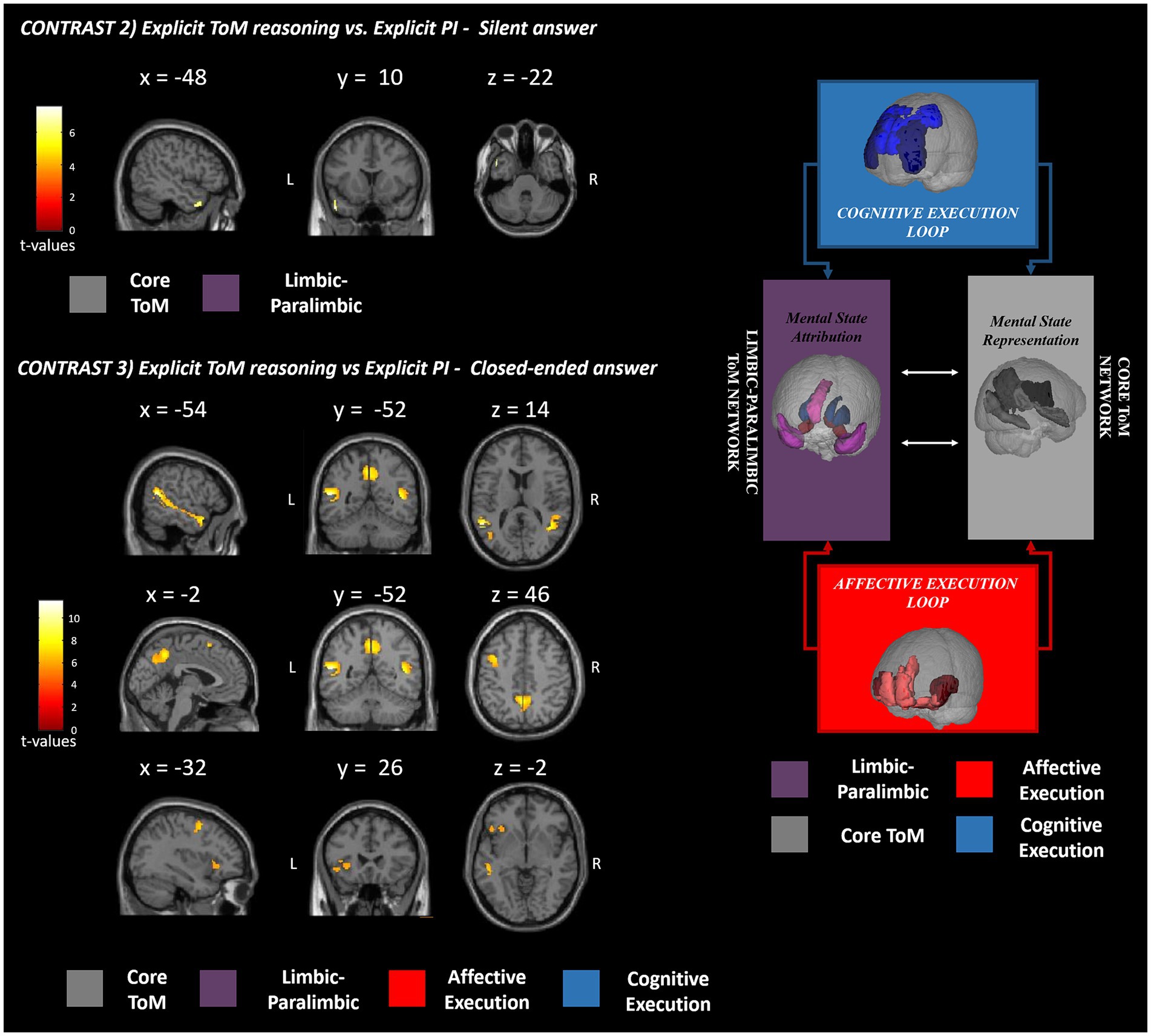
Figure 3 . fMRI GLM results. This figure shows the fMRI results observed in the explicit (contrasts 2 and 3) ToM reasoning with respect to the physical inference control condition. The significant clusters of activation are reported in red-yellow expressing the t-values according to the reported color bar. ToM, theory of mind; PI, physical inference; L, left; R, right. The significant clusters for each contrast are mapped according to the Abu-Akel and Shamay-Tsoory ToM model depicted on the right [adapted from Isernia et al. (2022b) ].
Explicit ToM reasoning was also investigated separately for the affective (contrasts 4 and 5) and cognitive (contrasts 6 and 7) ToM components through the use of silent open-ended questions and two-choice closed-ended questions.
The fourth contrast ( explicit aToM reasoning vs. explicit silent answer ) did not yield any significant supra-threshold activation, while the fifth contrast ( explicit cToM reasoning vs. explicit PI silent answer ) revealed significant functional activations in the bilateral TP (BA 38), precuneus (BA 7), right superior and middle temporal gyri (BA 21), the left inferior frontal cortex (BA 47), specifically the orbital and triangular pars ( Figure 4 ).
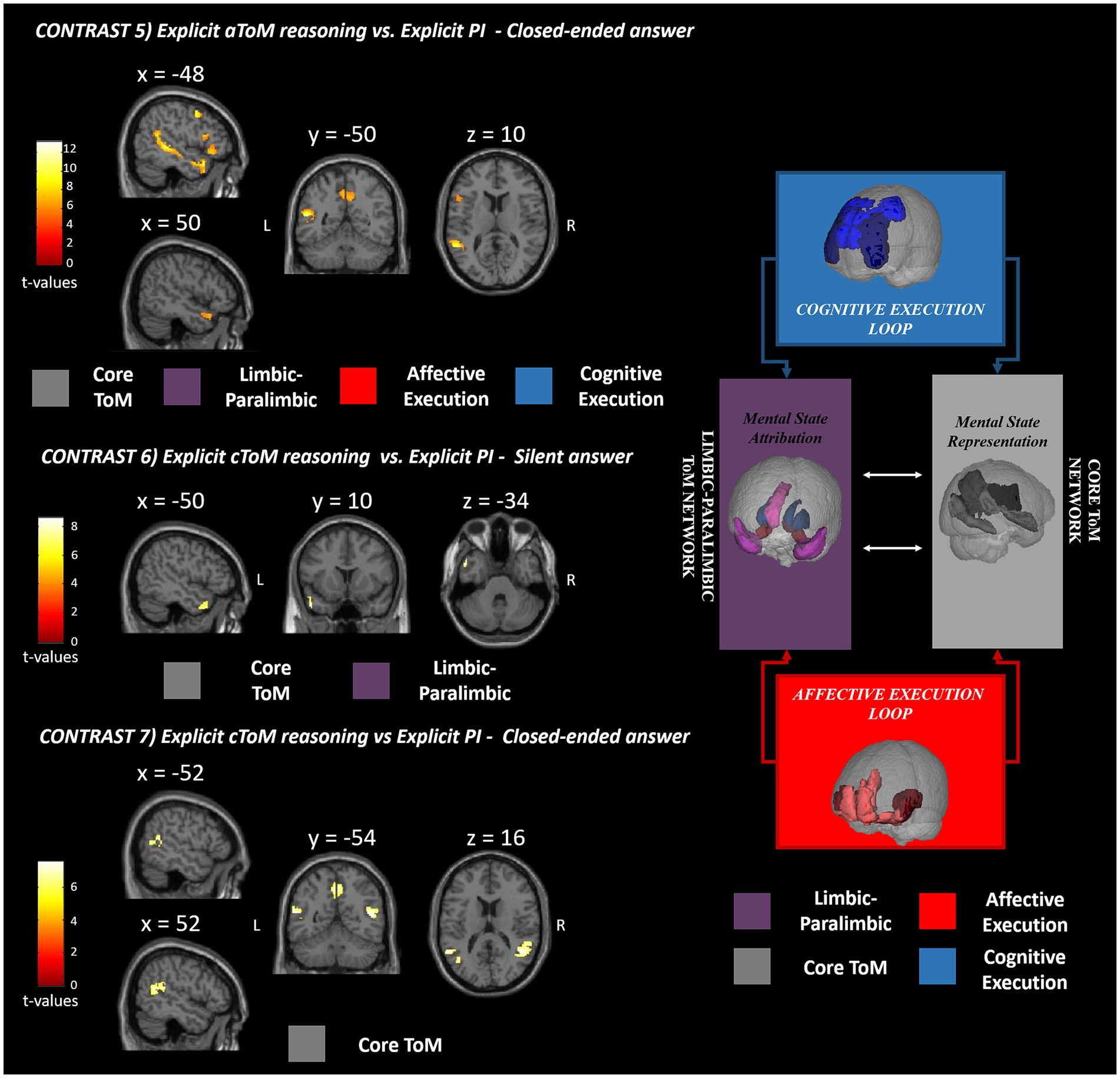
Figure 4 . fMRI GLM results. This figure shows the fMRI results observed for silent and closed-ended answering separately for affective (contrast 5) and cognitive (contrasts 6 and 7) ToM reasoning with respect to the physical inference control condition. The significant clusters of activation are reported in red-yellow expressing the t -values according to the reported color bar. ToM, theory of mind; PI, physical inference; L, left; R, right. The significant clusters for each contrast are mapped according to the Abu-Akel and Shamay-Tsoory ToM model depicted on the right [adapted from Isernia et al. (2022b) ].
As for the sixth contrast ( explicit cToM reasoning vs. explicit PI silent answer ), the activations were confined to the left superior and middle TP (BA 21, 38) ( Figure 4 ), while for the seventh contrast ( explicit cToM reasoning vs. explicit PI closed-ended answer ), activation was elicited in the bilateral precuneus (BA 7), bilateral superior temporal gyrus (BA 39), and left middle temporal gyrus (BA 39) ( Figure 4 ). As for the contrasts used to directly test for differences between the affective and cognitive ToM components, contrast 8 ( explicit aToM reasoning vs. explicit cToM reasoning silent answer ) yielded no significant supra-threshold activation, while contrast 9 ( explicit cToM reasoning vs. explicit aToM reasoning silent answer ) showed neural activation located in the bilateral TPJ (BA 39); contrast 10, namely, explicit aToM reasoning vs. explicit cToM reasoning closed-ended answer , showed significant neural activation in the left superior temporal sulcus, while contrast 11 (explicit cToM reasoning vs. explicit aToM reasoning closed-ended answer ) revealed no supra-threshold clusters. Detailed results and figures are reported in Supplementary Figure S1 .
The ROI-based individual variability analysis revealed low across-subjects consistency with respect to the neural activation elicited by the contrast testing for implicit ToM reasoning. Higher across-subjects consistency was instead observed for the other contrasts specifically in the left TP, left STS, left dorsolateral PFC, bilateral TPJ, and precunei.
The overlap maps and relative histograms are reported in Supplementary Figures S2–S8 .
4 Discussion
We aimed to explore the brain mechanisms involved in ToM reasoning during everyday social interactions. We adapted the Italian version of the ESCoT into an fMRI task to understand the engagement of ToM neural networks in healthy adults. We hypothesized finding neural activations in the core ToM network and the limbic–paralimbic network. In addition, we predicted finding a neural response in ToM ancillary circuits involving executive frontal loops.
Based on the ToM circuits depicted in the study by Abu-Akel and Shamay-Tsoory (2011) , we found no significant neural activation during implicit reasoning on the ESCoT social interaction animations. The lack of activation, which is in contrast with previous studies ( Wolf et al., 2010 ; Jacoby et al., 2016 ), may be related to the generic social instruction (“ focus on the social interactions” ) provided to participants before viewing the movie. In fact, previous studies ( Wolf et al., 2010 ; Jacoby et al., 2016 ) used a specific open-ended ToM question as their instruction (“ What is the character thinking?” ). It could be argued that the generic instruction we provided (“ focus on the social interactions ”) may not necessarily direct a participant’s attention to making inferences about mental states, but also different social cues, producing a consequent general social cognition neural pattern in both conditions (ToM and PI). Reversely, the generic instruction prompting physical inference “ focus on elements in the scene ” might not preclude ToM reasoning, and participants could have been similarly engaged in social cognition operations in both ToM and control conditions.
The results of the explicit ToM reasoning demonstrated different activation patterns related to the two response modalities: the open- (silent) and closed-ended answers. The neural activations related to ToM silent answers (contrast 2) were exclusively confined to the left hemisphere and captured significant activations located in the TP and STS (BA 21, 22, 38), suggesting the involvement of the core ToM and limbic–paralimbic networks, devoted to mental state representation and attribution ( Abu-Akel and Shamay-Tsoory, 2011 ). During the ToM closed-ended answering (contrast 3), the neural pattern extended bilaterally in the core and limbic–paralimbic ToM networks ( Abu-Akel and Shamay-Tsoory, 2011 ) and involved additional brain areas of the cognitive and affective execution loops. This brain pattern resembles and extends the one reported by Wolf et al. (2010) during naturalistic social movie tasks in fMRI. The wider neural pattern of closed-ended answers may be linked to the higher specificity of these items compared to the silent questions. In fact, in contrast with closed-ended ones, the silent questions do not prompt reference to a targeted mental state, with consequent interindividual differences in attentional focus and brain activation. In fact, by inviting the participants to silently answer, the spontaneous fluctuation of attention and brain activity (i.e., mind wandering, Braboszcz and Delorme, 2011 ) may be barely controlled and potentially explains the broader activation of closed-ended than silent answers in eliciting ToM neural patterns. The observed interindividual differences could also be explained by a broader search elicited by an open-ended question versus a closed-ended question, thus resulting in a more varied range of possible responses. The reduced specificity of the silent answer could explain the lack of activations in ToM networks observed during affective ToM answering (contrast 4) and the restricted extension of the neural pattern during cognitive ToM reasoning (contrast 6). Extended activation during multiple-choice answers might also reflect a major recruitment of cognitive processes than silent answers. As a previous fMRI study on mentalizing suggested ( Wolf et al., 2010 ), these answer modes require additional operations, such as interpreting the alternatives, selecting the correct answers, and extensive reading comprehension. These elements concur with higher cognitive demands and task difficulty, plausibly strengthening the brain response. We may assume that to study selective neural patterns within the ToM networks, multiple-choice answers, assuring a more effective neural response, are preferable.
The lack of activation during affective ToM (contrast 4), as opposed to a restricted minimal pattern during cognitive ToM silent answers, might be ascribed, according to our supplementary analyses, to higher inter-subject variability in affective ToM reasoning resulting in a more widespread and less consistent neural pattern component (see Supplementary Figures S5, S7 ). These distinct patterns may be partially explained by the different types of mental states on which the subject is invited to reflect emotions versus thoughts. In fact, a previous study ( Drobyshevsky et al., 2006 ) exploring the sensitivity of common fMRI tasks assessing different neurocognitive domains reported poor sensitivity for the task on emotional function, which did not monitor the subject’s performance.
We found that closed answering enabled distinct neural patterns related to affective and cognitive mental state reasoning. The neural activation of affective ToM (contrast 5) included bilaterally the core and limbic–paralimbic networks, including the precunei, anterior superior temporal sulci, and TPs (BA 7, 21, 38). The involvement of the core network extended to the posterior portion of the STS only in the left hemisphere. The significant cluster of activation included some regions of the affective and cognitive execution loops: the left inferior frontal gyrus (pars orbitalis and triangularis, BA 47), the insula, and the middle frontal gyrus. This pattern of activation resembles the brain network involved in affective mental state representation, attribution, and application ( Abu-Akel and Shamay-Tsoory, 2011 ). Interestingly, the neural activation cluster located in the insula confirms the specific involvement of emotional content and social behavior processing during the execution of an affective naturalistic ToM task ( Henry et al., 2016 ; de Oliveira-Souza and Moll, 2019 ).
Concerning the cognitive mental state neural patterns (contrast 7), the closed-ended answers yielded brain activations located only in the core ToM network, including the bilateral TPJ and precunei (BA 39, 7). Again, the ToM core network for mental state representation was effectively elicited as expected, but the limbic–paralimbic circuit and the frontal loop supporting cognitive mental state deployment were not included. The lack of involvement of the frontal brain areas may be related to several aspects. First, these areas may be recruited both in physical inference and ToM answering using a complex ecological stimulus. The absence of the cognitive frontal circuit could be ascribed to the preserved cognitive level of participants (healthy adults) included in the study, plausibly showing a high level of frontal executive functioning. Preserved and high social cognition processes may lead to selective involvement of specific ToM circuits but not the executive control ones. In addition, it is worth noting that the task itself requires reasoning on the mental states of social events that have already occurred, and, unlike other tests, it does not involve inferring or anticipating future intentions and behaviors of the characters, which is likely to engage the frontal loop. A recent meta-analysis ( Schurz et al., 2021 ) presented distinct patterns of neural activation based on social cognition test stimuli, proposing a three clustering solution: cognitive (ToM), affective (empathy), and intermediate domains. According to the present results, the ESCoT belongs to the intermediate cluster, bridging between the different domains of cognitive ToM and empathy. The intermediate positioning of ESCoT may be linked to its intrinsically ecological nature. In fact, by showing everyday life interactions, cognitive and affective ToM reasoning might be at least in part simultaneously elicited to properly interpret the social dynamics.
Finally, regarding the core ToM network activation during affective and cognitive ToM answering, a distinct neural pattern was also observed in the temporal areas. Especially the STS activation (BA 21/22) was found only during the affective ToM answering, while the TPJ involvement (BA 39) was observed exclusively in the cognitive ToM reasoning. These findings are further confirmed by explorinsg a direct comparison between the cognitive and affective ToM neural patterns (contrasts 8–11). In fact, affective ToM revealed neural activation located in the STS when explicit ToM reasoning was explored using closed-ended answering, while cognitive ToM reasoning relied on the activation of the bilateral TPJ when explicit ToM reasoning was tested using silent answering.
The TPJ has been widely reported as a crucial hub for the switching between self-perspective and others’ viewpoint during mentalizing tasks ( Decety and Sommerville, 2003 ; Uddin et al., 2005 ; Corbetta et al., 2008 ), playing a mediating role between self and others’ perspectives ( Schulte-Rüther et al., 2007 ). With the ESCoT scenarios, participants are invited to reflect on the mental states of characters who interact in a specific context, which is influenced by social norm adhesion or violation. When participants are prompted to reason on the affective mental states of the injured character in the scenario (e.g., the old woman who does not receive help from the young man), attention was easily directed to that character with whom the participant was prone to empathize, resulting in neural activation confined in the STS. Instead, when the participant is invited to reason on the thoughts and expectations of the injured character (i.e., in the previous example, did the young man behave as the old woman expected?), the level of complexity increases because it requires the switching to others’ viewpoints. In fact, the participant likely compares the characters’ violated expectations, in line with their own, with the intentions of the other character who violated those expectations. In this case, we observed major involvement of TPJ, as previously reported.
Another explanation might be related to previous studies supporting that the ToM circuit is prone to respond more to unpredicted (violation) than predicted (adhesion) information ( Cloutier et al., 2011 ; Dungan et al., 2016 ), in light of the predictive coding view of the social brain. In addition, evidence highlighted an enhanced activity of the bilateral TPJ when mental state information is relevant for moral judgment ( Young and Waytz, 2013 ). Especially, enhanced activations in the TPJ are observed when an immoral behavior is shown ( Kim et al., 2021 ) compared to a moral social comportment. A suggested explanation is the perception of immoral behavior as containing more intents than moral ones ( Brambilla et al., 2019 ), plausibly recruiting a broader neural circuit. Overall, the role of ToM in moral cognition has been reported in the literature ( Turiel, 1983 ). In fact, reasoning on people’s intentionality inflects the moral judgment when the individual is wondering whether to blame an agent’s behavior or not ( Knobe, 2005 ).
The adaptation of the ESCoT into an fMRI task was not without limitations which should be addressed in future studies, especially involving aging or clinical populations. First, the ToM silent answering resulted in poor neural activation as previously demonstrated ( Wolf et al., 2010 ). Therefore, only closed-ended questions should be included in future versions. The modification will also shorten the overall duration of the experiment. In addition, the high socio-educational level of participants may have partly prevented the generalization of the observed results. Moreover, the differentiation of the neural underpinnings related to the adhesion or violation of the social norms depicted in the social interaction animations was not investigated in this study, which may potentially affect ToM mechanisms. Future contributions should address these aspects to explore and deepen the knowledge of the neural correlates involved in social cognition deficits. Future studies could use this version of the ESCoT in clinical settings to study ToM network in different pathologies at risk or with social cognition deficits. This could include neurodegenerative conditions, schizophrenia, or developmental disorders. In addition, the task could be used to evaluate neuroplasticity or reorganization after ToM training such as those used in rehabilitative settings. Finally, one last aspect that should not be overlooked when exploring higher-level cognitive domains such as ToM reasoning is the issue of individual variability ( Saxe and Kanwisher, 2003 ; Saxe and Powell, 2006 ). To address this issue, standard GLM factorial analysis should be integrated with further analysis assessing the consistency of brain responses across subjects. This has been addressed in the present study by performing an additional analysis with the methods proposed in a study by Seghier and Price (2016) , and the findings corroborate the group-level results. Specifically, concerning the two ToM components, the differential involvement of TPJ in cToM and STS in aToM was substantiated.
Finally, it is worth mentioning that our results prevented us from investigating online mentalizing reasoning when the subject is involved in first person social interactions. Future contributions might consider adopting different acquisition settings such as hyper-scanning (e.g., Bazán and Amaro, 2022 for a review) to study neural patterns during social interaction.

5 Conclusion
In conclusion, the present study investigated the brain mechanisms involved in individual mentalizing reasoning in real-life social interactions. The ESCoT stimuli were particularly effective in evoking ToM core neural underpinnings and elicited executive frontal loops. These results support the application of the ESCoT as a sensitive test of social cognition and provide further insights into the neural regions involved in social cognition.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Fondazione Don Gnocchi-Milan Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SI: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AP: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. FR: Methodology, Writing – review & editing. DC: Formal analysis, Writing – review & editing. MC: Data curation, Writing – review & editing. VB: Methodology, Supervision, Writing – review & editing. RB: Writing – review & editing. SM: Writing – review & editing. FB: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—A multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Acknowledgments
We would like to thank all the participants of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1420122/full#supplementary-material
1. ^ https://pstnet.com/products/e-prime/
2. ^ https://www.nordicneurolab.com/
3. ^ https://www.fil.ion.ucl.ac.uk/spm/
4. ^ https://it.mathworks.com/products/matlab.html
5. ^ https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/
Aboulafia-Brakha, T., Christe, B., Martory, M. D., and Annoni, J. M. (2011). Theory of mind tasks and executive functions: a systematic review of group studies in neurology. J. Neuropsychol. 5, 39–55. doi: 10.1348/174866410x533660
PubMed Abstract | Crossref Full Text | Google Scholar
Abu-Akel, A., and Shamay-Tsoory, S. (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia 49, 2971–2984. doi: 10.1016/j.neuropsychologia.2011.07.012
Adolphs, R. (2009). The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716. doi: 10.1146/annurev.psych.60.110707.163514
Baez, S., García, A. M., and Ibanez, A. (2017). The social context network model in psychiatric and neurological diseases. Curr. Top. Behav. Neurosci. 30, 379–396. doi: 10.1007/7854_2016_443
Baglio, F., and Marchetti, A. (2016). Editorial: when (and how) is theory of mind useful? Evidence from life-span research. Front. Psychol. 7:1425. doi: 10.3389/fpsyg.2016.01425
Baksh, R. A., Abrahams, S., Auyeung, B., and MacPherson, S. E. (2018). The Edinburgh social cognition test (ESCoT): examining the effects of age on a new measure of theory of mind and social norm understanding. PLoS One 13:e0195818. doi: 10.1371/journal.pone.0195818
Baksh, R. A., Abrahams, S., Bertlich, M., Cameron, R., Jany, S., Dorrian, T., et al. (2021). Social cognition in adults with autism spectrum disorders: validation of the Edinburgh social cognition test (ESCoT). Clin. Neuropsychol. 35, 1275–1293. doi: 10.1080/13854046.2020.1737236
Baksh, R. A., Bugeja, T., and MacPherson, S. E. (2020). Executive functions do not underlie performance on the Edinburgh social cognition test (ESCoT) in healthy younger and older adults. J. Int. Neuropsychol. Soc. 26, 527–538. doi: 10.1017/s1355617719001450
Crossref Full Text | Google Scholar
Baksh, R. A., MacPherson, S. E., Auyeung, B., Pal, S., and Abrahams, S. (2023). The relationship between social cognitive processes and behaviour changes in people with dementia using the Edinburgh social cognition test (ESCoT). Neuropsychology . 38, 223–238. doi: 10.1037/neu0000929
Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y., and Plumb, I. (2001). The "Reading the mind in the eyes" test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry 42, 241–251. doi: 10.1111/1469-7610.00715
Bazán, P. R., and Amaro, E. Jr. (2022). “fMRI and fNIRS methods for social brain studies: Hyperscanning possibilities” in Social and affective neuroscience of everyday human interaction: From theory to methodology . ed. P. S. Boggio (Berlin: Springer), 231–254.
Google Scholar
Braboszcz, C., and Delorme, A. (2011). Lost in thoughts: neural markers of low alertness during mind wandering. NeuroImage 54, 3040–3047. doi: 10.1016/j.neuroimage.2010.10.008
Brambilla, M., Carraro, L., Castelli, L., and Sacchi, S. (2019). Changing impressions: moral character dominates impression updating. J. Exp. Soc. Psychol. 82, 64–73. doi: 10.1016/j.jesp.2019.01.003
Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F., and Venneri, A. (2002). A short version of the Stroop test: normative data in an Italian population sample. Nuova Riv. Neurol. 12, 111–115.
Canty, A. L., Neumann, D. L., Fleming, J., and Shum, D. H. K. (2017). Evaluation of a newly developed measure of theory of mind: the virtual assessment of mentalising ability. Neuropsychol. Rehabil. 27, 834–870. doi: 10.1080/09602011.2015.1052820
Charlton, R. A., Barrick, T. R., Markus, H. S., and Morris, R. G. (2009). Theory of mind associations with other cognitive functions and brain imaging in normal aging. Psychol. Aging 24, 338–348. doi: 10.1037/a0015225
Cloutier, J., Gabrieli, J. D., O'Young, D., and Ambady, N. (2011). An fMRI study of violations of social expectations: when people are not who we expect them to be. NeuroImage 57, 583–588. doi: 10.1016/j.neuroimage.2011.04.051
Conti, S., Bonazzi, S., Laiacona, M., Masina, M., and Coralli, M. V. (2015). Montreal cognitive assessment (MoCA)-Italian version: regression based norms and equivalent scores. Neurol. Sci. 36, 209–214. doi: 10.1007/s10072-014-1921-3
Corbetta, M., Patel, G., and Shulman, G. L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324. doi: 10.1016/j.neuron.2008.04.017
de Oliveira-Souza, R., and Moll, J. (2019). Moral conduct and social behavior. Handb. Clin. Neurol. 163, 295–315. doi: 10.1016/b978-0-12-804281-6.00016-1
Decety, J., and Sommerville, J. A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn. Sci. 7, 527–533. doi: 10.1016/j.tics.2003.10.004
Drobyshevsky, A., Baumann, S. B., and Schneider, W. (2006). A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. NeuroImage 31, 732–744. doi: 10.1016/j.neuroimage.2005.12.016
Dungan, J. A., Stepanovic, M., and Young, L. (2016). Theory of mind for processing unexpected events across contexts. Soc. Cogn. Affect. Neurosci. 11, 1183–1192. doi: 10.1093/scan/nsw032
Dziobek, I., Fleck, S., Kalbe, E., Rogers, K., Hassenstab, J., Brand, M., et al. (2006). Introducing MASC: a movie for the assessment of social cognition. J. Autism Dev. Disord. 36, 623–636. doi: 10.1007/s10803-006-0107-0
Frith, C. D. (2008). Social cognition. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 363, 2033–2039. doi: 10.1098/rstb.2008.0005
Giovagnoli, A. R., Del Pesce, M., Mascheroni, S., Simoncelli, M., Laiacona, M., and Capitani, E. (1996). Trail making test: normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 17, 305–309. doi: 10.1007/bf01997792
Happé, F., Cook, J. L., and Bird, G. (2017). The structure of social cognition: in(ter)dependence of sociocognitive processes. Annu. Rev. Psychol. 68, 243–267. doi: 10.1146/annurev-psych-010416-044046
Heavey, L., Phillips, W., Baron-Cohen, S., and Rutter, M. (2000). The awkward moments test: a naturalistic measure of social understanding in autism. J. Autism Dev. Disord. 30, 225–236. doi: 10.1023/a:1005544518785
Henry, J. D., Cowan, D. G., Lee, T., and Sachdev, P. S. (2015). Recent trends in testing social cognition. Curr. Opin. Psychiatry 28, 133–140. doi: 10.1097/yco.0000000000000139
Henry, J. D., Phillips, L. H., Ruffman, T., and Bailey, P. E. (2013). A meta-analytic review of age differences in theory of mind. Psychol. Aging 28, 826–839. doi: 10.1037/a0030677
Henry, J. D., von Hippel, W., Molenberghs, P., Lee, T., and Sachdev, P. S. (2016). Clinical assessment of social cognitive function in neurological disorders. Nat. Rev. Neurol. 12, 28–39. doi: 10.1038/nrneurol.2015.229
Hildebrandt, M. K., Jauk, E., Lehmann, K., Maliske, L., and Kanske, P. (2021). Brain activation during social cognition predicts everyday perspective-taking: a combined fMRI and ecological momentary assessment study of the social brain. NeuroImage 227:117624. doi: 10.1016/j.neuroimage.2020.117624
Isernia, S., MacPherson, S. E., Baksh, R. A., Bergsland, N., Marchetti, A., Baglio, F., et al. (2022a). Italian adaptation of the Edinburgh social cognition test (ESCoT): a new tool for the assessment of theory of mind and social norm understanding. Front. Psychol. 13:971187. doi: 10.3389/fpsyg.2022.971187
Isernia, S., Pirastru, A., Massaro, D., Rovaris, M., Marchetti, A., and Baglio, F. (2022b). Resting-state functional brain connectivity for human mentalizing: biobehavioral mechanisms of theory of mind in multiple sclerosis. Soc. Cogn. Affect. Neurosci. 17, 579–589. doi: 10.1093/scan/nsab120
Isernia, S., Rossetto, F., Shamay-Tsoory, S., Marchetti, A., and Baglio, F. (2022c). Standardization and normative data of the 48-item Yoni short version for the assessment of theory of mind in typical and atypical conditions. Front. Aging Neurosci. 14:1048599. doi: 10.3389/fnagi.2022.1048599
Jacoby, N., Bruneau, E., Koster-Hale, J., and Saxe, R. (2016). Localizing pain matrix and theory of mind networks with both verbal and non-verbal stimuli. NeuroImage 126, 39–48. doi: 10.1016/j.neuroimage.2015.11.025
Jenkinson, M., Pechaud, M., and Smith, S. (2005). BET2: MR-based estimation of brain, skull and scalp surfaces. Eleventh annual meeting of the organization for human brain mapping
Kim, M. J., Mende-Siedlecki, P., Anzellotti, S., and Young, L. (2021). Theory of mind following the violation of strong and weak prior beliefs. Cereb. Cortex 31, 884–898. doi: 10.1093/cercor/bhaa263
Knobe, J. (2005). Theory of mind and moral cognition: exploring the connections. Trends Cogn. Sci. 9, 357–359. doi: 10.1016/j.tics.2005.06.011
Krohn, S., Tromp, J., Quinque, E. M., Belger, J., Klotzsche, F., Rekers, S., et al. (2020). Multidimensional evaluation of virtual reality paradigms in clinical neuropsychology: application of the VR-check framework. J. Med. Internet Res. 22:e16724. doi: 10.2196/16724
Love, N., Ruff, G., and Geldmacher, D. (2015). Social cognition in older adults: a review of neuropsychology, neurobiology, and functional connectivity. Med Clin Rev 1:6. doi: 10.21767/2471-299X.1000006
Maddaluno, O., Aiello, E. N., Roncoroni, C., Prunas, A., and Bolognini, N. (2022). The Reading the mind in the eyes test, Iowa gambling task and interpersonal reactivity index: normative data in an Italian population sample. Arch. Clin. Neuropsychol. 37, 929–938. doi: 10.1093/arclin/acab100
Mathersul, D., McDonald, S., and Rushby, J. A. (2013). Understanding advanced theory of mind and empathy in high-functioning adults with autism spectrum disorder. J. Clin. Exp. Neuropsychol. 35, 655–668. doi: 10.1080/13803395.2013.809700
McDonald, S., Flanagan, S., Rollins, J., and Kinch, J. (2003). TASIT: a new clinical tool for assessing social perception after traumatic brain injury. J. Head Trauma Rehabil. 18, 219–238. doi: 10.1097/00001199-200305000-00001
Monaco, M., Costa, A., Caltagirone, C., and Carlesimo, G. A. (2013). Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol. Sci. 34, 749–754. doi: 10.1007/s10072-012-1130-x
Pantelis, P. C., Byrge, L., Tyszka, J. M., Adolphs, R., and Kennedy, D. P. (2015). A specific hypoactivation of right temporo-parietal junction/posterior superior temporal sulcus in response to socially awkward situations in autism. Soc. Cogn. Affect. Neurosci. 10, 1348–1356. doi: 10.1093/scan/nsv021
Parsey, C. M., and Schmitter-Edgecombe, M. (2013). Applications of technology in neuropsychological assessment. Clin. Neuropsychol. 27, 1328–1361. doi: 10.1080/13854046.2013.834971
Poveda, B., Abrahams, S., Baksh, R. A., MacPherson, S. E., and Evans, J. J. (2022). An investigation of the validity of the Edinburgh social cognition test (ESCoT) in acquired brain injury (ABI). J. Int. Neuropsychol. Soc. 28, 1016–1028. doi: 10.1017/s1355617721001223
Rao, M. S. (1990). Cognitive function study group, Nmss: A manual for the brief repeatable battery of neuropsychological tests in multiple sclerosis . New York, NY: National Multiple Sclerosis Society.
Redcay, E., and Moraczewski, D. (2020). Social cognition in context: a naturalistic imaging approach. NeuroImage 216:116392. doi: 10.1016/j.neuroimage.2019.116392
Roeyers, H., Buysse, A., Ponnet, K., and Pichal, B. (2001). Advancing advanced mind-reading tests: empathic accuracy in adults with a pervasive developmental disorder. J. Child Psychol. Psychiatry 42, 271–278. doi: 10.1111/1469-7610.00718
Santangelo, G., Siciliano, M., Pedone, R., Vitale, C., Falco, F., Bisogno, R., et al. (2015). Normative data for the Montreal cognitive assessment in an Italian population sample. Neurol. Sci. 36, 585–591. doi: 10.1007/s10072-014-1995-y
Saxe, R., and Kanwisher, N. (2003). People thinking about thinking people. The role of the temporo-parietal junction in "theory of mind". NeuroImage 19, 1835–1842. doi: 10.1016/s1053-8119(03)00230-1
Saxe, R., and Powell, L. J. (2006). It's the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 17, 692–699. doi: 10.1111/j.1467-9280.2006.01768.x
Schulte-Rüther, M., Markowitsch, H. J., Fink, G. R., and Piefke, M. (2007). Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J. Cogn. Neurosci. 19, 1354–1372. doi: 10.1162/jocn.2007.19.8.1354
Schurz, M., Radua, J., Tholen, M. G., Maliske, L., Margulies, D. S., Mars, R. B., et al. (2021). Toward a hierarchical model of social cognition: a neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol. Bull. 147, 293–327. doi: 10.1037/bul0000303
Seghier, M. L., and Price, C. J. (2016). Visualising inter-subject variability in fMRI using threshold-weighted overlap maps. Sci. Rep. 6:20170. doi: 10.1038/srep20170
Shamay-Tsoory, S. G., and Aharon-Peretz, J. (2007). Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia 45, 3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021
Sheridan, L. K., Fitzgerald, H. E., Adams, K. M., Nigg, J. T., Martel, M. M., Puttler, L. I., et al. (2006). Normative symbol digit modalities test performance in a community-based sample. Arch. Clin. Neuropsychol. 21, 23–28. doi: 10.1016/j.acn.2005.07.003
Smith, S. M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155. doi: 10.1002/hbm.10062
Strauss, E., Sherman, E. M., and Spreen, O. (2006). A compendium of neuropsychological tests: administration, norms, and commentary. Oxford University Press .
Szucs, D., and Ioannidis, J. P. (2020). Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990-2012) and of latest practices (2017-2018) in high-impact journals. NeuroImage 221:117164. doi: 10.1016/j.neuroimage.2020.117164
Turiel, E. (1983). The development of social knowledge: Morality and convention . Cambridge: Cambridge University Press.
Tustison, N. J., Avants, B. B., Cook, P. A., Zheng, Y., Egan, A., Yushkevich, P. A., et al. (2010). N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320. doi: 10.1109/tmi.2010.2046908
Uddin, L. Q., Kaplan, J. T., Molnar-Szakacs, I., Zaidel, E., and Iacoboni, M. (2005). Self-face recognition activates a frontoparietal "mirror" network in the right hemisphere: an event-related fMRI study. NeuroImage 25, 926–935. doi: 10.1016/j.neuroimage.2004.12.018
Wolf, I., Dziobek, I., and Heekeren, H. R. (2010). Neural correlates of social cognition in naturalistic settings: a model-free analysis approach. NeuroImage 49, 894–904. doi: 10.1016/j.neuroimage.2009.08.060
Young, L., and Waytz, A. (2013). “Mind attribution is for morality” in Understanding other minds: Perspectives from developmental social neuroscience (Oxford: Oxford University Press), 93–103.
Keywords: theory of mind, task fMRI, social cognition, mentalizing, rehabilitation
Citation: Isernia S, Pirastru A, Rossetto F, Cacciatore DM, Cazzoli M, Blasi V, Baksh RA, MacPherson SE and Baglio F (2024) Human reasoning on social interactions in ecological contexts: insights from the theory of mind brain circuits. Front. Neurosci . 18:1420122. doi: 10.3389/fnins.2024.1420122
Received: 19 April 2024; Accepted: 01 July 2024; Published: 01 August 2024.
Reviewed by:
Copyright © 2024 Isernia, Pirastru, Rossetto, Cacciatore, Cazzoli, Blasi, Baksh, MacPherson and Baglio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alice Pirastru, [email protected]
† ORCID: Sara Isernia, orcid.org/0000-0002-0849-3984 Alice Pirastru, orcid.org/0000-0001-9474-2344 Alice Pirastru, orcid.org/0000-0001-9474-2344 Federica Rossetto, orcid.org/0000-0001-6336-2648 Diego Michael Cacciatore, orcid.org/0009-0009-7238-2427 Marta Cazzoli, orcid.org/0000-0002-9213-0667 Valeria Blasi, orcid.org/0000-0002-8395-0452 Asaad Baksh, orcid.org/0000-0001-6596-2145 Sarah E. MacPherson, orcid.org/0000-0001-8676-6514 Francesca Baglio, orcid.org/0000-0002-6145-5274
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Psychology Unlocked
The free online psychology textbook, social psychology research topics.
January 24, 2017 Daniel Edward Blog , Social Psychology 0

Whether you’re looking for social psychology research topics for your A-Level or AP Psychology class, or considering a research question to explore for your Psychology PhD, the Psychology Unlocked list of social psychology research topics provides you with a strong list of possible avenues to explore.
Where possible we include links to university departments seeking PhD applications for certain projects. Even if you are not yet considering PhD options, these links may prove useful to you in developing your undergraduate or masters dissertation.
Lots of university psychology departments provide contact details on their websites.
If you read a psychologist’s paper and have questions that you would like to learn more about, drop them an email.
Lots of psychologists are very happy to receive emails from genuinely interested students and are often generous with their time and expertise… and those who aren’t will just overlook the email, so no harm done either way!
Psychology eZine
Subscribe to our weekly newsletter for videos, articles, news and more.
We use Sendinblue as our marketing platform. By Clicking below to submit this form, you acknowledge that the information you provided will be transferred to Sendinblue for processing in accordance with their terms of use
- The Dunning-Kruger Effect: Why we think we know more than we do
- The Yale Food Addiction Scale: Are you addicted to food?
- Addicted to Pepsi Max? Understand addiction in six minutes (video)
- Functional Fixedness: The cognitive bias and how to beat it
- Summer Spending Spree! How Summer Burns A Hole In Your Pocket
What social factors are involved with the development of aggressive thoughts and behaviours? Is aggression socially-defined? Do different societies have differing definitions of aggression?
There has recently been a significant amount of research conducted on the influence of video games and television on aggression and violent behaviour.
Some research has been based on high-profile case studies, such as the aggressive murder of Jamie Bulger in 1993 by two children (Robert Thompson and Jon Venables). There is also a significant body of experimental research.
Attachment and Relationships
This is a huge area of research with lots of crossover into developmental psychology. What draws people together? How do people connect emotionally? What is love? What is friendship? What happens if someone doesn’t form an attachment with a parental figure?
This area includes research on attachment styles (at various stages of life), theories of love, friendship and attraction.
Attitudes and Attitude Change
Attitudes are a relatively enduring and general evaluation of something. Individuals hold attitudes on everything in life, from other people to inanimate objects, groups to ideologies.
Attitudes are thought to involve three components: (1) affective (to do with emotions), (2) behavioural, and (3) cognitive (to do with thoughts).
Research on attitudes can be closely linked to Prejudice (see below).
Authority and Leadership
Perhaps the most famous study of authority is Milgram’s (1961) Obedience to Authority . This research area has grown into a far-reaching and influential topic.
Research considers both positive and negative elements of authority, and applied psychology studies consider the role of authority in a particular social setting, such as advertising, in the workplace, or in a classroom.
The Psychology of Crowds (Le Bon, 1895) paved a path for a fascinating area of social psychology that considers the social group as an active player.
Groups tend to act differently from individuals, and specific individuals will act differently depending on the group they are in.
Social psychology research topics about groups consider group dynamics, leadership (see above), group-think and decision-making, intra-group and inter-group conflict, identities (see below) and prejudices (see below).
Gordon Allport’s (1979) ‘The Nature of Prejudice’ is a seminal piece on group stereotyping and discrimination.
Social psychologists consider what leads to the formation of stereotypes and prejudices. How and why are prejudices used? Why do we maintain inaccurate stereotypes? What are the benefits and costs of prejudice?
This interesting blog post on the BPS Digest Blog may provide some inspiration for research into prejudice and political uncertainty.
Pro- and Anti-Social Behaviour
Behaviours are only pro- or anti-social because of social norms that suggest so. Social Psychologists therefore investigate the roots of these behaviours as well as considering what happens when social norms are ignored.
Within this area of social psychology, researchers may consider why people help others (strangers as well as well as known others). Another interesting question regards the factors that might deter an individual from acting pro-socially, even if they are aware that a behaviour is ‘the right thing to do’.
The bystander effect is one such example of social inaction.
Self and Social Identity
Tajfel and Turner (1979) proposed Social Identity Theory and a large body of research has developed out of the concepts of self and social identity (or identities).
Questions in this area include: what is identity? What is the self? Does a social identity remain the same across time and space? What are the contributory factors to an individual’s social identity?
Zimbardo’s (1972) Stanford Prison Experiment famously considered the role of social identities.
Research in this area also links with work on groups (see above), social cognition (see below), and prejudices (see above).
Social Cognition
Social cognition regards the way we think and use information. It is the cross-over point between the fields of social and cognitive psychology.
Perhaps the most famous concept in this area is that of schemas – general ideas about the world, which allow us to make sense of new (and old) information quickly.
Social cognition also includes those considering heuristics (mental shortcuts) and some cognitive biases.
Social Influence
This is one of the first areas of social psychology that most students learn. Remember the social conformity work by Asch (1951) on the length of lines?
Other social psychology research topics within this area include persuasion and peer-pressure.
Social Representations
Social Representations (Moscovici, 1961) ‘make something unfamiliar, or unfamiliarity itself, familiar’ (Moscovici, 1984). This is a theory with its academic roots in Durkheim’s theory of collective representations.
Researchers working within this framework consider the social role of knowledge. How does information translate from the scientific realm of expert knowledge to the socially accessible realm of the layperson? How do we make sense of new information? How do we organise separate and distinct facts in a way that make sense to our needs?
One of the most famous studies using Social Representations Theory is Jodelet’s (1991) study of madness.
- Social Article
- Social Psychology
Copyright © 2024 | WordPress Theme by MH Themes
Cognitive Psychology Research Paper Topics

This page provides a comprehensive list of cognitive psychology research paper topics , curated to inspire and assist students in their exploration of how humans perceive, remember, think, speak, and solve problems. Cognitive psychology, a discipline pivotal to understanding the intricacies of the human mind, encompasses a wide array of fascinating topics that delve into the mental processes underlying our daily functioning and well-being. From investigating the mechanisms of memory and the complexities of language acquisition to exploring the influence of emotion on cognition and the application of cognitive principles in technology, these topics offer students a rich terrain for academic inquiry. Designed to cater to a broad spectrum of interests and academic objectives, this list serves as a starting point for students aiming to contribute meaningful insights into the cognitive processes that define human experience.
100 Cognitive Psychology Research Paper Topics
Cognitive psychology stands at the forefront of exploring the vast capabilities and intricacies of the human mind, offering profound insights into our thoughts, emotions, and behaviors. This branch of psychology delves into how people understand, diagnose, and interact with the world around them, influencing various aspects of human functioning and societal development. The research topics within cognitive psychology are as varied as they are dynamic, reflecting the continuous evolution of the field in response to new scientific discoveries and technological advancements. From the fundamental processes of perception and memory to the complex interplay between emotion and cognition, these topics not only contribute to our scientific knowledge but also have practical applications in education, mental health, artificial intelligence, and beyond.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% off with 24start discount code.
- The psychology of visual illusions
- Cross-modal perception and sensory integration
- The impact of aging on sensory processing
- Auditory perception and its cognitive implications
- The role of attention in shaping perception
- Taste, smell, and flavor perception
- Sensory deprivation and its effects on cognition
- Perception of pain and its cognitive modulation
- The neuroscience of touch
- Multisensory experiences and their cognitive effects
- Short-term versus long-term memory processes
- The effects of sleep on memory consolidation
- Autobiographical memory and self-identity
- Cognitive strategies to enhance memory retention
- The role of emotion in memory formation and recall
- False memories and their implications
- The cognitive neuroscience of working memory
- Memory disorders and cognitive rehabilitation
- The impact of technology on memory skills
- Eyewitness memory and cognitive psychology
- Models of attention and cognitive processing
- The impact of multitasking on cognitive performance
- Attentional biases and their psychological implications
- Cognitive load theory and information processing
- The role of attention in learning and memory
- Neural mechanisms underlying attention
- Distraction and cognitive control mechanisms
- The psychology of vigilance and sustained attention
- Attention deficits and hyperactivity disorders
- Selective attention and perceptual filtering
- The cognitive basis of language development
- Bilingualism and cognitive flexibility
- Language disorders and cognitive psychology
- The relationship between thought and language
- Cognitive neuroscience of reading and literacy
- Language processing in the brain
- Pragmatics and cognitive implications of language use
- The role of language in categorization and concept formation
- Sign language and cognitive processing
- Cognitive aspects of language evolution
- Cognitive strategies in problem-solving
- Decision-making processes and biases
- The psychology of judgment and choice
- Heuristics and cognitive shortcuts
- The role of intuition in decision-making
- Problem-solving in groups versus individually
- Cognitive biases and their impact on decision quality
- Risk assessment and decision-making under uncertainty
- The neuroscience of decision-making
- Creativity and cognitive processes in problem-solving
- Stages of cognitive development in children
- Cognitive theories of learning and instruction
- The role of play in cognitive development
- Adolescent cognitive development and risk-taking behavior
- Adult learning and cognitive change
- The impact of cognitive styles on learning outcomes
- Cognitive development in aging populations
- The role of technology in cognitive learning processes
- Cognitive enhancers and their impact on learning
- Metacognition and self-regulated learning
- Cognitive aspects of Alzheimer’s disease
- The neuropsychology of Parkinson’s disease
- Cognitive impairments in traumatic brain injury
- Neurocognitive deficits in schizophrenia
- Attention deficit hyperactivity disorder (ADHD) in adults
- Autism spectrum disorders and cognitive functioning
- The impact of stroke on cognitive functions
- Dementia and cognitive interventions
- Mild cognitive impairment and its progression
- Cognitive rehabilitation techniques for neurocognitive disorders
- The influence of emotion on cognitive processes
- Cognitive appraisal theories of emotion
- The role of cognition in emotional regulation
- Emotional intelligence and cognitive abilities
- The neuroscience of emotions and feelings
- Mood disorders and cognitive functioning
- The impact of stress on cognitive performance
- Emotion-cognition interactions in decision-making
- The cognitive psychology of happiness and well-being
- Emotional memory and its persistence
- Cognitive biases in social judgment and perception
- Theory of mind and perspective-taking
- Social cognition in interpersonal relationships
- The role of stereotypes in cognitive processing
- Cognitive underpinnings of prejudice and discrimination
- Social identity and cognition
- Moral reasoning and cognitive psychology
- The cognitive basis of empathy and altruism
- Social cognition and group dynamics
- Cognitive approaches to understanding social influence
- Cognitive psychology in human-computer interaction
- Virtual reality and its cognitive implications
- The impact of social media on cognition and social behavior
- Cognitive psychology principles in user experience design
- Artificial intelligence and cognitive modeling
- Gaming and cognitive skill development
- Cognitive training apps and their effectiveness
- Neurotechnology and cognitive enhancement
- The role of cognitive psychology in digital education
- Wearable technology and cognitive monitoring
The exploration of cognitive psychology research paper topics presents an unparalleled opportunity to delve into the mechanisms that underpin human cognition and behavior. Each category and topic not only contributes to the rich tapestry of cognitive psychology but also holds the potential for groundbreaking research that can influence educational practices, therapeutic approaches, and policy development. Students are encouraged to engage deeply with these topics, leveraging their curiosity and analytical skills to advance the field and contribute valuable insights into the complex world of human cognition.
What is Cognitive Psychology
Cognitive Psychology as a Discipline

The development of cognitive psychology marked a significant shift from the behaviorist perspective that dominated psychology for much of the early 20th century, which largely ignored mental processes. Instead, cognitive psychology focuses on understanding internal mental states and processes, utilizing this understanding to explain behavioral patterns. This focus on the internal workings of the mind has not only expanded the scope of psychological research but has also had practical applications in various fields such as education, mental health, artificial intelligence, and more, demonstrating the discipline’s broad impact.
The Importance of Research in Expanding Our Understanding of Cognitive Processes
Research in cognitive psychology plays a crucial role in expanding our understanding of the human mind and behavior. Through empirical studies, experiments, and longitudinal research, cognitive psychologists seek to build a body of knowledge about how cognitive processes work, how they change over time, and how they can be improved or altered. This research is fundamental to developing new theories of cognition that can explain complex human behaviors and cognitive anomalies.
One of the key contributions of cognitive psychology research is the development of models that describe various cognitive processes. For example, research on memory has led to the formulation of the multi-store model, which outlines how information flows from sensory memory to short-term memory and finally to long-term memory. Similarly, studies on decision-making and problem-solving have introduced several cognitive biases that influence human judgment, such as confirmation bias and availability heuristic. These models and theories are crucial for understanding the limitations and capabilities of human cognition, informing approaches in education, cognitive therapy, and even interface design in technology.
Moreover, cognitive psychology research has a significant impact on diagnosing and treating cognitive disorders. Studies on neurocognitive disorders, such as Alzheimer’s disease and attention deficit hyperactivity disorder (ADHD), provide insights into their cognitive underpinnings, leading to better diagnostic criteria and treatment options. Research in this field also supports the development of cognitive rehabilitation techniques and cognitive-behavioral therapies, demonstrating its vital role in improving mental health and cognitive function.
The Variety of Research Topics within Cognitive Psychology and Their Relevance to Real-World Applications
Cognitive psychology encompasses a wide array of research topics, each with direct implications for real-world applications. For instance, research in perception and sensation enhances our understanding of how sensory information is interpreted by the brain, influencing fields such as marketing, design, and even virtual reality development. Studies on attention and information processing have led to improvements in educational strategies, helping to develop teaching methods that align with cognitive load theory and the attentional needs of students.
Language and cognition research has profound implications for language teaching methodologies, speech therapy, and understanding language disorders. Insights from this research help in designing interventions for individuals with dyslexia or aphasia, facilitating better communication and learning outcomes. Additionally, the study of problem-solving and decision-making is pivotal for the development of artificial intelligence, providing algorithms with models of human cognition that can be simulated in computational systems.
The exploration of memory and recall has applications in legal settings, especially in eyewitness testimony and the reliability of memory. Cognitive psychology’s findings on the malleability of human memory and the conditions under which memories are accurately or inaccurately recalled are crucial for informing judicial processes and policies. Furthermore, the study of social cognition, which examines how individuals perceive, think about, and interact with others, is essential for understanding social behavior, improving interpersonal relationships, and addressing societal issues such as prejudice and discrimination.
Recent Advancements in Cognitive Psychology Research
Recent advancements in cognitive psychology research have been facilitated by technological innovations, allowing for more sophisticated exploration of cognitive processes. Neuroimaging techniques such as fMRI and PET scans have provided insights into the neural substrates of various cognitive functions, bridging the gap between cognitive psychology and neuroscience. These advancements have led to a deeper understanding of how different brain regions are involved in specific cognitive tasks, such as memory recall or language processing.
Additionally, the integration of machine learning and artificial intelligence in cognitive research has opened new avenues for analyzing large datasets, leading to more nuanced understandings of cognitive patterns and anomalies. This intersection of cognitive psychology and computational modeling has also advanced the development of intelligent systems capable of mimicking human cognitive functions, from language understanding to pattern recognition.
Another significant advancement is in the realm of cognitive enhancement, where research is exploring ways to improve cognitive functions through pharmacological means, cognitive training exercises, and even non-invasive brain stimulation techniques. These studies hold the potential for significant impacts on education, mental health treatment, and the general enhancement of cognitive abilities in healthy individuals.
Ethical Issues Inherent in Cognitive Psychology Research
Cognitive psychology research, while offering vast potential for understanding and enhancing human cognition, also presents several ethical considerations. Issues such as informed consent, privacy, and the potential for misuse of cognitive data are paramount concerns. The use of neuroimaging and other biometric data, for instance, raises questions about the privacy of mental states and the potential for such information to be used in ways that could infringe on individual rights or autonomy.
Additionally, the ethical implications of cognitive enhancement and the potential societal impacts of creating disparities between those who have access to cognitive enhancement technologies and those who do not are areas of ongoing debate. Cognitive psychology researchers must navigate these ethical waters carefully, ensuring that their work promotes the welfare and dignity of all individuals while advancing scientific knowledge.
Future Directions for Research in Cognitive Psychology
The future of cognitive psychology research promises further integration with neuroscience, the application of advanced computational models, and the exploration of how cognitive processes evolve in a rapidly changing digital world. An exciting direction for future research is the investigation of how digital technologies, such as smartphones and social media, are affecting cognitive development, attention spans, and social cognition. Understanding these impacts is crucial for developing strategies to mitigate potential negative effects while harnessing technology’s power to enhance cognitive function.
Another area of future research is the exploration of individual differences in cognition, understanding how genetic, environmental, and cultural factors contribute to the diversity of cognitive processes among individuals. This line of research holds the promise of personalizing educational and therapeutic approaches to cater to individual cognitive profiles.
The Transformative Potential of Research in Cognitive Psychology
Research in cognitive psychology holds transformative potential for numerous aspects of human life, from education and mental health to technology and social interaction. By continuing to explore the intricacies of cognitive processes and their neural underpinnings, cognitive psychology can contribute to a deeper understanding of what it means to be human. The ongoing exploration of cognitive phenomena not only enriches our knowledge of the mind but also translates into practical applications that can improve individual well-being and societal health. As cognitive psychology advances, its research continues to shape our world, demonstrating the enduring power of understanding the human mind.
iResearchNet’s Writing Services
In the intricate and evolving field of cognitive psychology, where the depth and breadth of research topics extend far into the understanding of the human mind, iResearchNet stands as a beacon of support for students embarking on their academic journey. Recognizing the challenges students face in navigating the complex landscape of cognitive psychology research, iResearchNet offers bespoke writing services tailored to meet the unique needs of each research endeavor. Our mission is to facilitate your academic success by providing customized, high-quality research papers that reflect the latest advancements and ethical standards in cognitive psychology.
- Expert Writers Holding Advanced Degrees in Cognitive Psychology : Our team comprises seasoned professionals who not only hold advanced degrees in cognitive psychology but also bring a wealth of research and practical experience to your project.
- Customized Papers That Precisely Meet Academic and Research Needs : Every paper is crafted with the utmost attention to detail, ensuring that it meets your specific academic guidelines and research objectives.
- In-Depth Research Leveraging the Latest Cognitive Psychology Studies : We conduct comprehensive research, utilizing the most current studies and findings in cognitive psychology to enrich your paper with cutting-edge insights.
- Strict Adherence to Academic Formatting Standards : Whether you require APA, MLA, Chicago/Turabian, or Harvard formatting, our writers are well-versed in all academic formatting guidelines, guaranteeing that your paper meets the highest scholarly standards.
- Commitment to Delivering Top-Quality Scholarly Work : Quality underpins everything we do. We’re committed to producing scholarly work that not only meets but exceeds academic expectations.
- Tailored Solutions Addressing Specific Research Questions : Recognizing the uniqueness of each research question, we offer tailored writing solutions that directly address your specific research focus.
- Competitively Priced Services for Students : Understanding the financial constraints faced by many students, our services are priced competitively, providing access to quality writing services without breaking the bank.
- Capability to Meet Tight Deadlines, Ensuring Timely Submissions : We pride ourselves on our ability to handle tight deadlines, ensuring that your project is delivered on time, every time, without compromising quality.
- Pledge of Punctual Delivery for Every Project : Timeliness is key in academic submissions. We pledge to deliver your project on or before the deadline, helping you avoid any last-minute stress.
- Continuous Support Available Any Time of the Day : Our support team is available 24/7, ready to answer your questions, provide updates, and offer the assistance you need at any stage of your project.
- Guarantee of Absolute Privacy for All Client Details : Your privacy is paramount. We adhere to strict confidentiality policies, ensuring that all your personal and project details remain private and secure.
- User-Friendly Platform for Effortless Order Tracking : Our online platform is designed for ease of use, allowing you to track your order’s progress with ease and confidence.
- Money-Back Guarantee for Unsatisfactory Results : While we strive for perfection, we offer a money-back guarantee if the final product does not meet your expectations, ensuring your complete satisfaction.
At iResearchNet, our unwavering dedication to supporting students in their cognitive psychology research endeavors is matched only by our commitment to excellence. By choosing our customized writing services, you’re not just getting a research paper; you’re gaining a partner dedicated to helping you succeed academically and professionally. We understand the transformative potential of cognitive psychology research and are here to ensure that your academic journey in this fascinating field is both successful and rewarding. Trust iResearchNet to be your ally in navigating the complexities of cognitive psychology research.
Unlock the Potential of Your Cognitive Psychology Research with iResearchNet!
Dive into the depths of cognitive psychology with confidence and let iResearchNet be your guide to academic excellence. Our expert writing services are specifically designed to cater to your cognitive psychology research paper needs, ensuring that your exploration into the human mind is not only insightful but also academically rewarding. Whether you’re unraveling the complexities of memory, perception, decision-making, or any other area within this fascinating field, our team is here to support your academic journey every step of the way.
Embrace the opportunity to elevate your research with the backing of iResearchNet’s seasoned professionals, who bring a wealth of knowledge and expertise to your project. Our customized writing solutions are tailored to your unique research questions and academic requirements, ensuring that your paper stands out in both depth and quality. With iResearchNet, navigating the intricate world of cognitive psychology research becomes a seamless and stress-free experience.
We understand the pressures of academic deadlines and the demand for high-quality research. That’s why our ordering process is designed to be as straightforward as possible, allowing you to quickly secure the expert assistance you need without any hassle. From the moment you reach out, you’ll enjoy comprehensive support, detailed updates, and continuous communication, ensuring a smooth and successful completion of your project.
Don’t let the challenge of crafting a top-notch cognitive psychology research paper hold you back. Choose iResearchNet and unlock the full potential of your academic endeavors. Our commitment to quality, combined with competitive pricing and a user-friendly platform, makes us the ideal partner for your cognitive psychology research needs. Start your journey to academic success today and experience the difference that professional, customized writing services can make.
ORDER HIGH QUALITY CUSTOM PAPER

- How it works

Useful Links
How much will your dissertation cost?
Have an expert academic write your dissertation paper!
Dissertation Services

Get unlimited topic ideas and a dissertation plan for just £45.00
Order topics and plan
Get 1 free topic in your area of study with aim and justification
Yes I want the free topic

32 Cognitive Psychology Dissertation Topics
Published by Owen Ingram at January 3rd, 2023 , Revised On August 11, 2023
The study of cognitive psychology focuses on how the brain processes and stores information. The underlying mechanisms are investigated using experimental methods, computer modelling, and neuropsychology.
The goal of brain theories is to understand how information is encoded at the macro and micro levels. Since this is a vast subject, there are numerous possible research areas you can choose from. You may further explore our selection if you wish to focus on cognitive psychology for your dissertation.
Related Academic Links: Neuro Psychology Dissertation Topics , Clinical Psychology Topics , Counselling Psychology Dissertation Topics , Forensic Psychology Dissertation Topics
Below Are Some Selected Cognitive Psychology Dissertation Topics
- Describe the consequences of autism.
- Using fMRI measures, can misleading information be accurately identified and separated from guilty knowledge?
- How does colour psychology work in research on cognitive development?
- How is attention span measured, and what does it mean?
- How do memories impact how people behave?
- According to the Network Neuroscience Theory, is general human intelligence a result of individual variances in brain network architecture and structure?
- What elements can help kids’ problem-solving skills develop?
- How does the development of cognition impact speech disorders?
- Effective cognition involves choosing the proper information at the proper time and in the proper order.
- Does subliminal perception exist, or does it only apply to certain circumstances?
- Information flow and parallel distributed processing hierarchy explained.
- The applicability of cognitive psychology research findings to actual behaviour and cognition, as well as their reliability, validity, and utility.
- Factors that may cause a child’s mental development to be delayed.
- What is the single parenting style best for a child’s mental development? The impact of romantic movies on children?
- The gradual activation of forwarding brain regions is necessary for attention.
- View-dependent theories of vision outperform view-independent theories in explaining natural perception.
- Computer simulations of vision can cause people to misunderstand how the mechanisms of perception truly work.
- How visual illusions to aid in the understanding of perception.
- Evidence for the hippocampus’s function in memory encoding and consolidation: applicability to dementia and other neurodegenerative diseases.
- Working memory and attention bias: working memory and attention in the visual domain.
- Describe the extent to which plasticity plays a role in the development of visual cognitive abilities.
- Examine automated priming effects’ consequences on complex behaviour in real life
- Discuss the importance of facial stimuli in assessing how the ventral pathway of the human body develops from childhood to adulthood.
- Analyze the growth of out-group and in-group associations in implicit intergroup cognition.
- What Are the Hierarchical Explanations of Information Flow and Parallel Processing Distribution?
- Are the abilities of children with dyscalculia not impacted by the disorder, or are they comparatively independent?
- Does the evidence support the idea that neural network theories can explain some lower-order brain operations but cannot explain the representations in higher areas?
- Investigating Human Cognitive Development as A Stand-In for Understanding Human Brain Evolution.
- Describe how the executive functions of the frontal brain distinguish humans.
- An analysis of Fodor’s modular theory of the brain in the context of contemporary neuroscientific evidence.
- Do You Know What a Cheater Detection Module Is, And Is It Real Or Just a Phrase?
- Evaluating the accuracy of Gibson’s direct perception theory in light of constructivist explanations and other modern cognitive theories.
Dissertation Experts
Orders completed by our expert writers are
- Formally drafted in an academic style
- Free Amendments and 100% Plagiarism Free – or your money back!
- 100% Confidential and Timely Delivery!
- Free anti-plagiarism report
- Appreciated by thousands of clients. Check client reviews
It is essential for your cognitive psychology dissertation that you take advantage of the opportunity to make your presence felt in psychology. To help you with your study, also look for intriguing dissertation topics that contain a wealth of information. Consult your supervisor about improving your dissertation. Research is always more powerful when based on a good and comprehensive topic.
Free Dissertation Topic
Phone Number
Academic Level Select Academic Level Undergraduate Graduate PHD
Academic Subject
Area of Research
Frequently Asked Questions
How to find cognitive psychology dissertation topics.
To find cognitive psychology dissertation topics:
- Study recent research trends.
- Explore cognitive disorders or therapies.
- Investigate memory, perception, learning.
- Analyze brain-imaging techniques.
- Consider AI’s impact on cognition.
- Select a topic resonating with your passion and research goals.
You May Also Like
One of the challenging things that demand a lot of creativity is coming up with original journalism dissertation topics. In contrast to other disciplines, journalism dissertations are judged based on the interviewee’s quality of information.
Even though event management seems easy, it is actually quite complex once you study it. If you study event management with an instructor who is committed to teaching you with integrity, it can be manageable.
We have collected a list of 35 dissertation topic ideas on tort law curated by professionals of the subject to help you with a tort law dissertation.
USEFUL LINKS
LEARNING RESOURCES

COMPANY DETAILS

- How It Works
Mounting research shows that COVID-19 leaves its mark on the brain, including significant drops in IQ scores
Chief of Research and Development, VA St. Louis Health Care System. Clinical Epidemiologist, Washington University in St. Louis
Disclosure statement
Ziyad Al-Aly receives funding from the U.S. Department of Veterans Affairs.
View all partners
From the very early days of the pandemic, brain fog emerged as a significant health condition that many experience after COVID-19.
Brain fog is a colloquial term that describes a state of mental sluggishness or lack of clarity and haziness that makes it difficult to concentrate, remember things and think clearly.
Fast-forward four years and there is now abundant evidence that being infected with SARS-CoV-2 – the virus that causes COVID-19 – can affect brain health in many ways .
In addition to brain fog, COVID-19 can lead to an array of problems , including headaches, seizure disorders, strokes, sleep problems, and tingling and paralysis of the nerves, as well as several mental health disorders .
A large and growing body of evidence amassed throughout the pandemic details the many ways that COVID-19 leaves an indelible mark on the brain. But the specific pathways by which the virus does so are still being elucidated, and curative treatments are nonexistent.
Now, two new studies published in the New England Journal of Medicine shed further light on the profound toll of COVID-19 on cognitive health .
I am a physician scientist , and I have been devoted to studying long COVID since early patient reports about this condition – even before the term “long COVID” was coined. I have testified before the U.S. Senate as an expert witness on long COVID and have published extensively on this topic.
How COVID-19 leaves its mark on the brain
Here are some of the most important studies to date documenting how COVID-19 affects brain health:
Large epidemiological analyses showed that people who had COVID-19 were at an increased risk of cognitive deficits , such as memory problems.
Imaging studies done in people before and after their COVID-19 infections show shrinkage of brain volume and altered brain structure after infection .
A study of people with mild to moderate COVID-19 showed significant prolonged inflammation of the brain and changes that are commensurate with seven years of brain aging .
Severe COVID-19 that requires hospitalization or intensive care may result in cognitive deficits and other brain damage that are equivalent to 20 years of aging .
Laboratory experiments in human and mouse brain organoids designed to emulate changes in the human brain showed that SARS-CoV-2 infection triggers the fusion of brain cells . This effectively short-circuits brain electrical activity and compromises function.
Autopsy studies of people who had severe COVID-19 but died months later from other causes showed that the virus was still present in brain tissue . This provides evidence that contrary to its name, SARS-CoV-2 is not only a respiratory virus, but it can also enter the brain in some individuals. But whether the persistence of the virus in brain tissue is driving some of the brain problems seen in people who have had COVID-19 is not yet clear.
Studies show that even when the virus is mild and exclusively confined to the lungs, it can still provoke inflammation in the brain and impair brain cells’ ability to regenerate .
COVID-19 can also disrupt the blood brain barrier , the shield that protects the nervous system – which is the control and command center of our bodies – making it “leaky.” Studies using imaging to assess the brains of people hospitalized with COVID-19 showed disrupted or leaky blood brain barriers in those who experienced brain fog.
A large preliminary analysis pooling together data from 11 studies encompassing almost 1 million people with COVID-19 and more than 6 million uninfected individuals showed that COVID-19 increased the risk of development of new-onset dementia in people older than 60 years of age.
Drops in IQ
Most recently, a new study published in the New England Journal of Medicine assessed cognitive abilities such as memory, planning and spatial reasoning in nearly 113,000 people who had previously had COVID-19. The researchers found that those who had been infected had significant deficits in memory and executive task performance.
This decline was evident among those infected in the early phase of the pandemic and those infected when the delta and omicron variants were dominant. These findings show that the risk of cognitive decline did not abate as the pandemic virus evolved from the ancestral strain to omicron.
In the same study, those who had mild and resolved COVID-19 showed cognitive decline equivalent to a three-point loss of IQ. In comparison, those with unresolved persistent symptoms, such as people with persistent shortness of breath or fatigue, had a six-point loss in IQ. Those who had been admitted to the intensive care unit for COVID-19 had a nine-point loss in IQ. Reinfection with the virus contributed an additional two-point loss in IQ, as compared with no reinfection.
Generally the average IQ is about 100. An IQ above 130 indicates a highly gifted individual, while an IQ below 70 generally indicates a level of intellectual disability that may require significant societal support.
To put the finding of the New England Journal of Medicine study into perspective, I estimate that a three-point downward shift in IQ would increase the number of U.S. adults with an IQ less than 70 from 4.7 million to 7.5 million – an increase of 2.8 million adults with a level of cognitive impairment that requires significant societal support.
Another study in the same issue of the New England Journal of Medicine involved more than 100,000 Norwegians between March 2020 and April 2023. It documented worse memory function at several time points up to 36 months following a positive SARS-CoV-2 test.
Parsing the implications
Taken together, these studies show that COVID-19 poses a serious risk to brain health, even in mild cases, and the effects are now being revealed at the population level.
A recent analysis of the U.S. Current Population Survey showed that after the start of the COVID-19 pandemic, an additional 1 million working-age Americans reported having “serious difficulty” remembering, concentrating or making decisions than at any time in the preceding 15 years. Most disconcertingly, this was mostly driven by younger adults between the ages of 18 to 44.
Data from the European Union shows a similar trend – in 2022, 15% of people in the EU reported memory and concentration issues .
Looking ahead, it will be critical to identify who is most at risk. A better understanding is also needed of how these trends might affect the educational attainment of children and young adults and the economic productivity of working-age adults. And the extent to which these shifts will influence the epidemiology of dementia and Alzheimer’s disease is also not clear.
The growing body of research now confirms that COVID-19 should be considered a virus with a significant impact on the brain. The implications are far-reaching, from individuals experiencing cognitive struggles to the potential impact on populations and the economy.
Lifting the fog on the true causes behind these cognitive impairments, including brain fog, will require years if not decades of concerted efforts by researchers across the globe. And unfortunately, nearly everyone is a test case in this unprecedented global undertaking.
- Intelligence
- Long COVID-19
- SARS-CoV-2 virus
- Cognitive health
Want to write?
Write an article and join a growing community of more than 187,800 academics and researchers from 5,011 institutions.
Register now
Words like 'this' and 'that' act as attention tools across languages
All languages have words like 'this' and 'that' to distinguish between referents that are 'near' and 'far'. Languages like English or Hebrew have two of these 'demonstratives'. Languages like Spanish or Japanese use a three-word system. For instance, in Spanish, 'este' signals something close to the speaker, 'ese' signals something far from the speaker but close to the listener, and 'aquel' signals something far from both.
"The reason why we were interested in demonstratives is because of their connection to social cognition: demonstratives are used to direct the listener's attention to a referent and establish joint attention," says MPI's Paula Rubio-Fernández, senior investigator and co-author of the study. "Engaging in joint attention is a uniquely human capacity that links language to social cognition in communication. Because demonstratives are universal, emerged early in language evolution and are acquired early in child development, they offer an ideal test case for the interdependence between these two fundamentally human capacities."
There is debate about whether directing the listener's attention -- the 'mentalistic' representation -- is part of the meaning (semantics) of demonstratives, or whether it arises from general principles of social cognition (pragmatics). The researchers used computational modelling and experiments with speakers of ten different languages from eight different language groups to investigate this question.
In an online task, participants saw pictures of a 'speaker' requesting an object from a 'listener', who was standing on the other side of a long table. The participants were asked to take the role of the speaker, and select a demonstrative from their native language to request the object ("Now I need …"). In the pictures, the listener was either already looking at the intended object or looking at one of four other objects (closer or further from the target). If directing attention is part of the meaning of demonstratives, all speakers should be sensitive to a listener's initial attention when selecting a demonstrative. However, there should also be variation across languages.
Results showed that participants were not only sensitive to the location of the target but also to the listener's attention. As expected, the meaning of demonstratives varied within and across languages. For example, the 'near' demonstrative (such as English 'this one') sometimes had a spatial meaning ('the one close to me'). But it also had a joint attention meaning ('the one we are both looking at') or a 'mentalistic' meaning ('the one over here'), directing the listener's attention towards the speaker. Interestingly, speakers of languages with a three-word system used the medial word (such as Spanish 'ese') to indicate joint attention.
"Our work sheds light on the interface between social cognition and language. We show that representations of interlocutor attention are embedded into one of the most basic word classes that appear across all languages: demonstratives," concludes Rubio-Fernández. "Our work also shows through Bayesian computational modelling that this form of attention manipulation cannot be explained via pragmatic reasoning external to the linguistic system, suggesting that mentalistic representations are embedded in a universal component of language".
- Language Acquisition
- Learning Disorders
- ADD and ADHD
- Child Development
- Social Issues
- Mental confusion
- Hyperactivity
- Social cognition
- Adult attention-deficit disorder
- Social movement
Story Source:
Materials provided by Max Planck Institute for Psycholinguistics . Note: Content may be edited for style and length.
Journal Reference :
- Julian Jara-Ettinger, Paula Rubio-Fernandez. Demonstratives as attention tools: Evidence of mentalistic representations within language . Proceedings of the National Academy of Sciences , 2024; 121 (32) DOI: 10.1073/pnas.2402068121
Cite This Page :
Explore More
- Enhancing Performance in Organic Semiconductors
- Half a Billion-Year-Old Spiny Slug
- Rising Earth in Antarctica: Sea Level Rise
- Origins of the Moon's Tenuous Atmosphere
- Sea Level Changes Shaped Early Life On Earth
- What Gave the First Molecules Their Stability?
- Fossil Shows How Penguins' Wings Evolved
- Global Warming and Rising Sea Levels
- Shifts in Vital Pacific Arctic Fisheries
- DNA Replication: Humans and Baker's Yeast
Trending Topics
Strange & offbeat.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Health Topics
- Brochures and Fact Sheets
- Help for Mental Illnesses
- Clinical Trials
Post-Traumatic Stress Disorder
What is post-traumatic stress disorder (ptsd).
Post-traumatic stress disorder (PTSD) is a disorder that develops in some people who have experienced a shocking, scary, or dangerous event.
It is natural to feel afraid during and after a traumatic situation. Fear is a part of the body’s “fight-or-flight” response, which helps us avoid or respond to potential danger. People may experience a range of reactions after trauma, and most people recover from initial symptoms over time. Those who continue to experience problems may be diagnosed with PTSD.
Who gets PTSD?
Anyone can develop PTSD at any age. This includes combat veterans and people who have experienced or witnessed a physical or sexual assault, abuse, an accident, a disaster, or other serious events. People who have PTSD may feel stressed or frightened, even when they are not in danger.
Not everyone with PTSD has been through a dangerous event. Sometimes, learning that a friend or family member experienced trauma can cause PTSD.
According to the National Center for PTSD , a program of the U.S. Department of Veterans Affairs, about six out of every 100 people will experience PTSD at some point in their lives. Women are more likely to develop PTSD than men. Certain aspects of the traumatic event and some biological factors (such as genes) may make some people more likely to develop PTSD.
What are the signs and symptoms of PTSD?
Symptoms of PTSD usually begin within 3 months of the traumatic event, but they sometimes emerge later. To meet the criteria for PTSD, a person must have symptoms for longer than 1 month, and the symptoms must be severe enough to interfere with aspects of daily life, such as relationships or work. The symptoms also must be unrelated to medication, substance use, or other illness.
The course of the disorder varies. Some people recover within 6 months, while others have symptoms that last for 1 year or longer. People with PTSD often have co-occurring conditions, such as depression, substance use, or one or more anxiety disorders.
After a dangerous event, it is natural to have some symptoms. For example, some people may feel detached from the experience, as though they are observing things rather than experiencing them. A mental health professional who has experience helping people with PTSD, such as a psychiatrist, psychologist, or clinical social worker, can determine whether symptoms meet the criteria for PTSD.
To be diagnosed with PTSD, an adult must have all of the following for at least 1 month:
- At least one re-experiencing symptom
- At least one avoidance symptom
- At least two arousal and reactivity symptoms
- At least two cognition and mood symptoms
Re-experiencing symptoms include:
- Experiencing flashbacks—reliving the traumatic event, including physical symptoms such as a racing heart or sweating
- Having recurring memories or dreams related to the event
- Having distressing thoughts
- Experiencing physical signs of stress
Thoughts and feelings can trigger these symptoms, as can words, objects, or situations that are reminders of the event.
Avoidance symptoms include:
- Staying away from places, events, or objects that are reminders of the traumatic experience
- Avoiding thoughts or feelings related to the traumatic event
Avoidance symptoms may cause people to change their routines. For example, some people may avoid driving or riding in a car after a serious car accident.
Arousal and reactivity symptoms include:
- Being easily startled
- Feeling tense, on guard, or on edge
- Having difficulty concentrating
- Having difficulty falling asleep or staying asleep
- Feeling irritable and having angry or aggressive outbursts
- Engaging in risky, reckless, or destructive behavior
Arousal symptoms are often constant. They can lead to feelings of stress and anger and may interfere with parts of daily life, such as sleeping, eating, or concentrating.
Cognition and mood symptoms include:
- Having trouble remembering key features of the traumatic event
- Having negative thoughts about oneself or the world
- Having exaggerated feelings of blame directed toward oneself or others
- Having ongoing negative emotions, such as fear, anger, guilt, or shame
- Losing interest in enjoyable activities
- Having feelings of social isolation
- Having difficulty feeling positive emotions, such as happiness or satisfaction
Cognition and mood symptoms can begin or worsen after the traumatic event. They can lead a person to feel detached from friends or family members.
If you or someone you know is struggling or having thoughts of suicide, call or text the 988 Suicide and Crisis Lifeline at 988 or chat at 988lifeline.org . In life-threatening situations, call 911 .
How do children and teens react to trauma?
Children and teens can have extreme reactions to trauma, but some of their symptoms may not be the same as those seen in adults. In children younger than age 6, these symptoms can include:
- Wetting the bed after having learned to use the toilet
- Forgetting how to talk or being unable to talk
- Acting out the scary event during playtime
- Being unusually clingy with a parent or other adult
Older children and teens usually show symptoms more like those seen in adults. They also may develop disruptive, disrespectful, or destructive behaviors. Older children and teens may feel guilty for not preventing injury or deaths. They may also have thoughts of revenge.
Learn more about how to help children and adolescents cope with disasters and other traumatic events .
What are the risk factors for PTSD?
Not everyone who lives through a dangerous event develops PTSD—many factors play a part. Some of these factors are present before the trauma; others become important during and after a traumatic event.
Risk factors that may increase the likelihood of developing PTSD include:
- Being exposed to previous traumatic experiences, particularly during childhood
- Getting hurt or seeing people hurt or killed
- Feeling horror, helplessness, or extreme fear
- Having little or no social support after the event
- Dealing with extra stress after the event, such as loss of a loved one, pain and injury, or loss of a job or home
- Having a personal or family history of mental illness or substance use
Resilience factors that may reduce the likelihood of developing PTSD include:
- Seeking out support from friends, family, or support groups
- Learning to feel okay with one’s actions in response to a traumatic event
- Having a coping strategy for getting through and learning from the traumatic event
- Being prepared and able to respond to upsetting events as they occur, despite feeling fear
How is PTSD treated?
It is important for anyone with PTSD symptoms to work with a mental health professional who has experience treating PTSD. The main treatments are psychotherapy, medications, or a combination of psychotherapy and medications. A mental health professional can help people find the best treatment plan for their symptoms and needs.
Some people with PTSD, such as those in abusive relationships, may be living through ongoing trauma. In these cases, treatment is usually most effective when it addresses both the traumatic situation and the symptoms of PTSD. People who experience traumatic events or who have PTSD also may experience panic disorder , depression , substance use , or suicidal thoughts . Treatment for these conditions can help with recovery after trauma. Research shows that support from family and friends also can be an important part of recovery.
Psychotherapy
Psychotherapy (sometimes called talk therapy) includes a variety of treatment techniques that mental health professionals use to help people identify and change troubling emotions, thoughts, and behaviors. Psychotherapy can provide support, education, and guidance to people with PTSD and their families. Treatment can take place one on one or in a group and usually lasts 6 to 12 weeks but can last longer.
Some types of psychotherapy target PTSD symptoms, while others focus on social, family, or job-related problems. Effective psychotherapies often emphasize a few key components, including learning skills to help identify triggers and manage symptoms.
One common type of psychotherapy, called cognitive behavioral therapy, can include exposure therapy and cognitive restructuring:
- Exposure therapy helps people learn to manage their fear by gradually exposing them, in a safe way, to the trauma they experienced. As part of exposure therapy, people may think or write about the trauma or visit the place where it happened. This therapy can help people with PTSD reduce symptoms that cause them distress.
- Cognitive restructuring helps people make sense of the traumatic event. Sometimes people remember the event differently from how it happened. They may feel guilt or shame about something that is not their fault. Cognitive restructuring can help people with PTSD think about what happened in a realistic way.
Medications
The U.S. Food and Drug Administration (FDA) has approved two selective serotonin reuptake inhibitors (SSRIs), a type of antidepressant medication, for the treatment of PTSD. SSRIs may help manage PTSD symptoms such as sadness, worry, anger, and feeling emotionally numb. Health care providers may prescribe SSRIs and other medications along with psychotherapy. Some medications may help treat specific PTSD symptoms, such as sleep problems and nightmares.
People should work with their health care providers to find the best medication or combination of medications and the right dose. To find the latest information about medications, talk to a health care provider and visit the FDA website .
How can I find help for PTSD?
If you’re not sure where to get help, a health care provider can refer you to a licensed mental health professional, such as a psychiatrist or psychologist with experience treating PTSD. Find tips to help prepare for and get the most out of your visit and information about getting help .
The Substance Abuse and Mental Health Services Administration has a online treatment locator to help you find mental health services in your area.
Here are some things you can do to help yourself while in treatment:
- Talk with your health care provider about treatment options and follow your treatment plan.
- Engage in exercise, mindfulness, or other activities that help reduce stress.
- Try to maintain routines for meals, exercise, and sleep.
- Set realistic goals and focus on what you can manage.
- Spend time with trusted friends or relatives and tell them about things that may trigger symptoms.
- Expect your symptoms to improve gradually, not immediately.
- Avoid the use of alcohol or drugs.
How can I help a friend or relative who has PTSD?
If you know someone who may be experiencing PTSD, the most important thing you can do is to help that person get the right diagnosis and treatment. Some people may need help making an appointment with their health care provider; others may benefit from having someone accompany them to their health care visits.
If a close friend or relative is diagnosed with PTSD, you can encourage them to follow their treatment plan. If their symptoms do not get better after 6 to 8 weeks, you can encourage them to talk to their health care provider. You also can:
- Offer emotional support, understanding, patience, and encouragement.
- Learn about PTSD so you can understand what your friend is experiencing.
- Listen carefully. Pay attention to the person’s feelings and the situations that may trigger PTSD symptoms.
- Share positive distractions, such as walks, outings, and other activities.
How can I find a clinical trial for PTSD?
Clinical trials are research studies that look at new ways to prevent, detect, or treat diseases and conditions. The goal of clinical trials is to determine if a new test or treatment works and is safe. Although individuals may benefit from being part of a clinical trial, participants should be aware that the primary purpose of a clinical trial is to gain new scientific knowledge so that others may be better helped in the future.
Researchers at NIMH and around the country conduct many studies with patients and healthy volunteers. We have new and better treatment options today because of what clinical trials uncovered years ago. Talk to your health care provider about clinical trials, their benefits and risks, and whether one is right for you.
To learn more or find a study, visit:
- NIMH’s Clinical Trials webpage : Information about participating in clinical trials
- Clinicaltrials.gov: Current Studies on PTSD : List of clinical trials funded by the National Institutes of Health (NIH) being conducted across the country
Where can I learn more about PTSD?
Free brochures and shareable resources.
- Helping Children and Adolescents Cope With Traumatic Events : This fact sheet presents information on how children and adolescents respond to traumatic events, and what family, friends, and trusted adults can do to help. Also available en español .
- Post-Traumatic Stress Disorder : This brochure provides information about PTSD, including what it is, who develops PTSD, symptoms, treatment options, and how to find help for yourself or someone else who may have PTSD. Also available en español .
- Digital Shareables on PTSD : These digital resources, including graphics and messages, can be used to spread the word about PTSD and help promote awareness and education in your community.
- NIMH-Funded Researcher Discusses PTSD : In this video, an expert describes PTSD signs, symptoms, diagnosis, treatments, and the latest research on PTSD.
Federal resources
- National Center for PTSD : Part of the U.S. Department of Veterans Affairs, this website has information and resources for anyone interested in PTSD, including veterans, family, friends, researchers, and health care providers. The site offers videos, apps, online programs, and other tools to help people with PTSD and their loved ones.
- Medications for PTSD : This webpage from the U.S. Department of Veterans Affairs describes effective medications for treating PTSD and considerations for evaluating treatment options.
- PTSD (MedlinePlus – also en español )
Research and statistics
- Journal Articles : This webpage provides articles and abstracts on PTSD from MEDLINE/PubMed (National Library of Medicine).
- PTSD Statistics : This webpage provides the statistics currently available on the prevalence of PTSD among people in the United States.
- PTSD Brain Bank : Supported by the U.S. Department of Veterans Affairs, this human tissue bank collects, processes, stores, and gives out research specimens for future scientific studies. Veterans and non-veterans with or without PTSD or other mental health diagnoses may enroll in the PTSD Brain Bank to help future efforts in PTSD research and treatment.
Last Reviewed: May 2024
Unless otherwise specified, the information on our website and in our publications is in the public domain and may be reused or copied without permission. However, you may not reuse or copy images. Please cite the National Institute of Mental Health as the source. Read our copyright policy to learn more about our guidelines for reusing NIMH content.
Advancements in Recommender Systems: A Comprehensive Analysis Based on Data, Algorithms, and Evaluation
- Li, Mingyue
- Liu, Xuguang
Using 286 research papers collected from Web of Science, ScienceDirect, SpringerLink, arXiv, and Google Scholar databases, a systematic review methodology was adopted to review and summarize the current challenges and potential future developments in data, algorithms, and evaluation aspects of RSs. It was found that RSs involve five major research topics, namely algorithmic improvement, domain applications, user behavior & cognition, data processing & modeling, and social impact & ethics. Collaborative filtering and hybrid recommendation techniques are mainstream. The performance of RSs is jointly limited by four types of eight data issues, two types of twelve algorithmic issues, and two evaluation issues. Notably, data-related issues such as cold start, data sparsity, and data poisoning, algorithmic issues like interest drift, device-cloud collaboration, non-causal driven, and multitask conflicts, along with evaluation issues such as offline data leakage and multi-objective balancing, have prominent impacts. Fusing physiological signals for multimodal modeling, defending against data poisoning through user information behavior, evaluating generative recommendations via social experiments, fine-tuning pre-trained large models to schedule device-cloud resource, enhancing causal inference with deep reinforcement learning, training multi-task models based on probability distributions, using cross-temporal dataset partitioning, and evaluating recommendation objectives across the full lifecycle are feasible solutions to address the aforementioned prominent challenges and unlock the power and value of RSs.The collected literature is mainly based on major international databases, and future research will further expand upon it.
- Computer Science - Information Retrieval;
- Computer Science - Machine Learning

COMMENTS
Social Cognition Research Topics: Social cognition draws heavily on material within cognitive psychology and social psychology to examine the relationship between basic cognitive operations and fundamental social problems. In this respect, work in this domain has attempted to show that, during his or her lifetime, an individual's thoughts and ...
2. Three Main Domains of Social Cognition. The ability to establish appropriate social interactions entails several distinct processes. First, the social agent must recognize the others as "living persons," via the analysis of complex perceptual information including facial expressions, gestures, postures and body language, and voice, [].Once integrated, this information will represent the ...
Cognitive dysfunction in schizophrenia: An expert group paper on the current state of the art. Philip D. Harvey, ... Antonio Vita, in Schizophrenia Research: Cognition, 2022. 5.2 Social cognition. Social cognition represents the cognitive capability to perceive, categorize and interpret social behavior of other people and concerns the various psychological processes that enable individuals to ...
The scientific study of social cognition is a growing field which promises to deliver valuable insights into how the brain underpins human's social success. ... SUBMIT PAPER. Review of General Psychology. Impact Factor: 3.6 / 5-Year ... Ochsner K. (2011). Reintegrating the study of accuracy into social cognition research. Psychological ...
1. Introduction. Human beings navigate the world by perceiving, attending to, and remembering incoming information within a framework of concepts such as ''number'' and ''cause.''. While these general abilities form the bedrock of any theory of cognition, social life presents cognitive scien-tists with a unique set of questions.
The fundamental assumption of social cognition research is the idea that internal mental representations of other persons and of social situations play a key causal role in shaping behavior.
Since the publication of Premack and Woodruff's classic paper introducing the notion of a 'theory of mind' (Premack and Woodruff in Behav Brain Sci 1(4):515-526, 1978), interdisciplinary research in social cognition has witnessed the development of theory-theory, simulation theory, hybrid approaches, and most recently interactionist and perceptual accounts of other minds. The ...
The study of cognition in major psychiatric disorders is an important research topic, but there is a paucity of available literature that meticulously addresses these issues. ... One way to address the major themes and findings across areas of social cognitive research is to divide these domains into the understanding of others (e.g., ToM, ...
Over the past three decades, research from the field of social neuroscience has identified a myriad of brain regions that support social cognition—the process by which people understand, store and apply information about others (e.g. Mitchell, 2008; Adolphs, 2009; Kliemann and Adolphs, 2018).This research has provided fundamental insights into mapping discrete brain regions to specific ...
paper addresses the definition of social cognition, the specificity of neural systems underlying social cognition, and the implications of this view for future research. 1. A definition social cognition and its core processes 1.1. The domains of social cognition Social cognition broadly includesthe cognitive processesused
Choose a Sub-Topic. Social psychologists are interested in all aspects of social behavior. Some of the main areas of interest within the field include social cognition, social influence, and social relationships investigating subtopics such as conformity, groupthink, attitude formation, obedience, prejudice, and so on.
In the last 20 years, research on implicit social cognition has established that social judgments and behavior are guided by attitudes and stereotypes of which the actor may lack awareness. Research using the methods of implicit social cognition has produced the concept of implicit bias, which has generated wide attention not only in social, clinical, and developmental psychology, but also in ...
Social Cognition. François Ric, in International Encyclopedia of the Social & Behavioral Sciences (Second Edition), 2015. Definition and Features of Social Cognition Approach. Social cognition can be defined as a domain of research studying human thinking and of its relationships with social behavior. More specifically, social cognition refers to the study of the processes by which people ...
Current behavioural and neuroscientific research in cognitive psychology has started to provide empirical evidence, demonstrating the clear link between embodied cognition and cognitive processes dependent on social situations (see Leung et al. 2011 ). That is, sensorimotor experience with the environment can be shown to shape the acquisition ...
This includes studies on topics such as conformity, obedience, and social pressure. Social perception: Social perception refers to the ways in which we form impressions of other people. This includes research on topics including first impressions, stereotyping, and prejudice. Social interaction: Social interaction refers to the ways in which we ...
Social cognition is the encoding, storage, retrieval, and processing, in the brain, of information relating to conspecifics, or members of the same species. Questions (207) Publications (105,456 ...
Development of social skills in children: neural and behavioral evidence for the elaboration of cognitive models. Social skills refer to a wide group of abilities that allow us to interact and communicate with others. Children learn how to solve social situations by predicting and understanding other's behaviors.
Social cognition research has its own features, different from other study types (e.g. questionnaire study, cognitive study). Just as we mentioned above, Baron and Kenny's analysis is usually suitable ... one paper further discussed the topic whether power corrupts[13]. In study 1, they manipulated
Social Psychology Research Topics List. This list of social psychology topics performs two functions. One, the headings alone describe, at a broad level, the kinds of topics covered in the field of social psychology. Looking at the overarching categories, one can see that social psychology studies cognition (thought) and action, helpful and ...
Social cognition refers to the different psychological processes that influence how people process, interpret, and respond to social signals. These processes allow people to understand social behavior and respond in ways that are appropriate and beneficial. Social cognition is a sub-topic of social psychology that focuses on how people process ...
Introduction: The relationship between neural social cognition patterns and performance on social cognition tasks in daily life is a topic of debate, with key consideration given to the extent to which theory of mind (ToM) brain circuits share properties reflecting everyday social functioning. To test the efficacy of ecological stimuli in ...
Social cognition also includes those considering heuristics (mental shortcuts) and some cognitive biases. Social Influence. This is one of the first areas of social psychology that most students learn. Remember the social conformity work by Asch (1951) on the length of lines? Other social psychology research topics within this area include ...
100 Cognitive Psychology Research Paper Topics. Cognitive psychology stands at the forefront of exploring the vast capabilities and intricacies of the human mind, offering profound insights into our thoughts, emotions, and behaviors. This branch of psychology delves into how people understand, diagnose, and interact with the world around them ...
32 Cognitive Psychology Dissertation Topics. Published by Owen Ingram at January 3rd, 2023 , Revised On August 11, 2023. The study of cognitive psychology focuses on how the brain processes and stores information. The underlying mechanisms are investigated using experimental methods, computer modelling, and neuropsychology.
George Orwell famously said, "At 50, everyone has the face he deserves" ().Research supports Orwell's observation, suggesting that changes in facial appearance over the years might be affected by one's personality and behaviors (2-4).The current work aims to explicitly test the developmental aspect of facial appearance, with the focus on a social process by utilizing a recently ...
A large and growing body of evidence amassed throughout the pandemic details the many ways that COVID-19 leaves an indelible mark on the brain. But the specific pathways by which the virus does so ...
Words like 'this' and 'that' or 'here' and 'there' occur in all languages. Researchers show that such 'demonstrative' words are used to direct listeners' focus of attention and to establish joint ...
Learn more about NIMH newsletters, public participation in grant reviews, research funding, clinical trials, the NIMH Gift Fund, and connecting with NIMH on social media. Digital Shareables Use these free education and outreach materials in your community and on social media to spread the word about mental health and related topics.
Using 286 research papers collected from Web of Science, ScienceDirect, SpringerLink, arXiv, and Google Scholar databases, a systematic review methodology was adopted to review and summarize the current challenges and potential future developments in data, algorithms, and evaluation aspects of RSs. It was found that RSs involve five major research topics, namely algorithmic improvement, domain ...