research news

Study suggests promising gene therapy for FOXG1 syndrome

Soo-Kyung and Jae Lee are the co-lead authors of a new study that suggests a promising viral gene therapy for FOXG1 syndrome, a severe neurodevelopmental disorder. Photo: Douglas Levere
By TOM DINKI
Published June 17, 2024
A viral gene therapy developed by UB researchers has reversed some brain abnormalities in infant mice with FOXG1 syndrome, a significant step toward one day treating children with this severe neurodevelopmental disorder.
This mediated delivery of the FOXG1 gene via adeno-associated virus 9 (AAV9) is detailed in a study published June 5 in Molecular Therapy Methods & Clinical Development . A postnatal injection of the therapy in day-old mice rescued a wide range of abnormalities, the study found, including in parts of the brain responsible for language, memory and social interaction.
“Our findings highlight the efficacy of AAV9-based gene therapy as a viable treatment strategy for FOXG1 syndrome and potentially other neurodevelopmental disorders with similar brain malformations,” says Soo-Kyung Lee, Empire Innovation Professor and Om P. Bahl Endowed Professor in the Department of Biological Sciences, College of Arts and Sciences, who led the study with her husband, Jae Lee, professor in the department. “This research asserts the therapeutic relevance of our approach in postnatal stages, which is a critical time frame for intervention.”
The Lees’ teenage daughter, Yuna, was diagnosed with FOXG1 syndrome at the age of 2. The researchers have since established themselves as leading experts on the disorder and are the principal investigators of UB’s FOXG1 Research Center (FRC). The center, which launched earlier this year, as well as this recent study, are supported by the FOXG1 Research Foundation.
The study was co-led by Kathrin Meyer, principal investigator at Nationwide Children’s Hospital in Columbus, Ohio. Other contributions represent the University of Pennsylvania and Samsung Medical Center in Seoul, South Korea.

The length and apex angle of the dentate gyrus in a mice with FOXG1 syndrome was restored after a postnatal injection of the researchers' AAV9-based gene therapy. Image: Soo-Kyung and Jae Lee
Reversing structural abnormalities
A master regulator gene, FOXG1 is one of the most important genes for early brain development and its impairment can result in profound brain structure abnormalities.
The Lees previously established that the FOXG1 gene and protein remain active in mice after birth, so they wondered if restoring FOXG1 levels could reverse some of the abnormalities associated with FOXG1 syndrome.
These abnormalities include failure to fully develop the corpus callosum, the bundle of nerves that connects the brain’s two hemispheres and helps integrate sensory and motor information with social interaction, executive function and language.
It’s thought that correcting the corpus callosum postnatally would be extremely difficult, given that it develops before birth, but when injected into mice postnatally, the Lee team’s viral gene therapy reconnected the callosal axons and restored the callosal nerves, substantially recovering the corpus callosum.
The therapy also increased the size of the dentate gyrus, the primary gateway for input formation into the rest of the hippocampus that is crucial for memory. This is one of only a few areas of the brain that continues to produce new neurons as mammals age into adulthood, making it a crucial target for postnatal treatments.
In addition, the therapy rescued areas of the brain related to signal speed between neurons.
Oligodendrocytes are the cells primarily responsible for myelination, the process of insulating nerves so they can transmit information rapidly. Brains with FOXG1 often have high numbers of oligodendrocyte precursor cells (OPC) yet delayed myelination.
According to the study, the therapy normalized the number of OPCs while restoring myelination.
The study provides a solid foundation for advancing the gene therapy toward human clinical trials, the researchers say.
“We are thrilled by the full rescue of brain structure abnormalities observed in our mouse model through this study. It marks a significant step forward in our research. With these promising results, we are eager to advance this AAV9 compound toward human clinical trials, hopeful that we can extend these breakthroughs to benefit children with FOXG1 syndrome.”
November 1, 2021
Four Success Stories in Gene Therapy
The field is beginning to fulfill its potential. These therapies offer a glimpse of what’s to come
By Jim Daley

Design Cells Getty Images
After numerous setbacks at the turn of the century, gene therapy is treating diseases ranging from neuromuscular disorders to cancer to blindness. The success is often qualified, however. Some of these therapies have proved effective at alleviating disease but come with a high price tag and other accessibility issues: Even when people know that a protocol exists for their disease and even if they can afford it or have an insurance company that will cover the cost—which can range from $400,000 to $2 million—they may not be able to travel to the few academic centers that offer it. Other therapies alleviate symptoms but don’t eliminate the underlying cause.
“Completely curing patients is obviously going to be a huge success, but it’s not [yet] an achievable aim in a lot of situations,” says Julie Crudele, a neurologist and gene therapy researcher at the University of Washington. Still, even limited advances pave the way for ongoing progress, she adds, pointing to research in her patients who have Duchenne muscular dystrophy: “In most of these clinical trials, we learn important things.”
Thanks to that new knowledge and steadfast investigations, gene therapy researchers can now point to a growing list of successful gene therapies. Here are four of the most promising.
Gene Swaps to Prevent Vision Loss
Some babies are born with severe vision loss caused by retinal diseases that once led inevitably to total blindness. Today some of them can benefit from a gene therapy created by wife-and-husband team Jean Bennett and Albert Maguire, who are now ophthalmologists at the University of Pennsylvania.
When the pair first began researching retinal disease in 1991, none of the genes now known to cause vision loss and blindness had been identified. In 1993 researchers identified one potential target gene, RPE65 . Seven years later Bennett and Maguire tested a therapy targeting that gene in three dogs with severe vision loss—it restored vision for all three.
In humans, the inherited condition that best corresponds with the dogs’ vision loss is Leber congenital amaurosis (LCA). LCA prevents the retina, a layer of light-sensitive cells at the back of the eye, from properly reacting or sending signals to the brain when a photon strikes it. The condition can cause uncontrolled shaking of the eye (nystagmus), prevents pupils from responding to light and typically results in total blindness by age 40. Researchers have linked the disease to mutations or deletions in any one of 27 genes associated with retinal development and function. Until gene therapy, there was no cure.
Mutations in RPE65 are just one cause of inherited retinal dystrophy, but it was a cause that Bennett and Maguire could act on. The researchers used a harmless adeno-associated virus (AAV), which they programmed to find retinal cells and insert a healthy version of the gene, and injected it into a patient’s eye directly underneath the retina. In 2017, after a series of clinical trials, the Food and Drug Administration approved voretigene neparvovecrzyl (marketed as Luxturna) for the treatment of any heritable retinal dystrophy caused by the mutated RPE65 gene, including LCA type 2 and retinitis pigmentosa, another congenital eye disease that affects photoreceptors in the retina. Luxturna was the first FDA-approved in vivo gene therapy, which is delivered to target cells inside the body (previously approved ex vivo therapies deliver the genetic material to target cells in samples collected from the body, which are then reinjected).
Spark Therapeutics, the company that makes Luxturna, estimates that about 6,000 people worldwide and between 1,000 and 2,000 in the U.S. may be eligible for its treatment—few enough that Luxturna was granted “orphan drug” status, a designation that the FDA uses to incentivize development of treatments for rare diseases. That wasn’t enough to bring the cost down. The therapy is priced at about $425,000 per injection, or nearly $1 million for both eyes. Despite the cost, Maguire says, “I have not yet seen anybody in the U.S. who hasn’t gotten access based on inability to pay.”
Those treated show significant improvement: Patients who were once unable to see clearly had their vision restored, often very quickly. Some reported that, after the injections, they could see stars for the first time.
While it is unclear how long the effects will last, follow-up data published in 2017 showed that all 20 patients treated with Luxturna in a phase 3 trial had retained their improved vision three years later. Bennett says five-year follow-up with 29 patients, which is currently undergoing peer review, showed similarly successful results. “These people can now do things they never could have dreamed of doing, and they’re more independent and enjoying life.”
Training the Immune System to Fight Cancer
Gene therapy has made inroads against cancer, too. An approach known as chimeric antigen receptor (CAR) T cell therapy works by programming a patient’s immune cells to recognize and target cells with cancerous mutations. Steven Rosenberg, chief of surgery at the National Cancer Institute, helped to develop the therapy and published the first successful results in a 2010 study for the treatment of lymphoma.
“That patient had massive amounts of disease in his chest and his belly, and he underwent a complete regression,” Rosenberg says—a regression that has now lasted 11 years and counting.
CAR T cell therapy takes advantage of white blood cells, called T cells, that serve as the first line of defense against pathogens. The approach uses a patient’s own T cells, which are removed and genetically altered so they can build receptors specific to cancer cells. Once infused back into the patient, the modified T cells, which now have the ability to recognize and attack cancerous cells, reproduce and remain on alert for future encounters.
In 2016 researchers at the University of Pennsylvania reported results from a CAR T cell treatment, called tisagenlecleucel, for acute lymphoblastic leukemia (ALL), one of the most common childhood cancers. In patients with ALL, mutations in the DNA of bone marrow cells cause them to produce massive quantities of lymphoblasts, or undeveloped white blood cells, which accumulate in the bloodstream. The disease progresses rapidly: adults face a low likelihood of cure, and fewer than half survive more than five years after diagnosis.
When directed against ALL, CAR T cells are ruthlessly efficient—a single modified T cell can kill as many as 100,000 lymphoblasts. In the University of Pennsylvania study, 29 out of 52 ALL patients treated with tisagenlecleucel went into sustained remission. Based on that study’s results, the FDA approved the therapy (produced by Novartis as Kymriah) for treating ALL, and the following year the agency approved it for use against diffuse large B cell lymphoma. The one-time procedure costs upward of $475,000.
CAR T cell therapy is not without risk. It can cause severe side effects, including cytokine release syndrome (CRS), a dangerous inflammatory response that ranges from mild flulike symptoms in less severe cases to multiorgan failure and even death. CRS isn’t specific to CAR T therapy: Researchers first observed it in the 1990s as a side effect of antibody therapies used in organ transplants. Today, with a combination of newer drugs and vigilance, doctors better understand how far they can push treatment without triggering CRS. Rosenberg says that “we know how to deal with side effects as soon as they occur, and serious illness and death from cytokine release syndrome have dropped drastically from the earliest days.”
Through 2020, the remission rate among ALL patients treated with Kymriah was about 85 percent. More than half had no relapses after a year. Novartis plans to track outcomes of all patients who received the therapy for 15 years to better understand how long it remains effective.
Precision Editing for Blood Disorders
One new arrival to the gene therapy scene is being watched particularly closely: in vivo gene editing using a system called CRISPR, which has become one of the most promising gene therapies since Jennifer Doudna and Emmanuelle Charpentier discovered it in 2012—a feat for which they shared the 2020 Nobel Prize in Chemistry. The first results from a small clinical trial aimed at treating sickle cell disease and a closely related disorder, called beta thalassemia, were published this past June.
Sickle cell disease affects millions of people worldwide and causes the production of crescent-shaped red blood cells that are stickier and more rigid than healthy cells, which can lead to anemia and life-threatening health crises. Beta thalassemia, which affects millions more, occurs when a different mutation causes someone’s body to produce less hemoglobin, the iron-rich protein that allows red blood cells to carry oxygen. Bone marrow transplants may offer a cure for those who can find matching donors, but otherwise treatments for both consist primarily of blood transfusions and medications to treat associated complications.
Both sickle cell disease and beta thalassemia are caused by heritable, single-gene mutations, making them good candidates for gene-editing therapy. The method, CRISPR-Cas9, uses DNA sequences from bacteria (clustered regularly interspaced short palindromic repeats, or CRISPR) and a CRISPR-associated enzyme (Cas for short) to edit the patient’s genome. The CRISPR sequences are transcribed onto RNA that locates and identifies DNA sequences to blame for a particular condition. When packaged together with Cas9, transcribed RNA locates the target sequence, and Cas9 snips it out of the DNA, thereby repairing or deactivating the problematic gene.
At a conference this past June, Vertex Pharmaceuticals and CRISPR Therapeutics announced unpublished results from a clinical trial of beta thalassemia and sickle cell patients treated with CTX001, a CRISPR-Cas9-based therapy. In both cases, the therapy does not shut off a target gene but instead delivers a gene that boosts production of healthy fetal hemoglobin—a gene normally turned off shortly after birth. Fifteen people with beta thalassemia were treated with CTX001; after three months or more, all 15 showed rapidly improved hemoglobin levels and no longer required blood transfusions. Seven people with severe sickle cell disease received the same treatment, all of whom showed increased levels of hemoglobin and reported at least three months without severe pain. More than a year later those improvements persisted in five subjects with beta thalassemia and two with sickle cell. The trial is ongoing, and patients are still being enrolled. A Vertex spokesperson says it hopes to enroll 45 patients in all and file for U.S. approval as early as 2022.
Derailing a Potentially Lethal Illness
Spinal muscular atrophy (SMA) is a neurodegenerative disease in which motor neurons—the nerves that control muscle movement and that connect the spinal cord to muscles and organs—degrade, malfunction and die. It is typically diagnosed in infants and toddlers. The underlying cause is a genetic mutation that inhibits production of a protein involved in building and maintaining those motor neurons.
The four types of SMA are ranked by severity and related to how much motor neuron protein a person’s cells can still produce. In the most severe or type I cases, even the most basic functions, such as breathing, sitting and swallowing, prove extremely challenging. Infants diagnosed with type I SMA have historically had a 90 percent mortality rate by one year.
Adrian Krainer, a biochemist at Cold Spring Harbor Laboratory, first grew interested in SMA when he attended a National Institutes of Health workshop in 1999. At the time, Krainer was investigating how RNA mutations cause cancer and genetic diseases when they disrupt a process called splicing, and researchers suspected that a defect in the process might be at the root of SMA. When RNA is transcribed from the DNA template, it needs to be edited or “spliced” into messenger RNA (mRNA) before it can guide protein production. During that editing process, some sequences are cut out (introns), and those that remain (exons) are strung together.
Krainer realized that there were similarities between the defects associated with SMA and one of the mechanisms he had been studying—namely, a mistake that occurs when an important exon is inadvertently lost during RNA splicing. People with SMA were missing one of these crucial gene sequences, called SMN1 .
“If we could figure out why this exon was being skipped and if we could find a solution for that, then presumably this could help all the [SMA] patients,” Krainer says. The solution he and his colleagues hit on, antisense therapy, employs single strands of synthetic nucleotides to deliver genetic instructions directly to cells in the body [see “ The Gene Fix ”]. In SMA’s case, the instructions induce a different motor neuron gene, SMN2 , which normally produces small amounts of the missing motor neuron protein, to produce much more of it and effectively fill in for SMN1 . The first clinical trial to test the approach began in 2010, and by 2016 the FDA approved nusinersen (marketed as Spinraza). Because the therapy does not incorporate itself into the genome, it must be administered every four months to maintain protein production. And it is staggeringly expensive: a single Spinraza treatment costs as much as $750,000 in the first year and $375,000 annually thereafter.
Since 2016, more than 10,000 people have been treated with it worldwide. Although Spinraza can’t restore completely normal motor function (a single motor neuron gene just can’t produce enough protein for that), it can help children with any of the four types of SMA live longer and more active lives. In many cases, Spinraza has improved patients’ motor function, allowing even those with more severe cases to breathe, swallow and sit upright on their own. “The most striking results are in patients who are being treated very shortly after birth, when they have a genetic diagnosis through newborn screening,” Krainer says. “Then, you can actually prevent the onset of the disease—for several years and hopefully forever.”
This article is part of “ Innovations In: Gene Therapy ,” an editorially independent special report that was produced with financial support from Pfizer .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Transl Med

Gene therapy for cystic fibrosis: new tools for precision medicine
1 Program in Developmental and Stem Cell Biology, Hospital for Sick Children, 686 Bay Street, PGCRL 16-9420, Toronto, ON M5G0A4 Canada
2 Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada
Elena N. Huang
Henry quach.
3 Program in Translational Medicine, Hospital for Sick Children, Toronto, ON M5G0A4 Canada
Amy P. Wong
Associated data.
Not applicable.
The discovery of the Cystic fibrosis (CF) gene in 1989 has paved the way for incredible progress in treating the disease such that the mean survival age of individuals living with CF is now ~58 years in Canada. Recent developments in gene targeting tools and new cell and animal models have re-ignited the search for a permanent genetic cure for all CF. In this review, we highlight some of the more recent gene therapy approaches as well as new models that will provide insight into personalized therapies for CF.
Introduction
Cystic fibrosis.
Cystic fibrosis (CF) is the most common life-limiting fatal genetic disorder, affecting approximately 90,000 individuals worldwide [ 1 ]. It is an autosomal recessive disorder that requires mutations in the CF gene in both genetic alleles [ 2 ]. The CF gene encodes for a protein the cystic fibrosis transmembrance conductance regulator (CFTR) which is a protein chloride channel that belongs to the family of adenosine triphosphate (ATP)-binding cassette (ABC) transporters. It consists of two membrane-spanning domains (MSD1, MSD2), two nucleotide-binding domains (NBD1, NBD2) and the functional regulatory domain (R) with multiple phosphorylation consensus sites, which when phosphorylated, undergoes conformational change and opening of the chloride channel [ 3 ]. Mutations in the CF gene affecting CFTR expression, protein levels or function, now known as CFTR variants, affect multiple organ systems including the lung, pancreas, liver, gut and reproductive organs. Changes in chloride and bicarbonate transportation across this channel impairs epithelial cell functions including mucociliary transport of foreign agents out of the airways, elevated concentrations sweat chloride, impairment in pancreatic hormone regulation, and intestinal obstruction [ 4 – 6 ]. In the lungs, CFTR-mediated export of chloride and bicarbonate ions across the epithelium into airway surface liquid (ASL) plays a vital role in maintaining the ASL pH and airway secreted protein composition (i.e. mucins). Dehydration of the ASL thickens mucus secretions and impairs mucociliary clearance, antimicrobial enzyme activity and promotes a pro-inflammatory environment mediated by recurrent infections leading to lung damage [ 7 ].
Classes of CFTR variants
In 1989, CFTR was identified and localized on the long arm of chromosome 7 (1q.31.2), consisting of 27 exons spanning about 215 kb of the genomic sequence [ 8 – 11 ]. While there have been > 2000 CF mutations identified to date ( http://www.genet.sickkids.on.ca/cftr/ ), over 360 are CF disease-causing variants ( www.cftr2.org ). Recently, these variants have been categorized into 7 classes based on CFTR protein dysfunction and/or gene expression [ 12 ] (Fig. 1 ): Class I are protein production variants that result in no functional CFTR protein with roughly 22% of CF patients harboring at least 1 mutant allele; Class II are protein processing variants that create misfolded CFTR protein and reduced expression on the cell membrane to function. Approximately 88% of CF patients have at least 1 mutant allele and the main variant p.Phe508del (F508del)-CFTR caused by a mutational deletion of the amino acid phenylalanine at the position 508 of the protein; Class III are gating variants that impair CFTR gate opening and encompasses roughly 6% of CF patients; Class IV result in defective ion channel conduction and approximately 6% of CF patients harbour this variant; Class V are insufficient protein variants and results in a reduced amount of CFTR at the surface membrane capturing 5% of CF patients; Class VI affects the stability of CFTR that causes a reduction in membrane retention and 5% of CF patients harbor at least one allele of this variant, and finally Class VII affects CFTR mRNA expression resulting in no mRNA and includes large deletions such as the dele2,3(21 Kb) mutation.

Classes of CFTR variants and CFTR modulators and the impact it has in CFTR expression and processing
Advantages and disadvantages of CFTR modulator therapy
Over the past 30 years, tremendous advances in clinical interventions and CF research have allowed for transformative advances in CF therapy. Prior to the development of small molecules targeting the CFTR protein (CFTR modulators), treatment of CF was solely aimed at alleviating the symptoms associated with the disease [ 13 ]. In recent years, CFTR modulators capable of directly correcting the genetic defect are paving the way for a cure for CF [ 14 ]. Here, we briefly touch on some current CFTR modulators that have been approved or are currently in clinical trials.
CFTR modulators are classified into 4 groups (Fig. 1 ): correctors, potentiators, stabilizers and amplifiers. Small molecules aimed at stabilizing the misfolded protein in the cytosol to prevent degradation are known as correctors (examples include lumacaftor (VX-809), tezacaftor (VX-661), and elexacaftor (VX-445) from Vertex Pharmaceuticals and posenacaftor (PTI-801) from Proteostasis). Small molecules that bind to the NBD domain of the CFTR channel to facilitate its opening are known as potentiators, (examples include ivacaftor (VX770) and dirocaftor (PTI-808)). Stabilizers such as cavosonstat ( {"type":"entrez-nucleotide","attrs":{"text":"N91115","term_id":"1444442"}} N91115 from Nivalis) rescues the protein stability on the plasma membrane, promotes CFTR maturation and is currently in phase II clinical trials. Amplifiers increase the amount of CFTR production and include nesolicaftor (PTI-428), a current candidate in phase III clinical trials in combination with PTI-801 and PTI-808. Finally, for CF-causing variants where in-frame nonsense, frameshift, and splicing variants that introduce a premature termination codon (PTC) into the CFTR mRNA (i.e. W1282X and G542X), read through agents such as ELX-02 developed by Eloxx Pharmaceuticals and Ataluren PTC-124 by PTC Therapeutics were designed to restore functional protein production by overriding PTC signals [ 15 ]. However, early clinical trials currently underway for ELX-02 and PTC-124 failed to show significant improvement in FEV1 measurements in patients with at least 1 mutant allele in a phase III clinical trial [ 16 ]. The number of transcripts differ considerably depending on the site of the PTC, the cell type and the patient’s genetic background [ 15 , 17 ]. Other small molecule inhibitors of the nonsense mediated decay (NMD) pathway such as SMG1 inhibitor (SMGi) can restore CFTR expression and function in cells harboring W1282X CFTR [ 18 ]. Therefore, combining small molecules to improve CFTR transcript production and/or stability with CFTR modulators may provide better clinical outcomes.
The approved CFTR modulator therapies ORKAMBI™ (a combination of VX-770 and VX-809) and SYMDEKO™ (a combination of VX-661 and VX-809) are combination treatments that has shown improved clinical benefits for some patients harboring F508del- CFTR . However, there are wide variations in responses to the drugs which suggest while the drugs may be used to treat the same genetic defect, other factors such as environmental [ 19 – 21 ] and gene modifiers [ 22 – 25 ] may influence therapy response. A recently approved drug, TRIKAFTA™ is a combination of 2 correctors (VX445 and VX661) and 1 potentiator (VX770) drugs that have shown incredible promise in improving lung function, sweat chloride conductance and lowering pulmonary exacerbations in F508del-CFTR individuals [ 26 ]. The short-term effectiveness of these modulators offer hope for restoring basic lung functions. However, the efficacy of this drug in effectively curing all CF individuals harboring at least 1 F508del allele remains unknown. Many rare CF variants are not eligible for current modulator treatment as these drugs are not expected to work such as for Class I production variants. Moreover, the long-term potential side effects of modulator treatment remain unclear [ 27 ] and with the costs for CFTR modulator therapy averaging over $300,000/year/patient [ 28 ], many CF individuals will not receive potential life-saving therapies without financial support or reimbursements. Therefore, new therapy approaches are still needed to treat all CF.
Gene therapy approaches for CF
Gene therapy offers great hope for the treatment of genetic diseases/disorders. By replacing the genetic mutation with a “correct version” of the CFTR gene, this method offers a potentially permanent cure. Indeed, since the discovery of the CF gene, many studies have attempted to correct the CFTR mutations through gene therapy approaches. While gene correction showed limited success in both cell and animal models [ 29 – 31 ], therapy for patients had proven to be more difficult. In-vitro studies have suggested that not all cells need to express normal CFTR to effect normal epithelial functions. In a mixing experiment where normal cells were mixed with CF mutant cells, only 6–10% of the epithelium needed to contain epithelial cells expressing normal CFTR to restore chloride transport similar to normal epithelia [ 32 ]. Conversely, in a gene targeting study, up to 25% gene correction could restore mucus transport in homozygous F508del human airway epithelial cells [ 33 ]. The number of cells harboring wild-type CFTR that is needed to translate into clinical benefit in patience remains unknown. However, theoretically correcting a stem cell population within the airways may provide a renewable and long-term source of endogenous cells capable of renewing the damaged epithelia with cells that express wild-type CFTR . Yet surprisingly, with the exception of a Phase I and II clinical trial for MRT5005 [ https://www.cff.org/Trials/Pipeline/details/10157/MRT5005 ], a drug that delivers CFTR -encoded mRNA to the lungs (RESTORE-CF), there are no other clinical trials for CF gene therapy. This may largely be due to several reasons: 1. The need for repeated delivery due to the inability to target stem/progenitor cells of the airways to sustain expression during cell turnover, 2. Suboptimal delivery or low efficiency of targeting of the donor plasmid/gene to the CF airways due to the highly inflammatory microenvironment, 3. The inability to deliver large DNA fragments of the CFTR gene effectively with current delivery methods, 4. Concerns of off-target safety that can result in insertional mutagenesis, and 5. Immune barriers limiting effective delivery of viral vectors. In this review, we briefly touch on some of the more recent genetic approaches that can rejuvenate CF gene therapy and touch on new cell and animal models that are enabling the testing of current gene targeting strategies and providing insight into personalized approaches for CF therapy.
Gene editing approaches
Gene editing tools can provide new gene therapy strategies to achieve permanent correction. Here we list a few editing tools used to date to test the efficacy of genetic correction for CF in-vitro.
Zinc Finger Nucleases (ZFNs) and transcription activator-like effector nucleases (TALEN)
Early developments of gene editing approaches included use of artificial restriction enzymes, Zinc Finger Nucleases (ZFNs) and transcription activator-like effector nucleases (TALEN) [ 34 , 35 ], (Table (Table1; 1 ; Fig. 2 ). These gene modification tools enabled precise genome editing through targeted nucleases cleavages and renewed hope for gene therapy. ZFNs are composed of specific pairs of oligos attached to a FokI restriction enzyme that facilitate a precise double-strand break (DSB) at the target site [ 36 ]. TALENs are composed of TALE repeats that bind and recognize extended DNA sequences and are also attached with a FoKI restriction enzyme to create a DSB [ 37 , 38 ]. In both instances, the DSB induces DNA repair mechanisms by either non homologous end joining (NHEJ), or homology-directed repair (HDR) [ 39 , 40 ]. Neither ZFN and TALENs technology have been used in CF gene therapies and in the advent of CRISPR-Cas systems, gene editing using the latter tool is more flexible making it the editing tool of choice for many researchers. The specific requirement of a pair of ZFNs reduces the number of target sites that can be identified for gene correction. Moreover, the low binding affinity of the ZFN creates undesirable off-target mutations in the genome [ 41 ]. TALEN has shown less off-target and better binding affinity than ZFN, however, the size for cDNA encoding a TALEN (3 kb) can be an issue for delivery into cells with a limited cargo size [ 42 ].
Advantages and disadvantages of gene editing tools
| ZFN | TALEN | CRISPR/Cas9 | Base Editing | Prime editing | |
|---|---|---|---|---|---|
| Mechanism | Type IIs restriction enzyme, FokI endonuclease, fused to pair of ZFN DNA binding domains Recognize 18-36 bp of DNA sequence Target DNA sequence break by protein-DNA interaction | Type IIs restriction enzyme, FokI endonuclease, fused to pair of TALEN DNA binding domains Recognize 30-40 bp of DNA sequence Target DNA sequence break by protein-DNA interaction | Few Cas endonuclease options for broader specificity and flexibility (Cas9, Cas12) PAM sequence require to design sgRNA Target DNA sequence break by DNA-RNA interaction | Direct conversion of a DNA base to another without DSBs at a target locus Permanent conversion of C-G to T-A base pairs by cytosin base editor (CBEs) Enzymatically convert A-T base pairs into G-C base pairs by adenine base editors (ABEs) | Fusion complex composed of a catalytically impaired Cas9 protein and an engineered reverse transcriptase Can recognize DNA of any sequence size |
| Efficiency | Low | Low | High | High | High |
| Advantages | Currently being used in clinical trials for HIV and Hunter’s syndrome Low immunity and Small protein size | Target any DNA sequence Less cytotoxic effects | Highly predictable target sequence Easy to design and possible to target only 1 bp of target sequence Potentially target multiple genes simultaneously | No random insertion and deletions because do not require DNA break High A > G and C > T conversion | No random insertion and deletions because do not require DNA break Can be used to generate different mutation types (insertions, deletions, and point mutations) |
| Limitations | Difficult due to extensive cloning needed to link two zinc finger modules together and expensive to design | Sensitive to DNA methylation Require pair of TALEN with two independent DNA binding sites | Require PAM site near the target DNA sequence to design gRNA Off-target effect observed Cas9 protein too large for AAV-based delivery | Only accounts for 4 out of 12 possible base-to-base conversions Too large for AAV-based delivery Difficult to edit DNA sequence that several A or C residues are nearby | High targeting efficiency but may depend on cell type Too large for AAV-based delivery Detection of undesired off-target effects and on-target mutation |

Graphics of gene editing technologies
CRISPR gene editing
In 2013, a new gene-editing tool used by bacteria to fend off bacteriophages by called clustered regularly interspaced short palindromic repeats (CRISPR) and it’s enzyme CRISPR associated protein 9 (Cas9) [ 43 ] was shown to be useful in editing the genomes of cultured mammalian cells [ 44 ]. The precise editing of the CRISPR-Cas9 system along with the versatile use of the system to silence genes by removing part of the gene or substituting the gene with desired ones has made the CRISPR-Cas9 system the preferred editing tool for gene editing. Moreover, the relative ease in designing a specific target site and low cost allows efficient gene editing to be done within a relatively short period of time [ 45 ]. The CRISPR-Cas9 is composed of two main modules: the guide RNA (gRNA), and the Cas9 protein enzyme. The gRNA is designed to recognize a specific sequence motif near the target site and recruits the Cas9 protein to cut and create a double-stranded DNA break (DSB). The cell’s natural DNA repair mechanisms are then activated to repair the cleaved DNA through NHEJ or HDR [ 39 , 40 ]. NHEJ directly ligates the broken ends, and can create “indels” or insertion or deletions of genes effectively creating mutants [ 46 ]. However, with a repair template, the HDR response will enable homologous recombination. This method is useful for introducing a desired gene (or a wild-type version of a gene). However, the frequency of HDR is very low [ 47 ] and therefore efficiency of “repairing” or replacing a mutant gene remains a challenge.
(i) Base editing : The CRIPSR-Cas9 system’s classical reliance on introducing DSBs poses an efficiency problem since undesirable random insertions or deletions (indels) occur more often at DNA cleavage sites than HDR. Base editing was thus pioneered to increase the efficiency of the CRISPR-Cas9 system by circumventing the need for DSBs altogether, allowing for the direct conversion of a DNA base to another without DSBs at a target locus [ 48 ]. Cytosine base editors (CBEs) facilitate the permanent conversion of C-G to T-A base pairs, while adenine base editors (ABEs) enzymatically convert A-T base pairs into G-C base pairs [ 49 ]. In the contexts of CF, base editing could then be an attractive new tool in treating CF, since many CFTR variants could be rescued with a single base pair change. Accordingly, Geurts et al. recently provided support to the efficacy and feasibility of utilizing such base editing tools safely within human cells to potentially treat CF with two respective ABEs [ 50 ]. A caveat of base editing is the limitation of only 4 possible base-to-base conversions and is too large for certain gene delivery vectors.
(ii) Prime editing : Prime editing has recently become an attractive advancement in the CRISPR toolbox [ 51 ]. This gene editing technology makes it possible to edit a specified DNA sequence, of variable lengths at a target site, with a fusion complex composed of a catalytically impaired Cas9 protein and an engineered reverse transcriptase [ 51 ]. A prime editing guide RNA (pegRNA) encodes the desired gene edit and directs the fusion complex to the target site [ 51 ]. As a possible gene replacement therapeutic technology, prime editing is very promising in the context of CF, given the most common CFTR variant (CFTR-F508del) has been repaired by prime editing in patient-derived intestinal organoids [ 52 ]. However, prime editing did result in varying degrees of targeting efficiency and undesired off-target mutations were also observed [ 52 ]. Nevertheless, since the CFTR gene is large, and a complete replacement of a mutant gene with wild-type CFTR would likely be inefficient, prime editing is leading method to address the vast number of CF disease-causing variants.
Gene delivery
There are several gene delivery methods to introduce a therapeutic gene or gene targeting. Both non-viral and viral delivery vectors have been tested in CF gene therapy research.
(i) Non-viral vectors : Non-viral vectors were developed as a strategy to deliver the CFTR gene. These non-integrating gene delivery methods do not disrupt the host genome and thus the risk of causing mutagenesis are low. Non-viral vectors are not restricted in the cargo load enabling larger donor DNA fragments to be used for gene repair. However, the efficacy of gene delivery is comparatively lower than viral methods. To enhance gene transfer into the nucleus, a cationic lipid is used to formulate the plasmid DNA [ 53 ] complexed with CFTR enhanced chloride transport by 20% in CF patients compared to non-CF levels [ 54 ]. Using a nebulized cationic lipid pGM169/GL67A to deliver the donor DNA, up to 3.7% increase in CFTR function in the lungs of CF patients was observed [ 55 , 56 ]. The drawback of the cationic liposome-mediated approach is the need for repeated delivery as transient expression of CFTR did not have a lasting effect [ 57 ]. Despite these efforts, non-viral based methods of gene delivery cannot permanently restore lung functions.
(ii) Viral vectors : To improve efficacy of targeting the cells, several viral based delivery methods have been tested to including adenovirus (Ad), adeno-associated virus (AAV), and retroviral vector in pre-clinical and clinical trials to deliver the corrected CFTR gene.
Adenovirus (Ad)
Based vectors were once the preferred delivery vectors for gene delivery [ 58 , 59 ]. Mutational deletion of viral replication genes and host immune cell evasion genes early region 1 and 3 (E1/E3) respectively, removed the ability of the virus to self-replicate making these viral vectors attractive for gene therapy. However, leaky expression of viral genes from E1 deleted vectors, in addition to capsid proteins, could elicit host immune responses to the Ad vectors [ 60 – 62 ]. The first clinical trial (in 1993) for CF gene therapy using an adenovirus vector failed to restore CFTR expression in CF patient’s nasal epithelia [ 63 , 64 ]. This led to the identification and testing of other adenovirus serotypes 2 and 5 in CF clinical trials which resulted in transient restoration of chloride transport in the nasal and bronchial epithelium [ 65 , 66 ]. However, evidence of a pro-inflammatory response was found with these Ad vectors which required repeated administration for effective gene delivery [ 63 , 65 ]. Even so, the trials have only demonstrated limited clinical benefits in CF patients [ 66 ].
Adeno-associated virus
AAV-based vectors have been tested as another gene delivery tool. With the ability to transduce terminally differentiated and non-dividing cells, AAV can also persist longer in-vivo [ 67 ] compared to its Ad counterpart. Transient immunosuppression can improve re-administration of AAV vectors in mouse lungs up to 8 months [ 68 ]. In 1998, the first successful human clinical trial with repeated delivery of AAV2-CFTR into the maxillary sinuses [ 69 ] demonstrated restoration of CFTR function without noticeable toxicity or an elevated immune response after 2 weeks of delivery. However, other clinical trial studies performed years later failed to show sufficient CFTR functional correction by AAV-CFTR [ 70 , 71 ]. One caveat of the AAV vectors is the limited target gene size (less than 4.6 kb) that can be inserted into the viral vector for efficient expression.
Helper-dependent adenoviruses (Hd-Ad)
To avoid the harmful immune response of Ad, the Helper-dependent Adenovirus (Hd-Ad) was developed [ 72 ]. Deletion of all viral coding sequences allows Hd-Ad to deliver large DNA cargo (to 37 kb) without eliciting host immune responses [ 73 , 74 ]. One unique feature of the Hd-Ad vectors is that they can be used to deliver both a gene editing endonuclease system and donor DNA in a single vector to achieve site-specific gene integration without expressing the endonuclease following gene correction [ 75 – 77 ]. Gene correction using Hd-Ad in CF mouse and pig airway basal cells can restore CFTR function similar to levels observed in normal wild-type cells as measured by fluorescence imaging plate reader (FLiPR) assay [ 30 , 72 , 78 – 81 ]. HD-Ad vectors have also been shown to be effective in correcting the CFTR gene in the lungs of CF knockout mice [ 82 ]. However, a major challenge remains for in-vivo gene therapy as the ability to sustain therapeutic effects is lost due to airway cell turnover. Therefore, targeting a stem cell compartment within the airways has become an attractive goal for permanent CF gene correction.
Retroviruses and lentiviruses
Retroviral and lentiviral vectors have been used for gene delivery methods as early as the late 1990s. Retroviruses harboring human CFTR gene transduced into rabbit tracheal epithelial cells showed persistent expression in the airways for up to 3 weeks. However, the transduced capacity by retroviruses were low and transduction occurred only in wounded areas [ 83 ]. Lentiviral vectors have been effective in delivering CFTR transgene into the airway epithelium [ 84 ] with potential to target the lung stem cell population for sustained and persistent CFTR expression [ 85 ]. While both retroviruses and lentiviruses can efficiently target host cells and integrate into the host genome, there remains significant concerns over their use as a delivery vector for gene therapy. The host immune responses remain a significant barrier in efficacious delivery of exogenous genetic materials by viral methods. In the context of CF airway disease, the proinflammatory milieu of the diseased airways compounded by the mucosal obstructions poses a challenge for any gene delivery methods. Second, there are concerns of insertional mutagenesis, epigenetic silencing, and secondary impact of altered expression levels derived by using viral promoters to drive the un-regulated expression of the transgene [ 86 , 87 ].
Therefore, while new gene editing approaches may increase the targeting efficiency of gene correction, precise and efficient delivery of the genetic tools to the right cell type for permanent gene correction remains a barrier to clinical use. To study this, new animal and advanced stem cell-based models may enable research into cell delivery and targeting strategies.
Animal models of CF
Animal models of CF are valuable tools that may be utilized to further understand disease pathogenesis and test new therapeutics. There are two fundamental issues that remain to be resolved before gene therapy can become viable for patients, and animal models provide a relevant platform through which these obstacles may be safely addressed. First, in-vivo efficiencies of gene targeting need to achieve a level that will translate to therapeutic outcome. Second, the efficacy of gene targeting must outweigh concerns of off-target mutagenesis from the gene editing tools. Animal models have traditionally been useful models to understand basic mechanisms of disease pathogenesis. Recent animal models for CF, especially those harboring human CF variants offer opportunities to test new emerging CFTR modulators for which these modulators are designed to specifically target the specific functional outcome. Here we briefly touch on several of these animal models and their use in CF therapy discovery (Fig. 3 ).

CF animal models compared to human disease phenotypes
(i) Mouse model : With a 78% amino acid sequence conservation between mouse and human CFTR (h CFTR ) [ 88 ], the use of mice for disease modelling comes as no surprise when also considering practical factors like costs, breeding time, and ease of maintenance. However, CF mouse models only exhibit mild pancreatic disease [ 89 , 90 ] if any, present variable gallbladder abnormalities [ 90 – 92 ], and liver pathologies are largely only observed in mice studied later in life [ 89 ]. While new humanized mouse models have become available, and can be used to study CFTR modulator efficacies, they possess a major limitation in harboring ~ 6 copies of the h CFTR gene [ 93 – 95 ]. Therefore, it remains unclear how effective these humanized models are for gene therapy testing but may be a good model for CFTR modulator testing.
(ii) Rat model : CF rat models present similar phenotypes with CF mice. Like the CF mice, the rat models do not recapitulate spontaneous lung infection or pancreatic and liver disease [ 96 , 97 ] though some models have displayed exocrine pancreas histopathology [ 98 ]. Nevertheless, rats possess a 76% amino acid sequence identity to h CFTR [ 99 ] and have submucosal glands in the large airways [ 97 , 100 ]. Rat models have also provided the groundwork for exploring new genetic advancements in CF modelling, like the generation of the first G542X CF nonsense mutation rat model with CRISPR-Cas9 [ 101 ], and a new F508del rat models that may be invaluable in the development of therapeutics [ 97 ].
(iii) Rabbit model : Rabbit models of CF are rather new to the field [ 102 , 103 ] thus the relevance to human CF disease remains to be seen. However, rabbits present as a very promising model for the study of lung diseases in general, due to their airway anatomy and inflammatory responses [ 104 ]. Further, there is a 92% amino acid sequence conservation between rabbit and human CFTR [ 88 ]. A caveat of the rabbit model is they lack submucosal glands within their airways [ 100 , 104 ] which contain CFTR-expressing cells in human airways.
(iv) Ferret model : Due to the highly conserved anatomy between human and ferret lungs [ 105 , 106 ], ferret CF models accurately mirror the key disease phenotypes of CF, including those unable to be recapitulated in other models [ 107 – 109 ] With a sequence homology of 92% with h CFTR [ 88 ], and an abundance of submucosal glands throughout their airways [ 110 ], ferrets are an attractive translational model of CF [ 111 ]. A caveat of the ferret model is the costs associated with maintaining these animal colonies and current CF ferret models require CFTR modulators to survive, making long-term study of the disease pathogenesis difficult.
(v) Pig model : Pig models share a 92% amino acid sequence identity with h CFTR [ 88 ], and arguably offer the highest translational potential for CF research due to their comparable genetics, physiology, and anatomy to humans [ 112 – 114 ]. However, porcine CF models present an even larger practical and cost challenge than ferrets. Their sheer size, while beneficially comparable to humans, calls for much consideration regarding labor costs and maintenance. For testing new drugs, the pig model can become astronomically expensive. Nevertheless, CF pig models recapitulate all key CF disease phenotypes, though notably with more severe manifestations than in humans [ 113 – 117 ].
Cell models for studying CF disease pathogenesis and therapy.
(i) Current gold-standard lung cell models : Cell models have played instrumental roles in understanding the biophysical properties of CFTR, the mechanistic cause of the defects and evaluating novel therapeutic strategies (Fig. 4 ). Human primary epithelial cell lines have been the main tool for assessing ion channel functions and for drug development [ 118 – 121 ]. While recent improvements in culture conditions have improved the expansion potential of primary cells, this expansive ability is limited [ 122 ] and primary cells enter senescence shortly in culture. To circumvent this, immortalized epithelial cell lines, such as A549, BEAS-2B, Calu-3 and 16HBE14o, are commonly used to study drug transport, metabolism, and epithelial integrity [ 123 – 127 ]. However, these immortalized cell lines are derived from lung tumour cells or have been transformed, and thus do not show original lung cell characteristics or reflect the repertoire of epithelial cell types found in the native lungs. Primary nasal cells are an alternative cell type to study CF airway disease due to the ease of generating nasal epithelial cultures from patients. The pros of these cells are the relative ease of obtaining samples from patients and they can be sampled several times (if needed). Studies have suggested nasal epithelial cells are a good surrogate of airway bronchial epithelial cells [ 128 , 129 ]. However, like primary bronchial cells, the ability to expand these cells in culture for sufficient use without re-sampling remains a problem. In addition, sampling variability can impact CFTR protein expression and function of the epithelium. Recently, lung stem cells isolated from bronchoalveolar lavage fluid can generate renewable airway organoids for multiple passages in cultures [ 130 ]. It remains to be seen whether a method of airway organoid generation can be achieved from individuals with airway diseases for disease modeling. Nonetheless, generation of a renewable source of patient-specific lung airway cells is a key enabler for identifying patient-specific therapies for lung diseases.

Cell models to study CF disease and therapies. For gene editing approaches, green “✓” indicates research data supporting the use of these approaches in the cell models for CF gene correction. Red “X” indicates no information available. For advantages/limitations section, green “✓” indicates possible and red “X” indicates not possible
(ii) Human pluripotent stem cell (PSC) models for personalized medicine : Human embryonic stem (hES) cells were discovered in 1998 and hold enormous promise to repair disease and regenerate tissues [ 131 ]. With the ability to self-renew and differentiate into cells of all three embryonic germ layers endoderm, ectoderm and mesoderm, hES became an intriguing source of cells for regenerative medicine. However, research in the use of hES for regeneration faced paucity due to the growing ethical concerns associated with the use of “embryonic/fetal” tissue. In 2006, the first discovery of induced pluripotent stem cells (iPSC) was made and revealed these cells shared similar characteristics to mouse ES [ 132 ]. By 2007, the first human iPSC was made by introducing four transcription factors associated with pluripotency to fibroblasts [ 133 ]. Since this discovery, therapeutic applications of human iPSC have led to > 65 market competitors offering iPSC-based products. Indeed, iPSC are a great source of cells for patient-specific disease modeling, drug discovery and personalized regenerative medicine. Biobanks of iPSC from individuals with various genetic mutations have become a useful resource for disease modeling. The Hospital for Sick Children in Toronto has now acquired over 100 CF patient cells harboring various CFTR variants and generated iPSC from each individual including some gene-corrected isogenic iPSC lines for benchmarking patient-specific “normal” responses [ 134 ]. This will undoubtedly enable research in modelling CF organ and patient-specific disease and therapy discoveries.
Differentiation of human iPSC into multiple tissue cell types has now been achieved albeit with varying efficiencies. Most directed differentiation methods use a stepwise approach of activating and/or inhibiting pathways known to affect developmental growth in animal models, especially the mouse. Indeed, we and others have identified key developmental pathways required to generate lung epithelial cells from human iPSC [ 135 – 138 ]. Moreover, airway and intestinal cells derived from homozygous F508del CF iPSC model CF phenotype (lack of CFTR membrane expression) can be used to screen for CFTR small molecule correctors [ 136 , 139 , 140 ]. We have shown that CF iPSC-derived airway cells are amenable to high throughput CFTR functional screens—a step towards using these cells for personalized medicine [ 139 – 143 ]. Recently, we have improved the generation of lung cells from human PSC and demonstrate the utility of capturing CFTR expression and function in the differentiated cells modeling development [ 136 , 144 ]. Understanding the impact of mutant CFTR during development remains poorly understood and these new PSC models will advance our understanding of the prenatal origins of disease mechanisms.
Another benefit of using iPSC models is the ability to determine both patient and tissue-specific responses. This is important as CFTR expression and activity levels differ in different tissues. Correction of CF mutations have been tested in iPSC, however the efficacy of these gene-editing strategies in-vivo remains to be seen [ 141 – 143 ]. Ultimately, establishing predictive patient and tissue specific models to predict patient outcome is key to advancing precision medicine.
New models, new gene editing tools, new targets?
One of the biggest challenges in generating treatment strategies for CF is the sheer number of CF-causing variants. Even among patients with the same variant, there are vast differences in severity of symptoms and responses to treatments. To date, treatment options for CF are mutation-dependent, and no viable options exist to universally address all CF patients. Though recent advancements in gene editing have fostered hope for personalized treatments, this is neither viable nor practical for treating all CF.
Recently, Kemaladewi et al. demonstrated a novel mutant-independent therapeutic approach to treat congenital muscular dystrophy type 1A (MDC1A) [ 145 ]. Using CRISPR, the feasibility of treating inherited diseases by looking beyond the singular disease-causing gene, and instead targeting compensatory modifier genes, was illustrated. In the context of CF, ion channels aside from CFTR have been implicated in CF disease severity and responses to modulator therapy. Therefore, targeting other ion channels known to also affect CF disease severity such as the sodium channel ENaC [ 146 ] or alternative ion channels TMEM16A ( ANO1 [ 147 , 148 ]) and SLC26A9 [ 149 , 150 ] may need to be assessed to find effective therapies for all individuals with CF.
Since the discovery of the CF gene over 30 years ago, it has become apparent that finding an effective therapy to treat all CF remains a challenge. While the discoveries of new small molecule modulators have greatly advanced treatment for some CF, the effectiveness of these lifesaving drugs have not been universally effective and rather limited to specific classes of mutations. Rare CFTR variants remain uncured. Now, with recent advances in new gene editing tools coupled with both iPSC-derived tissue models and new animal models, new precise gene targeting methods to treat CF disease will emerge and lead to potential effective personalized therapies. Classical approaches of targeting the disease-causing variant may also be replaced or coupled with mutation-agnostic approaches to treat complex CF phenotypes and with improved pre-clinical models, this can now be tested. With new advancements in gene editing technologies coupled with advanced cell models to test gene engineering approaches, this will lead to rapid developments of new therapies for all CF.
Acknowledgements
This work was supported by the SickKids Foundation-CIHR IHDCYH NI20-1070 and Medicine by Design (University of Toronto) grants. AC is a recipient of the University of Toronto Summer Studentship Award (2021); All figures were prepared with BioRender.com.
Abbreviations
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| F598del | P.Phe508del |
| ABC transporters | Adenosine triphosphate (ATP)-binding cassette transporters |
| MSD1 and MSD2 | Membrane-spanning domain 1 and 2 |
| NBD1 and MBD2 | Nucleotide-binding domain 1 and 2 |
| R domain | Regulatory domain |
| ASL | Airway surface liquid |
| PTC | Premature termination codon |
| NMD | Nonsense mediated decay |
| ZFNs | Zinc Finger Nucleases |
| TALEN | Transcription activator-like effector nucleases |
| DSB | Double-strand break |
| NHEJ | Non homologous end joining |
| HDR | Homology-directed repair |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| Cas9 | CRISPR associated protein 9 |
| gRNA | Guide RNA |
| indels | Insertions or deletions |
| CBEs | Cytosine base editors |
| ABEs | Adenine base editors |
| pegRNA | Prime editing guide RNA |
| Ad | Adenovirus |
| AAV | Adeno-associated virus |
| Ad | Adenovirus |
| E1 | Early region 1 |
| E3 | Early region 3 |
| AAV | Adeno-associated virus |
| Hd-Ad | Helper-dependent adenoviruses |
| FLIPR | Fluorescence imaging plate reader |
| h | Human cystic fibrosis transmembrane conductance regulator |
| hES | Human embryonic stem |
| iPSC | Induced pluripotent stem cells |
| MDC1A | Muscular dystrophy type 1A |
| ENaC | Epithelial sodium channel |
| TMEM16A | Transmembrane member 16A |
| ANO1 | Anoctamin-1 |
| SLC26A9 | Solute carrier family 26 member 9 |
Authors’ contributions
J-AL: Review of literature and manuscript preparation, Figure preparation. AC: Review of literature and manuscript preparation. EH: Manuscript and figure preparation. YX: Review of literature and manuscript preparation. HQ: Review of literature and manuscript preparation. JH: Review and editing of manuscript. APW: Review of literature and manuscript preparation, Review and editing of manuscript. All authors read and approved the final manuscript.
Applicable.
Availability of data and materials
Declarations.
Not applicable
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alex Cho and Elena N. Huang contributed equally to the paper
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection

Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
New gene therapy strategies for cancer treatment: a review of recent patents
Affiliation.
- 1 Department of Health Science, University of Jaén, Jaén; 23071, Spain.
- PMID: 22339358
- DOI: 10.2174/157489212801820093
Cancer is the second leading cause of death in the Western world. The limited successes of available treatments for cancer mean that new strategies need to be developed. The possibility of modifying the cancer cell with the introduction of genetic material opens the way to a new approach based on gene therapy. There are still many technical difficulties to be overcome, but recent advances in the molecular and cellular biology of gene transfer have made it likely that gene therapy will soon start to play an increasing role in clinical practice, particularly in the treatment of cancer. Gene therapy will probably be the therapeutic option in cases in which conventional treatments such as surgery, radiotherapy and chemotherapy have failed. The development of modified vectors, and an improved understanding of interactions between the vector and the human host, are generating inventions that are being protected by patents due to the considerable interest of industry for their possible commercialization. We review the latest strategies, patented and/or under clinical trial, in cancer gene therapy. These include patents that cover the use of modified vectors to increase the security and specificity, recombining adenovirus that leads to loss or gain of gene function, activation of the patient's own immune cells to eliminate cancer cells by expression of molecules that enhance immune responses, silencing genes related to the development of drug resistance in patients, inhibition of angiogenesis of solid tumors by targeting the tumor vasculature, and the development of enzymes that destroy viral or cancerous genetic material.
PubMed Disclaimer
Similar articles
- Cancer suicide gene therapy: a patent review. Navarro SA, Carrillo E, Griñán-Lisón C, Martín A, Perán M, Marchal JA, Boulaiz H. Navarro SA, et al. Expert Opin Ther Pat. 2016 Sep;26(9):1095-104. doi: 10.1080/13543776.2016.1211640. Epub 2016 Jul 20. Expert Opin Ther Pat. 2016. PMID: 27424657 Review.
- Virus-Based RNA Silencing Agents and Virus-Derived Expression Vectors as Gene Therapy Vehicles. Venkataraman S, Ahmad T, AbouHaidar MG, Hefferon KL. Venkataraman S, et al. Recent Pat Biotechnol. 2017;11(2):141-151. doi: 10.2174/1872208311666170301103722. Recent Pat Biotechnol. 2017. PMID: 28260520 Review.
- INGN 201: Ad-p53, Ad5CMV-p53, adenoviral p53, p53 gene therapy--introgen, RPR/INGN 201. [No authors listed] [No authors listed] Drugs R D. 2007;8(3):176-87. doi: 10.2165/00126839-200708030-00005. Drugs R D. 2007. PMID: 17472413 Review.
- Use of suicide genes for cancer gene therapy: study of the different approaches. Vassaux G, Martin-Duque P. Vassaux G, et al. Expert Opin Biol Ther. 2004 Apr;4(4):519-30. doi: 10.1517/14712598.4.4.519. Expert Opin Biol Ther. 2004. PMID: 15102601 Review.
- Viral-mediated gene transfer for cancer treatment. Wilson DR. Wilson DR. Curr Pharm Biotechnol. 2002 Jun;3(2):151-64. doi: 10.2174/1389201023378445. Curr Pharm Biotechnol. 2002. PMID: 12022258 Review.
- Hierarchically tumor-activated nanoCRISPR-Cas13a facilitates efficient microRNA disruption for multi-pathway-mediated tumor suppression. Liu X, Yang S, Wang L, Wu X, Wang X, Ou C, Yang J, Song L, Zhou S, Wu Q, Gong C. Liu X, et al. Theranostics. 2023 May 8;13(9):2774-2786. doi: 10.7150/thno.81776. eCollection 2023. Theranostics. 2023. PMID: 37284454 Free PMC article.
- A Winning New Combination? Toward Clinical Application in Oncology. Maqsood Q, Sumrin A, Iqbal M, Hussain N, Mahnoor M, Zafar Saleem M, Perveen R. Maqsood Q, et al. Cancer Control. 2023 Jan-Dec;30:10732748231175240. doi: 10.1177/10732748231175240. Cancer Control. 2023. PMID: 37166227 Free PMC article. Review.
- Efficient gene transfection to lung cancer cells via Folate-PEI-Sorbitol gene transporter. Cho KS, Kim S, Chun HB, Cheon JH, Cho MH, Lee AY, Arote RB. Cho KS, et al. PLoS One. 2022 May 4;17(5):e0266181. doi: 10.1371/journal.pone.0266181. eCollection 2022. PLoS One. 2022. PMID: 35507584 Free PMC article.
- Tumor progression-dependent angiogenesis in gastric cancer and its potential application. Hsieh HL, Tsai MM. Hsieh HL, et al. World J Gastrointest Oncol. 2019 Sep 15;11(9):686-704. doi: 10.4251/wjgo.v11.i9.686. World J Gastrointest Oncol. 2019. PMID: 31558974 Free PMC article. Review.
- Anti-Colorectal Cancer Effects of Probiotic-Derived p8 Protein. An BC, Hong S, Park HJ, Kim BK, Ahn JY, Ryu Y, An JH, Chung MJ. An BC, et al. Genes (Basel). 2019 Aug 19;10(8):624. doi: 10.3390/genes10080624. Genes (Basel). 2019. PMID: 31430963 Free PMC article.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Ingenta plc
Other Literature Sources
- The Lens - Patent Citations
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Latest News >
Study suggests promising gene therapy for FOXG1 syndrome
research news

Soo-Kyung and Jae Lee are the co-lead authors of a new study that suggests a promising viral gene therapy for FOXG1 syndrome, a severe neurodevelopmental disorder. Photo: Douglas Levere
By TOM DINKI
Published June 17, 2024
A viral gene therapy developed by UB researchers has reversed some brain abnormalities in infant mice with FOXG1 syndrome, a significant step toward one day treating children with this severe neurodevelopmental disorder.
This mediated delivery of the FOXG1 gene via adeno-associated virus 9 (AAV9) is detailed in a study published June 5 in Molecular Therapy Methods & Clinical Development . A postnatal injection of the therapy in day-old mice rescued a wide range of abnormalities, the study found, including in parts of the brain responsible for language, memory and social interaction.
“Our findings highlight the efficacy of AAV9-based gene therapy as a viable treatment strategy for FOXG1 syndrome and potentially other neurodevelopmental disorders with similar brain malformations,” says Soo-Kyung Lee, Empire Innovation Professor and Om P. Bahl Endowed Professor in the Department of Biological Sciences, College of Arts and Sciences, who led the study with her husband, Jae Lee, professor in the department. “This research asserts the therapeutic relevance of our approach in postnatal stages, which is a critical time frame for intervention.”
The Lees’ teenage daughter, Yuna, was diagnosed with FOXG1 syndrome at the age of 2. The researchers have since established themselves as leading experts on the disorder and are the principal investigators of UB’s FOXG1 Research Center (FRC). The center, which launched earlier this year, as well as this recent study, are supported by the FOXG1 Research Foundation.
The study was co-led by Kathrin Meyer, principal investigator at Nationwide Children’s Hospital in Columbus, Ohio. Other contributions represent the University of Pennsylvania and Samsung Medical Center in Seoul, South Korea.

The length and apex angle of the dentate gyrus in a mice with FOXG1 syndrome was restored after a postnatal injection of the researchers' AAV9-based gene therapy. Image: Soo-Kyung and Jae Lee
Reversing structural abnormalities
A master regulator gene, FOXG1 is one of the most important genes for early brain development and its impairment can result in profound brain structure abnormalities.
The Lees previously established that the FOXG1 gene and protein remain active in mice after birth, so they wondered if restoring FOXG1 levels could reverse some of the abnormalities associated with FOXG1 syndrome.
These abnormalities include failure to fully develop the corpus callosum, the bundle of nerves that connects the brain’s two hemispheres and helps integrate sensory and motor information with social interaction, executive function and language.
It’s thought that correcting the corpus callosum postnatally would be extremely difficult, given that it develops before birth, but when injected into mice postnatally, the Lee team’s viral gene therapy reconnected the callosal axons and restored the callosal nerves, substantially recovering the corpus callosum.
The therapy also increased the size of the dentate gyrus, the primary gateway for input formation into the rest of the hippocampus that is crucial for memory. This is one of only a few areas of the brain that continues to produce new neurons as mammals age into adulthood, making it a crucial target for postnatal treatments.
In addition, the therapy rescued areas of the brain related to signal speed between neurons.
Oligodendrocytes are the cells primarily responsible for myelination, the process of insulating nerves so they can transmit information rapidly. Brains with FOXG1 often have high numbers of oligodendrocyte precursor cells (OPC) yet delayed myelination.
According to the study, the therapy normalized the number of OPCs while restoring myelination.
The study provides a solid foundation for advancing the gene therapy toward human clinical trials, the researchers say.
“We are thrilled by the full rescue of brain structure abnormalities observed in our mouse model through this study. It marks a significant step forward in our research. With these promising results, we are eager to advance this AAV9 compound toward human clinical trials, hopeful that we can extend these breakthroughs to benefit children with FOXG1 syndrome.”
- News Center >
- News Releases >
Unproven stem cell, gene therapies: UB-led team authors guide to protect consumers

By Laurie Kaiser
Release Date: November 28, 2023

Laertis Ikonomou
BUFFALO, N.Y. — While stem cell therapy has been used to successfully generate and repair tissues that have been damaged due to certain conditions and diseases, such as leukemia, it is far from a cure-all.
Direct-to-consumer businesses, however, have increasingly been promoting unproven stem cell and gene-based interventions to unsuspecting members of the public across the globe as the answer to a litany of problems.
Through slick websites, television advertisements and social media postings, the businesses frame their so-called therapies as legitimate treatment for conditions ranging from musculoskeletal diseases and injuries to neurological disorders, said Laertis Ikonomou , PhD, associate professor of oral biology in the University at Buffalo School of Dental Medicine.
“These presumed therapies do not have substantive evidence of safety or efficacy,” he said.
As the immediate past chair of the International Society for Cell & Gene Therapy (ISCT) Committee on the Ethics of Cell and Gene Therapy (ECGT), which is composed of academic researchers, clinicians and regulatory policy and bioethics experts, Ikonomou has studied this unsavory practice for several years.
He recently served as the lead author in a guide on direct-to-consumer businesses that offer these unproven therapies. It was published in the September issue of Cytotherapy , the official peer-reviewed journal of ISCT.
“We broke down what is legitimate and what is not,” Ikonomou said. “We also provided an overview of reporting mechanisms for patients who believe they have been harmed by these unapproved and unproven products and suggested practical strategies to address and counteract the widespread marketing of such products.”
Desperate consumers seek unregulated interventions
Examples of interventions that direct-to-consumer clinics offer include cell-based products and cell-derived products such as extracellular vesicles and perinatal tissue products, and rarely, gene-based therapies including gene-modified cell therapies.
The clinics offer treatments for numerous conditions, including progressive ones such as Parkinson’s or Alzheimer’s disease that can significantly diminish quality of life. Often, patients come to the clinics from a place of desperation.
“When a physician has told them, ‘This is all we can do for you and you’re not going to get better,’ people are absolutely within their rights to look for alternative treatment,” Ikonomou said. “The problem is what a lot of these clinics offer is a total scam.”
In some cases, after undergoing the unregulated interventions, patients have ended up with physical harms, such as infections, or worsened conditions, including blindness.
Some of the clinics are led by medical doctors and some aren’t.
For instance, a clinic for orthopedic care may offer interventions for other conditions in which the providers are not trained.
“We have seen chiropractors and homeopathic doctors who decide to make stem cell therapy part of their business,” Ikonomou said. “Some businesses claim that the products they are selling are safe and effective. Others acknowledge the investigational nature of what they are selling but charge patients to access products in pay-to-participate studies. These purported studies are generally poorly designed, unblinded, unrandomized and uncontrolled.”
Typically, such studies have not been reviewed and authorized by national regulators, such as the U.S. Food and Drug Administration (FDA).
Ikonomou and his colleagues have been monitoring letters the FDA is addressing to these clinics.
“The products that they offer don’t fall within their practice of medicine,” he said. “For instance, you can’t use umbilical cord tissue to treat all kinds of conditions, yet certain clinics advertise it as if you can.”
Clinics often sell ‘pricey placebo effect’
Quick financial gain is often the primary motivator behind this nefarious practice, he said.
“Sometimes the clinic operators receive letters from regulatory agencies and then stop and switch to something else,” Ikonomou said. “There is a lot of movement in and out of this marketplace.”
Some musculoskeletal conditions, including unproven treatments for pain, are often offered in academic centers as a kind of auxiliary treatment to other types of treatments, he said.
“The mentality is that it won’t hurt and it may help,” he said. “The motive is a bit murkier than the private clinics.”
Ikonomou pointed out it isn’t just people diagnosed with serious illnesses or those in pain who are seeking therapy; clinics also target individuals wanting to just feel better, as part of the “total wellness” trend.
“It’s very expensive treatment,” he said. “Even if it’s safe, there’s no evidence of any efficacy. Most of the time, it’s just a very pricey placebo effect.”
Aggressive marketing tactics in sunbelt states
As an experiment, Ikonomou put his name on the mailing list of one clinic, saying he wanted to sign up for the newsletter.
“Within minutes,” he said, “I started getting calls.”
The practice mirrors that of predatory loans.
“Some people say it’s a buyer-beware situation,” he said. “I don’t agree with that. We don’t espouse that approach for tobacco products or predatory lending. There is regulatory action, legislation, and push back. We should follow the same approach with these businesses.”
Consumers in some states experience more aggressive marketing from these clinics than others. The worst offenders are primarily found in California, Florida, Texas and Arizona, according to published studies, Ikonomou said.
“There is a heavy concentration of retirees in those states, but it’s more complicated than that,” he said. “More research is needed to find out why.”
Not an easy fix
There is no easy solution to this situation, Ikonomou noted. Even if a patient is harmed, the clinics are not bound to report it. Also, it’s hard to prove causality, especially if enough time has passed, and some businesses require patients to sign non-disclosure agreements.
While the FDA and Federal Trade Commission (FTC) have issued numerous warnings to the offending clinics, the U.S. marketplace has become way too big, and it’s hard to contain, he said.
“Even if they’re threatened with lawsuits, the clinics get their own legal representation and fight back,” he said. “This can take years.”
This is why creating the consumer guide was so important.
“We’re trying to make this a starting point to reach out to patients and their families, so that they can make informed decisions,” he said. “It’s important to reach out to health practitioners, too, because they can also inform patients of these practices and warn them.”
Media Contact Information
Laurie Kaiser News Content Director Dental Medicine, Pharmacy Tel: 716-645-4655 [email protected]
Read the latest in your favorite channels.

Take UB With You. Wherever.

Case Study: Gene Therapy for Enhancement Purposes
Dr. Anderson specializes in a particular type of gene therapy that targets Alzheimer’s Disease (AD).  Neural degeneration and synapse loss in the brain are characteristic of AD. Therefore, this gene therapy aims to protect neurons from degeneration and enhance the function of any neurons that are remaining. Dr. Anderson has two patients request her services. However, after an initial meeting with them, she is unsure whether she should treat them both.
Alexis is a 50 year-old woman who has a family history of AD and is already beginning to experience very mild symptoms of what she thinks is AD. She tells Dr. Anderson that her mother was afflicted with AD. So, she knows first-hand the sadness and frustration the family of an AD patient has to experience. Alexis has a husband and three children and does not want to put them through the same difficult journey. Therefore, she is requesting the gene therapy to reverse the small-scale symptoms she already has and prevent the onset of the disease.
Kelly is a 21 year-old college student who is applying for medical school in the very near future. Her academic history is strong but not exceptional. For this reason, Kelly fears that she will not be accepted to the top medical schools. Kelly wants to attend medical school so she can help underserved populations and work in impoverished areas that lack good healthcare. She tells Dr. Anderson that she would like to receive the Alzheimer’s gene therapy in hopes it will boost her memory and enhance neural function. Kelly believes a good score on the MCAT will strengthen her application and enable her to fulfill her dream of providing medical aid to the world’s neediest people.
Dr. Anderson decides to treat Alexis, as she feels that Alexis is the type of patient that the therapy is designed for. However, she conflicted about offering the treatment for Kelly. She doesn’t like the idea of withholding medical treatment from a patient, but the treatment was not originally intended for enhancement purposes.
Should Dr. Anderson treat Kelly?
- Yes. It is not the role of a doctor to make value judgments on who should and should not receive treatment. Ultimately, treating Kelly will benefit mankind when she becomes a doctor
- No. The treatment was designed to help patients that have AD to regain their normal function. Regardless of the reason, gene therapy should not be used for enhancement purposes.
View Results
Safeguard your viral vector tech transfer: considerations and case studies

Uncover common challenges and considerations for seamless viral vector tech transfer and gain valuable insights gleaned through more than two decades of gene therapy CDMO experience.
Learn how offering phase-appropriate modular and fast track technology transfer frameworks can safeguard viral vector supply and minimize the need for comparability studies, leading to more or better quality product to facilitate program continuity.
Whatever stage of development you’re in, set your manufacturing strategy up for success and learn:
- Tech transfer approaches to bolster your program
- MSAT best practices and considerations to avoid roadblocks
- Real-world case study examples

James has worked in the viral vector CDMO space since 2018 and is currently a member of Charles River’s gene therapy CDMO business development team. James obtained a PhD and completed postdoctoral training in cancer gene therapy at the University of Alabama at Birmingham (UAB), studying oncolytic viruses. Afterward, he worked as a research scientist in virology, cell biology, cancer, and parasitology. Based at the viral vector manufacturing site in Rockville, MD, James provides technical support to the business development team, helping to guide client discussions and onboard new projects by collaborating with subject matter experts across various functional groups.
You might also like

Current limitations & potential innovations in developing IND-enabling rAAV- & lentivirus-based gene therapy drug products
- {{get this @root.labelField}}
Please note all fields marked with an * are mandatory
To personalise your emails with journal updates relevant to you, please use the tick boxes below to select journals of interest:
To further personalise your emails with interest updates relevant to you, please use the tick boxes below to select areas of interest:
If you have any problem accessing our content, please email [email protected]
Welcome back to Bioinsights, please log in to access this content
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- INNOVATIONS IN
- 26 October 2021
The Quest to Overcome Gene Therapy’s Failures
- Tanya Lewis 0
Tanya Lewis is senior editor for health and medicine at Scientific American .
You can also search for this author in PubMed Google Scholar

Illustration by Luisa Jung
Audrey was six months old when her parents first noticed something wasn’t right. Without warning, her body stiffened, and her eyes rolled into the corners of their sockets for hours at a time. Despite visits to multiple specialists, no one knew what was wrong. Her doctors prescribed seizure medication—lots of it—which sedated her but did not stop the eye-rolling. Finally, they confessed that they did not know how to help and sent Audrey and her parents home with a handful of pamphlets about living with a disability.
Genetic tests later diagnosed Audrey with a condition known as aromatic l -amino acid decarboxylase (AADC) deficiency, caused by mutations in a single gene. The extremely rare disorder manifests in infancy and lowers the activity of AADC, an enzyme that is critical for making the brain-signaling chemicals dopamine and serotonin. It causes severe developmental and motor disabilities, as well as sleep and mood problems. Most children with the condition are unable to talk, sit up or support their own weight.
After years of frustration, Audrey’s parents enrolled her in a clinical trial led by Krystof Bankiewicz, a professor of neurosurgery at the University of California, San Francisco, and the Ohio State University College of Medicine. The gene therapy Bankiewicz and his colleagues were testing uses a harmless virus as a vector to introduce an intact version of the gene responsible for making the AADC enzyme. Seven children participated in the trial. The researchers injected the virus directly into each child’s brain near neurons they hoped would start making AADC and, subsequently, dopamine.
Part of Innovations In Gene Therapy
The children ranged in age from four through nine years old at the beginning of the trial (Audrey was six at the time). The results were dramatic: by three months after surgery, six of the seven children stopped having oculogyric crises—the distinctive eye-rolling that is a hallmark of the disease. The seventh child also improved initially but died seven months later from complications of the disease itself, Bankiewicz says. A year postsurgery all six surviving children could control their heads normally, and four could sit independently. After a year and a half, Audrey and one other child were walking with hand support and learning to use muscles they had previously been unable to command. So far none of the children has shown any serious side effects.
Outcomes like Audrey’s would not have been possible without decades of research and patients who volunteered for experimental treatments, knowing they could be risking their lives, to help move gene therapy forward. Serious side effects, some deadly, threatened to derail the field in its early years, prompting researchers to step back and reconsider their approach. Convinced of the promise of genetic cures and of the potential to find safer, more precise gene delivery methods, they persisted.
Since then, gene therapy has yielded some notable successes . Yet the quest to control side effects is far from over. As in any pioneering field of medical science, researchers must strike a balance between advancing knowledge that could help cure devastating diseases and proceeding with caution to protect patients.
First Do No Harm
Jesse Gelsinger was 18 years old in 1999, when he joined one of the first clinical trials of gene therapy . Gelsinger suffered from an inherited genetic disorder called ornithine transcarbamylase (OTC) deficiency, which causes toxic levels of ammonia to build up in the blood. Untreated, that buildup can lead to vomiting, lethargy and, in severe cases, death. The condition affects up to one in 50,000 infants and is caused by mutations in the OTC gene. Standard treatment for the condition involves a restricted diet and supplementation known as alternative pathway therapy. Gelsinger was being treated for the condition and had a mild case, but he occasionally experienced episodes of high ammonia levels, known as hyperammonemia, once even slipping into a coma.
The gene therapy trial he enrolled in used a type of cold virus known as an adenovirus that had been engineered to deliver a working version of the OTC gene to his liver cells. Gelsinger was one of two participants receiving the highest dose. Within days of the treatment, however, his condition declined rapidly. His body launched a severe inflammatory response that led to organ failure and, ultimately, brain death.
A few years later several children who had been treated with gene therapy for a severe immune disease developed cancer.
Research funding dried up, and many investigators abandoned the field. But those who remained began to make improvements in both the safety and the efficacy of viral vectors. They also began exploring a gene-editing method called CRISPR, which could enable more targeted therapies but came with a new set of risks.
Gene therapy has come a long way since Gelsinger died—Audrey is living proof of that. Yet researchers remain vigilant about the specter of side effects. “We’re in a very different place now,” says Mark Batshaw, the physician who helped to lead the trial involving Gelsinger more than 20 years ago. “We know a lot more about vectors. We know a lot more about the immunity that is associated with that. And I think there’s a lot more care.”
After Gelsinger’s death, the U.S. Food and Drug Administration banned James Wilson, the scientist whose laboratory developed the therapy Gelsinger received, and his institution, the University of Pennsylvania’s Institute for Human Gene Therapy, from conducting human trials for at least five years. An FDA notice cited repeated and deliberate violations of the trial protocol for an investigational drug. The agency suspended all research at Wilson’s institute, too.
But it was not the end for gene therapy or for Wilson’s career. “There was a precipitous decline in enthusiasm in supporting the field,” Wilson says. Nevertheless, “there were a few of us who continued to work on gene therapy,” he says. “We pivoted from clinical applications to basic science around the delivery of genes.” Wilson and his colleagues turned back to the lab bench to understand what went wrong in Gelsinger’s death. Their best hypothesis is that he had antibodies to adenovirus from a previous exposure to the virus and that these relics of a former infection supercharged his immune system’s response to the adenovirus vector.
Wilson and other researchers took a hard look at the issue of side effects and how to minimize them. Because the viral vector seemed to be the biggest risk, they switched to adeno-associated viruses (AAVs), which proved far safer. Today AAVs are used in numerous therapies, including an approved drug for spinal muscular atrophy. “I’m glad we stayed with it,” Wilson says.

Audrey, with her mother, Carrie, three years after her successful gene therapy. Credit: Timothy Archibald
Learning from Failure
Around the same time as the Gelsinger trial, scientists in France and England were working on a therapy for severe combined immunodeficiency syndrome (SCID), a genetic condition that affects at least one in 50,000 babies. It is sometimes referred to as “bubble boy disease” because those afflicted with it, primarily boys, are born without an immune system and must live in isolated, sterile environments to keep from getting sick. It can be cured with a bone marrow transplant from a matched donor, but only about a quarter of affected children find such a match. Without treatment, children with SCID usually die within the first year of life.
For their vector, the researchers turned to a group of viruses called gammaretroviruses because they believed them to be efficient at delivering genetic material to cells. In a pair of clinical trials, they targeted a form of SCID that is passed down from a mother to her baby on an X chromosome, known as SCID-X1. It is caused by errors in a gene that encodes a protein called IL2RG. In both trials, the patients’ own bone marrow stem cells were collected and isolated. The researchers used a gammaretroviral vector to insert a working copy of the IL2RG gene into them, then reinfused the modified cells. Initially the therapy appeared somewhat successful: most of the 10 children who were treated started producing functional T cells—an important component of a working immune system. But within three to six years half the subjects developed leukemia, and one died. The viral vectors are believed to have activated a known cancer-causing gene. The FDA halted all U.S. trials involving a retroviral vector aimed at modifying bone marrow stem cells.
“Our knowledge came from animal models,” says Marina Cavazzana, a pediatrician and hematologist at Paris Descartes University’s Necker Hospital, who wrote the clinical protocol and handled patient follow-up for the clinical trial in France. The problem, she says, is that the animal models were unable to predict human toxicity. “I stopped the clinical trial, we came back to the bench, and we tried to explain the reason for these side effects. And we came back again to the clinic,” she says.
David Williams, chief of hematology and oncology at Boston Children’s Hospital, was involved in those early SCID trials. “In the end,” he says of both the SCID trials and Gelsinger’s trial, “you have to try these things in human beings to completely understand the benefits versus the risks.”
When Williams and his colleagues resumed their work on SCID a decade later, they created a modified version of their gammaretrovirus to avoid activating cancer-causing oncogenes. It still prompted the development of just one type of immune cell, however, and recipients required continued intravenous injections to maintain production. But nearly a decade later none of the subjects has shown signs of leukemia or other side effects.
It was yet another viral vector that helped to push the SCID effort across the finish line. In 2016 a team led by Ewelina Mamcarz, a bone marrow transplant specialist at St. Jude Children’s Research Hospital in Memphis, launched a trial for SCID-X1 using a lentivirus (a virus related to HIV) as a vector. Researchers built a “firewall” into it that would prevent the activation of any parts of the genome that might cause leukemia. Mamcarz and her colleagues also pretreated patients with chemotherapy to make room for the modified bone marrow stem cells.
Mamcarz’s team has treated a total of 18 infants with this gene therapy. To date, about five years post-treatment, none has developed leukemia. “We are hopeful we’re kind of out of the woods now, but we will continue to monitor patients closely,” Mamcarz says. “My anxiety level was much higher when we started [the trial] because there was so much unknown,” she says. “I think I can sleep at peace now, years into this gene therapy in infants, but we never rest.”
Concerns about gene therapy’s side effects have also been front of mind for researchers working on other conditions. Sickle cell disease, which affects about 300,000 infants born every year and occurs more commonly among people of African descent, has long been a prime target for gene therapy because it, too, is caused by a single-gene defect. This condition causes red blood cells to take on a sickle shape and clump together, making them unable to transport oxygen efficiently. People with the disease experience debilitating pain crises, strokes and other problems, and it can be fatal. Although treatments exist, the only cure is a risky bone marrow or stem cell transplant.
Bluebird Bio, a biotech company in Cambridge, Mass., reported promising results from a clinical trial of its sickle cell gene therapy in late 2020. Nineteen patients were treated with a lentiviral vector containing a working version of the gene that encodes a component of adult hemoglobin—all 19 stopped having severe pain crises within six months. But more than five years later two patients in a different cohort developed a rare blood cancer called acute myeloid leukemia.
The FDA placed a clinical hold on the Bluebird Bio study, as well as several similar trials, while the company investigated these cases. Bluebird Bio’s own investigation found that the leukemia was unlikely to be related to gene therapy. According to Rich Colvin, Bluebird Bio’s chief medical officer, in one of the cases the viral vector was not found in the cancer cells, and in the other, viral DNA was present but had not integrated into any gene known to be involved in leukemia development. In June 2021 the FDA lifted its hold on the trials, which have since resumed.
Bluebird Bio is also testing a gene therapy for patients with X-linked adrenoleukodystrophy (ALD), a devastating disease that primarily affects boys and gives them only a five- to 10-year life expectancy. In that trial, one of the 67 patients developed myelodysplastic syndrome, a condition that can lead to leukemia, and this time it was found to be related to the viral vector. The FDA has now placed the trial on hold. Colvin says the benefits of the therapy still outweigh the risk of ALD, which would have proved fatal. But he knows it is a delicate balance: “I think you have to have humility when you’re manipulating the human genome.”
Risk Vs. Benefit
Viral vectors, by their very nature, can insert themselves into an undesired part of the target cell’s genome. But newer technology is enabling much more precise edits to a gene. The CRISPR technique is already being used in some gene therapies. Although there is a potential risk of so-called off-target effects on other parts of the genome, these have not been observed in the early clinical trials.
In a trial sponsored by Cambridge, Mass.–based CRISPR Therapeutics and Boston-based Vertex Pharmaceuticals involving CRISPR gene therapy, two patients with sickle cell disease and 20 patients with a related condition called beta thalassemia saw near-complete improvement of their symptoms, according to unpublished data. Although longer-term follow-up is needed, David Altshuler, chief scientific officer at Vertex Pharmaceuticals, calls the results a “medical and scientific milestone.”
With all new therapies, the risk of side effects must be considered in the context of the diseases being treated. A condition such as AADC deficiency can be fatal, and Audrey’s mother, Carrie, knew that when she enrolled her daughter in the U.C.S.F. clinical trial. She was desperate and figured any improvement would be better than the status quo.
Three years after enrolling Audrey in the trial, Carrie says that her daughter is a “totally different kid.” She doesn’t have the eye-rolling anymore. She is learning to eat food by mouth and to speak some words. Thanks to her talking device—a touch-pad machine that allows her to activate spoken, computer-generated phrases—she can communicate. Carrie says that before the treatment, her daughter could understand what people were saying, but she could not express herself. “Now she can just really speak her mind,” Carrie says.
Audrey continues to struggle with some things, including balance and speech. But her life today is far from what it might otherwise have looked like. And in that one gene therapy’s success, Carrie says, other families can find hope. “If we don’t do it, we know the end result,” she says. But “if it can do anything, even a little bit, it’s already a win.”
doi: https://doi.org/10.1038/d41586-021-02734-w
This article is part of Innovations In Gene Therapy , an editorially independent supplement produced with the financial support of third parties. About this content .
Related Articles

- Gene therapy

Make gene therapies more available by manufacturing them in lower-income nations
Comment 17 JUL 24
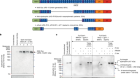
Split intein-mediated protein trans-splicing to express large dystrophins
Article 17 JUL 24

Scientists edit the genes of gut bacteria in living mice
News 10 JUL 24
Lecturer/Senior Lecturer at the Dyson School of Design Engineering
About the role: Do you want to change the world for the better, and do you believe this can be done through research and education? If so, grab you...
South Kensington, London (Greater) (GB)
Imperial College London (ICL)
Faculty Positions in Westlake University
Founded in 2018, Westlake University is a new type of non-profit research-oriented university in Hangzhou, China, supported by public a...
Hangzhou, Zhejiang, China
Westlake University
Call for Global Talents, Recruitment Information of Nankai University
Nankai University welcomes global outstanding talents to join for common development.
Tianjin, China
Nankai University
Director for Signature Research Programme in Cancer and Stem Cell Biology, DukeNUS Medical School &
The successful candidate will demonstrate their ability of growing, leading and mentoring faculty as well as developing and operationalising strategy.
Singapore (SG)
DukeNUS Medical School
Scientist / Postdoc (m/f/d): Analysis of Microscopic BIOMedical Images (AMBIOM)
A new project area in the institute is the development of artificial intelligence (AI)
Dortmund, Nordrhein-Westfalen (DE)
Leibniz-Institut für Analytische Wissenschaften – ISAS – e.V.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Interactive Resources for Schools
- Page 1 - Genetic engineering
- Page 2 - What is genetic engineering?
- Page 3 - How does genetic engineering work?
- Page 4 - Ways of moving genes
- Page 5 - CRISPR-Cas9: a game-changer
- Page 6 - Genetic engineering: hopes and fears I
- Page 7 - Genetic engineering: hopes and fears II
- Page 8 - Transgenic plants – food for the future
- Page 9 - Gene therapy – case studies
- Page 10 - Gene silencing
- Page 11 - Recombinant hormones
- Page 12 - Minimising the risks of genetic engineering
- Page 13 - Ethics, laws and religion
- Page 14 - Activity: genetic engineering in the media
- Page 15 - Activity: genetic engineering in commercial agriculture
Help and information
This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
Glossary: Any word with a glossary entry is highlighted like this. Moving the mouse over the highlighted word will show a definition of that word.
Animations: This topic has features with which you can interact, these are usually animations. Most of the animations can be expanded to full screen size, on a new window, ideal for showing on an interactive whiteboard. The animations will play all the way through or can be viewed one section at a time.
Downloads: Teachers can download individual diagrams, animations and other content from the Download Library area of the website. Terms and Conditions apply.
Activity: We propose an activity to help the students further consolidate their understanding of the topic.
Topic last updated: 18 Nov 2021
You may also be interested in these related topics, biotechnology, genes and inheritance, genetic engineering, gene therapy – case studies, this page is recommended for 16+ students. 14-16 might consider this page as a stretch & challenge , as it is quite advanced..
SCID – also known as severe combined immunodeficiency – is a very rare genetic disorder which only affects between 1 in 50,000 and 1 in 100,000 births. Children born with SCID do not have an effective immune system , so they are extremely vulnerable to any form of infection. In many instances, all of the problems result from a single defective gene coding for the enzyme adenosine deaminase. Boys are more often affected than girls because at least one form of the disease is sex-linked (carried on the X chromosome ).
In the past, the only way of keeping these children alive was to bring them up in a completely sterile environment, with all their food, water and air sterilised and with no direct contact with other people. Even then, affected children rarely lived into their teens as the slightest contamination could kill them.
Another alternative is a bone marrow transplant if a suitable donor can be found. Although the affected child has no immune system to cause rejection , the transplanted marrow can attack the patient’s cells. What is more, the donor cells may be infected with a virus – and this can kill the recipient very quickly. Patients can also be regularly injected with the enzyme they need, but this involves a lifetime of carefully managed therapy.

Life for children with SCID without treatment is very limited.
So gene therapy , inserting a healthy gene into the DNA using a vector such as a specially modified virus, offers the exciting possibility of a normal life for children who otherwise have a limited life expectancy and relatively poor quality of life.
The first ever attempts at gene therapy were carried out on children with SCID. Different variations of the technique were tried on children in several countries, including Britain. The trials had considerable success – the children treated all developed functioning immune system s which enabled them to fight off infections and to make antibodies when they were given vaccines. They could leave hospital, and their sterile environments, and live normal lives.
Then came the news that, about 3 years after their treatment, first one and then two of the nine children with SCID treated successfully using gene therapy in France developed leukaemia -like symptoms. They responded well to chemotherapy , but both the French and the American governments halted trials of gene therapy for SCID until more was known about why these boys fell ill and whether it was linked to the gene therapy.
The UK government decided differently, feeling that the potential benefits outweighed the possible risks. This view was backed up both by doctors carrying out the therapy at Great Ormond Street Hospital and by the mother of Rhys Evans, the first British boy to be given gene therapy. He received the treatment in 2001, when he was an infant, and he is now a healthy young man, enjoying normal life with a functioning immune system. Great Ormond Street has had many success stories treating this extremely rare condition with gene therapy. They are now considering ways to use the same techniques to tackle other genetic diseases.
Professor Nevin, who chaired the UK committee which made the decision that work should continue commented: "As with all innovative treatments, there will always be the potential for side-effects."
Dr Bobby Gaspar of Great Ormond Street Hospital said: "If we stop these studies now we will be denying extremely effective therapy to children and they may suffer as a result of not receiving this therapy. Ethically we believe it is the right thing to go on."
Marie Evans, the mother of Rhys who has undergone the treatment, also had an opinion.
"If they stop something just because one child has an adverse effect at the end of the day medicine and the world just doesn't go on," she said.
Gene therapy isn’t suitable for all patients, but at Great Ormond Street a number of children have now been successfully treated, without developing leukaemia, and trials into other uses of the technology are underway.
Sickle cell disease
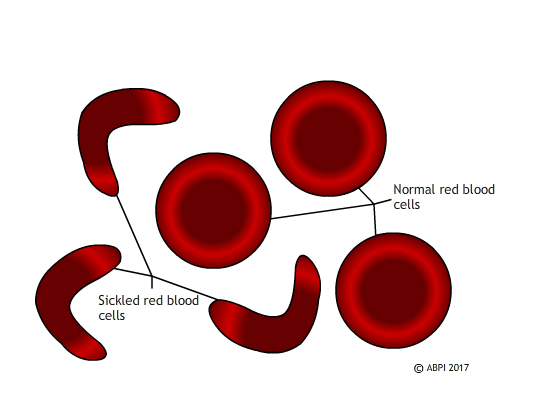
Sickled red blood cells do not carry oxygen effectively and they block small blood vessels. Gene therapy holds out the hope of dealing with both problems in one solution.
Unlike SCID, which is extremely rare, sickle cell disease affects millions of people around the world. In sickle cell disease, a mutation in a single gene affects the formation of one of the two types of protein chain which make up haemoglobin . This changes the shape of the haemoglobin molecule and reduces its ability to carry oxygen. The mutated haemoglobin also makes the red blood cells take on a sickle shape instead of the normal biconcave discs. These sickled red blood cells tend to stick together. They block small blood vessels , causing terrible pain and often tissue damage as well. People who are affected need regular blood transfusions, and often strong painkillers. Bone marrow transplant s can treat the disease, but only about 10% of the millions of people affected globally ever find a matching donor. Ultimately - and especially if untreated – sickle cell disease can kill.
In 2017 French scientists announced that they had reversed the progress of sickle cell disease in a teenage boy, by genetic modification of his bone marrow. The boy was very severely affected. By 13 he had had his spleen removed and his hips replaced, and he needed opioid painkillers to deal with the pain. Scientists took bone marrow stem cell s, genetically modified them using a viral vector so they could make functioning haemoglobin, and replaced the stem cells in the patient. For 15 months the boy has been making normal haemoglobin, and his red blood cells have functioned perfectly normally. He does not need transfusions or painkillers.
Scientists are always wary of claiming to have found a cure – and this patient is the first to succeed in the clinical trials. It will require many more years of testing – and success in other patients – before the procedure can be declared a complete success but this appears to be a major step forward. Seven other patients have been treated by the French team and they are also showing promising progress.
This is a very exciting development which could potentially help huge numbers of people – for example, 100,000 people are affected by sickle cell disease in the US alone. However, it also raises some ethical questions. The majority of people affected by sickle cell disease live in relatively poor countries, with limited health infrastructure. They do not have the resources to offer gene therapy to everyone – or even a minority – of the people affected. So at the moment, even if gene therapy does provide a cure for sickle cell disease, it will be a cure which is only available to affected people in the richer countries of the world. Perhaps as gene editing becomes more common and more successful it will become easier and cheaper and therefore available globally.
Perhaps we will need to find other ways of treating this and other genetic diseases. Whatever the future holds, we need to consider both the science and the ethics of the treatments we develop.
You can find out more about SCID and the use of gene therapy here: Treating the bubble babies: gene therapy in use, Your Genome Severe combined immunodeficiency , Great Ormond Street Hospital for Children Gene therapy success, Great Ormond Street Hospital for Children
Find out more about gene therapy and sickle cell disease here: Gene therapy ‘cures’ boy of blood disease that affects millions, New Scientist Teenager’s sickle cell reversed with world-first therapy, BBC News website
Muscular dystrophy – the importance of animal models
Duchenne muscular dystrophy (DMD) is the most severe form of muscular dystrophy. It affects about one in every 3500 boys who are born – about 100 boys a year in the UK. It is a sex-linked genetic condition which means the boys cannot make a protein called dystrophin, a protein vitally important for maintaining healthy muscles. Without it the muscles weaken and waste away, being replaced by fat, so that by their early teens most affected boys are confined to a wheelchair and their life expectancy is only to early adulthood.
The faulty gene is very large, which makes normal gene therapy techniques difficult. However researchers in the United States and in Britain have found ways of using parts of a healthy gene, called mini-genes, to repair the damaged DNA, enabling the muscles to produce dystrophin and to function in a much more normal way. What is more, the effect has been long term – the protein was still being made a year after the gene was inserted. The only problem is that the gene therapy technique has so far only been tried in mice and golden retrievers, which have a natural mutation similar to muscular dystrophy .
Much of this research depends on knockout mice. To produce knockout mice researchers genetically modify some embryo nic stem cell s to inactivate or ‘knock out’ a healthy gene. These cells are then injected into mouse embryos which are then implanted into a surrogate mother. The mice which result have some knockout cells and some normal cells, and they are then implanted to produce homozygous knockout mice.
Knockout mice often show changes in their phenotype which mimic human genetic problems , helping scientists understand exactly what the gene does.
Knockout mice are also useful for studying the impact of different therapies. We have many of our genes in common – of 4000 genes studied in mice and humans, only ten of them are found in one species but not in the other. This, along with the fact that mice reproduce rapidly, have large litters, and are easy and cheap to keep means that knockout mice are incredibly useful in our search to understand gene functions and to find cures for many diseases.
The problem with the mdx mice (a popular model for studying DMD) is that they only display relatively mild symptoms. Several breeds of domestic dog have also been found to have a natural mutation in the dystrophin gene and some work has been done on golden retrievers. Dogs are not ideal laboratory animals for many reasons – they are intelligent and emotive, they are not easy to manipulate genetically, and they take time and effort to breed. However, dogs affected by the canine form of Duchenne muscular dystrophy do have symptoms which are very similar to humans. Now a team at the Royal Veterinary College have discovered a line of King Charles spaniels which appear to have the same mutation in the same gene as humans. A research project began in 2015 looking at the progression of the disease in this breed of dog. This may in future lead to improved therapies for humans and dogs alike.

Many of the current trials on possible treatments for DMD still involve the use of medicines to alleviate symptoms, but there have been some promising results recently with genetic modification in both mice and dogs. A few phase 1 human clinical trials are in progress and more are expected soon. Some scientists are attempting to replace small regions of the faulty gene, others are trying to replace the whole thing. Gene therapy has not yet been fully successful in overcoming any genetic diseases, so any patients who take part in early trials of a possible new treatment – and their parents – are very brave. New technologies such as CRISPR-Cas9 hold out hope for new therapies including editing muscle-forming stem cell s rather than trying to change the whole organism. There is a long way to go, but muscular dystrophy is another disease where gene therapy may eventually result in a treatment or even a cure.
See: Knockout Mice Fact Sheet, National Human Genome Research Institute Why Mouse Matters, 2000 Mouse Sequencing Consortium, National Human Genome Research Institute A new animal model of Duchenne muscular dystrophy, Muscular Dystrophy UK
Discussion Point
Animals are frequently used in scientific research.
What are some arguments for and against this?
Transgenic plants – food for the future
Gene silencing.

IMAGES
COMMENTS
University at Buffalo College of Arts and Sciences. BUFFALO, N.Y. — A $1.5 million grant has been awarded to University at Buffalo researcher Soo-Kyung Lee to study the rare neurodevelopmental disorder FOXG1 Syndrome. Lee, Empire Innovation Professor and Om P. Bahl Endowed Professor in the Department of Biological Sciences, received the award ...
A viral gene therapy developed by UB researchers has reversed some brain abnormalities in infant mice with FOXG1 syndrome, a significant step toward one day treating children with this severe neurodevelopmental disorder. This mediated delivery of the FOXG1 gene via adeno-associated virus 9 (AAV9) is detailed in a study published June 5 in ...
This mediated delivery of the FOXG1 gene via adeno-associated virus 9 (AAV9) is detailed in a study published June 5 in Molecular Therapy Methods & Clinical Development. A postnatal injection of the therapy in day-old mice rescued a wide range of abnormalities, the study found, including in parts of the brain responsible for language, memory ...
This article was originally published with the title " Four Success Stories in Gene Therapy " in Scientific American Magazine Vol. 325 No. 5 (November 2021) doi:10.1038 ...
Soo-Kyung and Jae Lee are the co-lead authors of a new study that suggests a promising viral gene therapy for FOXG1 syndrome, a severe neurodevelopmental disorder. ... According to the study, the therapy normalized the number of OPCs while restoring myelination. ... Graduate School of Education 367 Baldy Hall Buffalo, NY 14260-1000 716-645-2110 ...
Gene therapy has made inroads against cancer, too. ... helped to develop the therapy and published the first successful results in a 2010 study for the treatment of lymphoma. ... In SMA's case, ...
Gene Therapy - Successes and challenges in clinical gene therapy. ... Smith B, Nayak S, et al. B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study ...
Documented in studies of gene therapy for X-linked SCID, 1,2 Wiskott-Aldrich ... as is the case for other classes of therapeutics. 76 The other development is the issuance in 2018 of six ...
Metrics. Gene therapy is at an inflection point. Recent successes in genetic medicine have paved the path for a broader second wave of therapies and laid the foundation for next-generation ...
Ten patients received one of four FLT180a doses of vector genomes (vg) per kilogram of body weight: 3.84×10 11 vg, 6.40×10 11 vg, 8.32×10 11 vg, or 1.28×10 12 vg. After receiving the infusion ...
Introduction. Gene therapy offers a novel approach to treating monogenic diseases that, rather than only treating symptoms, targets the root cause of a disease by introducing a vector coding for a gene that compensates for a mutated or absent gene. 1 The pathological consequences of a disease may be prevented or substantially delayed after only a single gene therapy treatment. 2, 3, 4 Because ...
BUFFALO, N.Y. — A viral gene therapy developed by University at Buffalo researchers has reversed some brain abnormalities in infant mice with FOXG1 syndrome, a significant step toward one day treating children with this severe neurodevelopmental disorder. This mediated delivery of the FOXG1 gene via adeno-associated virus 9 (AAV9) is detailed ...
Gene therapy approaches for CF. Gene therapy offers great hope for the treatment of genetic diseases/disorders. By replacing the genetic mutation with a "correct version" of the CFTR gene, this method offers a potentially permanent cure. Indeed, since the discovery of the CF gene, many studies have attempted to correct the CFTR mutations
GENE THERAPY Success Stories Gene therapy is beginning to fulfill its potential. Four therapies offer a glimpse of what's to come By Jim Daley After numerous setbacks at the turn of the century, gene therapy is treating diseases ranging from neuromuscular disorders to cancer to blindness. The success is often qualified, however. Some of these ...
Abstract. Cancer is the second leading cause of death in the Western world. The limited successes of available treatments for cancer mean that new strategies need to be developed. The possibility of modifying the cancer cell with the introduction of genetic material opens the way to a new approach based on gene therapy.
Toggle Navigation Menu. 12/18/23 About. 11/22/23 Academics
BUFFALO, N.Y. — While stem cell therapy has been used to successfully generate and repair tissues that have been damaged due to certain conditions and diseases, such as leukemia, it is far from a cure-all. ... Committee on the Ethics of Cell and Gene Therapy (ECGT), which is composed of academic researchers, clinicians and regulatory policy ...
Case Study: Gene Therapy for Enhancement Purposes. Dr. Anderson specializes in a particular type of gene therapy that targets Alzheimer’s Disease (AD).  Neural degeneration and synapse loss in the brain are characteristic of AD. Therefore, this gene therapy aims to protect neurons from degeneration and enhance the function of any ...
On 16 February 2021, Bluebird Bio suspended phase 1/2 and phase 3 clinical trials of its LentiGlobin gene therapy for sickle-cell disease after two patients were diagnosed with cancer, 5 years ...
Background and Problem. Allucent's client requested support to bring an AAV gene therapy for a rare neurodegenerative disease into the clinic for a Phase 1b study in patients. Dose scaling from pharmacology and toxicology studies by brain volume alone for administration directly into the brain by MRI-Guided Convection-Enhanced Delivery led to ...
James has worked in the viral vector CDMO space since 2018 and is currently a member of Charles River's gene therapy CDMO business development team. James obtained a PhD and completed postdoctoral training in cancer gene therapy at the University of Alabama at Birmingham (UAB), studying oncolytic viruses.
Tanya Lewis. Illustration by Luisa Jung. Audrey was six months old when her parents first noticed something wasn't right. Without warning, her body stiffened, and her eyes rolled into the ...
Gene therapy gene therapy. A new, experimental method of fighting disease by replacing a defective gene with a healthy gene. has not yet been fully successful in overcoming any genetic diseases, so any patients who take part in early trials of a possible new treatment - and their parents - are very brave.