- Open access
- Published: 08 May 2014

Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity
- Deepak Bhardwaj 1 ,
- Mohammad Wahid Ansari 1 ,
- Ranjan Kumar Sahoo 1 &
- Narendra Tuteja 1
Microbial Cell Factories volume 13 , Article number: 66 ( 2014 ) Cite this article
101k Accesses
639 Citations
24 Altmetric
Metrics details
Current soil management strategies are mainly dependent on inorganic chemical-based fertilizers, which caused a serious threat to human health and environment. The exploitation of beneficial microbes as a biofertilizer has become paramount importance in agriculture sector for their potential role in food safety and sustainable crop production. The eco-friendly approaches inspire a wide range of application of plant growth promoting rhizobacteria (PGPRs), endo- and ectomycorrhizal fungi, cyanobacteria and many other useful microscopic organisms led to improved nutrient uptake, plant growth and plant tolerance to abiotic and biotic stress. The present review highlighted biofertilizers mediated crops functional traits such as plant growth and productivity, nutrient profile, plant defense and protection with special emphasis to its function to trigger various growth- and defense-related genes in signaling network of cellular pathways to cause cellular response and thereby crop improvement. The knowledge gained from the literature appraised herein will help us to understand the physiological bases of biofertlizers towards sustainable agriculture in reducing problems associated with the use of chemicals fertilizers.
Introduction
Conventional agriculture plays a significant role in meeting the food demands of a growing human population, which has also led to an increasing dependence on chemical Fertilizers and pesticides [ 1 ]. Chemical fertilizers are industrially manipulated, substances composed of known quantities of nitrogen, phosphorus and potassium, and their exploitation causes air and ground water pollution by eutrophication of water bodies [ 2 ]. In this regard, recent efforts have been channelized more towards the production of ‘nutrient rich high quality food’ in sustainable comportment to ensure bio-safety. The innovative view of farm production attracts the growing demand of biological based organic fertilizers exclusive of alternative to agro-chemicals [ 3 ]. In agriculture, encourage alternate means of soil fertilization relies on organic inputs to improve nutrient supply and conserve the field management [ 4 ]. Organic farming is one of such strategies that not only ensures food safety but also adds to the biodiversity of soil [ 5 ]. The additional advantages of biofertilizers include longer shelf life causing no adverse effects to ecosystem [ 6 ].
Organic farming is mostly dependent on the natural microflora of the soil which constitutes all kinds of useful bacteria and fungi including the arbuscular mycorrhiza fungi (AMF) called plant growth promoting rhizobacteria (PGPR). Biofertilizers keep the soil environment rich in all kinds of micro- and macro-nutrients via nitrogen fixation, phosphate and potassium solubalisation or mineralization, release of plant growth regulating substances, production of antibiotics and biodegradation of organic matter in the soil [ 7 ]. When biofertilizers are applied as seed or soil inoculants, they multiply and participate in nutrient cycling and benefit crop productivity [ 8 ]. In general, 60% to 90% of the total applied fertilizer is lost and the remaining 10% to 40% is taken up by plants. In this regard, microbial inoculants have paramount significance in integrated nutrient management systems to sustain agricultural productivity and healthy environment [ 9 ]. The PGPR or co-inoculants of PGPR and AMF can advance the nutrient use efficiency of fertilizers. A synergistic interaction of PGPR and AMF was better suited to 70% fertilizer plus AMF and PGPR for P uptake. Similar trend were also reflected in N uptake on a whole-tissue basis which shows that 75%, 80%, or 90% fertilizer plus inoculants were significantly comparable to 100% fertilizer [ 10 ]. This review is intended to cater to the needs of agriculturists and plant biologists whose work focuses on creating clean and efficient means to improve the quality of soil by nourishing and maintaining the useful and natural flora of microorganisms or PGPRs. Further, it presents recent developments in the area of field management that reveals the potential application of biofertilizers and increased nutrient profiles, plant growth and productivity, and improved tolerance to environmental stress with a particular emphasis on mechanism of the feat of biofertilizers.
The microbiome: potential significance of beneficial microbes in sustainable agriculture
The rhizosphere, which is the narrow zone of soil surrounding plant roots, can comprise up to 10 11 microbial cells per gram of root [ 11 ] and above 30,000 prokaryotic species [ 12 ] that in general, improve plant productivity [ 12 ]. The collective genome of rhizosphere microbial community enveloping plant roots is larger compared to that of plants and is referred as microbiome [ 13 ], whose interactions determine crop health in natural agro-ecosystem by providing numerous services to crop plants viz., organic matter decomposition, nutrient acquisition, water absorption, nutrient recycling , weed control and bio-control [ 14 ]. The metagenomic study provides the individual the core rhizosphere and endophytic microbiomes activity in Arabidopsis thaliana using 454 sequencing (Roche) of 16S rRNA gene amplicons [ 15 ]. It has been proposed that exploiting tailor-made core microbiome transfer therapy in agriculture can be a potential approach in managing plant diseases for different crops [ 16 ]. Rhizosphere microbial communities an alternative for chemical fertilizers has become a subject of great interest in sustainable agriculture and bio-safety programme.
A major focus in the coming decades would be on safe and eco-friendly methods by exploiting the beneficial micro-organisms in sustainable crop production [ 17 ]. Such microorganisms, in general, consist of diverse naturally occurring microbes whose inoculation to the soil ecosystem advances soil physicochemical properties, soil microbes biodiversity, soil health, plant growth and development and crop productivity [ 18 ]. The agriculturally useful microbial populations cover plant growth promoting rhizobacteria, N 2 -fixing cyanobacteria, mycorrhiza, plant disease suppressive beneficial bacteria, stress tolerance endophytes and bio-degrading microbes [ 8 ]. Biofertilizers are a supplementary component to soil and crop management traditions viz., crop rotation, organic adjustments, tillage maintenance, recycling of crop residue, soil fertility renovation and the biocontrol of pathogens and insect pests, which operation can significantly be useful in maintaining the sustainability of various crop productions [ 19 ]. Azotobacter , Azospirillum , Rhizobium , cyanobacteria, phosphorus and potassium solubilising microorganisms and mycorrhizae are some of the PGPRs that were found to increase in the soil under no tillage or minimum tillage treatment [ 20 , 21 ]. Efficient strains of Azotobacter , Azospirillum, Phosphobacter and Rhizobacter can provide significant amount of nitrogen to Helianthus annus and to increase the plant height, number of leaves, stem diameter percentage of seed filling and seed dry weight [ 22 ]. Similarly, in rice, addition of Azotobacter , Azospirillum and Rhizobium promotes the physiology and improves the root morphology [ 23 ].
Azotobacter plays an important role in the nitrogen cycle in nature as it possesses a variety of metabolic functions [ 18 ]. Besides playing role in nitrogen fixation, Azotobacter has the capacity to produce vitamins such as thiamine and riboflavin [ 24 ], and plant hormones viz., indole acetic acid (IAA), gibberellins (GA) and cytokinins (CK) [ 25 ]. A. chroococcum improves the plant growth by enhancing seed germination and advancing the root architecture [ 26 ] by inhibiting pathogenic microorganisms around the root systems of crop plants [ 27 ]. This genus includes diverse species, namely, A. chroococcum, A.vinelandii, A. beijerinckii, A. nigricans, A. armeniacus and A. paspali . It is used as a biofertilizer for different crops viz., wheat, oat, barley mustard, seasum, rice, linseeds, sunflower, castor, maize, sorghum, cotton, jute, sugar beets, tobacco, tea, coffee, rubber and coconuts [ 28 ]. Azospirillum is another free-living, motile, gram variable and aerobic bacterium that can thrive in flooded conditions [ 6 ] and promotes various aspects of plant growth and development [ 29 ]. Azospirillum was shown to exert beneficial effects on plant growth and crop yields both in greenhouse and in field trials [ 30 ]. Diverse species of the genus Azospirillum including A. lipoferum , A. brasilense , A. amazonense , A. halopraeferens and A. irakense have been reported to improve productivity of various crops [ 6 ]. Interestingly, it was observed that Azospirillum inoculation can change the root morphology via producing plant growth regulating substances [ 31 ] via siderophore production [ 6 ]. It also increases the number of lateral roots and enhances root hairs formation to provide more root surface area to absorb sufficient nutrients [ 32 ]. This improves the water status of plant and aids the nutrient profile in the advancement of plant growth and development [ 33 , 34 ]. Co-inoculation of Azospirillium brasilense and Rhizobium meliloti plus 2,4D posed positive effect on grain yield and N,P,K content of Triticum aestivum [ 35 ]. Rhizobium has been used as an efficient nitrogen fixer for many years. It plays an important role in increasing yield by converting atmospheric nitrogen into usable forms [ 36 ]. Being resistant to different temperature ranges Rhizobium normally enters the root hairs, multiplies there and forms nodules [ 37 ]. Rhizobium inoculants in different locations and soil types were reported to significantly increase the grain yields of bengal gram [ 38 ], lentil [ 39 ], pea, alfalfa and sugar beet rhizosphere [ 40 ], berseem [ 41 ], ground nut [ 36 ] and soybean [ 42 ]. These Rhizobium isolates obtained from wild rice have been reported to supply nitrogen to the rice plant to promote growth and development [ 43 ]. One of the species of Rhizobium , Sinorhizobium meliloti 1021 infects plants other than leguminous plants like rice to promote growth by enhancing endogenous level of plant hormone and photosynthesis performance to confer plant tolerance to stress [ 44 ]. In groundnut, IRC-6 strain of Rhizobium has resulted in the enhancement of several useful traits such as increased number of pink coloured nodules, nitrate reductase activity and leghaemoglobin content in 50 DAI (days after inoculation) [ 36 ]. Rhizobial symbiosis provides defence to plants against pathogens and herbivores, such as example, Mexican bean beetle [ 45 ] and the green house whitefy Trialeurodes vaporariorum [ 46 ] (Figure 1 ).
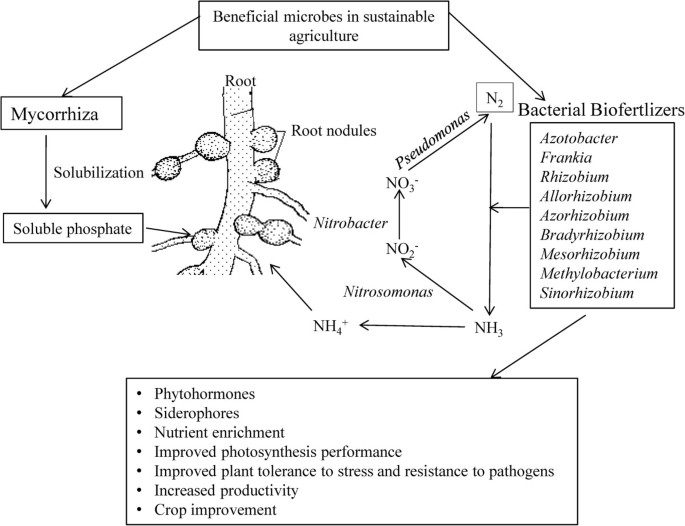
Potential use of soil microbes in sustainable crop production. The beneficial soil micro-organisms sustain crop production either as biofertilizers [ 19 ] or symbiont [ 17 ]. They perform nutrient solubilisation which facilitate nutrient availability and thereby uptake [ 20 , 21 ]. It improves the plant growth by advancing the root architecture [ 26 ]. Their activity provides several useful traits to plants such as increased root hairs, nodules and nitrate reductase activity and [ 36 ]. Efficient strains of Azotobacter , Azospirillum, Phosphobacter and Rhizobacter can provide significant amount of available nitrogen through nitrogen cycling [ 22 ]. The biofertilizers produced plant hormones, which include indole acetic acid (IAA), gibberellins (GA) and cytokinins (CK) [ 25 , 44 ]. Biofertilizers improve photosynthesis performance to confer plant tolerance to stress [ 44 ] and increase resistance to pathogens [ 45 ] thereby resulting in crop improvement [ 18 ].
Biofertlizers exploitation and nutrients profile of crops
A key advantage of beneficial microorganisms is to assimilate phosphorus for their own requirement, which in turn available as its soluble form in sufficient quantities in soil. Pseudomonas, Bacillus, Micrococcus, Flavobacterium, Fusarium, Sclerotium, Aspergillus and Penicillium have been reported to be active in the solubilisation process [ 47 ]. A phosphate-solubilizing bacterial strain NII-0909 of Micrococcus sp. has polyvalent properties including phosphate solubilisation and siderophore production [ 48 ]. Similarly, two fungi Aspergillus fumigatus and A. Niger were isolated from decaying cassava peels were found to convert cassava wastes by the semi-solid fermentation technique to phosphate biofertilizers [ 49 ]. Burkholderia vietnamiensis, stress tolerant bacteria, produces gluconic and 2-ketogluconic acids, which involved in phosphate solubilisation [ 50 ]. Enterobacter and Burkholderia that were isolated from the rhizosphere of sunflower were found to produce siderophores and indolic compounds (ICs) which can solubilize phosphate [ 51 ]. Potassium solubilising microorganisms (KSM) such as genus Aspergillus, Bacillus and Clostridium are found to be efficient in potassium solubilisation in the soil and mobilize in different crops [ 52 ]. Mycorrhizal mutualistic symbiosis with plant roots satisfies the plant nutrients demand [ 53 ], which leads to enhance plant growth and development, and protect plants from pathogens attack and environmental stress [ 54 ]. It leads to the absorption of phosphate by the hyphae from outside to internal cortical mycelia, which finally transfer phosphate to the cortical root cells [ 55 ]. Nitrogen fixing cyanobacteria such as Aulosira, Tolypothrix, Scytonema, Nostoc, Anabaena and Plectonema are commonly used as biofertilizers [ 56 , 57 ]. Besides the contribution of nitrogen, growth-promoting substances and vitamins liberated by these algae Cylindrospermum musicola increase the root growth and yield of rice plants [ 58 ]. Interestingly, genetic engineering was used to improve the nitrogen fixing potential of Anabaena sp. strain PCC7120 [ 59 ]. Constitutive expression of the hetR gene driven by a light-inducible promoter enhanced HetR protein expression, leading to higher nitrogenase activity in Anabaena sp. strain PCC7120 as compared with the wild-type strain. This in turn caused better growth of paddy when applied to the fields [ 60 ].
Biofertilizers relevance and plant tolerance to environmental stress
Abiotic and biotic stresses are the major constraints that are affecting the productivity of the crops. Many tools of modern science have been extensively applied for crop improvement under stress, of which PGPRs role as bio protectants has become paramount importance in this regard [ 61 ]. Rhizobium trifolii inoculated with Trifolium alexandrinum showed higher biomass and increased number of nodulation under salinity stress condition [ 41 , 62 ]. Pseudomonas aeruginosa has been shown to withstand biotic and abiotic stresses [ 63 ]. Paul and Nair [ 64 ] found that P. fluorescens MSP-393 produces osmolytes and salt-stress induced proteins that overcome the negative effects of salt. P. putida Rs-198 enhanced germination rate and several growth parameters viz., plant height, fresh weight and dry weight of cotton under condition of alkaline and high salt via increasing the rate of uptake of K + , Mg 2+ and Ca 2+ , and by decreasing the absorption of Na + [ 65 ]. Few strains of Pseudomonas conferred plant tolerance via 2,4-diacetylphloroglucinol (DAPG) [ 66 ]. Interestingly, systemic response was found to be induced against P. syringae in Arabidopsis thaliana by P. fluorescens DAPG [ 67 ]. Calcisol produced by PGPRs viz., P. alcaligenes PsA15, Bacillus polymyxa BcP26 and Mycobacterium phlei MbP18 provides tolerance to high temperatures and salinity stress [ 68 ]. It has been demonstrated that inoculation of plant with AM fungi also improves plant growth under salt stress [ 69 ]. Achromobacter piechaudii was also shown to increase the biomass of tomato and pepper plants under 172 mM NaCl and water stress [ 70 ]. Interestingly, a root endophytic fungus Piriformospora indica was found to defend host plant against salt stress [ 69 ]. In one of the studies it was found that inoculation of PGPR alone or along with AM like Glomus intraradices or G. mosseae resulted in the better nutrient uptake and improvement in normal physiological processes in Lactuca sativa under stress conditions. The same plant treated with P. mendocina increased shoot biomass under salt stress [ 71 ]. Mechanisms involved in osmotic stress tolerance employing transcriptomic and microscopic strategies revealed a considerable change in the transcriptome of Stenotrophomonas rhizophila DSM14405 T in response to salt stress [ 72 ] . Combination of AM fungi and N 2 -fixing bacteria helped the legume plants in overcoming drought stress [ 73 ]. Effect of A.brasilense along with AM can be seen in other crops such as tomato, maize and cassava [ 74 – 76 ]. A. brasilense and AM combination improved plant tolerance to various abiotic stresses [ 77 ]. The additive effect of Pseudomonas putida or Bacillus megaterium and AM fungi was effective in alleviating drought stress [ 78 ]. Application of Pseudomonades sp. under water stress improved the antioxidant and photosynthetic pigments in basil plants. Interestingly, combination of three bacterial species caused the highest CAT, GPX and APX activity and chlorophyll content in leaves under water stress [ 79 ]. Pseudomonas spp. was found to cause positive affect on the seedling growth and seed germination of A. officinalis L. under water stress [ 80 ]. Photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress were found to increase after inoculation of arbuscular mycorrhiza [ 81 ]. The beneficial effects of mycorrhizae have also been reported under both the drought and saline conditions [ 82 ]. Heavy metals such as cadmium, lead, mercury from hospital and factory waste accumulate in the soil and enter plants through roots [ 83 ]. Azospirillium spp, Phosphobacteria spp and Glucanacetobacter spp. isolated from rhizosphere of rice field and mangroves were found to be more tolerant to heavy metal specially iron [ 83 , 84 ]. P. potida strain 11 (P.p.11), P. potida strain 4 (P.p.4) and P. fluorescens strain 169 (P.f.169) can protect canola and barley plants from the inhibitory effects of cadmium via IAA, siderophore and 1-aminocyclopropane-1-carboxylate deaminase (ACCD) [ 85 ]. It was reported that rhizoremediation of petroleum contaminated soil can be expedited by adding microbes in the form of effective microbial agent (EMA) to the different plant species such as cotton, ryegrass, tall fescue, and alfalfa [ 86 ].
PGPRs as biological agents proved to be one of the alternatives of chemical agents to provide resistance to against various pathogen attacks [ 87 ]. Apart from acting as growth-promoting agents they can provide resistance against pathogens by producing metabolites [ 88 ]. Bacillus subtilis GBO3 can induce defense-related pathways viz., salicylic acid (SA) and jasmonic acid (JA) [ 89 ]. Application of PGPR isolates viz., B. amyloliquefaciens 937b and B. pumilus SE-34 provide immunity against tomato mottle virus [ 90 ]. B. megaterium IISRBP 17, characterized from stem of black pepper, acts against Phytophthor capsici [ 91 ]. Bacillus subtilis N11 along with mature composts was found to control Fusarium infestation on banana roots [ 92 ]. Similarly, B. subtilis (UFLA285) was found to provide resistance against R. solani and also it induced foliar and root growth in cotton plants [ 93 ]. In another interesting study Paenibacillus polymyxa SQR-21 was identified as a potential agent for the bio-control of Fusarium wilt in watermelon [ 94 ]. Further, the exploitation of PGPRs was found to be effective to manage the spotted wilt viruses in tomato [ 87 ], cucumber mosaic virus of tomato and pepper [ 90 ], and banana bunchy top virus in banana [ 95 ]. In some cases it was shown that along with bacteria, mycorrhizae can also confer resistant against fungal pathogens and inhibit the growth of many root pathogens such as R. solani , Pythium spp., F. Oxysporum, A. obscura and H. annosum [ 96 , 97 ] by improving plant nutrients profile and thereby productivity [ 69 ]. For instance Glomus mosseae was effective against Fusarium oxysporum f. sp. Basilica which causes root-rot disease of basil plants [ 98 ]. Medicago tranculata also showed induction of various defense-related genes with mycorrhizal colonization [ 99 ]. It was shown that addition of arbuscular mycorrhizal fungi and Pseudomonas fluorescens to the soil can reduce the development of root-rot disease and enhance the yield of Phaseolus vulgaris L. [ 100 ].
Mechanism of action of various biofertilizers
Mycorrhiza is the association of fungus with the roots of higher plants. While it remains an enigma, it serves as a model system to understand the mechanism behind stimulation of growth in the root cells as a result of mycorrhizal inhabitation. Genome sequencing of two EM fungi (ectomycorrhizae), the L. bicolor 13, and T. melanosporum (black truffle) 14, helps in the identification of factors that regulate the development of mycorrhiza and its function in the plant cell [ 101 ]. Fifteen genes that up-regulated during symbiosis were identified as putative hexose transporters in L. bicolor. Its genome lacked genes encoding invertases making it dependent on plants for glucose. However, melanosporum possesses one invertase gene, and unlike L. bicolor it can directly use the sucrose of the host [ 101 ]. The up-regulation of transporter genes during symbiosis indicated the action of transportation of useful compounds like amino acids, oligopeptides and polyamines through the symbiotic interface from one organism to other. Free living mycelium can take nitrate and ammonium from the soil. Subsequently, these compounds reach the mantle and hartig net and are then transferred to the plants. Cysteine-rich proteins (MISSP7) of fungus play an important role as effectors and facilitators in the formation of symbiotic interfaces [ 102 ]. Many genes related to auxin biosynthesis and root morphogenesis showed up-regulation during mycorrhizal colonization [ 69 , 103 , 104 ]. Further, G. versiforme possesses inorganic phosphate (Pi) transporters on its hyphae which help in the direct absorption of phosphate from the soil and a glutamine synthase gene was found in G. intraradice , which strengthens the possibility of nitrogen metabolism in fungal hyphe that can be transported later to the plant [ 105 ]. Bioactive compounds called Myc factors similar to Nod factors of Rhizobium are suggested to be secreted by mycorrhiza and Rhizobium and perceived by host roots for the activation of signal transduction pathway or common symbiosis (SYM) pathway [ 106 , 107 ]. The pathways that prepare plant for both AM and Rhizobium infection have some common points. The common SYM pathway prepares the host plant to bring about changes at the molecular and anatomical level with the first contact of fungal hyphae. So far, calcium is supposed to be the hub of secondary messengers via Ca 2+ spiking in the nuclear region of root hairs [ 108 ]. Rhizobium leguminosarum biovar viciae can induce various genes in the plants like pea, alfalfa and sugar beet as evident from the microarray studies [ 40 ]. PGPR produce IAA which, in turn, induces the production of nitric Oxide (NO), which acts as a second messenger to trigger a complex signaling network leading to improved root growth and developmental processes [ 109 ].
Expression of ENOD11 and many defense-related genes and root remodelling genes get up-regulated during entry. Subsequently, this allows the formation of a pre-penetration apparatus or PPA [ 110 ]. Though the biology behind the development of arbuscules is unknown, a gene called vapyrin when knocked down causes a decline in the growth of arbuscules [ 111 ]. Many other genes including subtilisin protease 65, phosphate transporter 66 or two ABC transporters 67 are known to be involved in arbuscules formation [ 112 , 113 ]. Nitrogen-fixation genes are popularly used by scientists today to create engineered plants that can fix atmospheric nitrogen. The induction of nif genes in case of nitrogen fixing bacteria takes place under low concentration of nitrogen and oxygen in the rhizosphere [ 1 ]. Interestingly, sugarcane plantlets inoculated with a wild strain of G. diazotrophicus , have demonstrated fixation of radioactive N 2 when compared with the G. diazotrophicus mutant that has mutant nif D gene which proved the significance of nif genes. Efficiency of nitrogen fixation is dependent on the utilization of carbon [ 114 , 115 ]. A bacterium like Bacillus subtilis (UFLA285) can differentially induce 247 genes in cotton plant as compared to control where no PGPR was supplied to the cotton plant [ 85 ]. Many disease resistance genes that work via jasmonate/ethylene signaling as well as osmotic regulation via proline synthesis genes were differentially expressed with UFLA285 induction [ 85 ]. Various differentially expressed genes were identified which include metallothionein-like protein type 1, a NOD26-like membrane integral protein, ZmNIP2-1, a thionin family protein, an oryzain gamma chain precursor, stress-associated protein 1 (OsISAP1), probenazole-inducible protein PBZ1 and auxin and ethylene-responsive genes [ 116 ]. The expression of the defense-related proteins PBZ1 and thionins were found to get repressed in the rice–H seropedicae association, suggesting the modulation of plant defense responses during colonisation [ 116 ].
Among the PGPR species, Azospirillum was suggested to secrete gibberellins, ethylene and auxins [ 117 ]. Some plant associated bacteria can also induce phytohormone synthesis, for example lodgepole pine when inoculated with Paenibacillus polymyxa had elevated levels of IAA in the roots [ 118 ]. Rhizobium and Bacillus were found to synthesize IAA at different cultural conditions such as pH, temperature and in the presence of agro waste as substrate [ 119 ]. Ethylene, unlike other phytohormones, is responsible for the inhibition of growth of dicot plants [ 69 ]. It was found by Glick et al . [ 120 ] that PGPR could enhance the growth of plant by suppressing the expression of ethylene. Interestingly, a model was suggested in which it was shown that ethylene synthesis from 1-aminocyclopropane-1-carboxylate (ACC), an immediate precursor of ethylene, which is hydrolyzed by bacterial ACC-deaminase enzyme in the need of nitrogen and carbon source is also one of the mechanisms of induction of conditions suitable for growth. ACC-deaminase activity was also found in the bacteria such as Alcaligenes sp., Bacillus pumilus, Pseudomonas sp . and Variovorax paradoxus [ 69 ]. The involvement of ACC deaminase in the indirect influence on the growth of plants was proved in Canola, where mutations in ACC deaminase gene caused the loss of effect of growth promoting Pseudomonas putida [ 29 ]. Interestingly, the potential of PGPRs was further enhanced by introducing genes involved in the direct oxidation (DO) pathway and mineral phosphate solubilisation (MPS) into some useful strains of PGPRs. Gene encoding glucose dehydrogenase (gcd) involved in the DO pathway was cloned and characterized from Acinetobacter calcoaceticus and E. coli and Enterobacter asburiae [ 121 ]. Also a soluble form of gcd has been cloned from Acinetobacter calcoaceticus and G. oxydans [ 122 ]. Furthermore there are reports of site-directed mutagenesis of glucose dehydrogenase (GDH) and gluconate dehydrogenase (GADH) that has improved the activity of this enzyme. Mere substitution of S771M provided thermal stability to E.coli while mutation of glutamate 742 to lysine improved the EDTA tolerance of E. coli PQQGDH. The application of this technology was achieved by transferring genes involved in the DO pathway viz., GDH, GADH and pyrroloquinoline quinine ( PQQ ) to rhizobacteria, and phosphoenolpyruvate carboxylase ( PPC ) to P. Fluorescens , provide the MPS trait [ 122 ] (Figure 2 ).
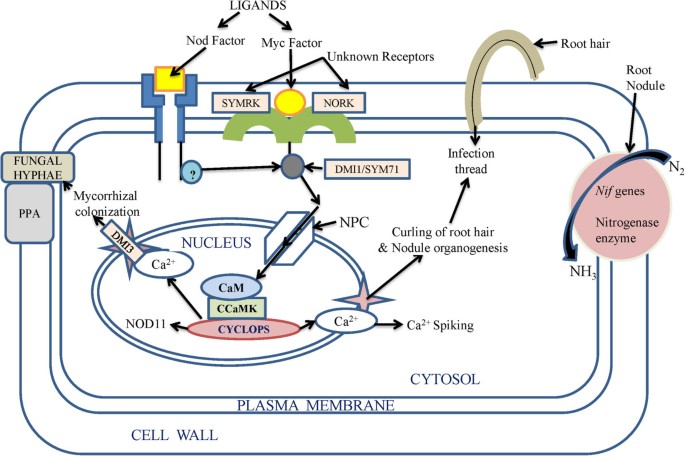
Hypothetical mechanism of action of biofertilizers in the root cell. Bioactive ligands called Myc factors and Nod factors secreted by mycorrhiza and Rhizobium were perceived by host roots to trigger the signal transduction pathway [ 106 , 107 ], which initiates further signal transduction pathway through unknown receptors (SYMRK and NORK) [ 101 ] which trigger release of Ca 2+ in the cytosol [ 108 ]. The whole pathway involves receptor like kinases or other kinase related proteins like DMI and SYM71 to phosphorylate their substrates [ 123 , 124 ]. Nuclear pore complex (NPC) and some of its proteins (NUP) play role in calcium spiking. DM1 proteins play role in maintaining periodic oscillation of calcium ions inside and outside the nucleus. Several channels proteins (Ca 2+ channel proteins) also facilitate this process with the help of various transporters [ 108 ]. CCaMK is a calcium calmodulin-dependent protein kinase, which phosphorylate the product of CYCLOPS protein thus initiating activation of various genes involving formation of structures like nodule and (PPA) pre-penetration apparatus [ 124 ].
Conclusions
Environmental stresses are becoming a major problem and productivity is declining at an unprecedented rate. Our dependence on chemical fertilisers and pesticides has encouraged the thriving of industries that are producing life-threatening chemicals and which are not only hazardous for human consumption but can also disturb the ecological balance. Biofertilizers can help solve the problem of feeding an increasing global population at a time when agriculture is facing various environmental stresses. It is important to realise the useful aspects of biofertilizers and implement its application to modern agricultural practices. The new technology developed using the powerful tool of molecular biotechnology can enhance the biological pathways of production of phytohormones. If identified and transferred to the useful PGPRs, these technologies can help provide relief from environmental stresses. However, the lack of awareness regarding improved protocols of biofertiliser applications to the field is one of the few reasons why many useful PGPRs are still beyond the knowledge of ecologists and agriculturists. Nevertheless, the recent progresses in technologies related to microbial science, plant-pathogen interactions and genomics will help to optimize the required protocols. The success of the science related to biofertilizers depends on inventions of innovative strategies related to the functions of PGPRs and their proper application to the field of agriculture. The major challenge in this area of research lies in the fact that along with the identification of various strains of PGPRs and its properties it is essential to dissect the actual mechanism of functioning of PGPRs for their efficacy toward exploitation in sustainable agriculture.
Santos VB, Araujo SF, Leite LF, Nunes LA, Melo JW: Soil microbial biomass and organic matter fractions during transition from conventional to organic farming systems. Geoderma. 2012, 170: 227-231.
Article CAS Google Scholar
Youssef MMA, Eissa MFM: Biofertilizers and their role in management of plant parasitic nematodes. A review. E3 J Biotechnol. Pharm Res. 2014, 5: 1-6.
Google Scholar
Raja N: Biopesticides and biofertilizers: ecofriendly sources for sustainable agriculture. J Biofertil Biopestici. 2013, 1000e112: 1000e112-
Araujo ASF, Santos VB, Monteiro RTR: Responses of soil microbial biomass and activity for practices of organic and conventional farming systems in Piauistate, Brazil. Eur J Soil Biol. 2008, 44: 225-230. 10.1016/j.ejsobi.2007.06.001.
Article Google Scholar
Megali L, Glauser G, Rasmann S: Fertilization with beneficial microorganisms decreases tomato defenses against insect pests. Agron Sustain Dev. 2013, doi:10.1007/s13593-013-0187-0
Sahoo RK, Ansari MW, Pradhan M, Dangar TK, Mohanty S, Tuteja N: Phenotypic and molecular characterization of efficient native Azospirillum strains from rice fields for crop improvement. Protoplasma. 2014, doi:10.1007/s00709-013-0607-7
Sinha RK, Valani D, Chauhan K, Agarwal S: Embarking on a second green revolution for sustainable agriculture by vermiculture biotechnology using earthworms: reviving the dreams of Sir Charles Darwin. Int J Agric Health Saf. 2014, 1: 50-64.
Singh JS, Pandey VC, Singh DP: Efficient soil microorganisms: a new dimension for sustainable agriculture andenvironmental development. Agric Ecosyst Environ. 2011, 140: 339-353. 10.1016/j.agee.2011.01.017.
Adesemoye AO, Kloepper JW: Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol. 2009, 85: 1-12.
Adesemoye AO, Torbert HA, Kloepper JW: Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Micro Ecol. 2009, 58: 921-929. 10.1007/s00248-009-9531-y.
Egamberdieva D, Kamilova F, Validov S, Gafurova L, Kucharova Z, Lugtenberg B: High incidence of plant growth stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ Microbiol. 2008, 10: 1-9.
CAS Google Scholar
Mendes R, Garbeva P, Raaijmakers JM: The rhizosphere microbiome: significance of plant beneficial plant pathogenic and human pathogenic microorganisms. FEMS Microbiol Rev. 2013, 37: 634-663.
Bulgarelli D, Schlaeppi K, Spaepen S, Loren V, van Themaat E, Schulze-Lefert P: Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol. 2013, 64: 807-838.
Berg G, Zachow C, Müller H, Phillips J, Tilcher R: Next-generation bio-products sowing the seeds of success for sustainable agriculture. Agronomy. 2013, 3: 648-656. 10.3390/agronomy3040648.
Hirsch PR, Mauchline TH: Who’s who in the plant root microbiome?. Nat Biotechnol. 2012, 30: 961-962.
Gopal M, Gupta A, Thomas GV: Bespoke microbiome therapy to manage plant diseases. Front Microbiol. 2013, 5: 15-
Nina K, Thomas WK, Prem SB: Beneficial organisms for nutrient uptake. VFRC report 2014/1, virtual fertilizer research center. 2014, 63-Washington, DC: Wageningen Academic Publishers
Sahoo RK, Ansari MW, Dangar TK, Mohanty S, Tuteja N: Phenotypic and molecular characterization of efficient nitrogen fixing Azotobacter strains of the rice fields. Protoplasma. 2013, doi:10.1007/s00709-013-0547-2
Sahoo RK, Bhardwaj D, Tuteja N: Biofertilizers: a sustainable eco-friendly agricultural approach to crop improvement. Plant Acclimation to Environmental Stress. Edited by: Tuteja N, Gill SS. 2013b, 403-432. LLC 233 Spring Street, New York, 10013, USA: Springer Science plus Business Media
Chapter Google Scholar
Dogan K, Kamail Celik I, Mustafa Gok M, Ali C: Effect of different soil tillage methods on rhizobial nodulation, biyomas and nitrogen content of second crop soybean. Afr J Microbiol Res. 2011, 5: 3186-3194.
Aziz G, Bajsa N, Haghjou T, Taule C, Valverde A, Mariano J, Arias A: Abundance, diversity and prospecting of culturable phosphate solubilizing bacteria on soils under crop–pasture rotations in a no-tillage regime in Uruguay. Appl Soil Ecol. 2012, 61: 320-326.
Dhanasekar R, Dhandapani R: Effect of biofertilizers on the growth of Helianthus annuus. Int J plant, Ani Environ Sci. 2012, 2: 143-147.
Choudhury MA, Kennedy IR: Prospects and potentials for system of biological nitrogen fixation in sustainable rice production. Biol Fertil Soils. 2004, 39: 219-227. 10.1007/s00374-003-0706-2.
Revillas JJ, Rodelas B, Pozo C, Martinez-Toledo MV, Gonzalez LJ: Production of B-Group vitamins by two Azotobacter strainswith phenolic compounds as sole carbon source under diazotrophicand adiazotrophic conditions. J Appl Microbiol. 2000, 89: 486-493.
Abd El-Fattah DA, Ewedab WE, Zayed MS, Hassaneina MK: Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculants. Ann Agric Sci. 2013, 58: 111-118.
Gholami A, Shahsavani S, Nezarat S: The Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Germination seedling Growth and Yield of Maize. Int J Biol Life Sci. 2009, 5: 1-
Mali GV, Bodhankar MG: Antifungal and phytohormone production potential of Azotobacter chroococcum isolates from Groundnut (Arachis hypogea L.) rhizosphere. Asian J Exp Sci. 2009, 23: 293-297.
Wani SA, Chand S, Ali T: Potential use of Azotobacter chroococcum in crop production: an overview. Curr Agric Res J. 2013, 1: 35-38. 10.12944/CARJ.1.1.04.
Bhattacharyya PN, Jha DK: Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012, 28: 1327-1350.
Saikia SP, Bora D, Goswami A, Mudoi KD, Gogoi A: A review on the role of Azospirillum in the yield improvement of non leguminous crops. Afr J Microbiol Res. 2013, 6: 1085-1102.
Bashan Y, Holguin G, Bashan LE: Azospirillum-plant relationships: agricultural, physiological, molecular and environmental advances (1997–2003). Can J Microbiol. 2004, 50: 521-577.
Mehdipour-Moghaddam MJ, Emtiazi G, Salehi Z: Enhanced auxin production by Azospirillum pure cultures from plant root exudates. J Agr Sci Tech. 2012, 14: 985-994.
Sarig S, Blum A, Okon Y: Improvement of the water status and yield of field-grown grain sorghum (Sorghum bicolor) by inoculation with Azospirillum brasilense. J Agric Sci. 1992, 110: 271-277.
Ilyas N, Bano A, Iqbal S, Raja NI: Physiological, biochemical and molecular characterization of Azospirillum spp. isolated from maize under water stress. Pak J Bot. 2012, 44: 71-80.
Askary M, Mostajeran A, Amooaghaei R, Mostajeran M: Influence of the co-inoculation Azospirillum brasilense and Rhizobium meliloti plus 2, 4-D on grain yield and N, P, K content of Triticum aestivum (cv. Baccros and Mahdavi). Am Eurasian J Agric Environ Sci. 2009, 5: 296-307.
Sharma P, Sardana V, Kandola SS: Response of groundnut (Arachishypogaea L.) to Rhizobium Inoculation. Libyan Agric Res Centre J Int. 2011, 2: 101-104.
Nehra K, Yadav SA, Sehrawat AR, Vashishat RK: Characterization of heat resistant mutant strains of Rhizobium sp. [ Cajanus ] for growth, survival and symbiotic properties. Indian J Microbiol. 2007, 47: 329-335.
Patil PL, Medhane NS: Seed inoculation studies in gram (Cicer arietinum) with different strains of Rhizobium sp. Plant Soil. 1974, 40: 221-223. 10.1007/BF00011425.
Rashid MH, Schafer H, Gonzalez J, Wink M: Genetic diversity of rhizobia nodulating lentil (Lens culinaris ) in Bangladesh. Syst Appl Microbiol. 2012, 35: 98-109.
Ramachandran VK, East AK, Karunakaran R, Downie JA, Poole SP: Adaptation of Rhizobium leguminosarum to pea, alfalfa and sugar beet rhizosphere investigated by comparative transcriptomics. Genome Biol. 2011, 12: 106-109.
Hussain N, Mujeeb F, Tahir M, Khan GD, Hassan NM, Bari A: Effectiveness of Rhizobium under salinity stress. Asian J Plant Sci. 2002, 1: 12-14.
Grossman JM, Schipanski ME, Sooksanguan T, Drinkwater LE: Diversity of rhizobia nodulating soybean Glycine max (Vinton)] varies under organic and conventional management. Appl Soil Ecol. 2011, 50: 14-20.
Peng G, Yuan Q, Li H, Zhang W, Tan Z: Rhizobium oryzae sp. nov., isolated from the wild rice Oryza alta. Int J Syst Evol Microbiol. 2008, 58: 2158-2163.
Chi F, Yang P, Han F, Jing Y, Shen S: Proteomic analysis of rice seedlings infected by Sinorhizobium meliloti 1021. Proteomics. 2010, 10: 1861-1874.
Thamer S, Schädler M, Bonte D, Ballhorn DJ: Dual benefit from a belowground symbiosis: nitrogen fixing rhizobia promote growth and defense against a specialist herbivore in a cyanogenic plant. Plant Soil. 2011, 34: 1209-1219.
Menjivar RD, Cabrera JA, Kranz J, Sikora RA: Induction of metabolite organic compounds by mutualistic endophytic fungi to reduce the greenhouse whitefly Trialeurodes vaporariorum (Westwood) infection on tomato. Plant Soil. 2012, 352: 233-241. 10.1007/s11104-011-0991-8.
Pindi PK, Satyanarayana SDV: Liquid microbial consortium- a potential tool for sustainable soil health. J Biofertil Biopest. 2012, 3: 4-
Dastager SG, Deepa CK, Pandey A: Isolation and characterization of novel plant growth promoting Micrococcus sp NII-0909 and its interaction with cowpea. Plant Physiol Biochem. 2010, 48: 987-992.
Ogbo FC: Conversion of cassava wastes for biofertilizer production using phosphate solubilizing fungi. Bioresour Technol. 2010, 101: 4120-4124.
Park J, Bolan N, Megharaj M, Naidu R: Isolation of Phosphate-Solubilizing Bacteria and characterization of their Effects on Lead Immobilization. Pedologist. 2010, 53: 67-75.
Ambrosini A, Beneduzi A, Stefanski T, Pinheiro F, Vargas L, Passaglia L: Screening of plant growth promoting Rhizobacteria isolated from sunflower Helianthus annuus L. Plant & Soil. 2012, 356: 245-264. 10.1007/s11104-011-1079-1.
Mohammadi K, Yousef Sohrabi Y: Bacterial Biofertilizers for sustainable crop production: A review. J Agric Biol Sci. 2012, 7: 307-316.
Kogel KH, Franken P, Huckelhovenl R: Endophyte or parasite – what decides?. Curr Opin Plant Biol. 2006, 9: 358-363.
Lamabam PS, Gill SS, Tuteja N: Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal Behav. 2011, 6: 175-191.
Smith S, Lakobsen I, Gronlund M, Smith FA: Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156: 1050-1057.
Abdel-Lateif K, Bogusz D, Hocher V: The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal Behav. 2012, 7: 636-641.
Roy M, Srivastava RC: Assembling BNF system in rice plant: frontier areas of research. Curr Sci. 2013, 104: 3-10.
Venkataraman GS, Neelakantan S: Effect of cellular constituents of the nitrogen fixing blue-green algae. Cylindrospermum nusciola on the root growth of rice seedlings. J General Appl Microbiol. 1967, 13: 53-61. 10.2323/jgam.13.53.
Pandey S, Shrivastava AK, Rai R, Rai LC: Molecular characterization of Alr1105 a novel arsenate reductase of the diazotrophic cyanobacterium Anabaena sp. PCC7120 and decoding its role in abiotic stress management in Escherichia coli. Plant Mol Biol. 2013, 83: 417-432.
Chaurasia AK, Apte SK: Improved eco-friendly recombinant Anabaena sp. strain PCC7120 with enhanced nitrogen biofertilizer potential. Appl Environ Microbiol. 2011, 77: 395-399.
Yang JW, Kloepper JW, Ryu CM: Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14: 1-4.
Antoun H, Prevost D: Ecology of plant growth promoting rhizobacteria. PGPR: Biocontrol and Biofertilization. Edited by: Siddiqui ZA. 2005, 1-38. Dordrecht: Springer
Pandey PK, Yadav SK, Singh A, Sarma BK, Mishra A, Singh HB: Cross-Species Alleviation of Biotic and Abiotic Stresses by the Endophyte Pseudomonas aeruginosa PW09. J Phytopathol. 2012, 160: 532-539. 10.1111/j.1439-0434.2012.01941.x.
Paul D, Nair S: Stress adaptations in a plant growth promoting Rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J Basic Microbiol. 2008, 48: 1-7.
Yao L, Wu Z, Zheng Y, Kaleem I, Li C: Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. European J Soil Biol. 2010, 46: 49-54. 10.1016/j.ejsobi.2009.11.002.
Schnider-Keel U, Seematter A, Maurhofer M, Blumer C, Duffy B, Gigot-Bonnefoy C, Reimmann C, Notz R, Defago G, Haas D, Keel C: Autoinduction of 2, 4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J Bacteriol. 2000, 182: 1215-1225.
Weller DM, Mavrodi DV, van Pelt JA, Pieterse CM, van Loon LC, Bakker PA: Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2, 4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology. 2012, 102: 403-412.
Egamberdiyeva D: The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. Soil Ecol. 2007, 36: 184-189. 10.1016/j.apsoil.2007.02.005.
Ansari MW, Trivedi DK, Sahoo RK, Gill SS, Tuteja N: A critical review on fungi mediated plant responses with special emphasis to Piriformospora indica on improved production and protection of crops. Plant Physiol Biochem. 2013, 70: 403-410.
Alavi P, Starcher MR, Zachow C, Müller H, Berg G: Root-microbe systems: the effect and mode of interaction of stress protecting agent (SPA) Stenotrophomonas rhizophila DSM14405 T . Front Plant Sci. 2013, 4: 141-
Kohler J, Caravaca F: An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa Roldan. Soil Biol Biochem. 2010, 42: 429-434. 10.1016/j.soilbio.2009.11.021.
Gao X, Lu X, Wu M, Zhang H, Pan R, Tian J, Li S, Liao H: Co-Inoculation with Rhizobia and AMF Inhibited Soybean Red Crown Rot: From Field Study to Plant Defense-Related Gene Expression Analysis. PLoS ONE. 2012, 7: e33977-doi:10.1371/journal.pone.0033977
Aliasgharzad N, Reza M, Neyshabouri Salimi G: Effects of arbuscular mycorrhizal fungi and Bradyrhizobium japonicum on drought stress of soybean. Biologia. 2006, 19: 324-328.
German MA, Burdman S, Okon Y, Kigel J: Effects of Azospirillum brasilense on root morphology of common bean (Phaseolus vulgaris L.) under different water regimes. Biol Fertil Soils. 2000, 32: 259-264. 10.1007/s003740000245.
Casanovas EM, Barassi CA, Sueldo RJ: Azospirillum inoculation mitigates water stress effects in maize seedlings. Cer Res Commun. 2002, 30: 343-350.
Creus CM, Graziano M, Casanovas EM, Pereyra MA, Simontacchi M, Puntarulo S: Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta. 2005, 221: 297-303.
Joe MM, Jaleel CA, Sivakumar PK, Zhao CX, Karthikeyan B: Co-aggregation in Azospirillum brasilensense MTCC-125 with other PGPR strains: Effect of physical and chemical factors and stress endurance ability. J Taiwan Inst Chem Engg. 2009, 40: 491-499. 10.1016/j.jtice.2009.02.006.
Marulanda A, Barea JM, Azcon R: Stimulation of Plant Growth and Drought Tolerance by Native Microorganisms (AM Fungi and Bacteria) from Dry Environments: Mechanisms Related to Bacterial Effectiveness. J Plant Growth Regul. 2009, 28: 115-124. 10.1007/s00344-009-9079-6.
Heidari M, Golpayegani A: Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agric Sci. 2012, 11: 57-61.
Liddycoat SM, Greenberg BM, Wolyn DJ: The effect of plant growth-promoting rhizobacteria on asparagus seedlings and germinating seeds subjected to water stress under greenhouse conditions. Can J Microbiol. 2009, 55: 388-394.
Ruiz-Sanchez M, Aroca R, Munoz Y, Polon R, Ruiz-Lozano JM: The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J Plant Physiol. 2010, 167: 862-869.
Aroca R, Ruiz-Lozano JM, Zamarreno AM, Paz JA, García-Mina JM, Pozo MJ, Lopez-Raez JA: Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol. 2013, 170: 47-55.
Gill SS, Khan NA, Tuteja N: Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress ( Lepidium sativum L.). Plant Sci. 2012, 182: 112-120.
Samuel S, Muthukkaruppan SM: Characterization of plant growth promoting rhizobacteria and fungi associated with rice, mangrove and effluent contaminated soil. Curr Bot. 2011, 2: 22-25.
Baharlouei K, Pazira E, Solhi M: Evaluation of Inoculation of plant Growth-Promoting Rhizobacteria on Cadmium. 2011, Singapore: International Conference on Environmental Science and Technology IPCBEE vol.6 IACSIT Press
Tang J, Wang R, Niu X, Wang M, Zhou Q: Characterization on the rhizoremediation of petroleum contaminated soil as affected by different influencing factors. Biogeosciences Discuss. 2010, 7: 4665-4688. 10.5194/bgd-7-4665-2010.
Murphy JF, Zehnder GW, Schuster DJ, Sikora EJ, Polstan JE, Kloepper JW: Plant growth promoting rhizobacteria mediated protection in tomato against tomato mottle virus. Plant Dis. 2000, 84: 79-84.
Backman PA, Sikora RA: Endophytes: an emerging tool for biological control. Biol Control. 2008, 46: 1-3.
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW: Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134: 1017-1026.
Murphy JF, Reddy MS, Ryu CM, Kloepper JW, Li R: Rhizobacteria mediated growth promotion of tomato leads to protection against cucumber mosaic virus. Phytopathology. 2003, 93: 1301-1307.
Aravind R, Kumar A, Eapen SJ, Ramana KV: Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Lett Appl Microbiol. 2009, 48: 58-64.
Zhang N, Kai W, He X, Li S, Zhang Z, Shen B, Yang X, Zhang R, Huang Q, Shen Q: A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil. 2011, 344: 87-97. 10.1007/s11104-011-0729-7.
Medeiros FHV, Souza RM, Medeiros FCL, Zhang H, Wheeler T, Payton P, Ferro HM, Paré PW: Transcriptional profiling in cotton associated with Bacillus subtilis (UFLA285) induced biotic-stress tolerance. Plant Soil. 2011, 347: 327-337. 10.1007/s11104-011-0852-5.
Ling N, Huang Q, Guo S, Shen Q: Paenibacillus polymyxa SQR-21 systemically affects root exudates of watermelon to decrease the conidial germination of Fusarium oxysporum f.sp. niveum. Plant Soil. 2011, 341: 485-493. 10.1007/s11104-010-0660-3.
Harish S, Kavino M, Kumar N, Balasubramanian P, Samiyappan R: Induction of defense-related proteins by mixtures of plant growth promoting endophytic bacteria against Banana bunchy top virus. Biol Control. 2009, 51: 16-25. 10.1016/j.biocontrol.2009.06.002.
Khalil S, Labuschagne I: Role of mycorrhizae, pathogens and weeds in sustainable pine Forest management Soil biology and biochemistry section, national agricultural research centre, Islamabad–Pakistan. Int J Agric Biol. 2002, 4: 1-
Riedlinger J, Schrey SD, Tarkka MT, Hampp R, Kapur M, Fiedler HP: Auxofuran, a novel substance stimulating growth of fly agaric, produced by the mycorrhiza helper bacterium Streptomyces AcH 505. Appl Environ Microbiol. 2006, 72: 3550-3557.
Toussaint JP, Kraml M, Nell M, Smith SE, Smith FA, Steinkellner S, Schmiderer H, Novak V: Effect of Glomus mosseae on concentrations of rosmarinic and caffeic acids and essential oil compounds in basil inoculated with Fusarium oxysporum f. sp. basilica. Plant Pathol. 2008, 57: 1109-1116. 10.1111/j.1365-3059.2008.01895.x.
Liu JY, Maldonado-Mendoza I, Lopez-Meyer M, Cheung F, Town CD, Harrison MJ: Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J. 2007, 50: 529-544.
Neeraj KS: Organic amendments to soil inoculated arbuscular mycorrhizal fungi and Pseudomonas fluorescens treatments reduce the development of root-rot disease and enhance the yield of Phaseolus vulgaris L. Eur J Soil Biol. 2011, 47: 288-295. 10.1016/j.ejsobi.2011.07.002.
Bonfante P, Genre A: Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat Commun. 2010, 27: 1-48.
Plett JM, Kemppainen M, Kale SD, Kohler A, Legue V, Brun A, Tyler BM, Pardo AG, Martin F: A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr Biol. 2011, 21: 1197-1203.
Splivallo R, Fischer U, Gobel C, Feussner I, Karlovsky P: Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 2009, 150: 2018-2029.
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM: Agricultural importance of algae. Afr J Biotechnol. 2012, 11: 11648-11658.
Salvioli A, Zouari I, Chalot M, Bonfante P: The arbuscular mycorrhizal status has an impact on the transcriptome profile and amino acid composition of tomato fruit. BMC Plant Biol. 2012, 12: 44-
Kosuta S: Diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific expression in roots of Medicago truncatula. Plant Physiol. 2003, 131: 952-962.
Roberts NJ, Morieri G, Kalsi G, Rose A, Stiller J, Edwards A, Xie F, Gresshoff PM, Oldroyd GE, Downie JA, Etzler ME: Rhizobial and mycorrhizal symbioses in Lotus japonicus require lectin nucleotide phosphohydrolase, which acts upstream of calcium signaling. Plant Physiol. 2013, 161: 556-567.
Sieberer BJ, Chabaud M, Timmers AC, Monin A, Fournier J, Barker DG: A nuclear-targeted cameleon demonstrates intranuclear Ca 2+ spiking in Medicago truncatula root hairs in response to rhizobial nodulation factors. Plant Physiol. 2009, 151: 1197-1206.
Molina-Favero C, Mónica Creus C, Luciana Lanteri M, Correa-Aragunde N, Lombardo MC, Barassi AC, Lamattina L: Nitric Oxide and Plant Growth Promoting Rhizobacteria: Common Features Influencing Root Growth and Development. Adv Bot Res. 2007, 46: 1-33.
Bucher M, Wegmüller S, Drissner D: Chasing the structures of small molecules in arbuscular mycorrhizal signalling. Curr Opin Plant Biol. 2009, 12: 500-507.
Bapaume L, Reinhardt D: How membranes shape plant symbioses: signaling and transport in nodulation and arbuscular mycorrhiza. Front Plant Sci. 2012, 3: 223-
Zhang Q, Blaylock LA, Harrison MJ: Two Medicago truncatula Half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell. 2010, 22: 1483-1497.
Tromas A, Parizot B, Diagne N, Champion A, Hocher V: Heart of endosymbioses: transcriptomics reveals a conserved genetic program among arbuscular mycorrhizal, actinorhizal and legume-rhizobial symbioses. PLoS ONE. 2012, 7: e44742-
Sevilla M, Burris RH, Gunapala N, Kennedy C: Comparison of benefit to sugarcane plant growth and 15n2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and Nif–mutant strains. Mol Plant-Microbe Interact. 2001, 14: 358-366. 10.1094/MPMI.2001.14.3.358.
Bertalan M, Albano R, de-Pádua V, Rouws L, Rojas C, Hemerly A, Teixeira K, Schwab S, Araujo J, Oliveira A, França L, Magalhães V, Alquéres S, Cardoso A, Almeida W, Loureiro MM, Nogueira E, Cidade D, Oliveira D, Simão T, Macedo J, Valadão A, Dreschse M, Freitas F, Vida M, Guedes H, Rodrigues E, Meneses C, Brioso P, Pozzer L: Complete genome sequence of the sugarcane nitrogen-fixing endophyte Gluconacetobacter diazotrophicus Pal5. BMC Genomics. 2009, 10: 450-
Brusamarello-Santos L, Pacheco F, Aljanabi S, Monteiro R, Cruz L, Baura V, Pedrosa F, Souza E, Wassem R: Differential gene expression of rice roots inoculated with the diazotroph Herbaspirillum seropedicae. Plant Soil. 2012, 356: 113-125. 10.1007/s11104-011-1044-z.
Perrig D, Boiero ML, Masciarelli OA, Penna C, Ruiz OA, Cassan FD, Luna MV: Plant-growth promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl Microbiol Biotechnol. 2007, 75: 1143-1150.
Bent E, Tuzun S, Chanway CP, Enebak S: Alterations in plant growth and in root hormone levels of lodgepole pines inoculated with rhizobacteria. Can J Microbiol. 2001, 47: 793-800.
Sudha M, Gowri RS, Prabhavati P, Astapriya P, Devi SY, Saranya A: Production and optimization of indole-acetic-acid by indigenous micro flora using agro waste as substrate. Pakistan J Biological Sci. 2012, 15: 39-43. 10.3923/pjbs.2012.39.43.
Glick BR, Penrose DM, Li J: A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol. 1998, 190: 63-68.
Tripura CB, Sudhakar Reddy P, Reddy MK, Sashidhar B, Podile AR: Glucose dehydrogenase of a rhizobacterial strain of Enterobacter asburiae involved in mineral phosphate solubilization shares properties and sequence homology with other members of enterobacteriaceae. Indian J Microbiol. 2007, 47: 126-131.
Sashidhar B, Podile AR: Mineral phosphate solubilisation by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol. 2010, 109: 1-12.
Ané JM1, Kiss GB, Riely BK, Penmetsa RV, Oldroyd GE, Ayax C, Lévy J, Debellé F, Baek JM, Kalo P, Rosenberg C, Roe BA, Long SR, Dénarié J, Cook DR: Medicago truncatula DMI1required for bacterial and fungal symbioses in legumes. Sci. 2004, 303: 1364-1367. 10.1126/science.1092986.
Maillet F, Poinsot V, André O, Puech-Pagès V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A, Martinez EA, Driguez H, Bécard G, Dénarié J: Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature. 2011, 469: 58-63.
Download references
Acknowledgments
Work on biofertilizers, signal transduction and plant stress tolerance in NT’s laboratory is partially supported by ICGEB, New Delhi, Department of Science and Technology (DST) and Department of Biotechnology (DBT), Government of India.
Author information
Authors and affiliations.
Plant Molecular Biology Group, International Centre for Genetic Engineering and Biotechnology (ICGEB), Aruna Asaf Ali Marg, New Delhi, 110067, India
Deepak Bhardwaj, Mohammad Wahid Ansari, Ranjan Kumar Sahoo & Narendra Tuteja
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Narendra Tuteja .
Additional information
Competing interests.
Authors declare that they have no competing interests.
Authors’ contributions
MWA and DB contributed with the paper writing, data researching and designs the Figures. RKS supported the paper writing, data researching and revised the changes made to this paper. NT approved the changes made, and also with data researching and formatted the review. All authors read and approved the final manuscript.
Deepak Bhardwaj, Mohammad Wahid Ansari contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/ .
The Creative Commons Public Domain Dedication waiver ( https://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bhardwaj, D., Ansari, M.W., Sahoo, R.K. et al. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact 13 , 66 (2014). https://doi.org/10.1186/1475-2859-13-66
Download citation
Received : 25 February 2014
Accepted : 30 April 2014
Published : 08 May 2014
DOI : https://doi.org/10.1186/1475-2859-13-66
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Biofertilizer
- Crop improvement
- Environmental stress
- Mode of action of biofertilizers
- Sustainable agriculture
Microbial Cell Factories
ISSN: 1475-2859
- Submission enquiries: [email protected]
Fermentation: A Process for Biofertilizer Production
- First Online: 08 December 2017
Cite this chapter

- Harish Suthar 6 ,
- Krushi Hingurao 7 ,
- Jaysukh Vaghashiya 6 &
- Jivabhai Parmar 8
Part of the book series: Microorganisms for Sustainability ((volume 6))
2549 Accesses
12 Citations
Biofertilizers are the product of fermentation process, constituting efficient living soil microorganisms. They improve plant growth and productivity through supply of easily utilizable nutrients. They are cost-effective and eco-friendly bioinoculants having great potential to enhance agricultural production in sustainable way. Biofertilizers are grouped into different types based on their functions such as nitrogen-fixing, phosphate-solubilizing, phosphate mobilizing, and other plant growth-promoting biofertilizers promoting plant growth by different mechanisms. Solid-state fermentation and submerged fermentation are two main types of fermentation, used for the production of biofertilizers. Each type of biofertilizer is prepared by selection of efficient microbial strain, its cultivation using specific nutrient medium, scale-up, and formulation using solid or liquid base. Knowledge about host specificity of the microbial strain and properties of soil and environmental conditions of the field are the important factors which determine the success of biofertilizer application. Recent developments in the field of microbial taxonomy, molecular biology, genetic engineering, metabolic engineering, computer science, and nanotechnology have played a significant role in the advancement of fermentation process of biofertilizer production. Hence, the production of biofertilizers having better efficiency, higher competitive ability, multiple functionality, and longer shelf life has become possible. Biofertilizers with such characteristics can be an effective substitute of chemical fertilizers. The present chapter deals with the types of biofertilizers, their applications and outcomes, types of fermentation processes used for biofertilizer production, and past and present status of fermentation technologies used for biofertilizer production.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Similar content being viewed by others

Unexploited potential of some biotechnological techniques for biofertilizer production and formulation
Recent Advancements in Fermentation Strategies for Mass Production and Formulation of Biofertilizers: Towards Waste Valorization
Biofertilizers: an advent for eco-friendly and sustainable agriculture development.
Abd El-Fattah DA, Eweda WE, Zayed MS et al (2013) Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculants. Ann Agric Sci 58(2):111–118
Google Scholar
Abd El-Lattief EA (2016) Use of Azospirillum and Azotobacter bacteria as biofertilizers in cereal crops: a review. Int J Res Eng Appl Sci 6(7):36–44
Abdel Ghany TM, Alawlaqi MM, Al Abboud MA (2013) Role of biofertilizers in agriculture: a brief review. Mycopathologia 11(2):95–101
Abi-Ghanem R, Carpenter-Boggs L, Smith JL et al (2012) Nitrogen fixation by US and middle eastern chickpeas with commercial and wild middle eastern inocula. ISRN Soil Sci 2012:1. https://doi.org/10.5402/2012/981842
Article CAS Google Scholar
Adholeya A, Das M (2012) Biofertilizers: potential for crop improvement under stressed conditions. In: Tuteja N, Gill SS, Tuteja R (eds) Improving crop productivity in sustainable agriculture. Wiley-VCH, Weinheim, pp 183–200
Chapter Google Scholar
Agarwal S, Ahmad Z (2010) Contribution of the Rhizobium inoculation on plant growth and productivity of two cultivars of Berseem ( Trifolium alexandrinum L.) in saline soil. Asian J Plant Sci 9:344–350
Article Google Scholar
Akhtar M, Siddiqui Z (2009) Effects of phosphate solubilizing microorganisms and Rhizobium sp. on the growth, nodulation, yield and root-rot disease complex of chickpea under field condition. Afr J Biotechnol 8(15):3489–3496
Alam S, Seth RK (2014) Comparative study on effect of chemical and biofertilizer on growth, development and yield production of paddy crop ( Oryza sativa ). Int J Sci Res 3(9):411–414
Alam S, Khalil S, Ayub N et al (2002) In vitro solubilization of inorganic phosphate by phosphate solubilizing microorganism (PSM) from maize rhizosphere. Int J Agric Biol 4:454–458
CAS Google Scholar
Albareda M, Rodríguez-Navarro DN, Camacho M et al (2008) Alternatives to peat as a carrier for rhizobia inoculants: solid and liquid formulations. Soil Biol Biochem 40:2771–2779
Allen EK, Allen ON (1950) The anatomy of nodular growths on roots of Tribulus cistoides L. Proc Soil Soc Am 14:179–183
Armada E, Portela G, Roldan A et al (2014) Combined use of beneficial soil microorganism and agro waste residue to cope with plant water limitation under semiarid conditions. Geoderma 232:640–648
Atieno M, Herrmann L, Okalebo R et al (2012) Efficiency of different formulations of Bradyrhizobium japonicum and effect of co-inoculation of Bacillus subtilis with two different strains of Bradyrhizobium japonicum . World J Microbiol Biotechnol 28:2541–2550
Article CAS PubMed Google Scholar
Auge RM, Toler HD, Saxton AM (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24
Article PubMed Google Scholar
Baby UI (2002) Biofertilizers in tea planters. Chronicle 98:395–396
Bakri Y, Akeed Y, Thonart P (2012) Comparison between continuous and batch processing to produce xylanase by Penicillium canescens 10-10c. Braz J Chem Eng 29(3):441–448
Baral RB, Adhikari P (2013) Effect of Azotobacter on growth and yield of maize. SAARC J Agri 11(2):141–147
Bashan Y, Holguin G, Lifshitz R (1993) Isolation and characterization of plant growth-promoting rhizobacteria. In: Glick BR, Thompson JE (eds) Methods in plant molecular biology and biotechnology. CRC Press, Boca Raton, pp 331–345
Bassey EE (2013) Trends in fermentation process, purification and recovering of biomolecules. Inter J Agri Biosci 2(6):340–343
Beijerinck MW (1888) Culture des Bacillus radicola aus den. Knöllchen. Bot Ztg 46:740–750
Beijerinck MW (1922) Azotobacter chroococcum als indikator van de vruchtbarrheid van den grond. K Ned Akad Wet Versl GewoneVergad Afd Natuurkd 30:431–438
Ben Rebah F, Tyagi RD, Prevost D (2002) Wastewater sludge as a substrate for growth and carrier for rhizobia: the effect of storage conditions on survival of Sinorhizobium meliloti . Bioresour Technol 83:145–151
Ben Rebah F, Prevost D, Yezza A et al (2007) Agro-industrial waste materials and wastewater sludge for rhizobial inoculant production: a review. Bioresour Technol 98:3535–3546
Berruti A, Lumini E, Balestrini R et al (2016) Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front Microbiol 6:1559. https://doi.org/10.3389/fmicb.2015.01559
Article PubMed PubMed Central Google Scholar
Bhardwaj D, Ansari MW, Sahoo RK et al (2014) Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Factories 13(1):1–9
Biederbeck VO, Geissler HJ (1993) Effect of storage temperatures on rhizobium meliloti survival in peat- and clay-based inoculants. Can J Plant Sci 73:101–110
Bissonnette N, Lalande R, Bordeleau LM (1986) Large-scale production of Rhizobium meliloti on Whey. Appl Environ Microbiol 52(4):838–841
CAS PubMed PubMed Central Google Scholar
Brimhall GH, Chadwick OA, Lewis CJ et al (1992) Deformational mass transport and invasive processes in soil evolution. Science 255:695–702
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154(2):275–304
Carnahan JE, Mortenson LE, Mower HF et al (1960) Nitrogen fixation in cell-free extracts of Clostridium pasteurian . Biochim Biophys Acta 44:520–535
Chanda S, Matai S, Chakrabatri S (1987) Deproteinized leaf juice as a medium for growth of Rhizobium . Indian J Exp Biol 25:573–575
CAS PubMed Google Scholar
Chang CH, Yang SS (2009) Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour Technol 100:1648–1658
Coelho LF, de Lima CJB, Rodovalho CM et al (2011) Lactic acid production by new Lactobacillus plantarum LMISM6 grown in molasses: optimization of medium composition. Braz J Chem Eng 28(1):201–203
Cooper J (2004) Multiple responses of rhizobia to flavonoids during legume root infection. In: Callow JA (ed) Advances in botanical research incorporating advances in plant pathology. Academic, London, pp 1–62
Demain AL (2000) Microbial biotechnology. Trends Biotechnol 18(1):26–31
Dobereiner J, Day JM (1976) Associative symbioses in tropical grasses: characterization of microorganisms and dinitrogen fixing sites. In: Newton WE, Nymans CJ (eds) Proceedings of the first international symposium on nitrogen fixation. Washington State University Press, Pullman, pp 518–538
Dodd IC, Ruiz-Lozano JM (2012) Microbial enhancement of crop resource use efficiency. Curr Opin Biotechnol 23:236–242
Doroshenko EV, Bulygina ES, Spiridonova EM et al (2007) Isolation and characterization of nitrogen-fixing bacteria of the genus Azospirillum from the soil of a sphagnum peat bog. Mikrobiologiia 76(1):107–115
Douds DD, Nagahashi G, Pfeffer PE et al (2005) On-farm production and utilization of Arbuscular mycorrhizal fungus inoculum. Can J Plant Sci 85:15–21
Duraraj P, Maniarasan U, Nagarajan N (2016) Study of growth and yield of cluster bean in alkaline soil using organic manure and biofertilizers. Int J Pl An and Env Sci 6(2):22–27
Estrella MJ, Pieckenstain FL, Marina M et al (2004) Cheese whey: an alternative growth and protective medium for Rhizobium loti cells. J Ind Microbiol Biotechnol 31:122–126
FCO (1985) Biofertilizers and Organic Fertilizers in Fertilizer (Control) Order, 1985, National Centre of Organic Farming, Department of Agriculture and Cooperation, Ministry of Agriculture, Govt of India. http://ncof.dacnet.nic.in/Training_manuals/Training_manuals_in_English/BF_and_OF_in_FCO.pdf . Accessed 2 Oct 2017
Fox AR, Soto G, Valverde C et al (2016) Major cereal crops benefit from biological nitrogen fixation when inoculated with the nitrogen-fixing bacterium Pseudomonas protegens Pf-5 X940. Environ Microbiol 18(10):3522–3534
Franche C, Lindstrom K, Elmerich C (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321(1–2):35–59
Gandora V, Gupta RD, Bhardwaj KKR (1998) Abundance of Azotobacter in great soil groups of North-West Himalayas. J Indian Soc Soil Sci 46(3):379–383
Ghany TMA, Alawlaqi MM, Al Abboud MA (2013) Role of biofertilizers in agriculture: a brief review. Mycopath 11(2):95–101
Ghosh N (2004) Promoting bio-fertilizers in Indian agriculture. Econ Polit Wkly 39(52):5617–5625
Glick BR, Todorovic B, Czarny J et al (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242
Gomare KS, Mese M, Shetkar Y (2013) Isolation of Azotobacter and cost effective production of biofertilizer. Indian J Appl Res 3(5):54–56
Gurikar C, Naik MK, Sreenivasa MY (2016) Azotobacter : PGPR activities with special reference to effect of pesticides and biodegradation. In: Singh DP, Singh HB, Prabha R (eds) Microbial inoculants in sustainable agricultural productivity, vol 1. Springer, New Delhi, pp 229–244
Hameed S, Yasmin S, Malik S et al (2004) Rhizobium , Bradyrhizobium and Agrobacterium strains isolated from cultivated legumes. Biol Fertil Soils 39(3):179–185
Harrison MJ, van Buuren ML (1995) A phosphate transporter from the mycorrhizal fungus Glomus versiforme . Nature 378:626–629
Hellriegel H, Wilfarth H (1888) Untersuchungenüber die Stickstoffnahrung der Gramineen und Leguminosen. Beilageheftzu der Zeitschrift des Vereinsfür die Rübenzucker-Industrie des DeutschenReiches, Bu’chdruckerei der “Post,” Kayssler & Co, Berlin
Herrmann L, Lesueur D (2013) Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol 97(20):8859–8873
Jaga PK, Upadhyay VB (2013) Effect of FYM, biofertilizer and chemical fertilizers on wheat. Asian J Soil Sci 8(1):185–188
Jain SK, Pathak DV, Sharma HR (2000) Alternate C substrate for mass production of Rhizobium inoculants. Haryana Agric Univ J Res 30:1–6
Javaid A (2009) Arbuscular mycorrhizal mediated nutrition in plants. J Plant Nutr 32(10):1595–1618
Jayaraj J, Yi H, Liang GH et al (2004) Foliar application of Bacillus subtilis AUBS 1 reduces sheath blight and triggers defense mechanisms in rice. J Plant Dis Protect 111(2):115–125
Kannaiyan S, Kumar K (2005) Azolla biofertilizers for sustainable rice production. Daya Publishing House, New Delhi
Kapulnic Y, Kigel J, Nur I et al (1981) Effect of Azospirillum inoculation on some growth parameters and n content of wheat, sorghum and panicum. Plant Soil 61:65–70
Kaushik BD (2014) Developments in Cyanobacterial biofertilizer. Proc Indian Nat Sci Acad 80(2):379–388
Kim KY, Jordan D, McDonald GA (1998) Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol Fertil Soils 26:79–87
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12:181–184
Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th international conference on plant pathogenic bacteria. Station de Pathologie Végétale et de Phytobactériologie, anger, 27 August−2 September
Kobiler D, Cohen-Sharon A, Tel-Or E (1981) Recognition between the N 2 -fixing Anabaena and the water fern Azolla . FEBS Lett 133:157–160
Kukreja K, Suneja S, Goyal S et al (2004) Phytohormone production by Azotobacter -a review. Agric Rev 25(1):70–75
Kumar A, Kumari B, Mallick MA (2016) Phosphate solubilizing microbes: an effective and alternative approach as biofertilizers. Int J Pharm Pharm Sci 8(2):37–40
Kure AM, Patil SR, Jadhao VG (2016) Performance evaluation of developed lab scale fermenter. Int J Agric Engg 9(2):202–209
Lavania M, Nautiyal CS (2013) Solubilization of tricalcium phosphate by temperature and salt tolerant Serratia marcescens NBRI1213 isolated from alkaline soils. Afr J Microbiol Res 7(34):4403–4413. https://doi.org/10.5897/AJMR2013.5773
Lazcano C, Barrios-Masias FH, Jackson LE (2014) Arbuscular mycorrhizal effects on plant water relations and soil greenhouse gas emissions under changing moisture regimes. Soil Biol Biochem 74:184–192
Leo Daniel AE, Venkateswarlu B, Suseelendra D et al (2013) Effect of polymeric additives, adjuvants, surfactants on survival, stability and plant growth promoting ability of liquid bioinoculants. J Plant Physiol Pathol 01(02):1–5. https://doi.org/10.4172/2329-955X.1000105
Li XX, Liu Q, Liu XM et al (2016) Using synthetic biology to increase nitrogenase activity. Microb Cell Factories 15:43
Maier RJ, Moshiri F (2000) Role of the Azotobacter vinelandii nitrogenase-protective shethna protein in preventing oxygen mediated cell death. J Bacteriol 182(13):3854–3857
Article CAS PubMed PubMed Central Google Scholar
Malusa E, Sas-Paszt L, Ciesielska J (2012) Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J 2012:1. https://doi.org/10.1100/2012/491206
Mia MAB, Shamsuddin ZH (2010) Rhizobium as a crop enhancer and biofertilizer for increased cereal production. Afr J Biotech 9:6001–6009
Mishra DJ, Singh R, Mishra UK et al (2013) Role of bio-fertilizer in organic agriculture: a review. Res J Recent Sci 2(1):39–41
Moore D, Robson GD, Trinci APJ (2011) 21st century guidebook to fungi. Cambridge University Press, New York
Book Google Scholar
Morgan JAW, Bending GD, White PJ (2005) Biological costs and benefits to plant–microbe interactions in the rhizosphere. J Exp Bot 56(417):1729–1739
Nagappan R (2013) Biopesticides and biofertilizers: ecofriendly sources for sustainable agriculture. J Biofertil Biopestici 4:1–2. https://doi.org/10.4172/2155-6202.1000e112
Nalawde AA, Bhalerao SA (2015) Comparative account of effect of biofertilizers on the growth and biochemical parameters of Vigna mungo (L. Hepper). Int J Adv Res Biol Sci 2(5):62–66
Nayak T, Patangray AJ (2015) Biofertilizer-beneficial for sustainable agriculture and improving soil fertility. Asia J Multidiscip Stud 3(2):189–194
Nehra V, Choudhary M (2015) A review on plant growth promoting rhizobacteria acting as bioinoculants and their biological approach towards the production of sustainable agriculture. J Appl Nat Sci 7:540–556
Nobbe F, Hiltner L (1895) Inoculation of the soil for cultivating leguminous plants. US Patent 5,70,813, 3 November 1896
Okon Y, Albercht SL, Burris RH (1977) Methods for growing Spirillum lipoferum and for counting it in pure culture and in association with plants. Appl Environ Microbiol 33:85–88
Pandey A, Selvakumar P, Soccol CR et al (1999) Solid state fermentation for the production of industrial enzymes. Curr Sci 77(1):149–162
Parthiban K, Manikandan S, Ganesapandian S (2011) Production of cellulose I microfibrils from Rhizobium sp. and its wound healing activity on mice. Asian J Applied Sci 4:247–254
Pathak DV, Kumar M (2016) Microbial inoculants as biofertilizers and biopesticides. In: Singh DP, Singh HB, Prabha R (eds) Microbial inoculants in sustainable agricultural productivity, vol 1. Springer, New Delhi, pp 197–209
Patil N, Choudhri SD, Gawade SS et al (2013) Effect of different C sources on production and stability of biofertilizer. Int J of Adv Biotec and Res 4(1):85–95
Paul D, Nair S (2008) Stress adaptations in a plant growth promoting Rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J Basic Microbiol 48:1–7
Peng X, Börner RA, Nges IA et al (2014) Impact of bioaugmentation on biochemical methane potential for wheat straw with addition of Clostridium cellulolyticum . Bioresour Technol 152:567–571
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiologia 17:362–370
Pindi PK, Satyanarayana SDV (2012) Liquid microbial consortium a potential tool for sustainable soil health. J Biofertil Biopestici 03:124. https://doi.org/10.4172/2155-6202.1000124
Podile AR, Kishore GK (2006) Plant growth promoting rhizobacteria. In: Gnanamanickam SS (ed) Plant associated bacteria. Springer, Dordrecht, pp 195–230
Ponmurugan P, Gopi C (2006) In vitro production of growth regulators and phosphatase activity by phosphate solubilizing bacteria. Afr J Biotechnol 5:348–350
Prasad MP (2014) Optimization of fermentation conditions of phosphate solubilization bacteria – a potential bio-fertilizer. Merit Res J Microbiol Biol Sci 2(2):31–35
Prasanna R, Nain L, Pandey AK et al (2012) Microbial diversity and multidimensional interactions in the rice ecosystem. Arch Agron Soil Sci 58(7):723–744
Rafael A, Juan Cesar FO, Leonardo C (2017) Metabolic engineering of a diazotrophic bacterium improves ammonium release and biofertilization of plants and microalgae. Metab Eng 40:59. https://doi.org/10.1016/j.ymben.2017.01.002
Reddy KR, Reddy GB, Reddy MR et al (1977) Effects of Azotobacter inoculation and nitrogen levels on yield of sorghum. Indian J Agron 22(4):203–205
Richter DD, Markewitz D (1995) How deep is soil? Bio Sci 45:600–609
Rillig MC, Aguilar-Trigueros CA, Bergmann J et al (2015) Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol 205:1385–1388
Rodrigues PE, Rodregues LS, Oliveira ALM et al (2008) Azospirillum amazonense inoculation: effects on growth, yield and N 2 -fixation of rice ( Oryza sativa L.) Plant Soil 302:249–261
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rosenberger RF, Elsden SR (1960) The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol 22:726–739
Roychowdhury D, Paul M, Kumar Banerjee S (2015) Isolation, identification and characterization of phosphate solubilising bacteria from soil and the production of biofertilizer. Int J Curr Microbiol App Sci 4(11):808–815
Saithi S, Borg J, Nopharatana M et al (2016) Mathematical modelling of biomass and enzyme production kinetics by Aspergillus niger in solid-state fermentation at various temperatures and moisture contents. J Microb Biochem Technol 8:123–130
Secilia J, Bagyaraj DJ (1987) Bacteria and actinomycetes associated with pot cultures of vesicular arbuscular mycorrhizas. Can J of Microbiol 33:1069–1073
Senoo K, Kaneko M, Taguchi R et al (2002) Enhanced growth and nodule occupancy of red kidney bean and soybean inoculated with soil aggregate-based inoculants. Soil Sci Plant Nutr 48(2):251–259
Sethi SK, Adhikary SP (2012) Cost effective pilot scale production of biofertilizer using Rhizobium and Azotobacter . Afr J Biotechnol 11(70):13490–13493
Shanware A, Kalkar S, Trivedi M (2014) Potassium solublisers: occurrence, mechanism and their role as competent biofertilizers. Int J Curr Microbiol App Sc 3(9):622–629
Sharma S, Pant D, Singh S et al (2007) Mycorrhizae in Indian agriculture. In: Hamel C, Plenchette C (eds) Mycorrhizae in crop production. Haworth Press, Binghampton, pp 239–291
Sharma SB, Sayyed RZ, Trivedi MH et al (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587. https://doi.org/10.1186/2193-1801-2-587
Article PubMed PubMed Central CAS Google Scholar
Singh JS, Pandey VC, Singh DP (2011) Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric Ecosyst Environ 140:339–353
Singh AK, Gauri C, Bhatt RP et al (2012) Comparative study of carrier based materials for rhizobium culture formulation. Indian J Agric Res 46(4):344–349
Singh S, Singh BK, Yadav SM et al (2014) Potential of biofertilizers in crop production in Indian agriculture. Amer J Plant Nutr Fertil Tech 4(2):33–40
Singh M, Dotaniya ML, Mishra A et al (2016a) Role of biofertilizers in conservation agriculture. In: Bisht JK, Meena VS, Mishra PK et al (eds) Conservation agriculture: an approach to combat climate change in Indian Himalaya. Springer, Singapore, pp 113–134
Singh JS, Kumar A, Rai AN et al (2016b) Cyanobacteria : a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front Microbiol 7:529. https://doi.org/10.3389/fmicb.2016.00529
PubMed PubMed Central Google Scholar
Stewart WDP (1969) Biological and ecological aspects of nitrogen fixation by free-living microorganisms. Proc R Soc 172:367–388
Subba Rao NS (1977) Soil microorganisms and plant growth. Oxford/IBH Publishing Co, New Delhi
Subramaniyam R, Vimala R (2012) Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int J Sci Nat 3(3):480–486
Tao GC, Tian SJ, Cai MY et al (2008) Phosphate solubilizing and mineralizing abilities of bacteria isolated from soils. Pedosphere 18(4):515–523
Tarrand JJ, Krieg NR, Döbereiner J (1978) A taxonomic study of the Spirillum lipoferum group, with description of a new genus, Azospirillum gen. nov., and two species, Azospirillum lipoferum ( Beijerinck ) com nov. and Azospirillum brasilense sp. nov. Can J Microbiol 24:967–980
Tensingh Baliah N, Jeeva P (2016) Isolation, identification and characterization of phosphate solubilizing bacteria isolated from economically important tree species. Int J Sci Nat 7(4):870–876
Trivedi P, Pandey A (2007) Application of immobilized cells of Pseudomonas putida to solubilise insoluble phosphate in broth and soil conditions. J Plant Nutr Soil Sci 170:629–631
Trivedi M, Kalkar S, Shanware A (2016) Isolation, characterization & development of liquid formulations of potassium solubilizing fungi. Int J Adv Res 4(9):999–1003
Vaishampayan A, Sinha RP, Hader DP et al (2001) Cyanobacterial biofertilizers in rice agriculture. Bot Rev 67(4):453–516
van Rhijn P, Vanderleyden J (1995) The Rhizobium -plant symbiosis. Microbiol Rev 59(1):124–142
van Rossum DV, Schuurmans FP, Gillis M et al (1995) Genetic and phenotypic analysis of Bradyrhizobium strains nodulating Peanut ( Arachis hypogaea L.) roots. Appl Environ Microbiol 61:1599–1609
Verma M, Sharma S, Prasad R (2011) Liquid biofertilizers: advantages over carrier- based biofertilizers for sustainable crop production. Newsl Intern Soc Environ Bot 17:2
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Waheed A, Afzal A, Sultan T et al (2014) Isolation and biochemical characterization of rhizobium from pea crop at Swabi. Int J Biosci 4(8):231–240
Wani SA, Chand S, Ali T (2013) Potential use of Azotobacter chroococcum in crop production: an overview. Curr Agri Res 1(1):35–38. 10.12944/CARJ.1.1.04
Wani SA, Chand S, Wani MA et al (2016) Azotobacter chroococcum– a potential biofertilizer in agriculture: an overview. In: Kakeem KR, Akhtar J, Sabir M (eds) Soil science: agricultural and environmental prospectives. Springer, Cham, pp 333–348
Wei GH (2002) Rhizobium indigoferae sp. nov. and Sinorhizobium kummerowiae sp. nov., respectively isolated from Indigofera spp. and Kummerowia stipulacea . Int J Syst Evol Microbiol 52(6):2231–2223
Weir BS (2016) The current taxonomy of rhizobia http://www.rhizobia.co.nz/taxonomy/rhizobia.html . Accessed 4 Feb 2017
Yadav AK, Chandra K (2014) Mass production and quality control of microbial inoculants. Proc Indian Natn Sci Acad 80(2):483–489
Yehya M, Hamze M, Mallat H et al (2013) Prevalence and antibiotic susceptibility of Bacteroides fragilis group isolated from stool samples in North Lebanon. Braz J Microbio l44(3):807–812
Zahran HH (1999) Rhizobium -legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63(4):968–89, table of contents
Zaiadan BK, Motorin DN, Baimakhanova GB et al (2014) Promising microbial consortia for producing biofertilizers for rice fields. Mikrobiologiia 83(4):467–474
Zohar-Perez C, Chet I, Nussinovitch A (2005) Mutual relationships between soils and biological carrier systems. Biotechnol Bioeng 92(1):54–60
Download references
Author information
Authors and affiliations.
Department of Post-Harvest Technology, ASPEE College of Horticulture and Forestry, Navsari Agricultural University, Navsari, Gujarat, 396 450, India
Harish Suthar & Jaysukh Vaghashiya
Department of Microbiology and Biotechnology Centre, Faculty of Science, The M. S. University of Baroda, Vadodara, Gujarat, 390 002, India
Krushi Hingurao
Department of Fruit Science, ASPEE College of Horticulture and Forestry, Navsari Agricultural University, Navsari, Gujarat, 396 450, India
Jivabhai Parmar
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Harish Suthar .
Editor information
Editors and affiliations.
Department of Agricultural Microbiology, B. A. College of Agriculture, Anand Agricultural University, Anand, Gujarat, India
Deepak G. Panpatte
Yogeshvari K. Jhala
Rajababu V. Vyas
Harsha N. Shelat
Rights and permissions
Reprints and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Suthar, H., Hingurao, K., Vaghashiya, J., Parmar, J. (2017). Fermentation: A Process for Biofertilizer Production. In: Panpatte, D., Jhala, Y., Vyas, R., Shelat, H. (eds) Microorganisms for Green Revolution. Microorganisms for Sustainability, vol 6. Springer, Singapore. https://doi.org/10.1007/978-981-10-6241-4_12
Download citation
DOI : https://doi.org/10.1007/978-981-10-6241-4_12
Published : 08 December 2017
Publisher Name : Springer, Singapore
Print ISBN : 978-981-10-6240-7
Online ISBN : 978-981-10-6241-4
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
REVIEW article
Plant-microbe interactions in the rhizosphere for smarter and more sustainable crop fertilization: the case of pgpr-based biofertilizers.

- Faculty of Agricultural, Environmental and Food Sciences, Free University of Bolzano, Bolzano, Italy
Biofertilizers based on plant growth promoting rhizobacteria (PGPR) are nowadays gaining increasingly attention as a modern tool for a more sustainable agriculture due to their ability in ameliorating root nutrient acquisition. For many years, most research was focused on the screening and characterization of PGPR functioning as nitrogen (N) or phosphorus (P) biofertilizers. However, with the increasing demand for food using far fewer chemical inputs, new investigations have been carried out to explore the potential use of such bacteria also as potassium (K), sulfur (S), zinc (Zn), or iron (Fe) biofertilizers. In this review, we update the use of PGPR as biofertilizers for a smarter and more sustainable crop production and deliberate the prospects of using microbiome engineering-based methods as potential tools to shed new light on the improvement of plant mineral nutrition. The current era of omics revolution has enabled the design of synthetic microbial communities (named SynComs ), which are emerging as a promising tool that can allow the formulation of biofertilizers based on PGPR strains displaying multifarious and synergistic traits, thus leading to an increasingly efficient root acquisition of more than a single essential nutrient at the same time. Additionally, host-mediated microbiome engineering (HMME) leverages advanced omics techniques to reintroduce alleles coding for beneficial compounds, reinforcing positive plant-microbiome interactions and creating plants capable of producing their own biofertilizers. We also discusses the current use of PGPR-based biofertilizers and point out possible avenues of research for the future development of more efficient biofertilizers for a smarter and more precise crop fertilization. Furthermore, concerns have been raised about the effectiveness of PGPR-based biofertilizers in real field conditions, as their success in controlled experiments often contrasts with inconsistent field results. This discrepancy highlights the need for standardized protocols to ensure consistent application and reliable outcomes.
1 Introduction
It is now a fact that the global request of agricultural food products is progressively rising because of the increase in the World population. In fact, as far as FAO projections are considered, human population inhabiting the planet is expected to reach more than 10 billion people by 2070 ( Daniel et al., 2022 ). In this scenario, the agricultural sector is urged to increase crops’ yield to meet the standard of food security. Indeed, since the green revolution (middle of 20th century), the increased inputs of chemical fertilizer proved itself as an efficient strategy to enhance agricultural productivity ( Fasusi et al., 2021 ). Unfortunately, due to the limited nutrient use efficiency, crops are able to exploit only a portion of the applied fertilizers, while the remaining part (generally much more than half) remains in the soil being then, very often, transformed in not available forms through different mechanisms (e.g., precipitation, adsorption, leaching, volatilization) ( Zhang et al., 2018 ). It is evident that this phenomenon, in addition to be less sustainable, particularly in a long period vision, poses serious risks for both the environment and human beings. In this regard as well as in the framework of the 17 sustainable development goals launched by the EU, 1 the implementation of innovative solutions for a more sustainable agriculture appears to be mandatory in the coming years. In this context, the exploitation of the potentialities lying in the so termed rhizosphere management seems extremely promising. The concept rhizosphere management refers to a series of actions aimed at increasing in this specific volume of soil the available fractions of the different nutrients for an equilibrate crop development and, then, production ( Zia et al., 2021 ; Kumawat et al., 2022 ). Interestingly, beneficial soil microorganisms, also accounted as plant growth-promoting rhizobacteria (PGPR), are known to be a promising tool in this sense thanks to their capability to significantly ameliorate the edaphic conditions of the rhizosphere soil ( Pii et al., 2015a ). In this regard, it should be highlighted that for crops a more balanced availability of nutrients, specifically in the rhizosphere, is not only strategic to guarantee the production (food security) in a more sustainable way, but also crucial for both the quality of primary production (food safety) ( Tomasi et al., 2015 ; Sambo et al., 2019 ) and the composition of its epiphytic microbial community ( Valentinuzzi et al., 2021 ), being this latter fundamental for the post-harvest characteristics of the edible plants tissues and their shelf life.
In a previous review, we have already postulated the need of deepening our understanding about the molecular and biochemical mechanisms underpinning plant-microbe interactions ( Pii et al., 2015a ) as a condition for a possible wider and more effective use of PGPRs in agriculture, particularly in a context of precision agriculture and its greater environmental sustainability. In these last years, many PGPR strains have been isolated and characterized for their potential usefulness in the management of the rhizosphere ( Fasusi et al., 2021 ). It is interesting to highlight that some of them are nowadays commonly used at the field scale as biofertilizers to achieve the objectives of higher food security and greater agricultural sustainability. Moreover, the results achievable with the application of these biofertilizers fit perfectly into the concept of a smart agriculture characterized by higher crop resilience and low external inputs that, even when essential, can be also tailored to the actual needs of the crops and to the variability of the agricultural land area ( Cesco et al., 2023 ).
Thus, considering recent scientific advancements in microbiology and biotechnology, this review incorporates insights from 158 relevant research papers published between 1996 and 2024. Notably, the majority of these papers (84%) were published in the last decade (2014–2024), reflecting the latest developments in the field. Accordingly, the review is organized into sections that highlight the crucial roles of plant-PGPR interactions in crop fertilization. It begins with a comprehensive exploration of PGPR as biofertilizers, focusing on their ability to enhance soil fertility and crop productivity by improving nutrient availability. The review then examines how essential nutrients (e.g., N, P, K, S, Zn, Fe) are mobilized and used by plants, discussing innovative biological strategies to enhance their availability, nutrient roles in plant metabolism, their soil forms, and uptake mechanisms. Additionally, the review highlights the potential of PGPR-based biofertilizers to boost nutrient availability and plant growth. It emphasizes the successes and challenges of these biofertilizers, underscoring the need for technologies like SynComs and genetic engineering to develop high-performing biofertilizers and engineered plants for enhanced nutrient acquisition and improved soil conditions. Finally, the review addresses skepticism about PGPR-based biofertilizers due to inconsistent field results compared to controlled experiments, and it calls for standardized protocols to ensure consistent application and reliability, facilitating their transition to practical agriculture.
In a view of an ever more efficient and eco-friendly agriculture, this research is especially important for farmer communities committed to sustainable agriculture systems. Adopting PGPR-based biofertilizers can enhance nutrient use efficiency, minimize environmental impacts, and boost both crop yield and quality ( Kumawat et al., 2023 ). This research supports the development of innovative and sustainable agricultural methods that address increasing food demand while protecting the environment and ensuring the long-term viability of farming operations.
2 Plant growth-promoting rhizobacteria as biofertilizers
About 20 years ago, the term biofertilizer was meant to define “a substance containing living microorganisms which, when applied to seeds, plant surfaces, or soil, colonizes the rhizosphere or the interior of the plant and promotes growth by increasing the supply or availability of primary nutrients to the host plant” ( Vessey, 2003 ). Through the years, several pieces of research have been carried out and, thanks to the knowledge acquired, one of the most recent definition, that is more in line with the content of this review, describes biofertilizer as “a product containing beneficial microorganisms with the potential to improve soil fertility and crop productivity by enhancing nutrient availability” ( Atieno et al., 2020 ). Since PGPR have been broadly reported as beneficial bacteria, they have been used worldwide as biofertilizers and characterized for their beneficial properties. The most reported effects are mainly ascribed to the involvement of members of the genera Azospirillum , Rhizobium , Bacillus , Pseudomonas , Burkholderia , Enterobacter , Herbaspirillum , Bradyrhizobium , Pantoea , Paenibacillus , Azotobacter , and Serratia ( Tabassum et al., 2017 ; Bhadrecha et al., 2023 ; Kumawat et al., 2024 ). However, to be considered an ideal biofertilizer with the potential to enhance plant nutrition and development upon inoculation, a PGPR strain should present the following characteristics:
1. It should possess a high rhizosphere colonization rate upon inoculation ( Backer et al., 2018 ).
2. It should be able to establish a stable and long-term colonization with the host plant ( Liu et al., 2019 ).
3. It should show a maximal, consistent and reproducible efficacy under a range of field conditions ( Herrmann and Lesueur, 2013 ).
4. It should be able to face intense competition with indigenous soil microbes ( Backer et al., 2018 ).
5. It should promote plant growth by improving nutrient availability at rhizosphere level ( Aloo et al., 2022 ), similarly to a localized fertilization.
6. It should present broad-spectrum versatility that encompasses a wide range of environmental adaptations ( Mitter et al., 2021 ).
7. It must be environmentally friendly and consistent with the requirements of sustainable practices ( Keswani et al., 2019 ).
8. It should be classified as safe, posing low threat to human health, soil and live communities ( Keswani et al., 2019 ) as desired in a context of One Health.
It is interesting to note that PGPR can also directly promote plant growth by providing phytohormones or signaling molecules, or they can indirectly influence plant health by synthesizing bioactive compounds with potential as antimicrobials or stress tolerance, hence earning the designation of biostimulants ( Kaushal et al., 2023 ). These compounds can modulate the root system architecture development, improve the photosynthetic capacity of a plant, or activate the antioxidant defense system ( Rosier et al., 2018 ; Grover et al., 2021 ). However, since the focus of this review is primarily on PGPR that can turn sparingly available fractions of essential nutrients into more accessible forms for plants, PGPR with biostimulating actions will not be considered and discussed.
For this reason, PGPR-based biofertilizers can be classified based on the specific mineral nutrient they contribute to promote the availability for plants ( Table 1 ). For instance, some PGPR strains are known to facilitate different processes including nitrogen (N) fixation (i.e., transforming atmospheric N 2 into NH 3 or NH 4 + ), solubilization of nutrients (i.e., turning insoluble forms of P into H 2 PO 4 − , insoluble K pools into the free ionic form [K + ], sparingly available Zn sources into the free ionic species [Zn 2+ ]), oxidation of substrates (i.e., producing SO 4 2− via oxidation of S 0 -containing sources), and/or metal chelation/complexation (e.g., exuding siderophores, phenolic compounds and/or organic acids to scavenge Fe 3+ and other nutrients) ( Colombo et al., 2014 ; Malusá and Vassilev, 2014 ; Mitter et al., 2021 ; Aloo et al., 2022 ). Moreover, it is important to mention that some strains of PGPR acting as biofertilizers, may have the ability to increase at the same time the supply to plants of more than one essential nutrient. Therefore, it is interesting to highlight that the action of these bacteria, whether single- or multi-element, is specifically localized in the rhizosphere, making them an extremely valuable tool for a precision agriculture context. In fact, the increasingly chance to use the endogenous nutritional resources of soil locally significantly reduces the need to broadcast synthetic fertilizers ( Vacheron et al., 2013 ; Pii et al., 2015a , 2016 ; Rosier et al., 2018 ). Furthermore, neglecting or not giving appropriate attention to the PGPR strains that exert direct effects on crop growth and health, particularly in the context of more efficient and more sustainable agriculture, would certainly represent a missed opportunity.
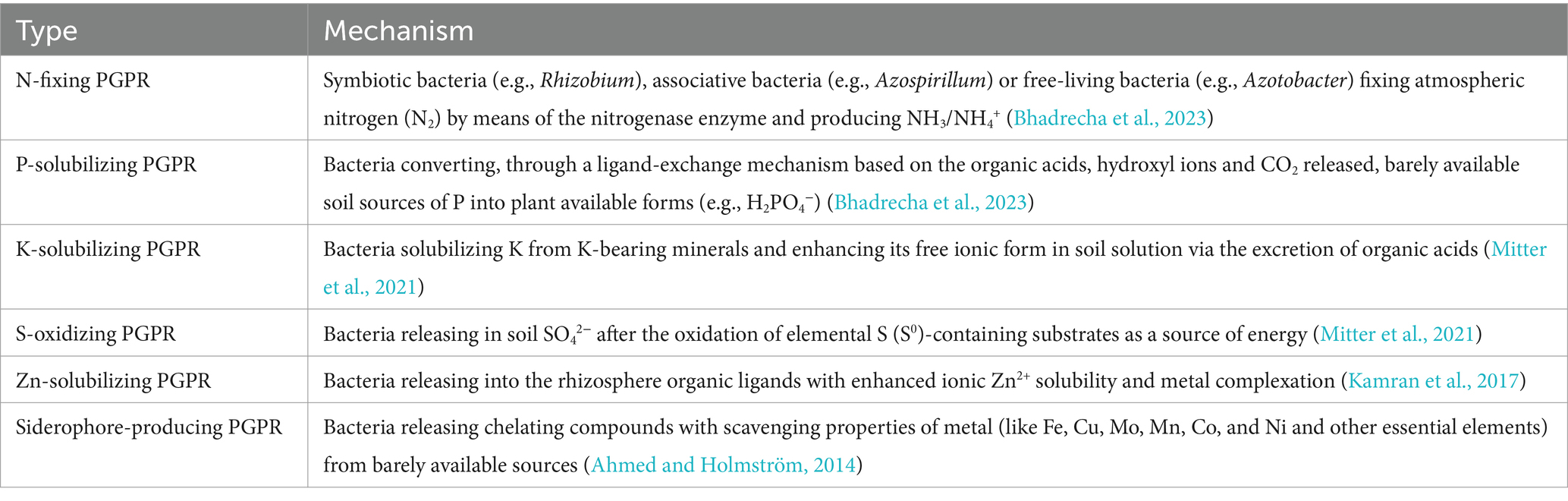
Table 1 . Classification of biofertilizers based on the mineral nutrient supplied.
3 Plant nutrient acquisition in a context of plant-biofertilizer interaction
3.1 the case study of nitrogen.
Nitrogen, accounting for 1–5% of the whole plant dry biomass, is a key component of amino acids, proteins, and chlorophyll being, therefore, essential for plant growth and development ( Jiang et al., 2020 ). The largest reservoir of N in the world is found in the atmosphere, mostly as nitrogen gas (N 2 ), which can only be used by plants after transformation through chemical [via lightning action, CNF, ( Barth et al., 2023 )] or biological N 2 fixation (BNF). This latter process can be performed by diazotrophs prokaryotes (N 2 fixers), including symbiotic bacteria, as well as free-living or associative bacteria ( Figure 1A ). These bacteria encompass nif genes encoding the nitrogenase enzyme, which catalyzes the reduction of N 2 to NH 4 + ( Soumare et al., 2020 ). Indeed, the bacteria-derived NH 4 + , if available in the rhizosphere, can then be also taken up by roots through plasma membrane transporters belonging to the AMT1 subfamily of the ammonium transporter/methylammonium permease family by exploiting either NH 4 + -uniport or NH 3 /H + co-transport ( Loque and Wirén, 2004 ; Neuhauser and Ludewig, 2014 ). In this regard, it should be mentioned that pieces of evidence showing alternative routes for NH 4 + movement across the plasma membrane have been gathered through the years. In particular, it was observed that NH 4 + can be also taken up by plants through non-selective cation channels (NSCC) and K + specific channels, as for instance AKT1 ( Coskun et al., 2013 ; Esteban et al., 2016 ). Nevertheless, it is worth mentioning that these not specific mechanisms have been suggested to play a predominant role in NH 4 + acquisition only when the external concentration of the nutrient is higher than 1–2 mM ( Muratore et al., 2021 ), being thus responsible for the onset of NH 4 + toxicity in plants.
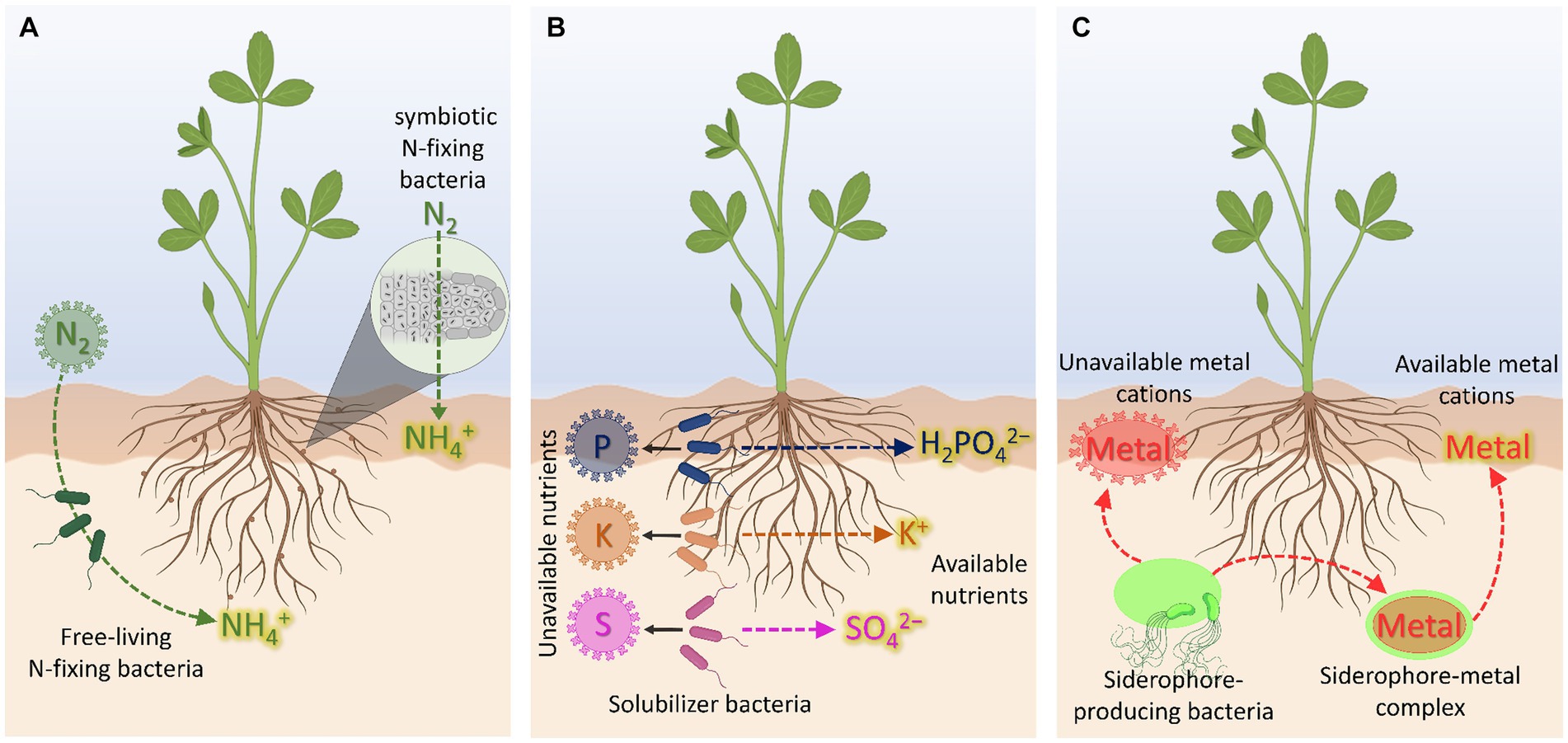
Figure 1 . Biofertilizer-mediated nutrient availability. (A) N-biofertilizers. (B) P-, K-, and S-biofertilizers. (C) Cation-chelating biofertilizers based on siderophore-producing bacteria. Created with BioRender ( https://biorender.com/ ).
The most studied symbiotic N 2 -fixing bacteria are rhizobia, which can establish symbiotic relationships with leguminous plants by forming specialized root nodule organs. Rhizobia, once enclosed in these nodules, experience a microaerobic environment, where nanomolar levels of oxygen (20–50 nM) are necessary for the nif gene expression and consequently, ensure high amounts of fixed N 2 ( Ledermann et al., 2021 ). It is estimated that the overall contribution of BNF in rhizobia-legumes systems is between 50 and 80% accounting for at least 70 million metric tons of N fixed per year ( Sindhu et al., 2019 ). For this reason, highly effective strains of rhizobia such as Rhizobium , Bradyrhizobium , and Sinorhizobium are often used as biofertilizers in most major legume crops thanks to their ability to fix N 2 in amounts as high as the amounts provided by synthetic N-fertilizers. In 2022, the global biofertilizer market was valued at USD 2.67 billion, whereby USD 1.92 billion was represented rhizobia-based biofertilizers, while the remaining USD 750 million constituted biofertilizers based on free-living N-fixing bacteria, P-solubilizing bacteria, K-solubilizing bacteria, and other PGPR. Moreover, their demand is estimated to grow by a further 12.3% compounding annual growth rate (CAGR) to reach about USD 6.97 billion by 2030. 2
Differently of symbiotic rhizobia, free-living associative bacteria inhabit the surfaces or the interstitial spaces of the host plant and use the root exudates as carbon sources to fix N 2 for their own use, providing the excess to the host ( Guo et al., 2023 ). Several free-living N-fixing bacteria, including members from the genera Azospirillum , Azotobacter , Herbaspirillum , Bacillus , Clostridium , Burkholderia , and Gluconobacter, have been reported to fix N 2 in the rhizosphere and bulk soil. These bacteria are particularly important for N nutrition in non-leguminous plants. Nonetheless, their contribution on BNF is more limited, providing up to 25% (13–22 kg N ha −1 yr. −1 ) of the total N in crops like rice, wheat and maize ( Ladha et al., 2016 ). In this context, an attractive avenue to improve BNF in non-leguminous crops has been the use of genetic approaches to engineer root nodulation with symbiotic bacteria also in cereals. However, none of the attempts performed to date has succeeded ( Pankievicz et al., 2019 ). On the other hand, notably progress has been made in genetic engineering strategies toward better N-fixing bacteria. Thus, by understanding the heterologous expression of the nif gene cluster in Klebsiella oxytoca , Azotobacter vinelandii , and Paenibacillus polymyxa , many scientists have been able to transfer their N-fixation trait to non-diazotrophic hosts ( Li and Chen, 2020 ). For instance, the metabolic engineering of A. vinelandii resulted in a constitutive expression of nif genes with an increased production of NH 4 + . Thanks to this improvement, an enhanced growth of not N-fertilized cucumber plants has been recorded in plants when root colonized by the engineered A. vinelandii strain ( Ambrosio et al., 2017 ). It is interesting to highlight that increased concentrations of NH 4 + were also reported by ( Pii et al., 2019 ) in the association between Azospirillum brasilense and maize plants. The authors also described that Azospirillum counteracted the plant response to NO 3 − uptake as a result of the activation of processes linked with NH 4 + fluxes and assimilation.
Recently, Rago et al. (2019) proposed to use N-fixing bacteria in association with electrochemical systems for the reduction of N 2 to NH 4 + at low energy cost, in a process called bioelectrochemical N fixation (e-BNF). This process aims at the production of microbial biomass through simultaneous N 2 and CO 2 fixation using electricity as only energy input, thus opening a new path toward local on-demand N-biofertilizer production, based on renewable power. In this regard, it is worth to mention that by using genetic manipulation in combination with e-BNF, ( Dong et al., 2021 ) integrated a modified nif gene cluster into the genome of the non-diazotrophic cyanobacterium Synechococcus elongatus and, thereafter an e-BNF system was used for NH 4 + production. The authors reported that the e-BNF led to an increased production of NH 4 + which was 21 times higher than that generated from solely photosynthesis-driven N fixation. Accordingly, these strategies provide novel technological feasibility to raise BNF inputs while alleviating the negative impacts of synthetic N-fertilizers (e.g., low crop N-efficiency, leaching, volatilization, use of not renewable energy for the synthesis).
3.2 The case study of phosphorus
Phosphorus is an essential nutrient representing the 0.1–0.8% of the dry matter and playing crucial roles in photosynthesis, energy transfer, respiration, and biosynthesis of molecules ( Vance et al., 2003 ). In soil, P is present in both inorganic (i.e., free ionic species and minerals) and organic forms (i.e., phosphates monoesters and diesters, polyphosphates, and phosphonates), being the mineral and organic fractions not usable by plants as they are ( Ducousso-Détrez et al., 2022 ). Moreover, it should be noted that the free ionic species have a strong affinity for soil particles being, therefore, readily retained in soil. As a consequence, only a minimal part of P in soil (about the 0.1%) is available for plant acquisition ( Lambers, 2022 ). At root level, P is taken up as inorganic phosphate (H 2 PO 4 − /HPO 4 2− , Pi) against the electrochemical gradient established at the plasma membrane ( Hinsinger, 2001 ). The acquisition of Pi from the soil solution is mediated by high affinity transporters, encoded by the gene family PHT1 ( Naureen et al., 2018 ), whose energy demand is sustained by the activity of plasma membrane H + -ATPase ( Młodzińska and Zboińska, 2016 ). On the other hand, it is well known that, with respect to the amount of P commonly supplied to a crop with chemical fertilizers, the majority undergoes precipitation and leaching processes, limiting then the fraction actually used by the crop to only 10–20% ( Ibrahim et al., 2022 ). In this context, the use of P-solubilizing bacteria (PSB) can nowadays represent a valid opportunity not only for an efficient mining and solubilization of P sources endogenously present in soil but also to limit the application of fertilizers characterized by such low use efficiency ( Figure 1B ). Moreover, if the localized effect of the strain (depending essentially on the root colonization by the bacteria) is considered, the sustainability of the entire process appears even more evident.
From the point of view of the mechanisms, the P solubilization mediated by PSB include the production and release of organic acids, phenolic compounds, siderophores, hydroxyl ions, protons, and CO 2 leading to soil acidification and/or ion exchange of P by carboxylates ( Alori et al., 2017 ). In fact, significant advancements including the isolation and characterization of bacterial genes have demonstrated that the glucose dehydrogenase (GDH, encoded by the gcd gene) and its cofactor pyrroloquinoline quinone (PQQ, encoded by the pqq operon) mediate the secretion of gluconic acid associated with P solubilization in Gram-negative bacteria ( Rasul et al., 2019 ). Other mechanisms governing the inorganic P-solubilization by PSB include the production of hydrolytic enzymes encoded by the pyrophosphatase ( ppa ) and exopolyphosphatase ( ppx ) genes ( Wu et al., 2022 ). However, with respect to the component of the mechanism connected with the organic acids exudation, it has been recently demonstrated, by using Leonard’s jars and Enterobacter sp., that the organic acids mainly involved in a soil–plant system (i.e., oxalic and citric acids) did not correspond to those released by the same bacterium in the highest concentrations under in vitro conditions (i.e., ketoglutaric and malic acids) ( Zuluaga et al., 2023b ). This experience clearly shows that the combination of the information acquired in in vitro experiments with that in conditions closer to the cultivated soil (i.e., in vivo ) is crucial for the identification of the bacteria with the best P-solubilizing attributes for the field level application ( Elhaissoufi et al., 2023 ).
Once solubilized, the crops benefit from the increased P availability enhancing their total biomass accumulation and productivity ( Zuluaga et al., 2021 ; Wang and Song, 2022 ; Elhaissoufi et al., 2023 ). A large number of PSB has been described in this sense, including species of Burkholderia , Enterobacter , Bacillus , Pseudomonas , Rhizobium , Ralstonia , Serratia , Azotobacter, Paenibacillu s, and Erwinia ( Zuluaga et al., 2020 ; Timofeeva et al., 2022 ). However, to better exploit the abilities of these bacteria, it becomes necessary a deeper understanding of their modes of action (including the regulation and elicitation of the interesting traits) and the development of strategies capable to boost their biofertilization properties. In this scenario, considering that the repeated (year after year) application of P fertilizers has led in the long period to a P accumulation in many agricultural soils (also known as legacy P, Doydora et al., 2020 ), the current strategies are focusing on the ability of PSB to mobilize legacy P supplying the nutrient to plants, either alone or in combination with external P inputs. The results have shown that PSB can enhance P uptake of plant by increasing the immediately plant-available P pool in rhizosphere soil ( Long and Wasaki, 2023 ). However, the supply of limited doses of exogenous P can potentiate the effect of PSB by solubilizing higher amounts of endogenous native Ca-, Fe- and Al-bound P ( Alam et al., 2023 ). The need for supplemental P with PSB is expected, since these bacteria also require P and other essential elements for their own metabolic processes ( Doydora et al., 2020 ).
Finally, it is important to mention what highlighted in a meta-analysis conducted by ( Zutter et al., 2022 ) considering 104 articles published between April 1976 and May 2021 related to “phosphate solubilizing bacteria” and “plant growth.” The authors raised the following issues:
i. biomass is generally chosen as the best indicative parameter to evaluate a plant P-status. However, the meta-analysis showed that plant growth promotion is not always related to improved P-uptake. Therefore, the use of multiple imaging techniques could also be implemented as a useful tool to assess P supply by PSB;
ii. the use of PSB consortia does not significantly provide an added benefit to plant growth and P-uptake compared to single strains. Thus, the combination of several PSB should be designed with care;
iii. strains of Burkholderia sp. and Enterobacter sp. have shown better effects on plant P-uptake and biomass than Bacillus sp. and Pseudomonas sp., but the use of these two strains is restricted due to their danger to human health. However, it has been assured that some species among these genera are unlikely to harm humans and, therefore, they should be used as safe P-biofertilizers in agriculture ( Sawana et al., 2014 ).
With respect to biotechnology application, few experiences of bioengineering PSB are described in literature. For instance, the incorporation of a pqq gene cluster in Herbaspirillum seropedicae resulted in the activation of gluconic acid secretion, which conferred the ability of P solubilization and the improvement of N and P status in inoculated rice plants ( Wagh et al., 2014 , 2016 ). In addition to its intrinsic ability to solubilize inorganic P, the phosphobacterium 9320-SD was transformed with a bacterial gene encoding phytase and this allowed the mineralization of organic phosphate into plant-available P ( Liu et al., 2015 ). In this regard, the manipulation of key genes through genome editing approaches could be a good strategy for enhancing the potential of PGPB as biofertilizers. Nonetheless, oncoming investigations should also include field trials under variable conditions to assess the real potential of genetically modified bacterial strains.
3.3 The case study of potassium
Potassium, ranging from 0.5 to 6% of the plant dry matter, is the third more represented essential nutrient in plants and it is implicated in crucial metabolic functions including photosynthesis, transport of nutrients and photosynthates, ATP production, and protein synthesis ( Szczerba et al., 2009 ; Hasanuzzaman et al., 2018 ). In soil, despite being one of the most abundant elements, only 2–3% is directly available for plant uptake in the free ionic form K + ( Olaniyan et al., 2022 ). At root level, K + uptake is achieved by the activity of High Affinity Transport Systems (HATS) and Low Affinity Transport Systems (LATS) ( Britto and Kronzucker, 2008 ). The high-affinity K + uptake is achieved through the activity of either the KT/HAK/KUP transporter family or the HKT transporter family when the external concentration of K + is in the μM range ( Santa-Maria et al., 2000 ). In this case, K + is taken up exploiting a symport mechanism along with H + , in an energy-dependent process ( Maathuis et al., 1997 ). As in the case of other mineral nutrients, the proton motive force across the plasma membrane is generated by the activity of ATPase enzymes, which hydrolyze ATP to extrude H + toward the rhizosphere ( Palmgren, 2001 ; Pardo et al., 2006 ). Conversely, the LATS is active when the external K + concentration is higher than 0.3–0.5 mM ( Wang and Wu, 2013 ), whereby it is taken up by non-selective and selective ion channels, as in the case of AKT1 transporter ( Hirsch et al., 1998 ). With respect to the soil-microorganism-plant interface, it is well known that one of the very effective strategies to increase the K available fraction is that relying on the use of K-solubilizing bacteria (KSB, Etesami et al., 2017 , Figure 1B ). The action of these bacteria is rather complex and involves the release of organic acids (OAs), the secretion of exopolysaccharides (EPS), and the formation of biofilms on mineral surfaces ( Sattar et al., 2019 ). The release of organic acids at the mineral surfaces, as well as the acidolysis and the carbonic acid production microbial respiration-dependent ( Basak et al., 2017 ), can favor the chemical weathering of K-bearing minerals, like mica, biotite, muscovite, feldspar, illite, and orthoclase ( Sattar et al., 2019 ). Differently, the secreted EPS can strongly adsorb organic acids, and the adhesion on the surface of K-bearing minerals will result in a locally higher concentration of OAs, with clear impact on weathering effects ( Liu et al., 2012 ). Similarly, when biofilm formation is considered, microbial cells are concentrated within a self-produced extracellular polymers matrix (composed by proteins, DNA and polysaccharides). These conditions offer the chance to boost the biochemical reactions underpinning K mobilization ( Nagaraju et al., 2017 ).
In this context there are several PGPR strains including Arthrobacter , Bacillus , Burkholderia , Enterobacter , Pseudomonas , Acidothiobacillus , Flavobacterium , and Thiobacillus that, exhibiting one or more of these actions at a time, can be defined as KSB bacteria ( Olaniyan et al., 2022 ). However, although the contribution of these bacteria in improving K acquisition in crops is out of any doubt, particularly in soils with impaired fertility, it should be highlighted that in general the extent of this contribution seems to not cover the entire plant requirement for an equilibrate development ( Ali et al., 2021 ; Aloo et al., 2022 ). Moreover, since the bacteria-based nutrient mobilization is generally relatively slow, the single inoculation of KSB may not be able to fulfill the nutrient plant needs. Accordingly, recent studies have shown that the use of biofertilizer bacterial consortia, composed of KSB in combination with other types of PGPR-based biofertilizers (i.e., integrating bacterial actions that are multiple, concurrent, and involving more than a single nutrient at a time), constitutes an innovative eco-friendly strategy to improve in a wider way the plant nutrient availability and agricultural production ( Jain and Hari, 2021 ; Sherpa et al., 2021 ).
Although any bioengineering applications to the KSB traits has been described in the literature yet, the whole genome sequencing of potential KSB strains [e.g., Bacillus aryabhattai ( Chen et al., 2022 ) and Priestia megaterium ( Wu et al., 2023 )] is increasingly being undertaken to possibly locate K solubilization-related genes that might be helpful in the development of biotechnological tools.
3.4 The case study of sulfur
Sulfur, representing up to 0.5% of the total plant dry biomass, is an essential macronutrient and crucial for the biosynthesis of the two S-containing amino acids (i.e., methionine and cysteine). These amino acids are then precursors for the synthesis of several other secondary metabolites (e.g., glutathione, phytochelatins, phytosiderphores) that are relevant for a balanced and healthy plant development ( Astolfi et al., 2021 ). Even though the total amount of S in soils ranges from 19 to 4,000 mg kg −1 , only a limited fraction represented by the inorganic sulfate form (SO 4 2− ) is readily available for plant uptake ( Ranadev et al., 2023 ). In fact, the 90–95% of the total S pool in soil is generally present in the organic and/or mineral forms, not directly acquirable by roots unless after mobilization/oxidation of S 0 to SO 4 2− ( Mitter et al., 2021 ). The remaining (5–10%) is mainly represented by the relatively mobile SO 4 2− fraction, whose 95% is adsorbed to the mineral soil fraction. Consequently, generally not more than 5% of the total SO 4 2− is present in the soil solution ranging from 0.15 to 1.2 g kg soil −1 ( Scherer, 2009 ).
At the root cells level, the SO 4 2− uptake is mediated by sulfate transporters (SULTR) family, which, depending on the plant species, can be formed by 12 to 16 members, subdivided in four distinct functional groups ( Takahashi et al., 2011 ). Functional studies in Arabidopsis thaliana have shown that SULTR1;1 and SULTR1;2 mediate the high affinity transport under SO 4 2− -limiting growth conditions ( Takahashi, 2019 ). The uptake of SO 4 2− is achieved through an active transport, whose motive force is suggested to be constituted by the proton gradient formed across the plasma membrane ( Buchner et al., 2004 ). Based on this background it appears clear that the majority of S in soil is present in forms and/or redox states that cannot be directly used by the plant. If on the one hand these aspects reveal how strategic for successful crop production is the practice of S fertilization, on the other they highlight how much the biological S-oxidation by sulfur-oxidizing bacteria (SOB) represent an interesting opportunity for developing new biofertilizers able to exploit more efficiently the endogenous soil S-sources ( Figure 1B ). In this context, it should be noted that, typically, the chemolithotrophic bacteria are the most dominant and efficient S-oxidizers in soils, known for converting the reduced forms of S (i.e., S 2− , S 0 , SO 3 2− , and S 2 O 3 2− ) into SO 4 2− ( Tourna et al., 2014 ). This group comprises several species of Thiobacillus , Acidithiobacillus , Sulfolobus , Thermothrix , Paracoccus , Thiomicrospira , Thiosphaera , and Acidianus ( Ranadev et al., 2023 ). However, the process of S oxidation can be also performed by heterotrophic bacteria belonging to the genera Burkholderia , Enterobacter , Klebsiella , Pseudomonas , and Xanthobacter , as described by ( Chaudhary et al., 2022 ). Regarding the mechanisms of S oxidation by the SOB, three oxidation processes have been proposed: (1) the Sox pathway, in which photo- and chemolithotrophic alpha-proteobacteria can directly oxidize all reduced forms of S into SO 4 2− (encoded by the sox operon); (2) the S4I pathway, where obligate chemolithotrophic beta- and gamma-proteobacteria produce S 4 O 6 2− as an intermediate; and (3) the branched thiosulfate oxidation pathway, that involves intracellular sulfur depositing under anaerobic conditions ( Ranadev et al., 2023 ). With reference to the field experiences, it has been well demonstrated with several agricultural crops that the inoculation of SOB in combination with elemental S sources promotes plant growth improving the yield ( Pourbabaee et al., 2020 ; Ranadev et al., 2023 ). Furthermore, the application of elemental S enriched with SOB seems not only to speed up the elemental S conversion into SO 4 2− , but to induce a rhizosphere soil acidification with the consequent effects on the availability of various other macro- and micro-nutrients ( Chaudhary et al., 2022 ; Nadeem et al., 2022 ).
With respect to the bioengineering applications to the interesting traits of these SOB, up to now no experiences are reported in literature. Nonetheless, several authors are carrying out efforts aimed at investigating the whole genome of SOB strains ( Koch and Dahl, 2018 ; Watanabe et al., 2019 ) in order to identify key genes and functions that might be used as valuable traits for genetically engineered strains as S biofertilizers in agriculture.
3.5 The case study of zinc
Zinc is an essential micronutrient whose concentration levels in plant tissues varies from 20 to 100 mg kg −1 (shoot dry matter). It is required for plant growth (e.g., integral component of lipids, proteins and carbohydrate synthesis via carbonic anhydrase), plant development (e.g., DNA replication, RNA biosynthesis, involvement in IAA synthesis), and plant resistance against biotic and abiotic stresses (e.g., detoxification of superoxide radicals) ( Hassan et al., 2020 ). In soil, depending on the geochemical composition and weathering of parent rocks, it is naturally present at concentrations ranging from 10 to 300 mg kg −1 ( Kabata-Pendias and Mukherjee, 2007 ). Sorption, complexation and, to a lesser extent, dissolution/precipitation mechanisms determine the solid–liquid partitioning of Zn in soil. When the soluble fraction is considered, Zn can occur in different forms, either as free divalent cation (Zn 2+ ) or complexed with organic ligands ( Cesco et al., 2022 ). Although Zn 2+ is the form mainly taken up at root level, the uptake of chelated forms (e.g., Zn-phytosiderophore or zinc-organic acids) across the plasma membrane is also reported in some plants ( von Wirén et al., 1996 ). The Zn 2+ transport across the root plasma membrane is predominantly mediated by members of the ZIP (Zn-regulated transporter proteins) family ( Greco et al., 2012 ). Nevertheless, several pieces of research have also highlighted that ZIP family protein iron-regulated transporters 1 and 3 (IRT1 and IRT3) can significantly contribute to Zn influx in root cells ( Dubeaux et al., 2018 ; Lee et al., 2021 ). Furthermore, members of the Yellow-Stripe1–like (YSL) family, the heavy metal ATPases (HMAs), and the cation diffusion facilitator (CDF) have also been shown to play a role in Zn homeostasis in plants ( Sinclair and Krämer, 2012 ; Cardini et al., 2021 ).
The highest values of Zn are generally found in soils rich in organic matter, clay minerals, and CaCO 3 . On the contrary, soils characterized by high pH values and low organic matter contents can often induce Zn deficiency phenomena, particularly in wet and cool conditions. To prevent in crops the consequences of this shortage (which include smaller leaves, chlorosis and stunted development), the exogenous application of ZnSO 4 as chemical fertilizer has been extensively used due to its high solubility and low cost. However, ZnSO 4 is rapidly converted into insoluble forms in soils, and only 2–5% of the total Zn applied can be actually used by plants ( Beig et al., 2023 ). In the frame of PGPR, Zn-solubilizing bacteria (ZnSB) are nowadays emerging as a potential eco-friendly alternative to enhance Zn availability in rhizosphere soil. Similarly to what described for PSB and KSB, the proposed mechanism underlying the ZnSB’s contribution to the Zn acquisition in crops is essentially based on a Zn-solubilizing action through a series of processes including acidification of the nearby soil (e.g., thanks to the exudation of organic acids and protons), the metal–ligand exchange (e.g., via the release of carboxylates, siderophores and other organic ligands) and/or the activity of redox systems on cell membranes ( Kamran et al., 2017 ). In this context, several pieces of literature have described that PGPR belonging to the genera Pseudomonas , Bacillus , Rhizobium , Enterobacter , Priestia and Pantoea , when applied to different crops (cereals and legumes), have demonstrated effectiveness in improving the Zn acquisition with beneficial effects both on crop development and its productivity ( Kamran et al., 2017 ; Yadav et al., 2022 ; Srithaworn et al., 2023 ). Moreover, there is recent evidence supporting the idea of an effectively use of ZnSB also to pursue the objective of biofortification of agricultural production, such as the case of wheat grain. In this specific case, a significant seed biofortification was obtained by using ZnSB in combination with Zn chemical fertilizers ( Yadav et al., 2022 ; Saleem et al., 2023 ).
With respect to innovations through genetic modifications, there is no evidence in literature describing programs to improve the Zn-solubilizing traits of these bacteria through biotechnological approaches. Furthermore, deeper insights into their Zn-solubilizing mechanisms are still necessary to maximize Zn accessibility in the rhizosphere environment.
3.6 The case study of iron and other micronutrients: bacterial siderophores
Iron (Fe) belongs to the group of essential micronutrients, being, among these, the one required in highest amounts. Due to its ability to easily switch between to oxidation states (Fe 2+ and Fe 3+ ), it is a cofactor of proteins involved in electron transfer and of many enzymes catalyzing redox reactions. For these reasons, Fe is involved in important metabolic pathways like photosynthesis, respiration, chlorophyll biosynthesis, and antioxidant defense system ( Murgia et al., 2022 ). In soil, although present at relatively high concentrations (20–40 mg kg −1 ) mainly as ferric (hydro)-oxides, its availability for plants is limited due to the poor solubility of the metal in the soil solution ( Zuluaga et al., 2023a ). Crops display at their root level the functionality of two different strategies of Fe acquisition: in dicots the Fe III -chelate reduction-based one while in monocots a strategy dependent on chelation by phytosiderophores with high affinity for binding Fe 3+ (for more details see also Morrisey and Guerinot, 2009 ).
With respect to the PGPR and plant Fe nutrition, several pieces of literature have demonstrated that these bacteria can significantly enhance in plants the Fe acquisition process in scenarios of restricted Fe availability. This effect is ascribed both to the synthesis and the release in the rhizosphere of huge amounts of organic acids, phenolic compounds, siderophores (favoring essentially the component of Fe solubilization), and to an enhanced activity of the plasma membrane ferric chelate reductase (FCR) of roots (enhancing the nutrient uptake step). In this regard, it is interesting to mention the experiences gained with the root inoculation with Azospirillum brasilense in cucumber plants. In these specific conditions, the PGPR’s effect appears to be ascribable to a rather complex of actions including not only an enhanced exudation of chelating compounds and an induced FCR activity as previously described, but also the modulation of the expression levels of key genes related to Fe acquisition and allocation ( Pii et al., 2015b , 2016 ; Marastoni et al., 2019 ). Moreover, it is worth to highlight that this beneficial effect was also evident in cucumber plants fed adequately with Fe resulting in contents of the nutrient in the tissues much higher than those observed in plants not inoculated with the PGPRs ( Pii et al., 2016 ).
In recent years, siderophore-producing bacteria (SPB) have been proposed as a sustainable alternative to synthetic fertilizers. Bacterial siderophores are low molecular weight organic compounds exhibiting cation-chelating properties and synthesized either under nutrient shortage in order to cope with the nutritional disorder or to alleviate the toxicity level of heavy metal ( Vijay et al., 2023 ). Although the term siderophore is more often used for the context of Fe nutrition and to indicate specifically a ligand able to form chelates with Fe, these compounds display affinity also for other elements like Cu, Mn, Mo, and Zn, all relevant for the plant and its metabolism ( Figure 1C ). Therefore, the root inoculation with SPB could be also envisaged as a strategy to improve the bioavailability not only of Fe but also of these other micronutrients. In this context, regarding specifically to the SPB contribution to Fe nutrition, Ferreira et al. (2019) demonstrated that a siderophore-based product prepared from Azotobacter vinelandii culture ameliorated Fe deficiency of soybean plants, thus representing a friendly Fe-fertilizer alternative for application in calcareous soils. Furthermore, ( McRose and Baars, 2017 ) provided evidence that, under Fe starvation, A. vinelandii produced higher amounts of vibrioferrin, an α-hydroxycarboxylate siderophore. However, under Mo limitation, this bacterial strain completely repressed the production of vibrioferrin, producing instead higher concentrations of protochelin, a siderophore belonging to the catechol class. These results suggest that the SPB A. vinelandii can selectively direct the production of siderophores depending on the element for which the limitation is sensed. However, it is important to highlight that the stability of the complex metal-siderophore (Me-S) can play a strategic role, both in a negative and positive sense. In fact, if on the one hand, when sufficiently strong, the Me-S complex stability ensures its persistence in the soil even in particularly disadvantaged conditions, on the other, when excessively strong, could represent a limit to its use by the roots, in particular for the strategy based on the ligand exchange like in monocots. In this regard it should be highlighted that the scientific debate is still wide ( Colombo et al., 2014 ). Therefore, for a more efficient use of root inoculation with SPB (and/or alternatively directly a product containing the siderophores) at the field scale, further investigations and knowledge, particularly at the mechanism scale, appear to be fundamental for the plant Fe nutrition context.
With respect to innovations through genetic modifications, there is no evidence in literature describing programs to improve the Fe-solubilizing traits of bacteria through biotechnological approaches.
4 Microbiome engineering-based approaches in biofertilizer development
Despite the well-documented beneficial effects of using biofertilizers based on single-strains or bacterial consortia, their adaptability to agricultural practices cannot be guaranteed, mainly because of their limited persistence in soil over time ( Mitter et al., 2021 ). Nowadays, different microbiome engineering-based approaches involving the manipulation of microbial communities have emerged as a promising technology to promote synergistic interactions that can provide collective benefits to the host plant. These approaches include the creation of synthetic communities ( SynComs ) and the host-mediated microbiome engineering (HMME) ( Yang et al., 2023 ). The key advantage of these approaches is not only the expected improvement of nutrient availability in the rhizosphere (with the consequent decrease in chemical fertilizer supplementation with the related environmental benefits), but also the positive effects of the rhizosphere microbiome to the plant health, amplifying the plant’s capability to cope with biotic and abiotic stresses.
4.1 The case study of biofertilizer based on synthetic communities
Synthetic microbial communities ( SynComs ) refer to artificial microbial consortia designed to recreate the core microbiome of a specific plant ( Mitter et al., 2021 ). The synthetic consortium is normally engineered with three or more strains used to get a more realistic understanding of the interactions between microorganisms, plants, and the environment ( Marín et al., 2021 ). According to their size, SynComs can be classified as a low- or high-complex consortia. Low-complex SynComs (<10 strains) are easier to design and can reduce costs and steps when scaling up bacterial growth in industrial processes. However, the small size of these consortia may not be taxonomically representative, thus neglecting important associations which are critical at the functional level. On the other hand, high-complex SynComs (>10 strains) can more efficiently mimic the autochthonous community in the rhizosphere with better chance of keeping associations intact, despite the limitations on their design ( Mitter et al., 2021 ).
Regarding the use of SynComs as a potential strategy to develop ecology-based biofertilizers that enhance nutrient acquisition of plants, some studies have been carried out on this area. For instance, ( Kaur et al., 2022 ) used a low-complex SynCom (4 strains) to investigate its impact on soil nutrient availability and growth of cotton plants. They found an increased nitrate availability in soils linked not only to the presence of the N-fixing Brevibacterium in the SynCom , but also to the enrichment of Cyanobacteria members induced by the consortium itself. Furthermore, after observing that indica rice recruited a higher proportion of bacteria related to N cycle as compared to japonica rice, Zhang et al. (2019) designed both a high-complex SynCom using16 indica -derived strains and a low-complex one using three japonicum -derived strains. The effects of both SynComs on the growth and N nutrition of IR24 rice plants were investigated and it was observed that the indica -derived high-complex SynCom was able to improve the transformation of organic N into NO 3 − and NH 4 + favoring, thus, in this crop higher values of N use efficiency (NUE). This result is perfectly coherent with historical observations of the higher NUE in indica varieties of rice than in the japonica ones ( Hu et al., 2015 ).
Concerning the possible application of SynComs concept to plants growing in soils characterized by variable P availability, Finkel et al. (2019) designed a high-complex consortium encompassing 185 strains. Interestingly, in this experience a clear link between the presence of the genus Burkholderia and the efficient use of the P sources has been highlighted. In particular, plants colonized by a SynCom lacking genus Burkholderia displayed a higher P allocation at the shoot level than those inoculated with the complete consortium containing Burkholderia strains. These results highlight that the rhizosphere of plants can be also colonized by latent opportunistic microbial competitors, which, under a nutrient shortage, may be capable to further exacerbate the nutritional disorder of the host plant. Despite these observations, the SynComs , once inoculated in plants exposed to a nutrient shortage, have always proven themselves efficient in bringing benefits to the host plants. Similarly, ( Harbort et al., 2020 ) developed a 115-strains SynCom to remediate Fe deficiency conditions in Arabidopsis plants. The research highlighted that the plant biosynthesis of coumarins and their exudation were required for SynCom -mediated rescue of plant development under Fe limitation. Interestingly, the same SynCom was not able to induce an over-accumulation of Fe, suggesting that the aiding activity of the consortium could be a plant-driven phenomenon, whose control was guaranteed by the root release of specific exudates. Overall, these pioneering studies, despite being at their beginning, clearly demonstrate the potential high functionality of the SynComs in enhancing the rhizosphere availability and the root acquisition of essential nutrients, yet overcoming some of the restraints of the single-strain biofertilizers currently available in the market.
4.2 The case study of host-mediated microbiome engineering
Plants generally influence the composition of the microbial community inhabiting their rhizosphere, where some microbial members are specifically recruited by the host, while other assemble opportunistically ( Pantigoso et al., 2022 ). Hence, host-mediated microbiome engineering (HMME) is a biotechnological approach that uses the plant host to indirectly select specialized microbiome assembly colonizing the rhizosphere and, there, able to enhance plant growth, nutrient acquisition, and resilience to stresses ( French et al., 2021 ). In some cases, such type of microbial selection has happened unintentionally after hundreds of years of domestication or during plant breeding programs, which led to the evolution of wild plant genotypes and, consequently, provoked an optimization in the rhizosphere microbiome of subsequent plants ( Escudero-Martinez and Bulgarelli, 2023 ). For instance, corn is one of the most farmed and fertilized crops in the world, and its domestication has led to the recruitment in the rhizosphere of N-cycling functional groups as compared with ancestral corn genotypes ( Favela et al., 2022 ). Moreover, it has been observed that, during nutrient starvation, corn ( Zea mays ) plants have evolved the ability to synthesize and release a variety of exudates into the rhizosphere. These organic compounds may have not only a role in influencing the biogeochemical cycles of nutrients in the rhizosphere, but also in promoting the recruitment of specific plant-beneficial taxa of microorganisms. In this context, it is interesting to mention the development of aerial roots secreting a huge amount of carbohydrate-rich mucilage observed in plants of a maize landrace grown under conditions of limited N availability (i.e., very limited or no fertilization) ( Deynze et al., 2018 ). The authors described that the high levels of N fixation observed were supported by the abundant secretion of the mucilage, which favored the assembling of a complex N-fixing microbiome by roots. Similarly, under N deprivation, ( Yu et al., 2021 ) observed that the flavonoids-releasing roots of maize plants specifically recruit in the rhizosphere bacteria of the taxon Oxalobacteraceae , that, in turn, facilitates N uptake through modulation of lateral root development. Based on these experiences, it appears evident that the reintroduction of alleles coding for beneficial compounds into crops by using HMME approaches could be a promising step toward the improvement of positive interactions between the plant rhizosphere and its microbiome.
Moreover, the increasing advances in plants-applied omics techniques has led to the identification of biosynthetic gene clusters (BGCs) responsible for the production of important secondary metabolites (e.g., terpenes, alkaloids, benzoxazinoids, cyanogenic glucosides, and polyketides). It is well known that these compounds are considerably involved in the assembly of rhizosphere microbiome ( Polturak et al., 2022 ). Therefore, plant genetic transformations targeted to these BCGs clusters by using HMME based on genetic techniques, can represent a promising and futuristic step to reinforce the ability of plants to recruit specific beneficial microbiota. However, further research in this host-mediated microbiota selection by roots is still necessary. In particular, different aspects, like the choice of host trait, transferability of the recruited microbiome, and the environment component that can interfere with the selection of the most promising microbiome, might be considered and deeper addressed ( French et al., 2021 ). Therefore, understanding the molecular and biochemical mechanisms governing the interactions between plant host and its associated microbiome, especially under nutritional disorders, would allow the design of crops with ability to produce/select “their own biofertilizers,” by reshaping specialized microbiomes that efficiently modulate (in their favor) the biogeochemical cycles of the nutrients in soil.

5 Current scenarios and future perspectives for PGPR-based biofertilizers
The global biofertilizer market has to date an estimated value of USD 3.1 billion and it is projected to increase to USD 7.0 billion in 2030 (markets&markets). Currently, most of the biofertilizers available in the market are based on either a single PGPR strain with single or multiple PGP activities, or microbial consortia with multiple PGP activities ( Figures 2A , B ). Both kinds of products have shown success in promoting plant growth and improving soil physiochemical properties at a relatively low production cost. In this context it is interesting to note that Bradyrhizobium -based biofertilizers applied to soybean plants are probably the most successful example of these products across the world, fully supplying the crop’s demand of N in countries like Brazil ( Santos et al., 2019 ). However, the efficacy of these biofertilizers often shows disparities due to (i) environmental variability, (ii) the intense competition with native microbial communities in the soil, and (iii) the application methods. Amazingly, due to the outstanding effects observed after the use of PGPR-based biofertilizers, some farmers have tried to produce their own biofertilizers using rudimentary bio-factories in a process called on-farm production ( Figure 2C ). This process consists of using makeshift equipment and infrastructure, including fermenters, open tanks, or even water tanks, without appropriate control of contaminations, which can lead to inconsistencies in production quality and efficiency ( Bocatti et al., 2022 ). Therefore, beyond the admirable intention of these farmers, this on-farm practice represents a real threat for the entire agriculture in a general sense since plant, animal, and human pathogens have been predominantly found in such products, with non-effective results for plant growth and health ( Bocatti et al., 2022 ). This aspect becomes even more relevant if the concept of One Health and its application in every context that concerns our planet, are considered.
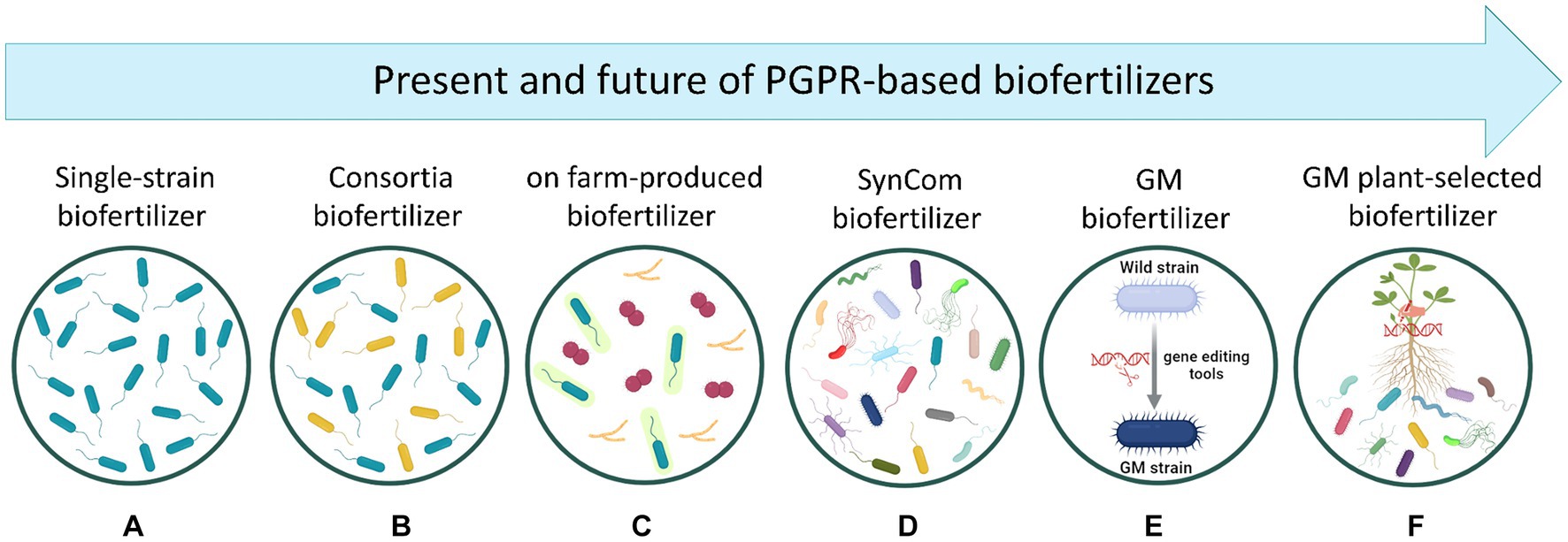
Figure 2 . Representation of the present and future of PGPR-based biofertilizer development. (A) Biofertilizers based on a single bacterial strain. (B) Biofertilizers containing multiple strains (two or more) which are selected by considering their ability in enhancing plant uptake of soil nutrients. (C) Biofertilizers produced by farmers in their own farms by using rudimentary bio-factories without appropriate control of contaminations, which may result in highly contaminated, non-effective products. (D) Biofertilizers containing multiple strains which are selected after analyzing the diversity profile of plant microbiome. Thus, SynComs are designed to recreate the core microbiome containing key microbial taxa carrying essential functional genes for the host plant. (E) Genetically modified biofertilizers where bacterial genes are modified with gene editing tools (e.g., CRISPR/Cas9, RNAi) to improve plant growth and nutrition. However, the incorporation of these products into farm systems remains controversial since their efficacy, survivability, and environmental hazards are not well understood ( Qiu et al., 2019 ). (F) Genetically modified plants driving the selection/recruitment of PGPR biofertilizers. Created with BioRender ( https://biorender.com/ ).
Following the line of future possibilities in the development of high-performing biofertilizers, the design of SynComs represents certainly an innovative and promising technology ( Figure 2D ), as already detailed in the previous section. However, when producing biofertilizers based on these SynComs , some challenges shall be considered. One of them pertains to the ability of designing effective SynComs with a minimal number of bacteria. In this regard, it should be noted that for commercial uses, the industrial production of multi-strain biofertilizers is commonly performed by separately fermenting different batches and mixed them afterwards. Thus, it appears clear that, to scale up bacterial growth at industrial level, theoretically one fermenter per bacterial strain is required. However, if this approach were also applied to the production of high-complex SynComs , its economic and structural non-sustainability appears evident. In this scenario, the selection of bacterial strains displaying multifarious and synergistic traits is urgently needed to ensure a feasible and simplified way to produce SynCom -based biofertilizers.
In recent times, it became possible the use of genetic engineering approaches to optimize bacterial genes, thus providing the chance to set up eco-friendly biofertilizers improved in their capacity to ameliorate the edaphic conditions of the crops’ rhizosphere ( Figure 2E ). The genetic editing on targeted PGPR genes holds the potential of being fast and reasonably effective, due to the direct introduction of specific traits into well-characterized bacteria. Therefore, in the field of PGPR-based biofertilizers, genetic engineering stands as a promising strategy by which a wild-type PGPR strain can be optimized to overexpress the molecular machinery underlying N, P, K, S, Fe, and/or Zn acquisition for itself and the host plant. In fact, the manipulation of nif , fix , and nod genes in N-fixing bacteria have been shown to improve their symbiotic or associative performance in chickpea plants, leading to an increased N content of the crop tissues and yield ( Silva et al., 2019 ). One other factor that could be exploited by using genetic manipulation is the insertion of genes related to PGP traits (one or more) into a bacterium that shows only one PGP mechanism. This approach may avoid the current need of mixing two bacterial strains when used in biofertilizer formulations. A hypothetical example of such types of transformation could be represented by a natural N-fixer that has been genetically strengthened with the ability to solubilize P and/or produce Fe-chelating compounds. Considering that for many years, engineering PGP traits has mostly focussed on N fixation and P solubilization, it is critical to enhance our knowledge about the molecular and functional mechanisms behind agronomically potential bacteria including KSB, SOB, and ZnSB to perform the most appropriated genome editing strategies toward more effective biofertilizers. Nevertheless, it cannot be ignored that the introduction into the environment of genetically manipulated bacterial strains might represent a potential risk of genetic pollution (i.e., horizontal genes transfer toward autochthonous microbiomes).
Besides the genetic modification of PGPR, another promising approach could be represented by engineering plants to either (i) increase their efficiency in nutrient acquisition and use, or (ii) recruit at the root level a more efficient microbial community. Considering the first point, and despite the genetic challenge, the development of plants able to directly fix atmospheric N without the help of bacterial partners would represent a feat without precedents, not only for alleviating adverse effects of synthetic N-fertilizers but also for the economic benefits derived from the higher harvest in system with low external inputs. In this regard it is interesting to note that current attempts are using the yeast Saccharomyces cerevisiae as model organism for initial testing of the functionality of eukaryotic nif proteins. In this attempt, chloroplasts and mitochondria are selected as candidate compartments for nitrogenase assembly and functioning ( Burén and Rubio, 2018 ). With reference instead to the second point, plants could be also manipulated to select a specific rhizosphere microbiome capable of improving the nutrient acquisition process ( Figure 2F ). In this respect, it is well known that different plant species with contrasting physiologies and traits can shape different microbial community structures via root exudation. Therefore, by identifying exuded molecules related to the signaling and recruitment of specialized biofertilizing microbial communities, plant genes encoding the production of these exudates could be potentially manipulated. To achieve this objective, a deeper understanding of the mechanisms underlying root exudation patterns under different nutritional status and environmental conditions is needed. In this context, the integration of multiple omics approaches including metabolomics, ionomics, genomics, and transcriptomics could help identifying and deciphering the key components necessary for successful plant manipulation to efficiently “self-select” the best biofertilizing microbiome.
6 Common scepticisms about biofertilizers: from the lab to the field
Despite their potential, there have been ongoing discussions and doubts regarding the overall efficacy of PGPR-based biofertilizers in enhancing plant growth and increasing crop yields in real-world field conditions. This skepticism arises from the significant gap between the effects demonstrated by PGPR-based biofertilizers in laboratory or greenhouse experiments and the results found in field trials. While controlled experiments offer precision and reproducibility ( Zuluaga et al., 2020 ; Scagliola et al., 2021 ), they often fail to capture the complex interactions between PGPR, plants, and soil microbiota present at field scale ( Thilakarathna and Raizada, 2017 ; Meyer et al., 2019 ). Moreover, several reports suggest that the viability and proliferation of PGPR-based biofertilizers introduced into fields vary and are greatly influenced by environmental factors such as temperature, rainfall, and soil composition, as well as interactions with the host plant and indigenous microorganisms ( Strigul and Kravchenko, 2006 ; Owen et al., 2015 ; Antoszewski et al., 2022 ). In addition, agronomical practices can significantly impact the composition of crops’ rhizosphere microbial community by modifying plant metabolism ( Cesco et al., 2021 ). Similarly, these practices influence the epiphytic microbial community on fruits, which is affected by the plant’s nutritional state ( Valentinuzzi et al., 2021 ). These findings underscore the complexity of the interactions among soil, microbes, and crops in the field, that may partly explain the varying levels of permanence and efficiency observed in the application of biostimulants.
In a meta-analysis conducted by Schütz et al. (2018) , 171 peer-reviewed field studies were evaluated to assess the impact of biofertilizers on crop yield and nutrient use efficiency of N and P. The authors found that biofertilizers performed best in dry climates, showing a yield increase of 20.0%, compared to a 14.9% increase in tropical climates. Additionally, the yield response to biofertilizers was generally small at low soil P levels, but their efficacy improved with higher soil P levels, demonstrating the high variability of microbial biofertilizers under different field and environmental conditions. Similarly, a meta-analysis of 28 research papers examined the effectiveness of different rhizobial inoculants on soybean traits under field conditions ( Thilakarathna and Raizada, 2017 ). It was shown that the effectiveness of the inoculants differed in terms of nodule count (−28 to +178 nodules) and grain-N yield (−6 to +176%) compared to uninoculated controls. This variability was attributed to various factors, including soybean genotype, interactions between soybean and rhizobia strains, inoculant formulation, concentration, and method of application. Adding to this complexity, research by Pandey et al. (1998) demonstrated that bacterial biofertilizers effective in subtropical climates struggled in temperate alpine regions due to their inability to establish and survive in lower temperatures. Furthermore, Sanz-sáez et al. (2015) found that inoculating soybean with Bradyrhizobium japonicum did not enhance photosynthesis, growth, or seed yield under ambient or elevated CO 2 levels in the field, likely due to competition from native rhizobia.
In scientific literature, the prevalence of failed field experiments is likely considerable, yet such instances are seldom disclosed. This discrepancy primarily stems from the scientific community’s focus on innovation over the repetition and validation of previously published studies ( Bashan et al., 2020 ; O’Callaghan et al., 2022 ). Moreover, the lack of standardized protocols for isolating, characterizing, and formulating PGPR strains exacerbates this issue, hindering the progression of biofertilizers from development to their ultimate field application. Standardized protocols would ensure consistency in application methods, such as the concentration of inoculants, timing, and techniques (e.g., seed coating versus soil drenching) allowing researchers and farming communities to draw more definitive conclusions about the efficacy of biofertilizers ( Khan et al., 2023 ). Recognizing this need, Neuhoff et al. (2024) recently proposed a comprehensive set of standards for agronomic field trials, summarized in Figure 3 , aimed at evaluating the efficacy of PGPR-based biofertilizers in arable crops within temperate climates. Thus, by adopting standardized protocols, the scientific community can address the gap in reliable field data, ultimately improving the progression of biofertilizers from experimental stages to widespread agricultural use. This approach will not only enhance the credibility of biofertilizer research but also support farmers in making informed decisions about their use, fostering broader acceptance and implementation.
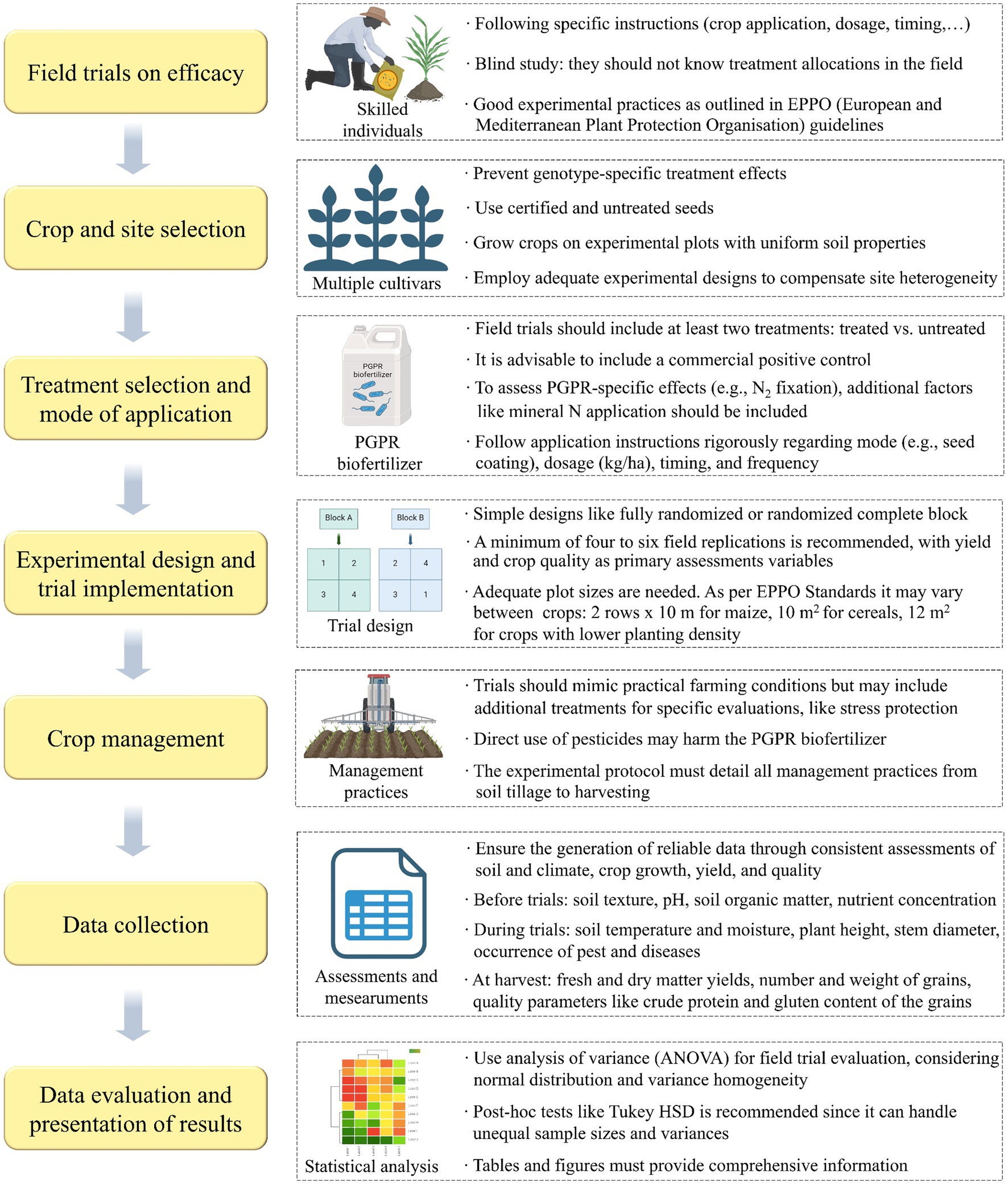
Figure 3 . Guidelines for PGPR-based biofertilizers field trial design and implementation as proposed by Neuhoff et al. (2024) .
7 Conclusion
It is evident that in the next future the current agriculture must face and meet important challenges whose success should consider the long-term environmental sustainability in a context of One Health paradigm and, properly of these last few years, the rapidly changing climate conditions. To achieve these objectives, all the tools and approaches that can contribute appear to be of crucial importance. The possibility of acting at a localized level at the rhizosphere ( precision farming ), allowing a more efficient exploitation of the endogenous nutrient resources already present in the soil as the PGPR-based biofertilizers seem to do, is certainly among the promising tools to contribute to the achievement of the general aim of a smarter and more sustainable agriculture. This review highlights how single strain and SynComs PGPR can impact soil fertility and nutrient availability for crop plants, how they influence the essential nutrient mobilization and uptake, and evaluates their effectiveness in controlled and field conditions. It also addresses skepticism about field results versus controlled experiments, advocating for standardized protocols and innovative approaches to improve biofertilizer performance. From this perspective, the potential of PGPR-based biofertilizers is evident, yet significant developments are needed regarding both the bacteria and the crops to optimize their use, especially in soils with altered fertility. Integrating advanced omics techniques, closely aligned with agricultural rhizosphere conditions, with cutting-edge bioengineering approaches could provide valuable insights. This integration is essential for advancing agriculture toward greater sustainability and resilience.
Author contributions
MA: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. RF: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. YP: Conceptualization, Data curation, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Free University of Bozen/Bolzano through the projects COMPETITIVE (coded TN202I); the Agritech National Research Center and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022). In particular, our study represents an original paper related to the Spoke 4 Multifunctional and resilient agriculture and forestry systems for the mitigation of climate change risks and in particular to the Tasks 4.1.2 titled Smart phenotyping platforms for the on-farm selection of resilient varieties and rootstocks (YP and SC). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^ https://sdgs.un.org/goals
2. ^ http://www.marketsandmarkets.com
Ahmed, E., and Holmström, S. J. M. (2014). Siderophores in environmental research: roles and applications. Microb. Biotechnol. 7, 196–208. doi: 10.1111/1751-7915.12117
PubMed Abstract | Crossref Full Text | Google Scholar
Alam, K., Barman, M., Prasad, S., Kannepalli, D., and Livleen, A. (2023). Modification of inorganic fractions of phosphorus by phosphate-solubilising microorganisms in conjunction with phosphorus fertilisation in a tropical inceptisol. J. Soil Sci. Plant Nutr. 23, 2488–2497. doi: 10.1007/s42729-023-01206-6
Crossref Full Text | Google Scholar
Ali, A., Awad, M., Hegab, S., Gawad, A., and Eissa, M. (2021). Effect of potassium solubilizing bacteria ( Bacillus cereus ) on growth and yield of potato. J. Plant Nutr. 44, 411–420. doi: 10.1080/01904167.2020.1822399
Aloo, B. N., Tripathi, V., Makumba, B. A., and Mbega, E. R. (2022). Plant growth-promoting rhizobacterial biofertilizers for crop production: the past, present, and future. Front. Plant Sci. 13:1002448. doi: 10.3389/fpls.2022.1002448
Alori, E. T., Glick, B. R., and Babalola, O. O. (2017). Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 8:971. doi: 10.3389/fmicb.2017.00971
Ambrosio, R., Cesar, J., Ortiz-marquez, F., and Curatti, L. (2017). Metabolic engineering of a diazotrophic bacterium improves ammonium release and biofertilization of plants and microalgae. Metab. Eng. 40, 59–68. doi: 10.1016/j.ymben.2017.01.002
Antoszewski, M., Mierek-Adamska, A., and Dąbrowska, G. B. (2022). The importance of microorganisms for sustainable agriculture: a review. Meta 12, 1–47. doi: 10.3390/metabo12111100
Astolfi, S., Celletti, S., Vigani, G., Mimmo, T., and Romera, F. J. (2021). Interaction between sulfur and iron in plants. Front. Plant Sci. 12:670308. doi: 10.3389/fpls.2021.670308
Atieno, M., Herrmann, L., Nguyen, H., Phan, H., Nguyen, N., Srean, P., et al. (2020). Assessment of biofertilizer use for sustainable agriculture in the Great Mekong region. J. Environ. Manag. 275, 1–9. doi: 10.1016/j.jenvman.2020.111300
Backer, R., Rokem, J. S., Ilangumaran, G., Lamont, J., Praslickova, D., Ricci, E., et al. (2018). Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 9:1473. doi: 10.3389/fpls.2018.01473
Barth, P., Stüeken, E. E., Helling, C., Rossmanith, L., Peng, Y., Walters, W., et al. (2023). Isotopic constraints on lightning as a source of fixed nitrogen in Earth’s early biosphere. Nat. Geosci. 16, 478–484. doi: 10.1038/s41561-023-01187-2
Basak, B. B., Sarkar, B., Biswas, D. R., Sarkar, S., and Sanderson, P. (2017). “Bio-intervention of naturally occurring silicate minerals for alternative source of potassium: challenges and opportunities” in Advances in Agronomy . ed. D. Sparks (Elsevier Inc), 115–145.
Google Scholar
Bashan, Y., Prabhu, S. R., de-Bashan, L. E., and Kloepper, J. W. (2020). Disclosure of exact protocols of fermentation, identity of microorganisms within consortia, formation of advanced consortia with microbe-based products. Biol. Fertil. Soils 56, 443–445. doi: 10.1007/s00374-020-01464-x
Beig, B., Bilal, M., Niazi, K., Jahan, Z., Haider, G., Zia, M., et al. (2023). Development and testing of zinc sulfate and zinc oxide nanoparticle-coated urea fertilizer to improve N and Zn use efficiency. Front. Plant Sci. 13:1058219. doi: 10.3389/fpls.2022.1058219
Bhadrecha, P., Singh, S., and Dwibedi, V. (2023). ‘A plant’s major strength in rhizosphere’: the plant growth promoting rhizobacteria. Arch. Microbiol. 205, 165–125. doi: 10.1007/s00203-023-03502-2
Bocatti, R. C., Ferreira, E., Ribeiro, R. A., Chueire, L., Delamuta, J., Kobayashi, R., et al. (2022). Microbiological quality analysis of inoculants based on Bradyrhizobium spp. and Azospirillum brasilense produced “on farm” reveals high contamination with non - target microorganisms. Braz. J. Microbiol. 53, 267–280. doi: 10.1007/s42770-021-00649-2
Britto, D. T., and Kronzucker, H. J. (2008). Cellular mechanisms of potassium transport in plants. Physiol. Plant. 133, 637–650. doi: 10.1111/j.1399-3054.2008.01067.x
Buchner, P., Takahashi, H., and Hawkesford, M. J. (2004). Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. J. Exp. Bot. 55, 1765–1773. doi: 10.1093/jxb/erh206
Burén, S., and Rubio, L. (2018). State of the art in eukaryotic nitrogenase engineering. FEMS Microbiol. Lett. 365, 1–9. doi: 10.1093/femsle/fnx274
Cardini, A., Pellegrino, E., White, P. J., Mazzolai, B., Mascherpa, M. C., and Ercoli, L. (2021). Transcriptional regulation of genes involved in zinc uptake, sequestration and redistribution following foliar zinc application to Medicago sativa . Plan. Theory 10, 1–19. doi: 10.3390/plants10030476
Cesco, S., Lucini, L., Miras-Moreno, B., Borruso, L., Mimmo, T., Pii, Y., et al. (2021). The hidden effects of agrochemicals on plant metabolism and root-associated microorganisms. Plant Sci. 311:1/9. doi: 10.1016/j.plantsci.2021.111012
Cesco, S., Sambo, P., Borin, M., Basso, B., Orzes, G., and Mazzetto, F. (2023). Smart agriculture and digital twins: applications and challenges in a vision of sustainability. Eur. J. Agron. 146, 1–9. doi: 10.1016/j.eja.2023.126809
Cesco, S., Terzano, R., Astolfi, S., Brunetto, G., Vigani, G., Pii, Y., et al. (2022). “Nutrient and elemental toxicieties” in Soil constraints on crop production . eds. Y. Dang, N. Menzies, and R. Dalal (Newcastle, UK: Cambridge Scholar Publishing), 218–243.
Chaudhary, S., Dhanker, R., Singh, K., Brar, B., and Goyal, S. (2022). Characterization of sulfur-oxidizing bacteria isolated from mustard ( Brassica juncea L.) rhizosphere having the capability of improving sulfur and nitrogen uptake. J. Appl. Microbiol. 133, 2814–2825. doi: 10.1111/jam.15742
Chen, Y., Yang, H., Shen, Z., and Ye, J. (2022). Whole-genome sequencing and potassium-solubilizing mechanism of Bacillus aryabhattai SK1-7. Front. Microbiol. 12:722379. doi: 10.3389/fmicb.2021.722379
Colombo, C., Palumbo, G., He, J. Z., Pinton, R., and Cesco, S. (2014). Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J. Soils Sediments 14, 538–548. doi: 10.1007/s11368-013-0814-z
Coskun, D., Britto, D. T., Li, M., Becker, A., and Kronzucker, H. J. (2013). Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiol. 163, 1859–1867. doi: 10.1104/pp.113.225961
Daniel, A. I., Fadaka, A. O., Gokul, A., Bakare, O. O., Aina, O., Fisher, S., et al. (2022). Biofertilizer: the future of food security and food safety. Microorganisms 10, 1–16. doi: 10.3390/microorganisms10061220
Deynze, A. V., Zamora, P., Delaux, P., Heitmann, C., Jayaraman, D., Rajasekar, S., et al. (2018). Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol. 16:e2006352. doi: 10.6084/m9.figshare.6534545
Dong, F., Lee, Y. S., Gaffney, E. M., Haddadin, H., Minteer, S. D., Chen, H., et al. (2021). An engineered, non-diazotrophic cyanobacterium and its application in bioelectrochemical nitrogen fixation. Cell Rep. Phys. Sci. 2, 1–16. doi: 10.1016/j.xcrp.2021.100444
Doydora, S., Gatiboni, L., Grieger, K., Hesterberg, D., Jones, J. L., McLamore, E. S., et al. (2020). Accessing legacy phosphorus in soils. Soil Syst. 4, 1–22. doi: 10.3390/soilsystems4040074
Dubeaux, G., Neveu, J., Zelazny, E., and Vert, G. (2018). Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Mol. Cell 69, 953–964. doi: 10.1016/j.molcel.2018.02.009
Ducousso-Détrez, A., Fontaine, J., Lounès-Hadj Sahraoui, A., and Hijri, M. (2022). Diversity of phosphate chemical forms in soils and their contributions on soil microbial community structure changes. Microorganisms 10:609. doi: 10.3390/microorganisms10030609
Elhaissoufi, W., Ibnyasser, A., Haddine, M., Zeroual, Y., Ghani, R., Barakat, A., et al. (2023). Screening of potential phosphate solubilizing bacteria inoculants should consider the contrast in P bio-solubilization rate along with plant growth promotion and P use efficiency. J. Appl. Microbiol. 134, 1–14. doi: 10.1093/jambio/lxac077
Escudero-Martinez, C., and Bulgarelli, D. (2023). Engineering the crop microbiota through host genetics. Annu. Rev. Phytopathol. 61, 257–277. doi: 10.1146/annurev-phyto-021621-121447
Esteban, R., Ariz, I., Cruz, C., and Fernando, J. (2016). Review: mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 248, 92–101. doi: 10.1016/j.plantsci.2016.04.008
Etesami, H., Emami, S., and Alikhani, H. A. (2017). Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects. A review. J. Soil Sci. Plant Nutr. 17, 897–911. doi: 10.4067/S0718-95162017000400005
Fasusi, O. A., Cruz, C., and Babalola, O. O. (2021). Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture 11, 1–19. doi: 10.3390/agriculture11020163
Favela, A., Bohn, M., and Kent, A. (2022). N-cycling microbiome recruitment differences between modern and wild Zea mays . Phytobiomes J. 6, 151–160. doi: 10.1094/PBIOMES-08-21-0049-R
Ferreira, C. M. H., López-rayo, S., Lucena, J. J., Soares, E. V., and Soares, H. M. V. M. (2019). Evaluation of the efficacy of two new biotechnological-based freeze-dried fertilizers for sustainable Fe deficiency correction of soybean plants grown in calcareous soils. Front. Plant Sci. 10:1335. doi: 10.3389/fpls.2019.01335
Finkel, O., Salas-Gonzalez, I., Castrillo, G., Spaepen, S., Law, T., Teixeira, P., et al. (2019). The effects of soil phosphorus content on plant microbiota are driven by the plant phosphate starvation response. PLoS Biol. 17:e3000534. doi: 10.1371/journal.pbio.3000534
French, E., Kaplan, I., Iyer-pascuzzi, A., Nakatsu, C. H., and Enders, L. (2021). Emerging strategies for precision microbiome management. Nat. Plants 7, 256–267. doi: 10.1038/s41477-020-00830-9
Greco, M., Chiappetta, A., Bruno, L., and Bitonti, M. B. (2012). In Posidonia oceanica cadmium induces changes in DNA methylation and chromatin patterning. J. Exp. Bot. 63, 695–709. doi: 10.1093/jxb/err313
Grover, M., Bodhankar, S., Sharma, A., Sharma, P., and Singh, J. (2021). PGPR mediated alterations in root traits: way toward sustainable crop production. Front. Sustain. Food Syst. 4:618230. doi: 10.3389/fsufs.2020.618230
Guo, K., Yang, J., Yu, N., Luo, L., and Wang, E. (2023). Biological nitrogen fixation in cereal crops: progress, strategies, and perspectives. Plant Commun. 4:100499. doi: 10.1016/j.xplc.2022.100499
Harbort, C. J., Hashimoto, M., Inoue, H., Sattely, E. S., Garrido-oter, R., and Schulze-lefert, P. (2020). Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis . Cell Host Microbe 28, 825–837. doi: 10.1016/j.chom.2020.09.006
Hasanuzzaman, M., Bhuyan, M., Nahar, K., Hossain, M., Mahmud, J., Hossen, M., et al. (2018). Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8:31. doi: 10.3390/agronomy8030031
Hassan, M. U., Aamer, M., Chattha, M. U., Haiying, T., Shahzad, B., Barbanti, L., et al. (2020). The critical role of zinc in plants facing the drought stress. Agriculture 10, 1–20. doi: 10.3390/agriculture10090396
Herrmann, L., and Lesueur, D. (2013). Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 97, 8859–8873. doi: 10.1007/s00253-013-5228-8
Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 173, 173–195. doi: 10.1023/A:1013351617532
Hirsch, R. E., Lewis, B. D., Spalding, E. P., and Sussman, M. R. (1998). A role for the AKT1 potassium channel in plant nutrition. Science 918, 1–5. doi: 10.1126/science.280.5365.918
Hu, B., Wang, W., Ou, S., Tang, J., Li, H., Che, R., et al. (2015). Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47, 834–838. doi: 10.1038/ng.3337
Ibrahim, M., Iqbal, M., Tang, Y. T., Khan, S., Guan, D. X., and Li, G. (2022). Phosphorus mobilization in plant–soil environments and inspired strategies for managing phosphorus: a review. Agronomy 12, 1–17. doi: 10.3390/agronomy12102539
Jain, D., and Hari, R. (2021). Effect of microbial consortia on growth and yield of wheat under typic haplustepts. Plant Physiol. Rep. 26, 570–580. doi: 10.1007/s40502-021-00607-y
Jiang, Q., Zhang, J., Xu, X., Liu, G., and Zhu, J. (2020). Effects of free-air CO 2 enrichment (FACE) and nitrogen (N) supply on N uptake and utilization of indica and japonica cultivars ( Oryza sativa L.). Ecol. Process. 9, 1–12. doi: 10.1186/s13717-020-00238-5
Kabata-Pendias, A., and Mukherjee, A. (2007). “Trace elements of group 12 (previously group IIb)” in Trace elements from soil to human . ed. A. Kabata-Pendias (Berlin, Heidelberg: Springer), 283–319.
Kamran, S., Shahid, I., Baig, D. N., Rizwan, M., and Malik, K. A. (2017). Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 8:2593. doi: 10.3389/fmicb.2017.02593
Kaur, S., Egidi, E., Trivedi, P., Macdonald, C. A., Prakash, J., Wang, J., et al. (2022). Synthetic community improves crop performance and alters rhizosphere microbial communities. J. Sustain. Agric. Environ. 1, 118–131. doi: 10.1002/sae2.12017
Kaushal, P., Ali, N., Saini, S., Pati, P. K., and Pati, A. M. (2023). Physiological and molecular insight of microbial biostimulants for sustainable agriculture. Front. Plant Sci. 14:1041413. doi: 10.3389/fpls.2023.1041413
Keswani, C., Prakash, O., Bharti, N., Vílchez, J. I., Sansinenea, E., Lally, R. D., et al. (2019). Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci. Total Environ. 690, 841–852. doi: 10.1016/j.scitotenv.2019.07.046
Khan, A., Singh, A. V., Gautam, S. S., Agarwal, A., Punetha, A., Upadhayay, V. K., et al. (2023). Microbial bioformulation: a microbial assisted biostimulating fertilization technique for sustainable agriculture. Front. Plant Sci. 14:1270039. doi: 10.3389/fpls.2023.1270039
Koch, T., and Dahl, C. (2018). A novel bacterial sulfur oxidation pathway provides a new link between the cycles of organic and inorganic sulfur compounds. ISME J. 12, 2479–2491. doi: 10.1038/s41396-018-0209-7
Kumawat, K. C., Razdan, N., and Saharan, K. (2022). Rhizospheric microbiome: bio-based emerging strategies for sustainable agriculture development and future perspectives. Microbiol. Res. 254:126901. doi: 10.1016/j.micres.2021.126901
Kumawat, K. C., Sharma, B., Nagpal, S., Kumar, A., Tiwari, S., and Nair, R. M. (2023). Plant growth-promoting rhizobacteria: salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 13:1101862. doi: 10.3389/fpls.2022.1101862
Kumawat, K. C., Sharma, P., Sirari, A., Sharma, B., Kumawat, G., Nair, R. M., et al. (2024). Co-existence of halo-tolerant Pseudomonas fluorescens and Enterococcus hirae with multifunctional growth promoting traits to ameliorate salinity stress in Vigna radiata . Chemosphere 349, 1–14. doi: 10.1016/j.chemosphere.2023.140953
Ladha, J. K., Tirol-pad, A., Reddy, C. K., Cassman, K. G., Verma, S., and Powlson, D. S. (2016). Global nitrogen budgets in cereals: a 50-year assessment for maize, rice, and wheat production systems. Sci. Rep. 6, 1–9. doi: 10.1038/srep19355
Lambers, H. (2022). Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 73, 17–42. doi: 10.1146/annurev-arplant-102720-125738
Ledermann, R., Schulte, C., and Poole, P. (2021). How rhizobia adapt to the nodule environment. J. Bacteriol. 203, 1–18. doi: 10.1128/JB.00539-20
Lee, S., Lee, J., Ricachenevsky, F. K., Punshon, T., Tappero, R., Salt, D. E., et al. (2021). Redundant roles of four ZIP family members in zinc homeostasis and seed development in Arabidopsis thaliana . Plant J. 108, 1162–1173. doi: 10.1111/tpj.15506
Li, Q., and Chen, S. (2020). Transfer of nitrogen fixation (nif) genes to non-diazotrophic hosts. Chembiochem 21, 1717–1722. doi: 10.1002/cbic.201900784
Liu, L., Du, W., Luo, W., Su, Y., Hui, J., and Ma, S. (2015). Development of an engineered soil bacterium enabling to convert both insoluble inorganic and organic phosphate into plant available phosphate and its use as a biofertilizer. Mol. Biotechnol. 57, 419–429. doi: 10.1007/s12033-014-9834-1
Liu, X., Jiang, X., He, X., Zhao, W., Cao, Y., Guo, T., et al. (2019). Phosphate-solubilizing Pseudomonas sp. strain P34-L promotes wheat growth by colonizing the wheat rhizosphere and improving the wheat root system and soil phosphorus nutritional status. J. Plant Growth Regul. 38, 1314–1324. doi: 10.1007/s00344-019-09935-8
Liu, D., Lian, B., and Dong, H. (2012). Isolation of Paenibacillus sp. and assessment of its potential for enhancing mineral weathering. Geomicrobiol J. 29, 413–421. doi: 10.1080/01490451.2011.576602
Long, H., and Wasaki, J. (2023). Effects of phosphate-solubilizing bacteria on soil phosphorus fractions and supply to maize seedlings grown in lateritic red earths and cinnamon soils. Microbes Environ. 38, 1–8. doi: 10.1264/jsme2.ME22075
Loque, D., and Wirén, N. (2004). Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 55, 1293–1305. doi: 10.1093/jxb/erh147
Maathuis, F. J. M., Sanders, D., and Gradmann, D. (1997). Kinetics of high-affinity K+ uptake in plants, derived from K+ −induced changes in current-voltage relationships. A modelling approach to the analysis of carrier-mediated transport. Planta 203, 229–236. doi: 10.1007/s004250050186
Malusá, E., and Vassilev, N. (2014). A contribution to set a legal framework for biofertilisers. Appl. Microbiol. Biotechnol. 98, 6599–6607. doi: 10.1007/s00253-014-5828-y
Marastoni, L., Pii, Y., Maver, M., Valentinuzzi, F., Cesco, S., and Mimmo, T. (2019). Role of Azospirillum brasilense in triggering different Fe chelate reductase enzymes in cucumber plants subjected to both nutrient deficiency and toxicity. Plant Physiol. Biochem. 136, 118–126. doi: 10.1016/j.plaphy.2019.01.013
Marín, O., González, B., and Poupin, M. J. (2021). From microbial dynamics to functionality in the rhizosphere: a systematic review of the opportunities with synthetic microbial communities. Front. Plant Sci. 12:650609. doi: 10.3389/fpls.2021.650609
McRose, D. L., and Baars, O. (2017). Siderophore production in Azotobacter vinelandii in response to Fe-, Mo- and V-limitation. Environ. Microbiol. 19, 3595–3605. doi: 10.1111/1462-2920.13857
Meyer, G., Maurhofer, M., Frossard, E., Gamper, H. A., Mäder, P., Mészáros, É., et al. (2019). Pseudomonas protegens CHA0 does not increase phosphorus uptake from 33P labeled synthetic hydroxyapatite by wheat grown on calcareous soil. Soil Biol. Biochem. 131, 217–228. doi: 10.1016/j.soilbio.2019.01.015
Mitter, E. K., Tosi, M., Obregón, D., Dunfield, K. E., and Germida, J. J. (2021). Rethinking crop nutrition in times of modern microbiology: innovative biofertilizer technologies. Front. Sustain. Food Syst. 5:606815. doi: 10.3389/fsufs.2021.606815
Młodzińska, E., and Zboińska, M. (2016). Phosphate uptake and allocation - A closer look at arabidopsis thaliana L. and Oryza sativa L. Front. Plant Sci. 7:1198. doi: 10.3389/fpls.2016.01198
Morrisey, J., and Guerinot, M. (2009). Iron uptake and transport in plants: the good, the bad, and the ionome. Chem. Rev. 109, 4553–4567. doi: 10.1021/cr900112r
Muratore, C., Espen, L., and Prinsi, B. (2021). Nitrogen uptake in plants: the plasma membrane root transport systems from a physiological and proteomic perspective. Plan. Theory 10, 1–26. doi: 10.3390/plants10040681
Murgia, I., Marzorati, F., Vigani, G., and Morandini, P. (2022). Plant iron nutrition: the long road from soil to seeds. J. Exp. Bot. 73, 1809–1824. doi: 10.1093/jxb/erab531
Nadeem, S. M., Hanif, A., Khan, M. Y., Waqas, M. R., Ahmad, Z., Ashraf, M. R., et al. (2022). Elemental sulphur with sulphur oxidizing bacteria enhances phosphorus availability and improves growth and yield of wheat in calcareous soil. Arch. Agron. Soil Sci. 69, 1–9. doi: 10.1080/03650340.2022.2099541
Nagaraju, Y., Triveni, S., Subhashreddy, R., and Jhansi, P. (2017). Biofilm formation of zinc solubilizing, potassium releasing bacteria on the surface of fungi. Int. J. Curr. Microbiol. App. Sci. 6, 2037–2047. doi: 10.20546/ijcmas.2017.604.241
Naureen, Z., Sham, A., Al Ashram, H., Gilani, S. A., Al Gheilani, S., Mabood, F., et al. (2018). Effect of phosphate nutrition on growth, physiology and phosphate transporter expression of cucumber seedlings. Plant Physiol. Biochem. 127, 211–222. doi: 10.1016/j.plaphy.2018.03.028
Neuhauser, B., and Ludewig, U. (2014). Uncoupling of ionic currents from substrate transport in the plant ammonium transporter AtAMT1;2. J. Biol. Chem. 289, 11650–11655. doi: 10.1074/jbc.C114.552802
Neuhoff, D., Neumann, G., and Weinmann, M. (2024). Testing plant growth promoting microorganisms in the field - a proposal for standards. Front. Plant Sci. 14:1324665. doi: 10.3389/fpls.2023.1324665
O’Callaghan, M., Ballard, R. A., and Wright, D. (2022). Soil microbial inoculants for sustainable agriculture: limitations and opportunities. Soil Use Manag. 38, 1340–1369. doi: 10.1111/sum.12811
Olaniyan, F. T., Alori, E. T., Adekiya, A. O., Ayorinde, B. B., Daramola, F. Y., and Osemwegie, O. O. (2022). The use of soil microbial potassium solubilizers in potassium nutrient availability in soil and its dynamics. Ann. Microbiol. 72, 1–12. doi: 10.1186/s13213-022-01701-8
Owen, D., Williams, A. P., Griffith, G. W., and Withers, P. J. A. (2015). Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 86, 41–54. doi: 10.1016/j.apsoil.2014.09.012
Palmgren, M. G. (2001). Plant plasma membrane H+ -ATPases: powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 817–845. doi: 10.1146/annurev.arplant.52.1.817
Pandey, A., Sharma, E., and Palni, L. M. S. (1998). Influence of bacterial inoculation on maize in upland farming systems of the Sikkim Himalaya. Soil Biol. Biochem. 30, 379–384. doi: 10.1016/S0038-0717(97)00121-1
Pankievicz, V. C. S., Irving, T. B., Maia, L. G. S., and Ané, J. (2019). Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 17:99. doi: 10.1186/s12915-019-0710-0
Pantigoso, H. A., Newberger, D., and Vivanco, J. M. (2022). The rhizosphere microbiome: plantmicrobial interactions for resource acquisition. J. Appl. Microbiol. 133, 2864–2876. doi: 10.1111/jam.15686
Pardo, M., Cubero, B., Leidi, E. O., and Quintero, F. J. (2006). Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J. Exp. Bot. 57, 1181–1199. doi: 10.1093/jxb/erj114
Pii, Y., Aldrighetti, A., Valentinuzzi, F., Mimmo, T., and Cesco, S. (2019). Azospirillum brasilense inoculation counteracts the induction of nitrate uptake in maize plants. J. Exp. Bot. 70, 1313–1324. doi: 10.1093/jxb/ery433
Pii, Y., Marastoni, L., Springeth, C., Chiara, M., Maria, G., Cesco, S., et al. (2016). Modulation of Fe acquisition process by Azospirillum brasilense in cucumber plants. Environ. Exp. Bot. 130, 216–225. doi: 10.1016/j.envexpbot.2016.06.011
Pii, Y., Mimmo, T., Tomasi, N., Terzano, R., Cesco, S., and Crecchio, C. (2015a). Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 51, 403–415. doi: 10.1007/s00374-015-0996-1
Pii, Y., Penn, A., Terzano, R., Crecchio, C., Mimmo, T., and Cesco, S. (2015b). Plant-microorganism-soil interactions influence the Fe availability in the rhizosphere of cucumber plants. Plant Physiol. Biochem. 87, 45–52. doi: 10.1016/j.plaphy.2014.12.014
Polturak, G., Liu, Z., and Osbourn, A. (2022). New and emerging concepts in the evolution and function of plant biosynthetic gene clusters. Curr. Opin. Green Sustain. Chem. 33:100568. doi: 10.1016/j.cogsc.2021.100568
Pourbabaee, A. A., Dinekaboodi, S. K., Seyed, H. M., Alikhani, H. A., and Emami, S. (2020). Potential application of selected sulfur-oxidizing bacteria and different sources of sulfur in plant growth promotion under different moisture conditions. Commun. Soil Sci. Plant Anal. 51, 735–745. doi: 10.1080/00103624.2020.1729377
Qiu, Z., Egidi, E., Liu, H., Kaur, S., and Singh, B. K. (2019). New frontiers in agriculture productivity: optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 37:107371. doi: 10.1016/j.biotechadv.2019.03.010
Rago, L., Zecchin, S., Villa, F., Goglio, A., Corsini, A., Cavalca, L., et al. (2019). Bioelectrochemical nitrogen fixation (e-BNF): electro-stimulation of enriched biofilm communities drives autotrophic nitrogen and carbon fixation. Bioelectrochemistry 125, 105–115. doi: 10.1016/j.bioelechem.2018.10.002
Ranadev, P., Ashwin, R., Bagyaraj, D. J., and Shinde, A. H. (2023). Sulfur oxidizing bacteria in agro ecosystem and its role in plant productivity - a review. J. Appl. Microbiol. 134, 1–15. doi: 10.1093/jambio/lxad161
Rasul, M., Yasmin, S., Suleman, M., Zaheer, A., Reitz, T., Tarkka, M. T., et al. (2019). Glucose dehydrogenase gene containing phosphobacteria for biofortification of phosphorus with growth promotion of rice. Microbiol. Res. 223–225, 1–12. doi: 10.1016/j.micres.2019.03.004
Rosier, A., Medeiros, F. H. V., and Bais, H. P. (2018). Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 428, 35–55. doi: 10.1007/s11104-018-3679-5
Saleem, S., Malik, A., and Khan, S. T. (2023). ZnO nanoparticles in combination with Zn biofertilizer improve wheat plant growth and grain Zn content without significantly changing the rhizospheric microbiome. Environ. Exp. Bot. 213:105446. doi: 10.1016/j.envexpbot.2023.105446
Sambo, P., Nicoletto, C., Giro, A., Pii, Y., Valentinuzzi, F., Mimmo, T., et al. (2019). Hydroponic solutions for soilless production systems: issues and opportunities in a smart agriculture perspective. Front. Plant Sci. 10:923. doi: 10.3389/fpls.2019.00923
Santa-Maria, G. E., Danna, C. H., and Czibener, C. (2000). High-affinity potassium transport in barley roots. Ammonium-sensitive and -insensitive pathways. Plant Physiol. 123, 297–306. doi: 10.1104/pp.123.1.297
Santos, M. S., Nogueira, M. A., and Hungria, M. (2019). Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 9, 1–22. doi: 10.1186/s13568-019-0932-0
Sanz-sáez, Á., Heath, K. D., Burke, P. V., and Ainsworth, E. A. (2015). Inoculation with an enhanced N2-fixing Bradyrhizobium japonicum strain (USDA110) does not alter soybean ( Glycine max Merr.) response to elevated CO2. Plant Cell Environ. 38, 2589–2602. doi: 10.1111/pce.12577
Sattar, A., Naveed, M., Ali, M., Zahir, Z. A., Nadeem, S. M., Yaseen, M., et al. (2019). Perspectives of potassium solubilizing microbes in sustainable food production system: a review. Appl. Soil Ecol. 133, 146–159. doi: 10.1016/j.apsoil.2018.09.012
Sawana, A., Adeolu, M., Gupta, R. S., Nierman, W. C., and Craig, J. (2014). Molecular signatures and phylogenomic analysis of the genus Burkholderia: proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front. Genet. 5:429. doi: 10.3389/fgene.2014.00429
Scagliola, M., Valentinuzzi, F., Mimmo, T., Cesco, S., Crecchio, C., and Pii, Y. (2021). Bioinoculants as promising complement of chemical fertilizers for a more sustainable agricultural practice. Front. Sustain. Food Syst. 4:622169. doi: 10.3389/fsufs.2020.622169
Scherer, H. W. (2009). Sulfur in soils. J. Plant Nutr. Soil Sci. 172, 326–335. doi: 10.1002/jpln.200900037
Schütz, L., Gattinger, A., Meier, M., Müller, A., Boller, T., Mäder, P., et al. (2018). Improving crop yield and nutrient use efficiency via biofertilization. A global meta-analysis. Front. Plant Sci. 8:2204. doi: 10.3389/fpls.2017.02204
Sherpa, M., Sharma, L., Bag, N., and Das, S. (2021). Isolation, characterization, and evaluation of native rhizobacterial consortia developed from the rhizosphere of rice grown in organic state Sikkim, India, and their effect on plant growth. Front. Microbiol. 12:713660. doi: 10.3389/fmicb.2021.713660
Silva, J., Menéndez, E., Eliziário, F., Mateos, P. F., Alexandre, A., and Oliveira, S. (2019). Heterologous expression of nifA or nodD genes improves chickpea- Mesorhizobium symbiotic performance. Plant Soil 436, 607–621. doi: 10.1007/s11104-019-03950-0
Sinclair, S. A., and Krämer, U. (2012). The zinc homeostasis network of land plants. Biochim. Biophys. Acta 1823, 1553–1567. doi: 10.1016/j.bbamcr.2012.05.016
Sindhu, S., Sharma, R., Sindhu, S., and Sehrawat, A. (2019). “Soil fertility improvement by symbiotic rhizobia for sustainable agriculture” in Soil fertility management for Sustainable development . eds. D. G. Panpatte and Y. K. Jhala (Gateway East, Singapore: Springer Nature Singapore), 101–166.
Soumare, A., Boubekri, K., Lyamlouli, K., Hafidi, M., Ouhdouch, Y., and Kouisni, L. (2020). From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: status and needs. Front. Bioeng. Biotechnol. 7:425. doi: 10.3389/fbioe.2019.00425
Srithaworn, M., Jaroenthanyakorn, J., Tangjitjaroenkun, J., Suriyachadkun, C., and Chunhachart, O. (2023). Zinc solubilizing bacteria and their potential as bioinoculant for growth promotion of green soybean ( Glycine max L. Merr.). PeerJ 11, 1–17. doi: 10.7717/peerj.15128
Strigul, N. S., and Kravchenko, L. V. (2006). Mathematical modeling of PGPR inoculation into the rhizosphere. Environ. Model Softw. 21, 1158–1171. doi: 10.1016/j.envsoft.2005.06.003
Szczerba, M. W., Britto, D. T., and Kronzucker, H. J. (2009). K+ transport in plants: physiology and molecular biology. J. Plant Physiol. 166, 447–466. doi: 10.1016/j.jplph.2008.12.009
Tabassum, B., Khan, A., Tariq, M., Ramzan, M., Saleem, M., Khan, I., et al. (2017). Bottlenecks in commercialisation and future prospects of PGPR. Appl. Soil Ecol. 121, 102–117. doi: 10.1016/j.apsoil.2017.09.030
Takahashi, H. (2019). Sulfate transport systems in plants: functional diversity and molecular mechanisms underlying regulatory coordination. J. Exp. Bot. 70, 4075–4087. doi: 10.1093/jxb/erz132
Takahashi, H., Kopriva, S., Giordano, M., Saito, K., and Hell, R. (2011). Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 62, 157–184. doi: 10.1146/annurev-arplant-042110-103921
Thilakarathna, M. S., and Raizada, M. N. (2017). A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol. Biochem. 105, 177–196. doi: 10.1016/j.soilbio.2016.11.022
Timofeeva, A., Galyamova, M., and Sedykh, S. (2022). Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plan. Theory 11, 1–23. doi: 10.3390/plants11162119
Tomasi, N., Pinton, R., Dalla, L., Cortella, G., Terzano, R., Mimmo, T., et al. (2015). New ‘solutions’ for floating cultivation system of ready-to-eat salad: a review. Trends Food Sci. Technol. 46, 267–276. doi: 10.1016/j.tifs.2015.08.004
Tourna, M., Maclean, P., Condron, L., Callaghan, M. O., and Wakelin, S. A. (2014). Links between sulphur oxidation and sulphur-oxidising bacteria abundance and diversity in soil microcosms based on soxB functional gene analysis. FEMS Microbiol. Ecol. 88, 538–549. doi: 10.1111/1574-6941.12323
Vacheron, J., Desbrosses, G., Bouffaud, M., Touraine, B., Moënne-Loccoz, Y., Muller, D., et al. (2013). Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 4:356. doi: 10.3389/fpls.2013.00356
Valentinuzzi, F., Pii, Y., Borruso, L., Mimmo, T., Puglisi, E., Trevisan, M., et al. (2021). Epiphytic microbial community and post-harvest characteristics of strawberry fruits as affected by plant nutritional regime with silicon. Agronomy 11, 1–13. doi: 10.3390/agronomy11122407
Vance, C. P., Uhde-Stone, C., and Allan, D. L. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157, 423–447. doi: 10.1046/j.1469-8137.2003.00695.x
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255, 571–586. doi: 10.1023/A:1026037216893
Vijay, K., Shibasini, M., Sivasakthivelan, P., and Kavitha, T. (2023). Microbial siderophores as molecular shuttles for metal cations: sources, sinks and application perspectives. Arch. Microbiol. 205, 1–17. doi: 10.1007/s00203-023-03644-3
von Wirén, N., Marschner, H., and Romheld, V. (1996). Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol. 111, 1119–1125. doi: 10.1104/pp.111.4.1119
Wagh, J., Chanchal, K., Sonal, S., Praveena, B., Archana, G., Kumar, G. N., et al. (2016). Inoculation of genetically modified endophytic Herbaspirillum seropedicae Z67 endowed with gluconic and 2-ketogluconic acid secretion, confers beneficial effects on rice (Oriza sativa). Plant Soil 409, 51–64. doi: 10.1007/s11104-016-2937-7
Wagh, J., Shah, S., and Bhandari, P. (2014). Heterologous expression of pyrroloquinoline quinone (pqq) gene cluster confers mineral phosphate solubilization ability to Herbaspirillum seropedicae Z67. Appl. Microbial. Cell Physiol. 98, 5117–5129. doi: 10.1007/s00253-014-5610-1
Wang, Z., and Song, Y. (2022). Toward understanding the genetic bases underlying plant - mediated “cry for help” to the microbiota. iMeta 1, 1–12. doi: 10.1002/imt2.8
Wang, Y., and Wu, W. (2013). Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 64, 451–476. doi: 10.1146/annurev-arplant-050312-120153
Watanabe, T., Kojima, H., Umezawa, K., Hori, C., and Scott, K. (2019). Genomes of neutrophilic sulfur-oxidizing chemolithoautotrophs representing 9 proteobacterial species from 8 genera. Front. Microbiol. 10:316. doi: 10.3389/fmicb.2019.00316
Wu, X., Rensing, C., Han, D., Xiao, K., Dai, Y., Tang, Z., et al. (2022). Genome-resolved metagenomics reveals distinct phosphorus acquisition strategies between soil microbiomes. mSystems 7, 1–13. doi: 10.1128/msystems.01107-21
Wu, X., Zhao, Z., Zhao, Z., Zhang, Y., Li, M., and Yu, Q. (2023). Analysis of the potassium-solubilizing Priestia megaterium strain NK851 and its potassium feldspar-binding proteins. Int. J. Mol. Sci. 24, 1–12. doi: 10.3390/ijms241814226
Yadav, R. C., Sharma, S. K., Varma, A., Rajawat, M., Khan, M., Sharma, P., et al. (2022). Modulation in biofertilization and biofortification of wheat crop by inoculation of zinc-solubilizing rhizobacteria. Front. Plant Sci. 13:777771. doi: 10.3389/fpls.2022.777771
Yang, S., Liu, H., Xie, P., Wen, T., Shen, Q., and Yuan, J. (2023). Emerging pathways for engineering the rhizosphere microbiome for optimal plant health. J. Agric. Food Chem. 71, 4441–4449. doi: 10.1021/acs.jafc.2c08758
Yu, P., He, X., Baer, M., Beirinckx, S., Tian, T., Moya, Y. A. T., et al. (2021). Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat. Plants 7, 481–499. doi: 10.1038/s41477-021-00897-y
Zhang, J., Liu, Y., Zhang, N., Hu, B., Jin, T., Xu, H., et al. (2019). NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 37, 676–684. doi: 10.1038/s41587-019-0104-4
Zhang, L., Yan, C., Guo, Q., Zhang, J., and Ruiz-Menjivar, J. (2018). The impact of agricultural chemical inputs on environment: global evidence from informetrics analysis and visualization. Int. J. Low-Carbon Technol. 13, 338–352. doi: 10.1093/ijlct/cty039
Zia, R., Shoib, M., Jawad, M., Hakim, S., and Imran, A. (2021). Plant survival under drought stress: implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 242, 1–16. doi: 10.1016/j.micres.2020.126626
Zuluaga, M. Y. A., Cardarelli, M., Rouphael, Y., Cesco, S., Pii, Y., and Colla, G. (2023a). Iron nutrition in agriculture: from synthetic chelates to biochelates. Sci. Hortic. 312:111833. doi: 10.1016/j.scienta.2023.111833
Zuluaga, M. Y. A., De Oliveira, A. L. M., Valentinuzzi, F., Jayme, N. S., Monterisi, S., Fattorini, R., et al. (2023b). An insight into the role of the organic acids produced by Enterobacter sp. strain 15S in solubilizing tricalcium phosphate: in situ study on cucumber. BMC Microbiol. 23:184. doi: 10.1186/s12866-023-02918-6
Zuluaga, M. Y. A., Milani, K. M. L., Gonçalces, L. S. A., and Oliveira, A. L. M. (2020). Diversity and plant growth-promoting functions of diazotrophic/N-scavenging bacteria isolated from the soils and rhizospheres of two species of Solanum. PLoS One 15:e0227422. doi: 10.1371/journal.pone.0227422
Zuluaga, M. Y. A., Oliveira, A. L. M., Valentinuzzi, F., Tiziani, R., Pii, Y., Mimmo, T., et al. (2021). Can inoculation with the bacterial biostimulant Enterobacter sp. strain 15S be an approach for the smarter P fertilization of maize and cucumber plants? Front. Plant Sci. 12:719873. doi: 10.3389/fpls.2021.719873
Zutter, N., Ameye, M., Bekaert, B., Verwaeren, J., Gelder, L., and Audenaert, K. (2022). Uncovering new insights and misconceptions on the effectiveness of phosphate solubilizing rhizobacteria in plants: a meta-analysis. Front. Plant Sci. 13:858804. doi: 10.3389/fpls.2022.858804
Keywords: beneficial bacteria, crop nutrition, nitrogen biofertilizer, phosphate biofertilizer, bacterial siderophores
Citation: Alzate Zuluaga MY, Fattorini R, Cesco S and Pii Y (2024) Plant-microbe interactions in the rhizosphere for smarter and more sustainable crop fertilization: the case of PGPR-based biofertilizers. Front. Microbiol . 15:1440978. doi: 10.3389/fmicb.2024.1440978
Received: 30 May 2024; Accepted: 29 July 2024; Published: 08 August 2024.
Reviewed by:
Copyright © 2024 Alzate Zuluaga, Fattorini, Cesco and Pii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica Yorlady Alzate Zuluaga, [email protected] ; Youry Pii, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Adoption of fertilizer-reduction and efficiency-increasing technologies in china: the role of information acquisition ability, 1. introduction, 2. theoretical analysis, 3. data and methods, 3.2. model set, 3.2.1. regression model, 3.2.2. mechanism testing model, 3.3. variable definitions and choices, 3.4. descriptive statistics, 4.1. results of the irt model, 4.1.1. results of the irt model parameter estimation, 4.1.2. results of iaa measurement, 4.2. impacts of iaa on decisions to adopt freits, 4.3. robustness testing, 4.4. mechanism testing, 5. discussion, 6. conclusions, author contributions, institutional review board statement, data availability statement, conflicts of interest.
| Combinations of Information Channel | IAA | Proportion of Farmers (%) | Combinations of Information Channel | IAA | Proportion of Farmers (%) |
|---|---|---|---|---|---|
| NP | −1.101 | 1.301 | AE+DB+AC+SH+NP | 0.215 | 0.186 |
| None | −0.882 | 7.249 | GD | 0.331 | 2.416 |
| AC+NP | −0.832 | 0.929 | AE+DB+AC+SH | 0.357 | 0.558 |
| SH+NP | −0.830 | 5.019 | GD+SH+NP | 0.367 | 0.372 |
| AC | −0.636 | 0.929 | GD+AC | 0.507 | 1.115 |
| SH | −0.634 | 20.260 | GD+SH | 0.509 | 7.063 |
| AC+SH+NP | −0.589 | 3.160 | GD+AC+SH+NP | 0.543 | 0.558 |
| AE+NP | −0.498 | 0.743 | GD+AC+SH | 0.689 | 5.948 |
| DB | −0.480 | 0.186 | AE+GD | 0.765 | 1.301 |
| AC+SH | −0.412 | 5.576 | AE+GD+SH+NP | 0.803 | 1.115 |
| AE | −0.327 | 2.230 | DB+GD+SH | 0.819 | 0.743 |
| AE+SH+NP | −0.286 | 0.558 | DB+GD+AC+SH+NP | 0.856 | 0.186 |
| DB+SH | −0.269 | 0.929 | AE+GD+AC | 0.958 | 0.929 |
| DB+AC+SH+NP | −0.231 | 0.186 | AE+GD+SH | 0.959 | 9.665 |
| AE+AC | −0.130 | 0.743 | AE+GD+AC+SH+NP | 0.998 | 0.558 |
| AE+SH | −0.129 | 5.390 | DB+GD+AC+SH | 1.015 | 0.558 |
| DB+AC+SH | −0.076 | 0.186 | AE+DB+GD+SH+NP | 1.142 | 0.558 |
| AE+DB+SH+NP | 0.036 | 0.186 | AE+GD+AC+SH | 1.167 | 5.019 |
| AE+AC+SH | 0.057 | 2.788 | AE+DB+GD+SH | 1.320 | 0.743 |
| AE+DB+SH | 0.181 | 0.186 | AE+DB+GD+AC+SH+NP | 1.365 | 0.743 |
| GD+NP | 0.189 | 0.558 | AE+DB+GD+AC+SH | 1.560 | 0.372 |
- Li, Y.X.; Zhang, W.F.; Ma, L.; Huang, G.Q.; Oenema, O.; Zhang, F.S.; Dou, Z.X. An Analysis of China’s Fertilizer Policies: Impacts on the Industry, Food Security, and the Environment. J. Environ. Qual. 2013 , 42 , 972–981. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010 , 327 , 1008–1010. [ Google Scholar ] [ CrossRef ]
- Smith, L.E.D.; Siciliano, G. A comprehensive review of constraints to improved management of fertilizers in China and mitigation of diffuse water pollution from agriculture. Agric. Ecosyst. Environ. 2015 , 209 , 15–25. [ Google Scholar ] [ CrossRef ]
- Li, X.W.; Lei, Z.W.; Qu, J.; Li, Z.; Zhou, X.W.; Zhang, Q.W. Synthesizing slow-release fertilizers via mechanochemical processing for potentially recycling the waste ferrous sulfate from titanium dioxide production. J. Environ. Manag. 2017 , 186 , 120–126. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Meng, F.Q.; Qiao, Y.H.; Wu, W.L.; Smith, P.; Scott, S. Environmental impacts and production performances of organic agriculture in China: A monetary valuation. J. Environ. Manag. 2017 , 188 , 49–57. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009 , 323 , 1014–1015. [ Google Scholar ] [ CrossRef ]
- Kuang, F.; Li, J.; Jin, J.; Qiu, X. Do Green Production Technologies Improve Household Income? Evidence from Rice Farmers in China. Land 2023 , 12 , 1848. [ Google Scholar ] [ CrossRef ]
- Khonje, M.G.; Manda, J.; Mkandawire, P.; Tufa, A.H.; Alene, A.D. Adoption and welfare impacts of multiple agricultural technologies: Evidence from eastern Zambia. Agric. Econ. 2018 , 49 , 599–609. [ Google Scholar ] [ CrossRef ]
- Chen, Y.H.; Xiang, W.; Zhao, M.J. Impacts of Capital Endowment on Farmers’ Choices in Fertilizer-Reduction and Efficiency-Increasing Technologies (Preferences, Influences, and Mechanisms): A Case Study of Apple Farmers in the Provinces of Shaanxi and Gansu, China. Agriculture 2024 , 14 , 147. [ Google Scholar ] [ CrossRef ]
- Hörner, D.; Wollni, M. Does integrated soil fertility management increase returns to land and labor? Plot-level evidence from Ethiopia. Agric. Econ. 2022 , 53 , 337–355. [ Google Scholar ] [ CrossRef ]
- Wang, X.; Drabik, D.; Zhang, J.B. How channels of knowledge acquisition affect farmers’ adoption of green agricultural technologies: Evidence from Hubei province, China. Int. J. Agric. Sustain. 2023 , 21 , 2270254. [ Google Scholar ] [ CrossRef ]
- Liu, Q.Q.; Yan, T.W. The effect of noncognitive abilities on promoting the adoption of soil testing and formula fertilization technology by farmers: Empirical insights from Central China. Environ. Dev. Sustain. 2023 , 15 , 1–33. [ Google Scholar ] [ CrossRef ]
- Tian, M.L.; Liu, R.F.; Wang, J.; Liang, J.H.; Nian, Y.F.; Ma, H.Y. Impact of Environmental Values and Information Awareness on the Adoption of Soil Testing and Formula Fertilization Technology by Farmers-A Case Study Considering Social Networks. Agriculture 2023 , 13 , 2008. [ Google Scholar ] [ CrossRef ]
- Zhou, Z.Y.; Liao, H.L.; Li, H. The Symbiotic Mechanism of the Influence of Productive and Transactional Agricultural Social Services on the Use of Soil Testing and Formula Fertilization Technology by Tea Farmers. Agriculture 2023 , 13 , 1696. [ Google Scholar ] [ CrossRef ]
- Kabunga, N.S.; Dubois, T.; Qaim, M. Heterogeneous information exposure and technology adoption: The case of tissue culture bananas in Kenya. Agric. Econ. 2012 , 43 , 473–485. [ Google Scholar ] [ CrossRef ]
- Zeng, Y.M.; Tian, Y.; He, K.; Zhang, J.B. Environmental conscience, external incentives and social norms in rice farmers’ adoption of pro-environmental agricultural practices in rural Hubei province, China. Environ. Technol. 2020 , 41 , 2518–2532. [ Google Scholar ] [ CrossRef ]
- Ren, Z.; Zhong, K. Driving mechanism of subjective cognition on farmers’ adoption behavior of straw returning technology: Evidence from rice and wheat producing provinces in China. Front. Psychol. 2022 , 13 , 922889. [ Google Scholar ] [ CrossRef ]
- Savari, M.; Damaneh, H.E.; Damaneh, H.E.; Cotton, M. Integrating the norm activation model and theory of planned behaviour to investigate farmer pro-environmental behavioural intention. Sci. Rep. 2023 , 13 , 5584. [ Google Scholar ] [ CrossRef ]
- Robertson, M.J.; Llewellyn, R.S.; Mandel, R.; Lawes, R.; Bramley, R.G.V.; Swift, L.; Metz, N.; O’Callaghan, C. Adoption of variable rate fertiliser application in the Australian grains industry: Status, issues and prospects. Precis. Agric. 2012 , 13 , 181–199. [ Google Scholar ] [ CrossRef ]
- Ju, X.T.; Gu, B.J.; Wu, Y.Y.; Galloway, J.N. Reducing China’s fertilizer use by increasing farm size. Global Environ. Change 2016 , 41 , 26–32. [ Google Scholar ] [ CrossRef ]
- Aregay, F.A.; Minjuan, Z. Impact of irrigation on fertilizer use decision of farmers in China: A case study in Weihe River Basin. J. Sustain. Dev. 2012 , 5 , 74–82. [ Google Scholar ] [ CrossRef ]
- Khonje, M.G.; Nyondo, C.; Chilora, L.; Mangisoni, J.H.; Ricker-Gilbert, J.; Burke, W.J. Exploring adoption effects of subsidies and soil fertility management in Malawi. J. Agric. Econ. 2022 , 73 , 874–892. [ Google Scholar ] [ CrossRef ]
- Feder, G.; Just, R.E.; Zilberman, D. Adoption of agricultural innovations in developing countries: A survey. Econ. Devel. Cult. Change 1985 , 33 , 255–298. [ Google Scholar ] [ CrossRef ]
- Ghadim, A.K.A.; Pannell, D.J.; Burton, M.P. Risk, uncertainty, and learning in adoption of a crop innovation. Agric. Econ. 2005 , 33 , 1–9. [ Google Scholar ] [ CrossRef ]
- Mumin, Y.A.; Abdulai, A. Social networks, adoption of improved variety and household welfare: Evidence from Ghana. Eur. Rev. Agric. Econ. 2022 , 49 , 1–32. [ Google Scholar ] [ CrossRef ]
- Beaman, L.; BenYishay, A.; Magruder, J.; Mobarak, A.M. Can network theory-based targeting increase technology adoption? Am. Econ. Rev. 2021 , 111 , 1918–1943. [ Google Scholar ] [ CrossRef ]
- Mumin, Y.A.; Abdulai, A.; Goetz, R. The role of social networks in the adoption of competing new technologies in Ghana. J. Agric. Econ. 2023 , 74 , 510–533. [ Google Scholar ] [ CrossRef ]
- Genius, M.; Koundouri, P.; Nauges, C.; Tzouvelekas, V. Information Transmission in Irrigation Technology Adoption and Diffusion: Social Learning, Extension Services, and Spatial Effects. Am. J. Agric. Econ. 2014 , 96 , 328–344. [ Google Scholar ] [ CrossRef ]
- Ramirez, A. The influence of social networks on agricultural technology adoption. Procedia-Soc. Behav. Sci. 2013 , 79 , 101–116. [ Google Scholar ] [ CrossRef ]
- Mannan, S.; Nordin, S.M.; Rafik-Galea, S.; Rizal, A.R.A. The ironies of new innovation and the sunset industry: Diffusion and adoption. J. Rural. Stud. 2017 , 55 , 316–322. [ Google Scholar ] [ CrossRef ]
- Liu, Y.Y.; Shi, R.L.; Peng, Y.T.; Wang, W.; Fu, X.H. Impacts of Technology Training Provided by Agricultural Cooperatives on Farmers’ Adoption of Biopesticides in China. Agriculture 2022 , 12 , 316. [ Google Scholar ] [ CrossRef ]
- Huang, J.; Xiang, C.; Jia, X.; Hu, R. Impacts of training on farmers’ nitrogen use in maize production in Shandong, China. J. Soil Water Conserv. 2012 , 67 , 321–327. [ Google Scholar ] [ CrossRef ]
- Jiang, W.J.; Yan, T.W.; Chen, B. Impact of media channels and social interactions on the adoption of straw return by Chinese farmers. Sci. Total Environ. 2021 , 756 , 144078. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, F.D.; Yang, P.; Zhang, K.J.; Yin, Y.S.; Zhang, Y.N.; Yin, C.B. The influence of smartphone use on conservation agricultural practice: Evidence from the extension of rice-green manure rotation system in China. Sci. Total Environ. 2022 , 813 , 152555. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, Z.; Li, X.J.; Xia, X.L.; Zhang, J.Z. The impact of social interaction and information acquisition on the adoption of soil and water conservation technology by farmers: Evidence from the Loess Plateau, China. J. Clean. Prod. 2024 , 434 , 139880. [ Google Scholar ] [ CrossRef ]
- Li, Z.; Zhang, D.S.; Yan, X.H. How Does Information Acquisition Ability Affect Farmers’ Green Production Behaviors: Evidence from Chinese Apple Growers. Agriculture 2024 , 14 , 680. [ Google Scholar ] [ CrossRef ]
- Khan, N.; Ray, R.L.; Sargani, G.R.; Ihtisham, M.; Khayyam, M.; Ismail, S. Current Progress and Future Prospects of Agriculture Technology: Gateway to Sustainable Agriculture. Sustainability 2021 , 13 , 4883. [ Google Scholar ] [ CrossRef ]
- Just, R.E.; Zilberman, D. The effects of agricultural development policies on income distribution and technological change in agriculture. J. Devel. Econ. 1988 , 28 , 193–216. [ Google Scholar ] [ CrossRef ]
- Atanu, S.; Love, H.A.; Schwart, R. Adoption of emerging technologies under output uncertainty. Am. J. Agric. Econ. 1994 , 76 , 836–846. [ Google Scholar ] [ CrossRef ]
- Ridier, A.; Ben El Ghali, M.; Nguyen, G.; Kephaliacos, C. The role of risk aversion and labor constraints in the adoption of low input practices supported by the CAP green payments in cash crop farms. Rev. Agric. Environ. Stud. Rev. D’etudes Agric. Environ. (RAEStud) 2013 , 94 , 195–219. [ Google Scholar ] [ CrossRef ]
- Davis, F.D.; Bagozzi, R.P.; Warshaw, P.R. User acceptance of computer technology: A comparison of two theoretical models. Manag. Sci. 1989 , 35 , 982–1003. [ Google Scholar ] [ CrossRef ]
- Xiuling, D.; Qian, L.; Lipeng, L.; Sarkar, A. The Impact of Technical Training on Farmers Adopting Water-Saving Irrigation Technology: An Empirical Evidence from China. Agriculture 2023 , 13 , 956. [ Google Scholar ] [ CrossRef ]
- Wozniak, G.D. Joint information acquisition and new technology adoption: Late versus early adoption. Rev. Econ. Stat. 1993 , 75 , 438–445. [ Google Scholar ] [ CrossRef ]
- Khataza, R.R.; Doole, G.J.; Kragt, M.E.; Hailu, A. Information acquisition, learning and the adoption of conservation agriculture in Malawi: A discrete-time duration analysis. Technol. Forecast. Soc. Change 2018 , 132 , 299–307. [ Google Scholar ] [ CrossRef ]
- Kabir, J.; Cramb, R.; Alauddin, M.; Gaydon, D.S.; Roth, C.H. Farmers’ perceptions and management of risk in rice/shrimp farming systems in South-West Coastal Bangladesh. Land Use Policy 2020 , 95 , 104577. [ Google Scholar ] [ CrossRef ]
- Alesina, A.; Zhuravskaya, E. Segregation and the Quality of Government in a Cross Section of Countries. Am. Econ. Rev. 2011 , 101 , 1872–1911. [ Google Scholar ] [ CrossRef ]
- Shen, L.; Wang, F. Can Market-Oriented Allocation of Land Factors Promote the Adoption of Cropland Quality Protection Behaviors by Farmers: Evidence from Rural China. Land 2024 , 13 , 665. [ Google Scholar ] [ CrossRef ]
- Abdul-Salam, Y.; Phimister, E. Efficiency Effects of Access to Information on Small-scale Agriculture: Empirical Evidence from Uganda using Stochastic Frontier and IRT Models. J. Agric. Econ. 2017 , 68 , 494–517. [ Google Scholar ] [ CrossRef ]
- Huang, W.H.; Yang, C.Y.; Liu, K.; Min, R. Information Acquisition Ability and Farmers’ Herd Behavior in Rice-Crayfish Coculture System Adoption. Agriculture 2023 , 13 , 1892. [ Google Scholar ] [ CrossRef ]
- Hall, T.J.; Dennis, J.H.; Lopez, R.G.; Marshall, M.I. Factors Affecting Growers’ Willingness to Adopt Sustainable Floriculture Practices. Hortscience 2009 , 44 , 1346–1351. [ Google Scholar ] [ CrossRef ]
- Zhou, Z.; Zhang, Y.; Yan, Z. Will Digital Financial Inclusion Increase Chinese Farmers’ Willingness to Adopt Agricultural Technology? Agriculture 2022 , 12 , 1514. [ Google Scholar ] [ CrossRef ]
- Guo, Z.; Chen, X.; Zhang, Y. Impact of environmental regulation perception on farmers’ agricultural green production technology adoption: A new perspective of social capital. Technol. Soc. 2022 , 71 , 102085. [ Google Scholar ] [ CrossRef ]
- Bandiera, O.; Rasul, I. Social networks and technology adoption in northern Mozambique. Econ. J. 2006 , 116 , 869–902. [ Google Scholar ] [ CrossRef ]
- Beethem, K.; Marquart-Pyatt, S.T.; Lai, J.N.F.; Guo, T. Navigating the information landscape: Public and private information source access by midwest farmers. Agric. Hum. Values 2023 , 40 , 1117–1135. [ Google Scholar ] [ CrossRef ]
- Tsakiris, P.; Damalas, C.A.; Koutroubas, S.D. Safety behavior in pesticide use among farmers of northern Greece: The role of information sources. Pest Manag. Sci. 2023 , 79 , 4335–4342. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khatoon-Abadi, A. Prioritization of Farmers’ Information Channels: A Case Study of Isfahan Province, Iran. J. Agric Sci. Tech. 2011 , 13 , 815–828. Available online: https://sid.ir/paper/62486/en (accessed on 15 May 2011).
- Robins, R.W.; Fraley, R.C.; Krueger, R.F. Handbook of Research Methods in Personality Psychology ; Guilford Press: New York, NY, USA, 2009. [ Google Scholar ]
- Edelen, M.O.; Reeve, B.B. Applying item response theory (IRT) modeling to questionnaire development, evaluation, and refinement. Qual. Life Res. 2007 , 16 , 5–18. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yue, S.M.; Xue, Y.; Lyu, J.; Wang, K.K. The Effect of Information Acquisition Ability on Farmers’ Agricultural Productive Service Behavior: An Empirical Analysis of Corn Farmers in Northeast China. Agriculture 2023 , 13 , 573. [ Google Scholar ] [ CrossRef ]
- Wainaina, P.; Tongruksawattana, S.; Qaim, M. Tradeoffs and complementarities in the adoption of improved seeds, fertilizer, and natural resource management technologies in Kenya. Agric. Econ. 2016 , 47 , 351–362. [ Google Scholar ] [ CrossRef ]
- Roodman, D. Fitting Fully Observed Recursive Mixed-process Models with cmp. Stata J. 2011 , 11 , 159–206. [ Google Scholar ] [ CrossRef ]
- Aldana, U.; Foltz, J.D.; Barham, B.L.; Useche, P. Sequential Adoption of Package Technologies: The Dynamics of Stacked Trait Corn Adoption. Am. J. Agric. Econ. 2011 , 93 , 130–143. [ Google Scholar ] [ CrossRef ]
- Ma, W.; Ma, C.; Su, Y.; Nie, Z. Organic farming: Does acquisition of the farming information influence Chinese apple farmers’ willingness to adopt? China Agric. Econ. Rev. 2017 , 9 , 211–224. [ Google Scholar ] [ CrossRef ]
- Oduniyi, O.S.; Tekana, S.S. Does Information Acquisition Influence the Adoption of Sustainable Land Management Practices? Evidence from Mpumalanga Province South Africa. Front. Sustain. Food Syst. 2021 , 5 , 769094. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Variable | Definition | Mean | Std. Dev. | Min. | Max. | |
|---|---|---|---|---|---|---|
| Dependent Variables | STFFT | Adoption of STFFT (1 = adopter, 0 = otherwise) | 0.125 | 0.330 | 0 | 1 |
| OFRT | Adoption of OFRT (1 = adopter, 0 = otherwise) | 0.260 | 0.439 | 0 | 1 | |
| Independent Variable | IAA | Parameters measured by the IRT model | 0.002 | 0.723 | −1.101 | 1.560 |
| Mechanism Variables | Technical Training | Whether farmers participated in formal training in FREITs (1 = yes, 0 = no) | 0.571 | 0.495 | 0 | 1 |
| Cognitive Level | Cognitive level for FREITs (1 = very low, 2 = low, 3 = moderate, 4 = high, 5 = very high) | 3.294 | 0.966 | 1 | 5 | |
| Instrument Variable | Cooperatives | Number of cooperatives in villages | 0.217 | 0.521 | 0 | 3 |
| Control Variables | Scale | Cropland area (hectares) | 5.507 | 11.897 | 0.133 | 193.43 |
| Plots | Number of arable plots (blocks) | 3.191 | 4.749 | 1 | 70 | |
| Cropping System | 1 = rice–crayfish coculture system, 0 = rice-only system | 0.699 | 0.459 | 0 | 1 | |
| Village Cadre | Village cadre in the household (1 = yes, 0 = no) | 0.230 | 0.422 | 0 | 1 | |
| Labor | Number of family agricultural laborers | 2.037 | 0.736 | 1 | 6 | |
| Age | Average age of agricultural laborers (years) | 52.090 | 7.622 | 26 | 73 | |
| Education | Average education years of agricultural laborers (years) | 7.268 | 2.825 | 0 | 20 | |
| Non-farm Income | Non-agricultural income of farm households as a proportion of total household income | 0.220 | 0.256 | 0 | 0.974 | |
| Financial Situation | Adequacy of financial resources for agricultural production (1 = adequacy, 0 = inadequacy) | 0.582 | 0.494 | 0 | 1 | |
| Knowledge | Knowledge of the hazards of chemical fertilizer application (1 = no knowledge, 2 = less knowledge, 3 = moderate, 4 = more knowledge, 5 = full knowledge) | 3.420 | 1.254 | 1 | 5 | |
| Jiangsu | Whether farmers were in Jiangsu Province (1 = yes, 0 = no) | 0.258 | 0.438 | 0 | 1 | |
| Response Variables | Agricultural Enterprises (AE) | Whether the farmer obtains information from agricultural enterprises (1 = yes, 0 = no) | 0.346 | 0.476 | 0 | 1 |
| Demonstration Bases (DB) | Whether the farmer obtains information from demonstration bases (1 = yes, 0 = no) | 0.065 | 0.247 | 0 | 1 | |
| Government Departments (GD) | Whether the farmer obtains information from government departments (1 = yes, 0 = no) | 0.405 | 0.491 | 0 | 1 | |
| Agricultural Cooperatives (AC) | Whether the farmer obtains information from agricultural cooperatives (1 = yes, 0 = no) | 0.312 | 0.464 | 0 | 1 | |
| Surrounding Households (SH) | Whether the farmer obtains information from surrounding households (1 = yes, 0 = no) | 0.794 | 0.405 | 0 | 1 | |
| Network Platforms (NP) | Whether the farmer obtains information from network platforms (1 = yes, 0 = no) | 0.169 | 0.375 | 0 | 1 |
| Information Channel | Differentiation Parameter | S.E. | Rank | Difficulty Parameter | S.E. | Rank |
|---|---|---|---|---|---|---|
| Agricultural Enterprises (AE) | 1.081 *** | 0.283 | 2 | 0.732 *** | 0.169 | 3 |
| Demonstration Bases (DB) | 0.758 ** | 0.307 | 3 | 3.838 *** | 1.335 | 1 |
| Government Departments (GD) | 2.671 * | 1.447 | 1 | 0.293 *** | 0.073 | 4 |
| Agricultural Cooperatives (AC) | 0.448 *** | 0.146 | 5 | 1.848 *** | 0.595 | 2 |
| Surrounding Households (SH) | 0.451 ** | 0.178 | 4 | −3.112 *** | 1.157 | 5 |
| Network Platforms (NP) | −0.361 ** | 0.176 | 6 | −4.522 ** | 2.115 | 6 |
| Variables | Probit | IV-Probit | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 STFFT | Model 2 ORFT | Model 3 STFFT | Model 4 ORFT | |||||
| Coef. | S.E. | Coef. | S.E. | Coef. | S.E. | Coef. | S.E. | |
| IAA | −0.098 | 0.111 | 0.097 | 0.088 | 1.110 *** | 0.235 | 1.216 *** | 0.212 |
| Scale | 0.012 | 0.008 | 0.007 | 0.007 | −0.004 | 0.007 | −0.007 | 0.005 |
| Plots | 0.007 | 0.018 | 0.004 | 0.015 | 0.039 *** | 0.014 | 0.038 *** | 0.011 |
| Cropping System | −0.447 *** | 0.168 | 0.805 *** | 0.172 | −0.467 *** | 0.136 | 0.297 | 0.238 |
| Village Cadre | 0.063 | 0.175 | −0.155 | 0.155 | −0.278 * | 0.159 | −0.410 *** | 0.135 |
| Labor | 0.264 *** | 0.093 | −0.043 | 0.094 | 0.020 | 0.098 | −0.164 ** | 0.072 |
| Age | 0.022 * | 0.012 | 0.003 | 0.009 | 0.012 | 0.010 | 0.000 | 0.008 |
| Education | 0.163 *** | 0.040 | 0.063 ** | 0.027 | 0.056 | 0.046 | −0.003 | 0.030 |
| Non-farm Income | −0.490 | 0.318 | −0.713 *** | 0.271 | −0.422 * | 0.245 | −0.567 ** | 0.234 |
| Financial Situation | −0.403 ** | 0.157 | 0.150 | 0.131 | −0.141 | 0.158 | 0.196 * | 0.109 |
| Knowledge | 0.066 | 0.065 | −0.058 | 0.051 | 0.084 * | 0.049 | 0.009 | 0.044 |
| Jiangsu | −0.265 | 0.213 | 0.629 *** | 0.155 | −0.164 | 0.161 | 0.379 ** | 0.169 |
| Constant | −3.840 *** | 0.831 | −1.730 *** | 0.648 | −1.661 | 1.027 | −0.415 | 0.727 |
| Results of the first-stage regression | ||||||||
| Cooperatives | 0.153 *** | 0.051 | 0.153 *** | 0.051 | ||||
| Control Variables | Yes | Yes | ||||||
| Log Pseudo Likelihood | −169.044 | −277.428 | −720.110 | −827.43 | ||||
| Chi-square | 54.684 *** | 49.176 *** | 193.620 *** | 245.056 *** | ||||
| Wald Test of Exogeneity | 7.40 *** | 7.19 *** | ||||||
| IV-Probit | CMP | ||||
|---|---|---|---|---|---|
| Model 5 | Model 6 | Model 7 | Model 8 | ||
| FREIT | STFFT | ORFT | STFFT | ORFT | |
| IAA | 1.197 *** (0.208) | 1.106 *** (0.241) | 1.217 *** (0.211) | ||
| Number of Channels | 0.615 *** (0.168) | 0.701 *** (0.152) | |||
| Control Variables | Yes | Yes | Yes | Yes | Yes |
| Cooperatives | 0.153 *** (0.051) | 0.331 *** (0.089) | 0.331 *** (0.089) | 0.153 *** (0.051) | |
| Control Variables | Yes | Yes | Yes | Yes | |
| Log Pseudo Likelihood | −870.068 | −971.727 | −1076.555 | −993.822 | |
| Chi-square | 222.282 *** | 132.224 *** | 167.872 *** | 189.960 *** | |
| Wald Test of Exogeneity | 7.68 *** | 6.40 ** | 6.34 ** | ||
| athrho2_1 | −1.094 *** (0.395) | −0.869 ** (0.343) | −0.787 ** (0.312) | ||
| atanhrho_12 | 0.797 ** (0.336) | ||||
| atanhrho_13 | −1.097 *** (0.413) | ||||
| atanhrho_23 | −1.085 *** (0.404) | ||||
| Probit | CMP | Oprobit | CMP | |
|---|---|---|---|---|
| Model 9 Technical Training | Model 10 FREIT | Model 11 Cognitive Level | Model 12 FREIT | |
| IAA | 1.244 *** (0.109) | 1.070 *** (0.245) | 0.399 *** (0.068) | 1.154 *** (0.229) |
| Technical Training | 0.360 ** (0.139) | |||
| Cognitive Level | 0.114 * (0.061) | |||
| Control Variables | Yes | Yes | Yes | Yes |
| Cooperatives | 0.153 *** (0.051) | 0.153 *** (0.051) | ||
| Control Variables | Yes | Yes | ||
| Log Pseudo Likelihood | −249.470 | −861.833 | −582.329 | −867.229 |
| Chi-square | 170.95 *** | 619.82 *** | 206.01 | 559.68 *** |
| atanhrho_12 | −1.103 *** (0.391) | −1.064 *** (0.399) |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Yang, C.; Huang, W.; Xiao, Y.; Qi, Z.; Li, Y.; Zhang, K. Adoption of Fertilizer-Reduction and Efficiency-Increasing Technologies in China: The Role of Information Acquisition Ability. Agriculture 2024 , 14 , 1339. https://doi.org/10.3390/agriculture14081339
Yang C, Huang W, Xiao Y, Qi Z, Li Y, Zhang K. Adoption of Fertilizer-Reduction and Efficiency-Increasing Technologies in China: The Role of Information Acquisition Ability. Agriculture . 2024; 14(8):1339. https://doi.org/10.3390/agriculture14081339
Yang, Caiyan, Weihong Huang, Yu Xiao, Zhenhong Qi, Yan Li, and Kun Zhang. 2024. "Adoption of Fertilizer-Reduction and Efficiency-Increasing Technologies in China: The Role of Information Acquisition Ability" Agriculture 14, no. 8: 1339. https://doi.org/10.3390/agriculture14081339
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Plant Sci
Overview of biofertilizers in crop production and stress management for sustainable agriculture
Parul chaudhary.
1 Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, India
Shivani Singh
Anuj chaudhary.
2 School of Agriculture and Environmental Science, Shobhit University, Gangoh, India
Anita Sharma
Govind kumar.
3 Department of Crop Production, Central Institute for Subtropical Horticulture, Lucknow, India
With the increase in world population, the demography of humans is estimated to be exceeded and it has become a major challenge to provide an adequate amount of food, feed, and agricultural products majorly in developing countries. The use of chemical fertilizers causes the plant to grow efficiently and rapidly to meet the food demand. The drawbacks of using a higher quantity of chemical or synthetic fertilizers are environmental pollution, persistent changes in the soil ecology, physiochemical composition, decreasing agricultural productivity and cause several health hazards. Climatic factors are responsible for enhancing abiotic stress on crops, resulting in reduced agricultural productivity. There are various types of abiotic and biotic stress factors like soil salinity, drought, wind, improper temperature, heavy metals, waterlogging, and different weeds and phytopathogens like bacteria, viruses, fungi, and nematodes which attack plants, reducing crop productivity and quality. There is a shift toward the use of biofertilizers due to all these facts, which provide nutrition through natural processes like zinc, potassium and phosphorus solubilization, nitrogen fixation, production of hormones, siderophore, various hydrolytic enzymes and protect the plant from different plant pathogens and stress conditions. They provide the nutrition in adequate amount that is sufficient for healthy crop development to fulfill the demand of the increasing population worldwide, eco-friendly and economically convenient. This review will focus on biofertilizers and their mechanisms of action, role in crop productivity and in biotic/abiotic stress tolerance.
Introduction
The world population will reach 9 billion by 2050 in accordance with Food and Agricultural Organization; as a result, there should be an enhancement in crop yield to meet the food demand. Soil is an important source of food production in human lifespan. In the last decades, due to the increase in agricultural practices such as pesticides and chemical fertilizers it has been degraded at a universal scale and causes lower fertility due to loss in biodiversity, water retention, and disturbance in biogeochemical cycles. Soil health and plant productivity are severely influenced by numerous interactions among plant, soil, and microorganisms (Harman et al., 2020 ). Soil microbes cooperate with one another and also with plant roots in numerous means providing a wide variety of essential acts which are valuable for sustaining the ecological balance in soil (Kumar et al., 2021c ). Plant microbial interactions are positive if they improve plant survival, nutritional status, and crop productivity and they are negative if they reduce plant growth. Soil fertility is inextricably linked to the balance of microorganisms and plants (Vishwakarma et al., 2020 ). The application of biofertilizers can be a probable approach to improve soil microbial status that stimulates the natural soil microbiota therefore influencing nutrient accessibility and decomposition of organic matter (Chaudhary et al., 2021 ). It was observed that the supply of biofertilizers in apricot modifies the microbial composition and degradation process which could be efficient in nutrient cycles in soil under field conditions (Agri et al., 2021 ; Baldi et al., 2021 ). The capability of biofertilizers to form a high-level microbial diversity in soil may outcome better crop productivity for sustainable agriculture (Agri et al., 2022 ). Recently, many studies reported the positive impact of beneficial soil microbes on crop productivity, but the role of consortium in agriculture is not entirely unstated. Usage of consortium has positive impact on nutrient uptake efficiency by plants, protection from pathogens, and stress conditions (Aguilar-Paredes et al., 2020 ). This review provided information on effective approaches such as biofertilizers which help in the restoration of agricultural soil thus improving crop health for sustainable agriculture. This can permit agriculturalists to enhance farming and reach a high standard of soil quality and subsequently lead to raised plant development.
Nutrients are required by every living creature in this world. A total of 17 essential plant nutrients are mandatory for the proper development of plants (Kumar et al., 2021a ). These 17 nutrients are divided into three classes based on the amount required such as major nutrients (carbon, hydrogen, oxygen, nitrogen, phosphorus, and potassium), minor nutrients such as sulfur, calcium, and magnesium, and micronutrients (nickel, zinc, molybdenum, manganese, iron, copper, chlorine, and boron). The plant takes up oxygen, hydrogen, and carbon from air and water, but the other nutrients are taken from soils in inorganic forms (Gong et al., 2020 ). Biofertilizer or biological fertilizer is a material that contains living or dormant microorganisms that colonize the rhizosphere or present inside the plants and directly or indirectly promotes the growth of plants by supplying nutrition (Malusa and Vassilev, 2014 ; Fasusi et al., 2021 ). Microorganisms present in soil used as biofertilizers can mobilize the nutrient from soil and convert them into a usable form from unusable form through biological processes like nitrogen fixation, phosphorus solubilization, zinc solubilization, siderophores production, and producing plant growth-promoting substances (Bhattacharjee and Dey, 2014 ; Mazid and Khan, 2015 ). Biofertilizers are applied to seed, root, soil, or by the foliar spray to enhance the microbial activity through their multiplication which then mobilizes the nutrients to target plants which remarkably improved the soil fertility and sooner increases the crop health and production (Pandey and Singh, 2012 ; Ismail et al., 2013 ).
Biotic stress is responsible to damage plants by pathogenic organisms like bacteria, fungi, viruses, parasites, and insects and by other harmful plants. They lead to declining the crop productivity by causing diseases such as vascular wilts, leaf spots, cankers, nutrient deficiency, systematic damage, chlorosis, stunting and reduce plant vigor, ultimately causing the death of the plants (Iqbal et al., 2021 ). Plant protects themselves to biotic stress via direct mechanisms like synthesis of secondary metabolite, hormones, cell-wall-degrading enzymes, and antioxidants (Kaur et al., 2022 ). The indirect mechanisms include the induction of acquired systematic resistance, plant pathogen molecular patterns (PAMPs) which in turn trigger the immunity and plant resistance proteins (Yu et al., 2022 ). Microorganisms solubilize the phosphorus and zinc, fixing the nitrogen and other macro- and micronutrients which promote the growth of the plants under biotic stress condition by providing nutrition (Singh et al., 2022a ). They also enhance the stress resistance in plants by expressing the gene of phytohormones and stress-related metabolite. Some microorganisms also produce the volatile organic compounds (VOCs) such as melatonin to protect the plant from pathogens (Moustafa-Farag et al., 2019 ). When pathogen attacks, the plant produces various compounds within the tissues that lead to the activation of defense mechanisms inside the plants such as induced systematic resistance, peroxidases, phenylalanine ammonia-lyase, polyphenol oxidase, and hypersensitivity (Kaur et al., 2022 ).
Climatic change is one of the major factors for enhancing abiotic stress on crops which results in reduced crop productivity (Liu et al., 2017a ). Climatic-related abiotic stresses included drought, waterlogging, excessive heat, and soil-related abiotic stresses are fertility, heavy metals, and salinity; all these are responsible for the poor yields of crops around the whole globe (Upadhyay et al., 2019 ). There is less water available to plants during drought conditions, and biofertilizers have the potential to produce cytokinin, gibberellins, abscisic acid, and IAA, which cause the plant to increase its growth, root length, total surface area, and the formation of root hairs and lateral roots, which increases water absorption from water-deficient soil (Kenneth et al., 2019 ; Raza et al., 2019 ). Pollutants released from industry without any further operation if released in the environment then they cause the accumulation of heavy metals such as copper, lead, nickel, zinc, etc., which have detrimental effects on the plants and animals (Popp et al., 2013 ). These heavy metals are removed from the environment by micro- and macro-nutrient solubilizing and mineralizing microorganisms (Bhojiya et al., 2021 ). Heat stress causes cellular changes like production of reactive oxygen species, reduction in cell turgidity, reduction in water uptake, reduction in growth of plants, ultimately leading to death of plant by showing initial symptoms like leaf senescence, damages to chloroplast, wilting of plant, and chlorosis (Ahluwalia et al., 2021 ), whereas low temperature causes the inactivation of protein and reduces the cell membrane fluidity leading to increases in photosynthesis, imbalance of water transport (Odoh et al., 2020 ). All these temperature-related stresses coped up by plants after the accumulation of the hydrophilic and osmolytes protein. Huang et al. ( 2015 ) reported that due to high salt concentration there is increased toxicity to cell due to accumulations of sodium and chloride ions inside the cell which in turn disturb the photosynthetic processes, stomatal opening and closing, shrinkage of cell within pant tissue. Various studies showed that bacteria and arbuscular mycorrhizae fungi help in surviving the plants under salinity stress condition by enhancing the plant growth and development. In this review, we will discuss about the biofertilizers and its mechanism for crop production and biotic/abiotic tolerance for sustainable agriculture.
Biofertilizers
In India, biofertilizer refers to the use of microorganisms to meet nutritional needs, whereas in other countries, the term microbial bioinoculant is used (Mitter et al., 2021 ). Biofertilizers are bio-based organic fertilizers that either could be from plant or animal sources or from living or dormant microbial cells that have the potential to improve the bioavailability and bioaccessibility of nutrient uptake in plants (Lee et al., 2018 ; Abbey et al., 2019 ). Bhardwaj et al. ( 2014 ) reported that live microbial mass is a major ingredient of biofertilizers. So biofertilizers are properly defined as “the preparations containing live microbes that help in enhancing soil fertility by fixing atmospheric nitrogen, solubilizing phosphorus or decomposing organic wastes or by elevating plant growth through the production of growth hormones with their biological activities” (Okur, 2018 ). Biofertilizers are generally applied in solid or dry forms, which are prepared after packing on suitable carriers such as clay minerals, rice bran, peat, lignite, wheat bran, humus, and wood charcoal. Carriers increase the shelf life and enable the easy handling of microbial inoculants (Bhattacharjee and Dey, 2014 ). The benefits of biofertilizers include low cost, enhanced nutrient availability, improved soil fertility, protect plants from soil-borne pathogens, sustainable agricultural production, enhanced biotic and abiotic stress tolerance, promote phytohormone production, improve soil health, causing less environmental pollution, and its continued use improves the fertility of soil considerably (Chaudhary et al., 2021 , 2022a ). Based on the source and raw material, global biofertilizer is marketed under two major categories like organic residue-based biofertilizer and microorganisms-based biofertilizer. Green manure, crop residues, treated sewage sludge, and farmyard manure are generally organic-based biofertilizers. While on the contrary, microorganism-based biofertilizers contain beneficial microorganisms like bacteria, fungi, and algae. Directly or indirectly, these biofertilizers mediate the performance of plant growth ( Figure 1 ). Direct mechanisms that act upon plants directly include nitrogen fixation, phosphate solubilization, micronutrient solubilization, and the production of phytohormones (Chaudhary et al., 2021 ). The indirect mechanism generally protects the plant from the deleterious effect of the pathogens by releasing lytic enzymes, antibiotics, siderophores, and cyanide production (Mahmud et al., 2021 ).

Types of biofertilizers on the basis of microorganism and functional characteristics.
Types of biofertilizers and their role in crop production and soil health maintenance
Various types of biofertilizers are classified based on microorganisms such as bacteria and fungi and function of the biofertilizers as shown in Figure 1 .
Nitrogen-fixing biofertilizers
Nitrogen is the vital macro-nutrient essential by plants because it improves the growth of the shoot system, helps in reproduction, is a constituent of chlorophyll responsible for the deep green color, and also increases the size of the grains (Sandhu et al., 2021 ). Although the nitrogen content in the atmosphere is 78% by a mass fraction, dinitrogen contains triple bonds and is an unavailable form of nitrogen present in the air for the plants. Dinitrogen should be first converted into soluble non-toxic form ammonia by the diazotrophs through the biological process of nitrogen fixations (Abbey et al., 2019 ). This ammonia is then converted to the nitrite and nitrate by the ammonia-oxidizing bacteria and by nitrifying bacteria, respectively (Roy et al., 2020 ). The unused nitrate is converted to the atmospheric nitrogen in the deeper soil horizons through the process of denitrification which will then escape to the atmosphere as dinitrogen gas. This is the typical path of the nitrogen cycle (Mahanty et al., 2017 ). Azotobacter and Bacillus sp. are involved in N fixation, growth promotion of maize plants, and forest crops (Etesami et al., 2014 ; Azeem et al., 2022 ). Inoculation of Bradyrhizobium japonicum in soybean plants improved plant biomass, nodulation, and N fixation (Htwe et al., 2019 ). Azotobacter chroococcum improved the plant height and chlorophyll content in maize plants (Jain et al., 2021 ). Bradyrhizobium sp. showed nitrogen fixation, IAA, and siderophores production and improved the yield of mung bean (Alkurtany et al., 2018 ). Nitrogen-fixing microbes are considered as symbiotic, free-living, and associative nitrogen-fixing bacteria (Aasfar et al., 2021 ). Jing et al. ( 2020 ) reported that the application of Pseudomonas protegens promoted plant growth in nitrogen-deficient conditions.
Symbiotic nitrogen-fixing microbes
In the process of symbiosis, macro-symbiont is the plant and microsymbionts are the prokaryotic bacteria. Rhizobium and legume symbiosis is one of the most studied mutualistic relationships between plant root nodules and nitrogen-fixing microorganisms. Mutualistic relationships are initiated when the plant began to secrete the flavonoids and iso-flavonoids in its rhizosphere, where it is recognized by Rhizobium (Hawkins and Oresnik, 2022 ). It started to do infection by differentiating root hairs, developing infection thread up to the root hair cell where infectious thread releases all its bacteria in the cytoplasmic region. Then, bacterial cell are terminally differentiated into the bacteroides, and the further development of bacteroides leads to the formation of symbiosome which is the site of nitrogen fixation (Cissoko et al., 2018 ; Jimenez-Jimenez et al., 2019 ; Suzaki et al., 2019 ). This atmospheric nitrogen fixation inside the nodule is carried out by the nitrogenase enzyme (Brahmaprakash and Sahu, 2012 ). Examples include Rhizobium associated with leguminous plants, Frankia (actinomycetes) associated with non-leguminous plants ( Alnus, Casuarina ), Azolla and the blue-green alga Anabaena azollae , and association of cyanobacteria with gymnosperms (Ghodhbane-Gtari et al., 2021 ). Fixation of N helps to improve the soil fertility and crop productivity. Mondal et al. ( 2020 ) reported that Rhizobium meliloti involved in N 2 fixation produced chitinase enzyme and improved the yield of peanut plants. The alfalfa-Rhizobium symbiotic system can stimulate plant N fixation, increase phytohormone production, and promote plant growth (Fang et al., 2020 ).
Free-living nitrogen-fixing bacteria
Mostly Azotobacter is studied because it is a free-living, non-symbiotic, and phototropic bacterium. Azotobacter chroococcum can be used as a biofertilizer because it has the potential to fix 10 mgN/g of carbon source supplied in-vitro (Mukherjee et al., 2022 ). Plant hormones such as indole acetic acids, gibberellic acids, naphthalene acetic acid, and vitamin B complex are produced by Azotobacter . It inhibits the root pathogens while promoting root growth, helps in mineral uptake, and improves soil fertility (Sumbul et al., 2020 ). Examples include Azotobacter, Bacillus, Clostridium , and Azospirillum . Application of Bacillus sp. significantly enhanced the growth of Arachis hypogea plant, protects plants from stress, and exhibits the production of ammonia and IAA (Gohil et al., 2022 ). Azospirillum brasilense reduces N fertilization, improves plant nutrition, and increases plant biomass and wheat grain yield as reported by Galindo et al. ( 2022 ).
Associative nitrogen-fixing bacteria
The Spirillum lipoferum was firstly isolated by M.W. Beijerinck in 1925. Spirillum was found associated with the roots of the grain which were also capable of fixing nitrogen (Soumare et al., 2020 ). Azospirillum is gram-negative, non-nodulating, aerobic-associative nitrogen-fixing bacteria with plants having a C4 dicarboxylic pathway of photosynthesis, such as sugarcane, maize, sorghum, bajra, and cereals like wheat, rice, barley (Yasuda et al., 2022 ). They also produce cytokinin, gibberellins, and indole acetic acid, which aid in the uptake of N, P, and K and promote the growth of roots. Examples such as Gluconobacter, Acetobacter, Herbaspirillum , and Azoarcus .
Phosphorus-solubilizing biofertilizers
Phosphorus is the second macro-nutrient that is responsible for limiting the growth of plants (Bechtaoui et al., 2021 ). It is an important constituent of organic and nucleic acids and is responsible for the synthesis of ATP and several amino acids. P helps in the nodulation process, amino acid synthesis, and proteins in leguminous plants (Wang et al., 2020 ). Soluble form of phosphorus is phosphate anion (orthophosphate), and their uptake is facilitated by rhizospheric microbes which help in plant nutrition. There are different microbes which can solubilize the remaining unavailable form of P into available form via organic acid production by bacteria which lowers the pH of the soil, leads to the dissolution of the phosphate compounds, and makes them available for the plant's nutrition (Mahanty et al., 2017 ). Examples of phosphate-solubilizing bacteria and fungi (PSB and PSF) are Bacillus, Rhizobium, Aerobacter, Burkholderia, Aspergillus , and Penicillium . Inoculation of Alcaligenes sp. improved plant growth parameters via P solubilization and IAA production (Abdallah et al., 2016 ). Rhizobium leguminosarum and Pseudomonas moraviensis enhanced the yield and growth of wheat plants and showed IAA and solubilization (Igiehon et al., 2019 ; Fahsi et al., 2021 ). Application of Arbuscular fungi can make greater availability of P in plants and protects them from stress condition as reported by Nacoon et al. ( 2020 ). Bacillus subtilis is also known as PSB which improved safflower growth and protects plants from salinity stress as reported by Zhang et al. ( 2019 ). NanoPhos containing phosphate-solubilizing bacteria enhanced the maize production via increasing the soil enzymes and bacteria population under field conditions (Chaudhary et al., 2021 ).
Phosphorus-mobilizing biofertilizers
They are beneficial bacteria that effectively mobilize the soluble phosphorus and mineralization of the organic phosphorus compound, both are unavailable form of phosphorus. Bacillus, Pseudomonas , and Rhizobium are representative phosphorus-mobilizing microorganisms (PMB) (Kirui et al., 2022 ). Three different mechanisms have been reported for this process. First, PMB is releasing the phosphatases enzyme. Second, PMB is producing organic acids. The last one added PMB may interact symbiotically with the other fungal mycorrhiza which mobilizes the soluble phosphorus from distant places where plant roots cannot reach by absorbing soluble phosphate by hyphae (Nassal et al., 2018 ; Etesami et al., 2021 ). One of the major advantages of Arbuscular mycorrhiza is transporting both inorganic and organic forms of phosphorus to plants. Examples of arbuscular mycorrhiza fungi (AMF) include Acaulospora sp., Glomus sp ., Entrophospora , and Paraglomus sp. and ectomycorrhiza include Amanita, Laccaria , and Boletus spp. Fungal endophyte ( Serendipita ) increased the K content in maize and protects plants from salinity stress (Haro and Benito, 2019 ).
Potassium-solubilizing biofertilizers
Subsequently, potassium (K) is the third major constituent of the macro-nutrients required by plants. It is mainly intricate in the regulation of stomatal closing and opening, nutrient uptake, protein synthesis improving the quality of products and provides resistance against stress environment (Santosh et al., 2022 ). K is present in different forms in soil depending upon the type of the soil composition like water-soluble, available form, and non-available form of the K (Basak et al., 2022 ). K is present in immobilized forms in silicate minerals like illite, orthoclase, biotite, illite, feldspar, etc. K solubilization occurs by both bacteria and fungi, and the major mechanism for solubilization of the unavailable form of K is acidification (means release of organic acids) (Varga et al., 2020 ). There are mechanisms also for solubilization of the K, namely, siderophores production, exchange reaction, and complexation (Sattar et al., 2019 ). Examples of potassium-solubilizing bacteria include Bacillus mucilaginous, B. edaphicus, B. circulans, Acidithiobacillus ferrooxidans, Frateuria aurantia, Herbaspirillum spp ., and Clostridium spp. , and potassium-solubilizing fungi include Aspergillus spp . and some arbuscular mycorrhiza fungi. Bacillus cereus showed K solubilization and improved potato plant health parameters and yield (Ali et al., 2021 ). Dal et al. ( 2020 ) reported that the combination of Rhizophagus irregularis and A. vinelandii improved soil enzyme activities and plant growth of wheat plants via P and K solubilization.
Potassium-mobilizing biofertilizers
The potassium-mobilizing microorganisms (PMMs) effectively release the unavailable potassium through the solubilization process (Patel et al., 2021 ). PMM is also recognized as potassium-dissolving bacteria or potassium-solubilizing bacteria. Ghaffari et al. ( 2018 ) observed that Frateuria and B. megaterium are efficient K-mobilizing bacteria used for crop farming purposes. Azotobacter showed K mobilization and solubilization in wheat plant and improved growth and soil microbial activities as reported by Game et al. ( 2020 ). Enterococcus and Pseudomonas aeruginosa also showed P and K solubilization and improved the maize height, yield, and nutrient acquisition (Kumar et al., 2021b ). Bacillus aryabhattai showed K solubilization, protects plants from stress, and improves their growth via the expression of K-solubilizing genes (Chen et al., 2022 ).
Sulfur-solubilizing biofertilizers
Sulfur helps in chlorophyll formation, activation of a certain enzyme, amino acid formation, vitamin formation and promotes nodulation, vital for the development of all plants (Wang et al., 2019 ). Sulfur solubilizers are also known as sulfur-oxidizing bacteria because they are transforming the most insoluble form of sulfur that is hydrogen sulfide (H 2 S) into an available form of sulfur known as sulfate (SO4 −2 ), and the reverse of this process is known assimilatory sulfate reduction which is mediated by sulfate-reducing bacteria (Wang et al., 2019 ). Sulfur transformation in the soil is primarily due to the microbial activity through the processes of mineralization, immobilization, oxidation, and reduction (Malik et al., 2021 ). Examples of aerobic sulfur-oxidizing bacteria include Bacillus, Beggiatoa, Aquifer, Paracoccus, Sulfolobus, Thiobacillus, Thermithiobacillus, Xanthobacter; phototropic anaerobic sulfur-oxidizing bacteria include Allochromatium, Chlorobium, Rhodobacter, Rhodopseudomonas; non-phototrophic obligate anaerobes include Wolinella succinogenes; and aerobic sulfur-oxidizing archaea include Sulfolobales members (Kusale et al., 2021 ). Thiobacillus thiooxidans and Bradyrhizobium japonicum are sulfur-oxidizing biofertilizers which showed better effect on cereal crops, medicinal plants, and forage crops (Zhang et al., 2018 ). Halothiobacillus bacteria tolerated the high salt concentration and improved crop production in saline soils (Boroujeni et al., 2021 ).
Zinc-solubilizing biofertilizers
Zinc is required during protein synthesis, DNA–protein interaction, growth hormone production, seed development, production of chlorophyll and protects plants from stress conditions (Umair Hassan et al., 2020 ). Insoluble forms of zinc are mostly ZnO, Zn 3 (PO 4 ) 2 , ZnCO 3 , and metallic Zn. The usable form of zinc by the plant is divalent cations (Ayoub et al., 2022 ). Zinc-solubilizing fertilizers contain the zinc solubilization bacteria which produce the organic acids to solubilize the insoluble zinc to Zn +2 , thereby enhancing zinc uptake in plants (Nitu et al., 2020 ). Examples of zinc-solubilizing bacteria and fungi are Bacillus subtilis, Pseudomonas striata, Serratia, Burkholderia cenocepacia, Aspergillus niger, A. nomius , and A. oryza which improved the soil enzyme activities and availability of Zn in crop plants (Batool et al., 2021 ). Leclercia adecarboxylata solubilizes Zn and produced siderophores which enhanced the Zn uptake in the roots of cucumber plants (Kang et al., 2021 ). Bacillus spp. and Pseudomonas taiwanensis showed a positive impact on the growth and chlorophyll content of maize plants (Chaudhary and Sharma, 2019 ; Hussain et al., 2020 ). Inoculation of Trichoderma longibrachiatum and Bacillus megaterium improved the seed germination of soybean plants in the pot experiment (Bakhshandeh et al., 2020 ). The application of PSB along with fertilizers improved the growth of faba bean in sandy soils (Ding et al., 2021 ).
Phytohormone-producing biofertilizers
Plant hormone or phytohormone plays a substantial role in plant development, secreted by both plants and microorganisms (Usman et al., 2022 ). Plant hormone production is an important feature of the beneficial microbes which is producing the indole-3-acetic acids, gibberellins, cytokinin, etc. (Eichmann et al., 2021 ). Auxin helped in the differentiation and division of plant cells. Cytokinin prevents the premature leaf senescence of plants (Wu et al., 2021a ). Abscisic acid is also identified as hormone which is produced by plants during stress conditions. Gibberellins are involved in seed germination, shoot elongation, flowering, and fruiting (Binenbaum et al., 2018 ). These hormones are generally secreted by microorganisms under environmental stress conditions to protect the plants by modulating the phytohormone level inside the host plants (Lopes et al., 2021 ). Bacillus thuringiensis has the genes required for IAA production which improved the growth of tomato plants (Batista et al., 2021 ). B. licheniformis is known for the production of IAA, ABA, and gibberellin which improved the growth of grapevine and protects plants from stress conditions (Salomon et al., 2014 ).
Siderophores producing biofertilizers
Iron (Fe) is a micronutrient that performs various functions like photosynthesis, respiration, chlorophyll, and many of the enzymatic reactions in plants (Gao et al., 2022 ). The unavailable form of iron in nature present under aerobic environment predominately is Fe +3 and is more probable form of insoluble oxyhydroxides and hydroxides complex. So, bacteria are producing the low-molecular weight iron-binding protein molecules called siderophores (Lurthy et al., 2020 ). Siderophores are water-soluble molecules that exist in two forms, namely, extracellular and intracellular. After capturing Fe +3 by siderophore inside bacteria, Fe +3 is reduced to the Fe +2 inside the cytoplasmic membrane which is then transported inside the cytoplasm by gating mechanisms (Gu et al., 2020 ). This available form of iron is given by the bacteria to the host plant for its development (Mahanty et al., 2017 ). Plant assimilates the iron with the help of siderophores by releasing the chelating agent via bacteria. Examples include Pseudomonas fluorescens C7 , Pseudomonas aeruginosa RSP5, and Pseudomonas aeruginosa RSP8. Application of siderophore-producing Bacillus sp. improves the growth of groundnut (Sarwar et al., 2020 ). Pseudomonas koreensis inoculation in maize plants inhibited the growth of plant pathogens via the production of siderophore and antioxidant enzymes (Ghazy and El-Nahrawy, 2021 ).
Organic matter decomposer biofertilizers
Soil organic matter is a mixture of living organisms consisting of bacteria, fungi, and insects, and the non-living part which includes fresh organic residues or waste, the dead and decaying matter of living organisms is generally known as humus (Lou et al., 2022 ). In organic matter generally, cellulose, lignin, hemicellulose, chitin, and lipids are present which are degraded by microbes such as bacteria, actinomycetes, and fungi. The organic-matter-degrading organisms break down the SOM into simpler or inorganic from which they derive energy and carbon for their growth. Examples of bacteriainclude Bacillus subtilis and Pseudomonas fluorescens and of fungi include ectomycorrhizal fungi. Trichoderma spp. involved in the degradation of litter at a faster rate releases antimicrobial compounds, improves the physicochemical properties of soil, and improves microbial diversity (Baldi et al., 2021 ). Bacillus subtilis and B. hisashii are involved in lignocellulose biodegradation by secreting the microbial enzymes as reported by Niu and Li ( 2022 ).
Endophytic bacteria as biofertilizers
Mutualistic microorganisms that employ the whole or part of their life cycle inside the plant tissues are known as endophytes (Fadiji and Babalola, 2020 ). Endophytes are of interest because they improve the nutritional requirements of the non-leguminous and leguminous plants by nitrogen fixation, phosphate solubilization, or by siderophores production (Janati et al., 2021 ). These bacteria have the potential to suppress pathogenic effects by activating the plant defense system (Dicko et al., 2021 ). Examples of endophytic bacteria include Klebsiella spp., Pseudomonas spp., Serratia spp., Bacillus spp., Burkholderia spp., Citrobacter spp . and endophytic fungi include Colletotrichum, Fusarium, Alternaria , and Aspergillus. Penicillium and Aspergillus isolated from roots of Taxus wallichiana solubilized P and produced phosphatase and phytase enzymes (Adhikari and Pandey, 2019 ). Kang et al. ( 2014a ) observed that Bacillus megaterium regulates the content of amino acids and carbohydrates to promote the growth of mustard plant. Endophytes isolated from rice such as Bradyrhizobium sp ., Paraburkholderia sp., showed acetylene reduction properties and high sugar content contributing to high nitrogen-fixing ability. High content of sugar in different crops such as sweet potato, pineapple, and sugar has known to assist endophytic N-fixing activity among non-leguminous plants (Okamoto et al., 2021 ).
Plant growth-promoting rhizobacteria
PGPR is used as biofertilizers; it represents the variation of soil bacteria that live in association with the rhizosphere, rhizoplane associated to root surface, and endophytes present inside the intercellular places (Vandana et al., 2021 ). PGPRs are soil bacteria which increase the growth and enhance the tolerance of plants toward stress conditions (Ghosh et al., 2019 ). There are diverse mechanisms shown by PGPR which support the plant growth such as N 2 fixation, macro- and micronutrient mineralization, secretion of exopolysaccharides, phytohormone production, siderophore, hydrogen cyanide to prevent the growth of phytopathogens, antibiotics, etc. (Gouda et al., 2018 ; Numan et al., 2018 ). Rhizobium lupini increased alfalfa growth and enhanced nutrient uptake efficiency (Duan et al., 2022 ). Application of biofertilizers such as Pseudomonas taiwanensis, Bacillus spp., and Pantoea agglomerans improved the maize growth, yield, and soil health parameters (Khati et al., 2018 ; Chaudhary et al., 2022b ). Application of Bacillus spp. improved the plant/soil health parameters and maize productivity as reported by Chaudhary et al. ( 2021 ). Kukreti et al. ( 2020 ) reported that Pseudomonas taiwanensis improved maize plant health and soil enzyme activities in the pot experiment.
Role of biofertilizers in biotic stress management
The outbreak of plant diseases in nature necessitates sustainable agriculture with minimum use of agrochemicals. For a long time, the use of chemicals has posed a significant risk to the environment and the agricultural sector (Akanmu et al., 2021 ). Long-term use of pesticides, on the other hand, harms both plant/soil health and eventually leads to significant crop loss. Thus, effective and eco-friendly phytopathogen control strategies such as biofertilizers are required. The exploitation of potential biofertilizers as endophytes could be useful to improve crop plants from various bacterial and fungal diseases (Collinge et al., 2022 ). Biological control of plant diseases occurs via destruction of pathogens via beneficial microbes such as Bacillus spp ., Pseudomonas spp ., Streptomyces, Pantoea spp., and several fungal spp. (Köhl et al., 2019 ; Chaudhary et al., 2021 ). Such endosymbiont group of biocontrol agents being friendly, they not only colonize internal plant tissue but also protect host plant throughout its life cycle without causing any apparent damage (Lahlali et al., 2022 ). The use of Bacillus sp. for crop growth promotion and biocontrol has a long history (Zhu et al., 2021 ). Bacillus thuringiensis (Bt), a producer of endotoxins that can be used as biopesticide and a source of genes for the creation of transgenic plants that are resistant to insects, is currently the most effective biopesticide on the market (Sujayanand et al., 2021 ).
Biofertilizers in the form of potential biocontrol agents represent a safe alternative to harmful chemicals like fertilizers, herbicides, pesticides, and insecticides (Hernández-Fernández et al., 2021 ). Consequently, the use of biofertilizers is receiving special attention for the management of phytopathogens that are comprised of bacteria, fungi, virus, aphids, and nematodes ( Table 1 ). Their ubiquitous nature and the ability to reside within plant tissues make them unique, showing multidimensional interactions within the host plant (Khare et al., 2018 ). The biodiversity of endophytes is hyperdiverse in almost every other plant species ranging from small non-vascular plants to large conifers like Pinus radiata (Liu et al., 2017b ). Some of the known endophytes are Burkholderia, Stenotrophomonas, Rhizobium, Microbacterium, and Bacillus spp . (Kandel et al., 2017 ).
Role of biofertilizers in biotic stress tolerance.
| sp. | Inhibit growth of pathogen and promote plant growth | You et al., | ||
| Regulates signaling pathway such as JA and MAPK | Nie et al., | |||
| Protects host plant from pathogen systemic resistance response | Rashid et al., | |||
| Enhanced the chitinase, hydrolytic, protease production and protects plants from pathogen | Hassan et al., | |||
| Protects plants from pathogen chitinase production | Mishra and Arora, | |||
| Protects from pathogen and activates defense response in plants | Rodriguez et al., | |||
| . | Provides immunity to plants and protect from disease increasing antioxidant enzymes | Hata et al., | ||
| . | Increased production of volatile fatty acids and antibiotics | Rybakova et al., | ||
| Increased expression of auxin-related genes and improved plant growth | Samaras et al., | |||
| Enhanced proline content and pathogen-related enzymes and inhibit the growth of pathogens | Taha et al., | |||
| Olive trees | Increased production of volatile fatty acids and improves seed germination | Sdiri et al., | ||
| Provides protection to plants from pathogen JA signaling and platelet-activating factor | Yu et al., | |||
| Viruses | Induced SA and JA signaling and protects plants from disease | Beris et al., | ||
| Increased secondary metabolite, phytohormone production and improved plant growth | Kousar et al., | |||
| Increased plant growth and suppress the growth of pathogens | Sundaramoorthy and Balabaskar, | |||
| . | Inhibit growth of pathogens production of cyanogens and lytic enzymes | Kumar et al., | ||
| . | . | Inhibit pathogens production of HCN and enzymes | Zain et al., | |
| . | Improved plant growth and increased chitinase, siderophore, and IAA production | Sendi et al., | ||
| Increased production of HCN against pathogens | Anand et al., | |||
| Increased production of volatile organic acids and inhibit pathogen growth | Don et al., |
There are several enzymes which protect the plants from stress conditions such as antioxidant enzymes like peroxidase (POD), polyphenol oxidase, phenylalanine ammonia-lyase (PAL), lipoxygenase, and chitinase (Cataldo et al., 2022 ). Lipoxygenase enzymes have its place to non-heme iron comprising dioxygenases which contribute to stress response via lipid oxidation. Also, it is found to act as signals for communication with the plant host, with associated endophytes and pathogens (Singh et al., 2022b ). In response to pathogen attack, endophytes boost plant immunity by priming induced systemic resistance (ISR) and systemic acquired resistance (SAR) via several phytohormones (Romera et al., 2019 ; Oukala et al., 2021 ). Pathogenesis-related proteins with antimicrobial properties are produced and accumulated by several endophytes symbiotically living with their host plants. Many endosymbionts have the capability to complement the inefficient antioxidative system of plants by different mechanisms (Shukla et al., 2022 ). In some strains, production of lipopeptides, surfactin, plipastatin, and mycosubtilin differentially activated the plant innate immune response (Kumar et al., 2021c ). Production of surfactin may have an important role in the suppression of Fusarium infestation on germinating seeds (Eid et al., 2021 ). Bacillus strains inhibited the verticillium wilt caused due to Verticillium dahliae by the production of secondary metabolites such as surfactin, fengycin, and bacillibactin, as well as expressing defense-related genes such as SOD and PAL (Hasan et al., 2020 ). Bacillus atrophaeus inhibits Meloidogyne incognita growth by producing volatile dimethyl disulfide and antioxidant enzymes (Ayaz et al., 2021 ). According to Nie et al. ( 2019 ), Bacillus cereus inhibits the growth of Pseudomonas syringae by producing antioxidant enzymes. Pseudomonas fluorescent controls the iron uptake genes and protects plants from phytopathogens as reported by Desrut et al. ( 2020 ). Acrophialophora jodhpurensis defends tomato plants from Rhizoctonia solani which causes crown root disease via the production of peroxidase enzyme, chitinase, and phenylalanine (Daroodi and Taheri, 2021 ). Plants are protected from Botrytis cinerea by Trichoderma atroviride via the production of glutamate and glyoxylate aminotransferase (González-López et al., 2021 ).
Secondary metabolites play the foremost role in defense mechanism toward pathogens, pests, and herbivores. Many plants microbiome especially endosymbionts regulate defense mechanisms through secreting various metabolites (Divekar et al., 2022 ). Secondary plant metabolites belonging to the family of steroids, alkaloids, phenolics, flavonoids, and terpenoids function in innate immunity and defense response signaling (Pang et al., 2021 ). Volatile compounds from endophytes modulate plant microbiome and possess antimicrobial properties. A variety of fungi, including Ascomycetes and Deuteromycetes , are inhibited by a mixture of VOCs produced by the fungal endophyte Phomopsis sp. (Hummadi et al., 2022 ). Three VOCs, including caryophyllene, 2-methoxy-4-vinylphenol, and 3,4-dimethoxystyrol, produced by endophytic fungi Sarocladium bravhiariae HND5 have been found to have antifungal activity against Fusarium oxysporum (Yang et al., 2021 ). Alkaloid produced by Epichloe sp. in a variety of grass species is one of the well-known secondary metabolites produced by endophytic fungi. Hennessy et al. ( 2022 ) reported Epichloe festucae colonized agricultural forage grasses and offered the plant defense against herbivorous insects. Streptomyces hydrogenans metabolites can be used as safe biocontrol agents against Meloidogyne incognita and plant growth promoters for Solanum lycopersicum (Sharma et al., 2020 ). Bacillus velezensis is a potential pesticide due to its strong biocontrol activity and ability to strengthen host defense against Magnaporthe oryzae fungi, which cause rice blast disease in plants (Chen et al., 2021 ). An isolate of Trichoderma asperellum increased the resistance in tomato seedling to the disease A. alternata leaf spot (Yu et al., 2021 ). Trichoderma asperellum also produces mycolytic enzymes such as chitinase and 1,3, glucanase which may be capable of destroying phytopathogens cell walls (Win et al., 2021 ). Trichoderma spp. also have biocontrol potential against V. dahliae , which causes olive tree wilting, and inhibit the pathogenic fungus mycelial growth (Reghmit et al., 2021 ). Trichoderma sp. has been shown successfully to suppress Sclerospora graminicola , the cause of pearl millet downy mildew disease, and develop systemic resistance (Nandini et al., 2021 ).
Some endophytes can also regulate stress management through SAR mediated by salicylic acid. SAR offers long-lasting stress management and broad-spectrum effectiveness against a variety of pathogens (Xia et al., 2022 ). It frequently involves the accumulation of chitinase and pathogenesis-related proteins (PR). In a study by Samain et al. ( 2019 ), Paenibacillus strain (PB2) used to control Mycosphaerella graminicola induced pathogenesis-related proteins (PR1) which is considered as marker of SAR. Application of Bacillus aryabhattai activated a durable defense response against pathogens facilitated through salicylic acid/ethylene pathways (Portieles et al., 2021 ). Trichoderma harzianum helps to improve plant immediate resistance against Nezara viridula feeding invasion via enhancing JA marker gene transcript levels (Alınç et al., 2021 ). Bacillus subtilis and Pseudomonas fluorescens mediated systemic alleviated the biotic stress in Solanum lycopersicum against Sclerotium rolfsii . Heat-killed endophytic strain B. aryabhattai (HKEB) induced defense-related genes protein (PR1) and phytoalexin-deficient 3 in A. thaliana . PR1 gene expression was found to be 20-fold higher in treated plants than control, and other genes found in the study were associated with jasmonic and salicylic acid pathways (Portieles et al., 2021 ). Endophytes exhibit different gene upregulation and a distinct signaling pathway in response to distinct colonization strategies (Morelli et al., 2020 ). Trichoderma spp. demonstrated antagonistic activity against phytopathogens such as B. cinerea, Fusarium solani , and Rhizoctonia solani used as biocontrol agent in greenhouse experiment (Sánchez-Montesinos et al., 2021 ).
Role of biofertilizers in abiotic stress management
Climate change is one of the major reasons for the increasing abiotic stresses on the crops, which results in reducing the world's agriculture productivity. Abiotic stresses like drought, salinity, waterlogging, and excessive heat are responsible for the poor yield of crops (He et al., 2018 ). In recent years, the abiotic stress has increased so fast, because of the fluctuation of climates or climate change, and it has caused an unusual rise in the weather conditions and incidents, which is responsible for the substantial losses of crops around the globe. These abiotic stresses induce several physiological, biochemical, and morphological changes in plant that finally affect the economic yield of crop plants, and it was reported that the yield loss from abiotic stress is about 51–82%, which if continues will affect the goal of sustainable food production (dos Santos et al., 2022 ).
The use of beneficial microbes such as endophytes capable of producing growth hormones, like IAA, ACC deaminase, augmented the K uptake in plant tissues but decreased the ethylene level which helps in tolerance of stress in diverse plants ( Figure 2 ). Biofertilizers as endophytes are found to have diverse associations with its host plant such as symbiotic, parasitic, and mutualistic and colonize plant tissues without causing any disease, thus benefiting for plants (Chaudhary et al., 2022c ). Endophytes may benefit from mutualistic associations as they obtain nutrients from the hosts, and they spread by host seed transmission. They are also able to enhance the nutrients uptake like nitrogen, magnesium, zinc, and phosphorus from soil and provide to the host plant for better growth and survival (Bamisile et al., 2018 ). It is well-identified that plant biofertilizers play a significant role in supporting the growth of crops under different abiotic stresses ( Table 2 ). Actinobacteria are well-known for plant growth via metabolite production and antibiotics under stress conditions (Yadav et al., 2018 ). Abd El-Daim et al. ( 2014 ) observed that the application of Pseudomonas sp. improved plant growth under heat stress via HSPs and ROS reduction. Paenibacillus sp. improved Phaseolus vulgaris growth via facilitating the siderophore, IAA and HCN production under salinity stress conditions (Gupta and Pandey, 2019 ). Trichoderma harzianum inoculation in rice plants improved root growth and protects from drought stress as reported by Shukla et al. ( 2012 ). Mukhtar et al. ( 2020a ) reported that Bacillus cereus enhanced the production of ACC deaminase and exopolysaccharide which protects Solanum lycopersicum plants from heat stress.

Role of biofertilizers for maintenance of crop productivity and soil health.
Role of biofertilizers in abiotic stress tolerance.
| Salinity, heavy metals | Improved salt tolerance ability exopolysaccharide production | Sultana et al., | ||
| Salt | Improved plant growth regulation of JA pathway and antioxidant enzymes | Liu et al., | ||
| Salt | Improved salt tolerance ability in stressed plants production of antioxidant enzymes and Fe attainment | Zhou et al., | ||
| Salt | Increased weight of roots and shoots production of IAA, proline, and glycine betaine | Mukhtar et al., | ||
| . | Salt | Increase chlorophyll content and protects plants from stress | Mokabel et al., | |
| . | Salt | Improved plant growth and photosynthesis antioxidant enzyme and AAC and siderophore production | Gupta et al., | |
| Drought and nitrogen | Increase plant biomass and chlorophyll content | Tufail et al., | ||
| Salt | Improved plant health parameters production antioxidant enzyme, proline contents in shoots and roots | Yasmin et al., | ||
| Drought | Improved photosynthesis increasing antioxidant enzymes | Qiang et al., | ||
| Salt | Increased antioxidant enzyme activity and chlorophyll content | Asaf et al., | ||
| Heat | Improved plant height, biomass, and chlorophyll content | Ismail et al., | ||
| Drought | Improved phenols, terpenoids and soil protein and enzyme activities | Cheng et al., | ||
| Salt | Improved shoot weight and growth | Meddich et al., | ||
| Nutrient | Improved Zn uptake and root and shoot biomass | Abadi et al., | ||
| Cold | Increased proline content and cold stress tolerance genes | Jiang et al., | ||
| Cold | Improved auxin production and cold-related gene expression | González-Pérez et al., | ||
| C3 plants | Salt | Improved chlorophyll content in plants | Chandrasekaran et al., | |
| and | Drought | Increased yield and protects from stress upregulation of CAT and POD activity | Sheteiwy et al., | |
| and spp. | Drought Salinity | Improved plant health and microbial diversity in soil Triggered CAT, proline, and IAA production | Igiehon et al., Fortt et al., |
Drought stress
Drought stress is one of the main abiotic stresses which causes water scarcity to meet the plant necessity and causes economic fatalities in agriculture production. The normal progress of plants is hindered due to decrease in water shortage in their cells. Drought stress decreased the rate of photosynthesis, germination in plants, and loss in crop productivity (Lata et al., 2018 ). Inoculation of beneficial biofertilizers (rhizospheric and endophytic microbes) improved plant growth and development via different direct/indirect mechanisms under stress situations. Stress can be overcome via using biofertilizers which produced growth hormones such as IAA and cytokinins and improved plant development (Fasusi et al., 2021 ). Inoculation of Pseudomonas putida boosted the flavonoids, salicylic, and abscisic acid production which protects soybean plants from drought stress (Kang et al., 2014b ). Inoculation of Pseudomonas spp. protects maize plants and improved biomass and sugar content in treated plants from drought stress via upregulation of dehydrin proteins and proline content (Sandhya et al., 2010 ). Khan et al. ( 2018 ) found that Bacillus thuringiensis improved chickpea growth under drought conditions via production of volatile organic compounds. Application of Microbacterium sp. improved maize plant growth, root length, photosynthetic rate, and yield under drought stress (Romera et al., 2019 ). Usage of Phoma improved the drought tolerance in Pinus tabulaeformis plants and increased seedling growth by improving the mechanism of water uptake, proline, and SOD (Zhou et al., 2021 ). Sheteiwy et al. ( 2021 ) reported that Bradyrhizobium japonicum and AMF improved the yield of soybean bacterial count and enzyme activities of soil via improving the nutrient accessibility in soil under drought stress. AMF and Rhizobium inoculation improved the Glycyrrhiza plant growth and phosphorus content in roots in drought stress (Hao et al., 2019 ). Combined inoculation of arbuscular fungi and bioinoculants improved plant biomass and chlorophyll content in date palm ( Phoenix dactylifera ) under water-deficit conditions via enhanced antioxidant enzyme activities, soluble sugars, and proteins (Anli et al., 2020 ). Inoculation of Glomus mosseae and Bacillus amyloliquefaciens in Phaseolus vulgaris significantly improved the photosynthetic rate and yield under water stress conditions (Salem and Al-Amri, 2021 ).
Salinity stress
Accumulation of salt in agricultural soil will have a negative impact on plants including its physiological, morphological, and molecular aspects. This affects plants via creating osmotic stress, ion toxicity and reducing the photosynthesis, CO 2 fixation, and transpiration rate in plants. Availability of nutrients and microbial diversity are also affected due to the salinity stress (Luo et al., 2021 ). Usage of bioinoculants is enormously supportive in countering the lethal properties of soil salinity via improving the soil physicochemical properties and thus improved crop production (Jiménez-Mejía et al., 2022 ). Interaction between microbes and plants can overcome stress problem. Gond et al. ( 2015 ) reported that inoculation of Pantoea agglomerans in tropical corn under salt stress (0–100 mM) improves tolerance and growth of plants due to the upregulation of aquaporins. Bacillus megaterium also regulates the aquaporin genes during salt stress in maize plants and improved root growth and leaf water content (Marulanda et al., 2010 ). Waqas et al. ( 2012 ) reported that Penicillium and Phoma glomerata improved the rice plant growth under salinity stress via increased production of CAT, POD, and IAA. Checchio et al. ( 2021 ) observed that Azospirillum brasilense improved resistance in corn plants via enhancing the production of antioxidant enzymes and glycine betaine. Application of Pseudomonas sp. improves Arabidopsis thaliana germination and growth via upregulation of lipoxygenase genes which are involved in tolerance mechanism via jasmonic pathway (Chu et al., 2019 ). The Arthrobacter nitroguajacolicus improved wheat growth under salt stress via upregulation of IAA, ACC, flavonoid, stilbenoid, terpenoids, and cytochrome P450 genes (Safdarian et al., 2019 ). Inoculation of Planococcus rifietoensis protects Cicer arietinum plants from salt stress (200 mM) via EPS and biofilm production (Qurashi and Sabri, 2012 ). Gupta and Pandey ( 2019 ) observed that inoculation of Paenibacillus sp. protects and improved Phaseolus vulgaris plant growth under salinity stress via the production of IAA and ACC deaminase. Meena et al. ( 2020 ) reported that Nocardioides sp. improved seedling growth of Triticum aestivum under salt stress (0–100 mM) via increasing the CAT and POD genes. Inoculation of Penicillium and Ampelomyces spp. improved drought and salinity stress tolerance in tomato plants via the production of osmolytes, stress-responsive genes, and antioxidant enzymes (Morsy et al., 2020 ). Inoculation of Piriformospora indica highly enhanced plant development and attenuated NaCl-induced lipid peroxidation which helps to build tolerance during salinity stress (Ghaffari et al., 2018 ). Studies show that the inoculation of Trichoderma longibrachiatum T6 in wheat increased the levels of antioxidant enzymes (SOD, POD, and CAT) which helped to improve the stress tolerance in plants during salt stress (Zhang et al., 2016 ). Agrobacterium and Raoultella showed production of IAA, HCN, and ACC under salt stress and improved growth of Tetragonia tetragonioides plants (Egamberdieva et al., 2022 ). Fortt et al. ( 2022 ) reported that the application of PGPR improved the growth of lettuce under salt stress via the production of IAA and antioxidant enzymes which provide protection to plants.
Temperature stress
Global warming is a serious risk to all living creatures and is becoming a worldwide concern. Temperature stress such as heat and cold greatly limits the growth and development of plants (Yadav et al., 2018 ). Heat stress causes modification in homeostasis, degradation of proteins, which have lethal effects on physiology of plants as it delays the seed germination, damages to seeds and affects agricultural production (Imran et al., 2021 ). Cold stress causes dehydration due to ice formation which is responsible for protein denaturation. It also causes plant leaves lesions, yellowing of leaves, and rotting. It also affects the seed germination and yield of crops (Wu et al., 2021b ). The application of several microbes alleviated the damaging effects of heat stress in various plants such as wheat, tomato, and sorghum via producing phytohormones, biofilm formation, and enhancing heat shock proteins (Issa et al., 2018 ; Sarkar et al., 2018 ). Bacillus cereus inoculation in tomato plants increased the production of HSPs, IAA, essential amino acids, and organic acids and protects plants from stress conditions (Khan et al., 2020 ). Inoculation of Azospirillum and B. amyloliquefaciens improved the heat tolerance via reducing oxidative damage in wheat seedling (Abd El-Daim et al., 2014 ). Duc et al. ( 2018 ) reported that Glomus sp. tolerates heat stress and protects tomato plants via scavenging ROS generation. Bacillus velezensis improved wheat plant survival under cold stress via increase in cold stress-related proteins as reported by Abd El-Daim et al. ( 2019 ). Zulfikar et al. ( 2011 ) reported that Pseudomonas putida also improved the growth of wheat plants under heat stress via enhanced production of proline, sugars, and antioxidant enzymes. R. irregularis and F. mosseae increased plant height, transpiration rate in maize, and nutrient composition in roots of triticum aestivum during heat stress (Cabral et al., 2016 ). Paraburkholderia phytofirmans having ACC deaminase-producing efficiency helps in normal growth of tomato plants under heat stress as reported by Esmaeel et al. ( 2018 ). Bacterial inoculants such as Rhodococcus and Burkholderia protect the medicinal plant Atractylodes lancea from heat stress and improved their growth via enriched root-associated microbes (Wang et al., 2022a ).
Heavy metal stress
Extreme usage of inorganic chemical fertilizers in agriculture system causes the accumulation of toxic metals such as nickel, manganese, cadmium, iron, and zinc in soil (Ghori et al., 2019 ). These metals are beneficial for plants at low level, but if their concentration increases cause stress via decrease in plant growth due to the decrease in photosynthesis, deprived nutrients, membrane integrity, and enzyme activities. It causes oxidative stress via ROS and H 2 O 2 generation and reduces plant growth and crop productivity (Ahmad et al., 2019 ; Gong et al., 2020 ). ROS generation occurs both under favorable and unfavorable circumstances, and it has a negative impact on vital macromolecules (Köhl et al., 2019 ). Rhizobium inoculation at nickel-contaminated site improves the chlorophyll content and increased lentil plant growth (Wani and Khan, 2013 ). Bradyrhizobium increased IAA production and siderophore production and improved the shoot weight of Lolium multiflorum at cadmium-contaminated site (Guo and Chi, 2014 ). Candida parapsilosis and B. cereus protect Trifolium repens plants from heavy metal stress conditions as reported by Azcón et al. ( 2010 ). Toxicity of arsenic in Brassica juncea is reduced by Staphylococcus arlettae via enhanced production of dehydrogenase and phosphatase enzyme in soil (Srivastava et al., 2013 ). Inoculation of Talaromyces pinophilus in Triticum aestivum plants stimulates plant growth via the production of gibberellic acid under heavy metal stress (El-Shahir et al., 2021 ). Paredes-Páliz et al. ( 2018 ) reported that inoculation of metal-resistant bacteria such as B. aryabhattai and Pantoea agglomerans brings production of phenylalanine ammonia-lyase enzyme and SOD which protects plants from metal stress. The addition of bioinoculants like P. aeruginosa and Burkholderia gladioli reduced Cd toxicity in Solanum lycopersicum by producing phenols, organic acids, and osmoprotectants (Khanna et al., 2019 ). Application of Serratia marcescens and E. bugandensis improved spinach ( Ipomoea aquatica ) growth via the production of polyamine under Pb and Cd toxicity (Wang et al., 2022b ). Citrobacter and Enterobacter cloacae mitigate the Cd and Pb toxicity, improve the wheat plant health parameters, and protect from stress via the generation of antioxidant enzymes (Ajmal et al., 2022 ). Oubohssaine et al. ( 2022 ) reported that Pseudarthrobacter oxydans improved Sulla spinosissima growth and can be used as biofertilizer at heavy metal-contaminated sites. Cadmium tolerance bacteria such as Curtobacterium ocenosedimentum having P-solubilizing, IAA, and siderophore-producing possessions improved chili growth and increased shoot/root length (Patel et al., 2022 ). Inoculation of Pseudoarthrobacter and Vibrio neocaledonicus improved the Salicornia ramosissima growth at As- and Cu-polluted sites (Mesa-Marín et al., 2020 ). Rhizobium inoculation can promote soil nutrient cycling by increasing enzyme activity in metal-contaminated soil, thereby providing more N and P for microbial activity and growth of plants (Ma et al., 2021 ; Duan et al., 2022 ). Heavy metal toxicity is a growing problem in the world; therefore, finding appropriate microbes proficient to depollution of the metals can benefit to improve the crop efficiency. Application of biofertilizers for sustainable food crop production and boosting various stress tolerance of plants are gaining popularity. Still, further studies are crucial to unravel the potential role of biofertilizers in responding to the impact of different stresses at molecular level.
Agriculture systems have to face the task of food production, stress management, and dependency on agrochemicals. The presence of pest and pathogen in crops causes decrease in crop yield and heavy crop losses every year. The occurrence of abiotic stresses due to the change in climatic conditions leads to difficult challenge to crop production worldwide. Different effective approaches should be employed to reduce crop output loss and control diseases. Hence, the necessity to implement the eco-friendly approaches such as biofertilizers is of great importance for sustainable agriculture. The application of biofertilizers not only improves plant heath parameters but also enhances the crop productivity, soil health and protects from stress environment. More research has been focused on physiological and molecular aspects under different conditions with different crops using biofertilizers under field conditions.
Author contributions
PC: conceptualization and wrote the manuscript. SS, AC, AS, and GK: editing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to acknowledge the Microbiology Department, Govind Ballabh Pant University of Agriculture and Technology.
- Aasfar A., Bargaz A., Yaakoubi K., Hilali A., Bennis I., Zeroual Y., Kadmiri M. I. (2021). Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability . Front. Microbiol. 12, 628379. 10.3389/fmicb.2021.628379 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Abadi V. A. J. M., Sepehri M., Khatabi B., Rezaei M. (2021). Alleviation of zinc deficiency in wheat inoculated with root endophytic fungus Piriformospora indica and Rhizobacterium, Pseudomonas putida . Rhizosphere 17 :100311. 10.1016/j.rhisph.2021.100311 [ CrossRef ] [ Google Scholar ]
- Abbey L., Abbey J., Leke-Aladekoba A., Iheshiulo E. M. A., Ijenyo M. (2019). Biopesticides and biofertilizers: types, production, benefits, and utilization . Byprod. Agri. Fisher. 2019 , 479–500. 10.1002/9781119383956.ch20 [ CrossRef ] [ Google Scholar ]
- Abd El-Daim I., Bejai S., Meijer J. (2014). Improved heat stress tolerance of wheat seedlings by bacterial seed treatment . Plant Soil . 379 , 337–350. 10.1007/s11104-014-2063-3 [ CrossRef ] [ Google Scholar ]
- Abd El-Daim I. A., Bejai S., Meijer J. (2019). Bacillus velezensis 5113 induced metabolic and molecular reprogramming during abiotic stress tolerance in wheat . Sci. Rep . 9 :16282. 10.1038/s41598-019-52567-x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Abdallah R. A. B., Trabelsi B. M., Nefzi A., Khiareddine H. J., Remadi M. D. (2016). Isolation of endophytic bacteria from Withania somnifera and assessment of their ability to suppress Fusarium wilt disease in tomato and to promote plant growth . J. Plant Pathol. Microbiol. 7 :352. 10.4172/2157-7471.1000352 [ CrossRef ] [ Google Scholar ]
- Adhikari P., Pandey A. (2019). Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots . Rhizosphere 9 , 2–9. 10.1016/j.rhisph.2018.11.002 [ CrossRef ] [ Google Scholar ]
- Agri U., Chaudhary P., Sharma A. (2021). In vitro compatibility evaluation of agriusable nanochitosan on beneficial plant growth-promoting rhizobacteria and maize plant . Natl. Acad. Sci. Lett. 44 , 555–559. 10.1007/s40009-021-01047-w [ CrossRef ] [ Google Scholar ]
- Agri U., Chaudhary P., Sharma A., Kukreti B. (2022). Physiological response of maize plants and its rhizospheric microbiome under the influence of potential bioinoculants and nanochitosan . Plant Soil 474 , 451–468. 10.1007/s11104-022-05351-2 [ CrossRef ] [ Google Scholar ]
- Aguilar-Paredes A., Valdés G., Nuti M. (2020). Agronomy ecosystem functions of microbial consortia in sustainable agriculture . Agronomy. 10. 10.3390/agronomy10121902 [ CrossRef ] [ Google Scholar ]
- Ahluwalia O., Singh P. C., Bhatia R. (2021). A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria . Res. Environ. Sustain . 2021 :100032. 10.1016/j.resenv.2021.100032 [ CrossRef ] [ Google Scholar ]
- Ahmad P., Tripathi D. K., Deshmukh R., Pratap S. V., Corpas F. J. (2019). Revisiting the role of ROS and RNS in plants under changing environment . Environ. Experi. Botany 161 , 1–398. 10.1016/j.envexpbot.2019.02.017 [ CrossRef ] [ Google Scholar ]
- Ajmal A. W., Yasmin H., Hassan M. N., Khan N., Jan B. L., Mumtaz S. (2022). Heavy metal-resistant plant growth-promoting Citrobacter werkmanii strain WWN1 and Enterobacter cloacae strain JWM6 enhance wheat ( Triticum aestivum L.) growth by modulating physiological attributes and some key antioxidants under multi-metal stress . Front. Microbiol . 6, 815704. 10.3389/fmicb.2022.815704 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Akanmu A. O., Babalola O. O., Venturi V., Ayilara M. S., Adeleke B. S., Amoo A. E., et al.. (2021). Plant disease management: leveraging on the plant-microbe-soil interface in the biorational use of organic amendments . Front. Plant Sci. 12 , 700507. 10.3389/fpls.2021.700507 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ali A. A., Awad M. Y. M., Hegab S. A., Abd El Gawad A. M., Eissa M. A. (2021). Effect of potassium solubilizing bacteria ( Bacillus cereus ) on growth and yield of potato . J. Plant. Nutr. 44 , 411–420. 10.1080/01904167.2020.1822399 [ CrossRef ] [ Google Scholar ]
- Alınç T., Cusumano A., Peri E., Torta L., Colazza S. (2021). Trichoderma harzianum strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity . J Chem Ecol. 47 , 455–462. 10.1007/s10886-021-01260-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alkurtany A., Ali S., Mahdi W. (2018). The efficiency of prepared biofertilizer from local isolate of Bradyrhizobium sp. on growth and yield of mungbean plant . Iraqi J. Agric. Sci. 49 , 722–730. [ Google Scholar ]
- Anand A., Chinchilla D., Tan C., Mène-Saffrané L., L'Haridon F., Weisskopf L. (2020). Contribution of hydrogen cyanide to the antagonistic activity of Pseudomonas strains against Phytophthora infestans . Microorganisms 8 :1144. 10.3390/microorganisms8081144 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anli M., Baslam M., Tahiri A., Raklami A., Symanczik S., Boutasknit A., et al.. (2020). Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm . Front. Plant Sci. 11 , 516818. 10.3389/fpls.2020.516818 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Asaf S., Hamayun M., Khan A. L., Waqas M., Khan M. A., Jan R., et al.. (2018). Salt tolerance of Glycine max . L induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system . Plant Physiol. Biochem. 128 , 13–23. 10.1016/j.plaphy.2018.05.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ayaz M., Ali Q., Farzand A., Khan A., Ling H., Gao X. (2021). Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita . Int. J. Mol. Sci . 22 :5049. 10.3390/ijms22095049 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ayoub I., Kumar V., Abolhassani R., Sehgal R., Sharma V., Sehgal R., et al.. (2022). Advances in ZnO: Manipulation of defects for enhancing their technological potentials . Nanotechnol. Rev . 11 , 575–619. 10.1515/ntrev-2022-0035 [ CrossRef ] [ Google Scholar ]
- Azcón R., Perálvarez M. C., Roldán A., Barea J. (2010). Arbuscular mycorrhizal fungi, Bacillus cereus , and Candida parapsilosis from a multicontaminated soil alleviate metal toxicity in plants . Microb. Ecol . 59 , 668–677. 10.1007/s00248-009-9618-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Azeem M., Haider M. Z., Javed S., Saleem M. H., Alatawi A. (2022). Drought stress amelioration in maize ( Zea mays L.) by inoculation of Bacillus spp. Strains under sterile soil conditions . Agriculture 12 :50. 10.3390/agriculture12010050 [ CrossRef ] [ Google Scholar ]
- Bakhshandeh E., Gholamhosseini M., Yaghoubian Y., Pirdashti H. (2020). Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress . Plant Growth Regul . 90 , 123–136. 10.1007/s10725-019-00556-5 [ CrossRef ] [ Google Scholar ]
- Baldi E., Gioacchini P., Montecchio D., Mocali S., Antonielli L., Masoero G., et al.. (2021). Effect of biofertilizers application on soil biodiversity and litter degradation in a commercial apricot orchard . Agronomy 11 :1116. 10.3390/agronomy11061116 [ CrossRef ] [ Google Scholar ]
- Bamisile B. S., Dash C. K., Akutse K. S., Keppanan R., Wang L. (2018). Fungal endophytes: beyond herbivore management . Front. Microbiol . 9, 544. 10.3389/fmicb.2018.00544 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Basak B. B., Maity A., Ray P., Biswas D. R., Roy S. (2022). Potassium supply in agriculture through biological potassium fertilizer: a promising and sustainable option for developing countries . Arch. Agronomy Soil Sci. 68 , 101–114. 10.1080/03650340.2020.1821191 [ CrossRef ] [ Google Scholar ]
- Batista B. D., Dourado M. N., Figueredo E. F. (2021). The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato ( Solanum lycopersicum cv. Micro-Tom) . Arch. Microbiol . 203 , 3869–3882. 10.1007/s00203-021-02361-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Batool S., Asghar H. N., Shehzad M. A., Yasin S., Sohaib M., Nawaz F., et al.. (2021). Zinc-solubilizing bacteria-mediated enzymatic and physiological regulations confer zinc biofortification in chickpea ( Cicer arietinum L.) . J. Soil Sci. Plant Nutr . 21 , 2456–2471. 10.1007/s42729-021-00537-6 [ CrossRef ] [ Google Scholar ]
- Bechtaoui N., Rabiu M. K., Raklami A., Oufdou K., Hafidi M., Jemo M. (2021). Phosphate-dependent regulation of growth and stresses management in plants . Front. Plant Sci. 12 , 679916. 10.3389/fpls.2021.679916 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beris D., Theologidis I., Skandalis N. (2018). Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y . Sci. Rep. 8 :10320. 10.1038/s41598-018-28677-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhardwaj D., Ansari M. W., Sahoo R. K., Tuteja N. (2014). Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity . Microb. Cell Fact. 13 , 1–10. 10.1186/1475-2859-13-66 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhattacharjee R., Dey U. (2014). Biofertilizer, a way towards organic agriculture: A review . Afr. J. Microbiol. Res. 8 , 2332–2343. 10.5897/AJMR2013.6374 [ CrossRef ] [ Google Scholar ]
- Bhojiya A. A., Joshi H., Upadhyay S. K., Srivastava A. K., Pathak V. V., Pandey V. C., et al.. (2021). Screening and optimization of zinc removal potential in Pseudomonas aeruginosa- HMR1 and its plant growth-promoting attributes . Bull. Environ. Contamination Toxicol . 2021 , 1–10. 10.1007/s00128-021-03232-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Binenbaum J., Weinstain R., Shani E. (2018). Gibberellin localization and transport in plants . Trends Plant Sci. 23 :5. 10.1016/j.tplants.2018.02.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Boroujeni S. M., Kalbasi M., Asgharzadeh A., Baharlouei J. (2021). Evaluating the potential of Halothiobacillus bacteria for sulfur oxidation and biomass production under saline soil . Geomicrobiol. J . 38 , 57–65. 10.1080/01490451.2020.1809571 [ CrossRef ] [ Google Scholar ]
- Brahmaprakash G. P., Sahu P. K. (2012). Biofertilizers for sustainability . J. Indian Institute Sci. 92 , 37–62. [ Google Scholar ]
- Cabral M., Cheng X., Singh S., Ivessa A. S. (2016). Absence of non-histone protein complexes at natural chromosomal pause sites results in reduced replication pausing in aging yeast cells . Cell Rep. 8 , 1747–1754. 10.1016/j.celrep.2016.10.050 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cataldo E., Fucile M., Mattii G. B. (2022). Biostimulants in viticulture: a sustainable approach against biotic and abiotic stresses . Plants 11 :162. 10.3390/plants11020162 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chandrasekaran M., Chanratana M., Kim K., Seshadri S., Sa T. (2019). Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress–a meta-analysis . Front. Plant Sci. 10 , 457. 10.3389/fpls.2019.00457 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chaudhary A., Chaudhary P., Upadhyay A., Kumar A., Singh A. (2022a). Effect of gypsum on plant growth promoting rhizobacteria . Environ. Ecol. 39 , 1248–1256. [ Google Scholar ]
- Chaudhary A., Parveen H., Chaudhary P., Khatoon H., Bhatt P. (2021). “Rhizospheric microbes and their mechanism,” in Microbial Technology for Sustainable Environment , eds P. Bhatt, S. Gangola, D. Udayanga, and G. Kumar (Singapore: Springer; ). 10.1007/978-981-16-3840-4_6 [ CrossRef ] [ Google Scholar ]
- Chaudhary P., Chaudhary A., Agri U., Khatoon H., Singh A. (2022b). “Recent trends and advancements for agro-environmental sustainability at higher altitudes,” In: Survival Strategies in Cold-adapted Microorganisms , eds R. Goel, R. Soni, D. C. Suyal, and M. Khan M. (Singapore: Springer; ). 10.1007/978-981-16-2625-8_19 [ CrossRef ] [ Google Scholar ]
- Chaudhary P., Chaudhary A., Bhatt P., Kumar G., Khatoon H., Rani A., et al.. (2022c). Assessment of soil health indicators under the influence of nanocompounds and Bacillus spp. in field condition . Front. Environ. Sci . 9, 769871. 10.3389/fenvs.2021.769871 [ CrossRef ] [ Google Scholar ]
- Chaudhary P., Sharma A. (2019). Response of nanogypsum on the performance of plant growth promotory bacteria recovered from nanocompound infested agriculture field . Environ. Ecol . 37 , 363–372. [ Google Scholar ]
- Checchio M. V., de Cássia Alves R., de Oliveira K. R., Moro G. V., Santos D. M. M. D., Gratão P. L. (2021). Enhancement of salt tolerance in corn using Azospirillum brasilense : An approach on antioxidant system . J. Plant Res . 24 :34302571. 10.1007/s10265-021-01332-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen Y., Yang H., Shen Z., Ye J. (2022). Whole-Genome Sequencing and Potassium-Solubilizing Mechanism of Bacillus aryabhattai SK1-7 . Front. Microbiol. 12 , 722379. 10.3389/fmicb.2021.722379 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen Z., Zhao L., Dong Y., Chen W., Li C., Gao X., et al.. (2021). The antagonistic mechanism of Bacillus velezensis ZW10 against rice blast disease: Evaluation of ZW10 as a potential biopesticide . PLoS ONE 16 , e0256807. 10.1371/journal.pone.0256807 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cheng X. F., Wu H. H., Zou Y. N., Wu Q. S., Kuča K. (2021). Mycorrhizal response strategies of trifoliate orange under well-watered, salt stress, and waterlogging stress by regulating leaf aquaporin expression . Plant Physiol. Biochem. 162 , 27–35. 10.1016/j.plaphy.2021.02.026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chu T. N., Tran B., Van Bui L., Hoang M. (2019). Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana . BMC Res. Notes 12 :11. 10.1186/s13104-019-4046-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cissoko M., Hocher V., Gherbi H., Gully D., Carré-Mlouka A., Sane S., et al.. (2018). Actinorhizal signaling molecules: Frankia root hair deforming factor shares properties with NIN inducing factor . Front. Plant Sci. 18 :1494. 10.3389/fpls.2018.01494 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Collinge D. B., Jensen D. F., Rabiey M., Sarrocco S., Shaw M. W., Shaw R. H. (2022). Biological control of plant diseases – What has been achieved and what is the direction? Plant Pathol . 71 , 1024–1047. 10.1111/ppa.13555 [ CrossRef ] [ Google Scholar ]
- Dal C.ortivo C., Ferrari M., Visioli G., Lauro M., Fornasier F., Barion G., et al.. (2020). Effects of seed-applied biofertilizers on rhizosphere biodiversity and growth of common wheat ( Triticum aestivum L.) in the field . Front. Plant Sci . 11, 72. 10.3389/fpls.2020.00072 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Daroodi Z., Taheri P. S. (2021). Direct antagonistic activity and tomato resistance induction of the endophytic fungus Acrophialophora jodhpurensis against Rhizoctonia solani . Biol. Control . 160 :104696. 10.1016/j.biocontrol.2021.104696 [ CrossRef ] [ Google Scholar ]
- Desrut A., Moumen B., Thibault F., Le Hir R., Coutos-Thévenot P., Vriet C. (2020). Beneficial rhizobacteria Pseudomonas simiae WCS417 induce major transcriptional changes in plant sugar transport . J. Exp. Bot. 71 , 7301–7315. 10.1093/jxb/eraa396 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dicko A. H., Nantoume D., Ouattara D., Kassogue A., Fane R., Malle I., et al.. (2021). Screening of rice endophytic natives biofertilizers with plant growth-promoting characteristics . Afr. J. Agri. Res. 17 , 1336–1342. 10.5897/AJAR2020.15191 [ CrossRef ] [ Google Scholar ]
- Ding Z., Ali E. F., Almaroai Y. A., Eissa M. A., Abeed A. H. A. (2021). Effect of potassium solubilizing bacteria and humic acid on faba bean ( Vicia faba L.) plants grown on sandy loam soils . J. Soil Sci. Plant Nutr . 21 , 791–800. 10.1007/s42729-020-00401-z [ CrossRef ] [ Google Scholar ]
- Divekar P. A., Narayana S., Divekar B. A., Kumar R., Gadratagi B. G., Ray A., et al.. (2022). Plant secondary metabolites as defense tools against herbivores for sustainable crop protection . Int. J. Mol. Sci . 23 :2690. 10.3390/ijms23052690 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Don Y., Schmidtke S. M., Gambetta L. M. (2020). Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro . Sci. Rep. 10 :4498. 10.1038/s41598-020-61471-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- dos Santos T. B, Ribas A. F., de Souza S. G. H., Budzinski I. G. F., Domingues D.S. (2022). Physiological responses to drought, salinity, and heat stress in plants: a review . Stresses 2 , 113–135. 10.3390/stresses2010009 [ CrossRef ] [ Google Scholar ]
- Duan C., Mei Y., Wang Q., Wang Y., Li Q., Hong M., et al.. (2022). Rhizobium inoculation enhances the resistance of alfalfa and microbial characteristics in copper-contaminated soil . Front. Microbiol. 12 , 781831. 10.3389/fmicb.2021.781831 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Duc N. H., Csintalan Z., Posta K. (2018). Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants . Plant Physiol. Biochem. 132 , 297–307. 10.1016/j.plaphy.2018.09.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Egamberdieva D., Alimov J., Shurigin V., Alaylar B., Wirth S., Bellingrath-Kimura S. D. (2022). Diversity and plant growth-promoting ability of endophytic, halotolerant bacteria associated with Tetragonia tetragonioides (Pall.) kuntze . Plants . 11 :49. 10.3390/plants11010049 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eichmann R., Richards L., Schafer P. (2021). Hormones as go-betweens in plant microbiome assembly . Plant J. 105 , 518–541. 10.1111/tpj.15135 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eid A. M., Fouda A., Abdel-Rahman M. A., Salem S. S., Elsaied A., Oelmüller R., et al.. (2021). Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview . Plants 10 :935. 10.3390/plants10050935 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- El-Shahir A. A., Noha A., El,-T, Omar M. A., Arafat A. H., Abdel L., et al.. (2021). The effect of endophytic Talaromyces pinophilus on growth, absorption and accumulation of heavy metals of Triticum aestivum grown on sandy soil amended by sewage sludge . Plants . 10 :2659. 10.3390/plants10122659 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Esmaeel Q., Miotto L., Rondeau M., Leclère V., Clément C., Jacquard C. (2018). Paraburkholderia phytofirmans PsJN-plants interaction: from perception to the induced mechanisms . Front. Microbiol . 9, 2093. 10.3389/fmicb.2018.02093 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Etesami H., Jeong B. R., Glick B. R. (2021). Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to p uptake by plant . Front. Plant Sci . 12, 699618. 10.3389/fpls.2021.699618 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Etesami H., Mirseyed Hosseini H., Alikhani H. A. (2014). Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions . Physiol. Mol. Biol. Plants. 20 , 425–434. 10.1007/s12298-014-0251-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fadiji A. E., Babalola O. O. (2020). Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects . Front. Bioeng. Biotechnol. 8 :467. 10.3389/fbioe.2020.00467 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fahsi N., Mahdi I., Mesfioui A., Biskri L., Allaoui A. (2021). Plant growth-promoting rhizobacteria isolated from the Jujube ( Ziziphus lotus ) plant enhance wheat growth, Zn uptake and heavy metal tolerance . Agriculture . 11 :316. 10.3390/agriculture11040316 [ CrossRef ] [ Google Scholar ]
- Fang L., Ju W., Yang C., Jin X., Zhang C. (2020). Exogenous application of signaling molecules to enhance the resistance of legume-rhizobium symbiosis in Pb/Cd-contaminated soils . Environ. Pollut . 365 :114744. 10.1016/j.envpol.2020.114744 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fasusi O. A., Cruz C., Babalola O. O. (2021). Agricultural sustainability: microbial biofertilizers in rhizosphere management . Agriculture 11 :163. 10.3390/agriculture11020163 [ CrossRef ] [ Google Scholar ]
- Fortt J., González M., Morales P., Araya N., Remonsellez F., Coba de la Peña T., et al.. (2022). Bacterial modulation of the plant ethylene signaling pathway improves tolerance to salt stress in lettuce ( Lactuca sativa L.) . Front. Sustain. Food Syst. 6 :768250. 10.3389/fsufs.2022.768250 [ CrossRef ] [ Google Scholar ]
- Galindo F. S., Pagliari P. H., Fernandes G. C., Rodrigues W. L., Boleta E. H. M., Jalal A., et al.. (2022). Improving sustainable field-grown wheat production with Azospirillum brasilense under tropical conditions: a potential tool for improving nitrogen management . Front. Environ. Sci . 10, 821628. 10.3389/fenvs.2022.821628 [ CrossRef ] [ Google Scholar ]
- Game B. C., Ilhe B. M., Pawar V. S., Khandagale P. P. (2020). Effect of Azotobacter , phosphate solubilizing bacteria and potash mobilizing bacteria inoculants on productivity of wheat ( Triticum aestivum L.) . Intern. J. Curr. Microbiol. Appl. Sci. 9 , 2800–2807. 10.20546/ijcmas.2020.903.322 [ CrossRef ] [ Google Scholar ]
- Gao D., Ran C., Zhang Y., Wang X., Lu S., Geng Y., et al.. (2022). Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions . Plant Physiol. Biochem . 185 , 112–122. 10.1016/j.plaphy.2022.05.021 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ghaffari H., Gholizadeh A., Biabani A., Fallah A., Mohammadian M. (2018). Plant growth promoting rhizobacteria (PGPR) application with different nitrogen fertilizer levels in rice ( Oryza sativa L.) . Pertanika J. Trop. Agric. Sci. 41 , 715–728. [ Google Scholar ]
- Ghazy N., El-Nahrawy S. (2021). Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant . Arch. Microbiol. 203 , 1195–1209. 10.1007/s00203-020-02113-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ghodhbane-Gtari F., D'Angelo T., Gueddou A., Ghazouani S., Gtari M., Tisa L. S. (2021). Alone yet not alone: frankia lives under the same roof with other bacteria in actinorhizal nodules . Front. Microbiol . 12, 749760. 10.3389/fmicb.2021.749760 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ghori N. H., Ghori T., Hayat M. Q., Imadi S. R., Gul A., Altay V., et al.. (2019). Heavy metal stress and responses in plants . Int. J. Environ. Sci. Technol . 16 , 1807–1828. 10.1007/s13762-019-02215-8 [ CrossRef ] [ Google Scholar ]
- Ghosh D., Gupta A., Mohapatra S. (2019). A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana . World J. Microbiol. Biotechnol. 35 , 1–15. 10.1007/s11274-019-2659-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gohil R. B., Raval V. H., Panchal R. R., Rajput K. N. (2022). Plant growth-promoting activity of Bacillus sp. PG-8 isolated from fermented panchagavya and its effect on the growth of arachis hypogea . Front. Agron . 4, 805454. 10.3389/fagro.2022.805454 [ CrossRef ] [ Google Scholar ]
- Gond S. K., Torres M. S., Bergen M. S., Helsel Z., White J. F. (2015). Induction of salt tolerance and up-regulation of aquaporin genes in tropical corn by rhizobacterium Pantoea agglomerans . Lett. Appl. Microbiol . 60 , 392–399. 10.1111/lam.12385 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gong Z., Xiong L., Shi H., Yang S., Herrera-Estrella L. R., Xu G., et al.. (2020). Plant abiotic stress response and nutrient use efficiency . Sci. China Life Sci . 63 , 635–674. 10.1007/s11427-020-1683-x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- González-López M. D. C., Jijón-Moreno S., Dautt-Castro M., Ovando-Vázquez C., Ziv T., Horwitz B. A., et al.. (2021). Secretome analysis of Arabidopsis-Trichoderma atroviride interaction unveils new roles for the plant glutamate:glyoxylate aminotransferase GGAT1 in plant growth induced by the fungus and resistance against botrytis cinerea . Int. J. Mol. Sci . 22 :6804. 10.3390/ijms22136804 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- González-Pérez E., Ortega-Amaro M. A., Salazar-Badillo F. B., Bautista E., Douterlungne D., Jiménez-Bremont J. F. (2018). The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction . Sci. Rep . 8 , 1–14. 10.1038/s41598-018-34500-w [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gouda S., Kerry R. G., Das G. (2018). Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture . Microbiol. Res. 206 , 131–140. 10.1016/j.micres.2017.08.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gu S., Wei Z., Shao Z., Friman V. P., Cao K., Yang T. (2020). Competition for iron drives phytopathogen control by natural rhizosphere microbiomes . Nat. Microbiol . 5 , 1002–1010. 10.1038/s41564-020-0719-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Guo J., Chi J. (2014). Effect of Cd-tolerant plant growth promoting Rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil . Plant Soil . 375 , 205–214. 10.1007/s11104-013-1952-1 [ CrossRef ] [ Google Scholar ]
- Gupta A., Rai S., Bano A., Khanam A., Sharma S., Pathak N. (2021). Comparative evaluation of different salt-tolerant plant growth-promoting bacterial isolates in mitigating the induced adverse effect of salinity in pisum sativum . Biointerface Res. Appl. Chem . 11 , 13141–13154. 10.33263/BRIAC115.1314113154 [ CrossRef ] [ Google Scholar ]
- Gupta S., Pandey S. (2019). ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean ( Phaseolus vulgaris ) plants . Front. Microbiol . 10, 1506. 10.3389/fmicb.2019.01506 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hao Z., Xie W., Jiang X., Wu Z., Zhang X., Chen B. (2019). Arbuscular mycorrhizal fungus improves Rhizobium–Glycyrrhiza seedling symbiosis under drought stress . Agronomy . 9 :572. 10.3390/agronomy9100572 [ CrossRef ] [ Google Scholar ]
- Harman G., Khadka R., Doni F., Uphoff N. (2020). Benefits to plant health and productivity from enhancing plant microbial symbionts . Front. Plant Sci . 11, 610065. 10.3389/fpls.2020.610065 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Haro R., Benito B. (2019). The role of soil fungi in K+ plant nutrition . Int. J. Mol. Sci. 20 :3169. 10.3390/ijms20133169 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hasan N., Farzand A., Heng Z., Khan I. U., Moosa A., Zubair M., et al.. (2020). Antagonistic potential of novel endophytic Bacillus strains and mediation of plant defense against Verticillium wilt in upland cotton . Plants 9 :1438. 10.3390/plants9111438 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hassan E. A., Yasser S., Mostafa A., Mohamed H., Nivien A. N. (2021). Biosafe management of Botrytis grey mold of strawberry fruit by novel bioagents . Plants 12 :2737. 10.3390/plants10122737 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hata E. M., Yusof M. T., Zulperi D. (2021). Induction of systemic resistance against bacterial leaf streak disease and growth promotion in rice plant by Streptomyces shenzhenesis TKSC3 and Streptomyces sp. SS8 . Plant Pathol. J. 37 , 173–181. 10.5423/PPJ.OA.05.2020.0083 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hawkins J. P., Oresnik I. J. (2022). The Rhizobium -legume symbiosis: co-opting successful stress management . Front. Plant Sci . 3, 796045. 10.3389/fpls.2021.796045 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- He M., He C.-Q., Ding N.-Z. (2018). Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance . Front. Plant Sci . 9, 1771. 10.3389/fpls.2018.01771 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hennessy L. M., Popay A. J., Glare T. R. (2022). Olfactory responses of Argentine stem weevil to herbivory and endophyte-colonisation in perennial ryegrass . J. Pest Sci . 95 , 263–277. 10.1007/s10340-021-01375-2 [ CrossRef ] [ Google Scholar ]
- Hernández-Fernández M., Cordero-Bueso G., Ruiz-Muñoz M., Cantoral J. M. (2021). Culturable yeasts as biofertilizers and biopesticides for a sustainable agriculture: a comprehensive review . Plants (Basel) 21 :822. 10.3390/plants10050822 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Htwe A. Z., Moh S. M., Moe K., Yamakawa T. (2019). Biofertilizer production for agronomic application and evaluation of its symbiotic effectiveness in soybeans . Agronomy 9 :162. 10.3390/agronomy9040162 [ CrossRef ] [ Google Scholar ]
- Huang C. J., Wei G., Jie Y. C., Xu J. J., Zhao S. Y., Wang L. C., et al.. (2015). Responses of gas exchange, chlorophyll synthesis and ROS-scavenging systems to salinity stress in two ramie ( Boehmeria nivea L.) cultivars . Photosynthetica 53 , 455–463. 10.1007/s11099-015-0127-0 [ CrossRef ] [ Google Scholar ]
- Hummadi E. H., Cetin Y., Demirbek M., Kardar N. M., Khan S., Coates C. J., et al.. (2022). Antimicrobial volatiles of the insect pathogen Metarhizium brunneum . J. Fungi 22 :326. 10.3390/jof8040326 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hussain A., Zahir Z. A., Ditta A., Tahir M. U., Ahmad M., Mumtaz M. Z., et al.. (2020). Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize ( Zea mays L.) production and biofortification under two cropping seasons . Agronomy 10 :39. 10.3390/agronomy10010039 [ CrossRef ] [ Google Scholar ]
- Igiehon N. O., Babalola O. O., Aremu B. R. (2019). Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress . BMC Microbiol. 19 :159. 10.1186/s12866-019-1536-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Igiehon N. O., Babalola O. O., Cheseto X., Torto B. (2021). Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress . Microbiol. Res . 242 :126640. 10.1016/j.micres.2020.126640 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Imran Q. M., Falak N., Hussain A., Mun B.-G., Yun B.-W. (2021). Abiotic stress in plants; stress perception to molecular response and role of biotechnological tools in stress resistance . Agronomy 11 :1579. 10.3390/agronomy11081579 [ CrossRef ] [ Google Scholar ]
- Iqbal Z., Iqbal M. S., Hashem A., Abd_Allah E. F., Ansari M. I. (2021). Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions . Front. Plant Sci. 12 , 631810. 10.3389/fpls.2021.631810 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ismail A. H., Mehmood A., Qadir M., Husna A. I., Hamayun M., Khan N. (2020). Thermal stress alleviating potential of endophytic fungus Rhizopus oryzae inoculated to sunflower ( Helianthus annuus L.) and soybean ( Glycine max L.) . Pak. J. Bot . 52 , 1857–1865. 10.30848/PJB2020-5(10) [ CrossRef ] [ Google Scholar ]
- Ismail E. G., Mohamed W. W., Khattab S., Sherif F. E. (2013). Effect of manure and bio-fertilizers on growth, yield, silymarin content, and protein expression profile of Silybum marianum . Int. J. Med. Arom. Plants. 3 , 430–438. [ Google Scholar ]
- Issa A. Esmaeel Q. Sanchez L. Courteaux B. Guise and, J.-F. Gibon Y. (2018). Impacts of Paraburkholderia phytofirmans strain PsJN on tomato ( Lycopersicon esculentum L.) under high temperature . Front. Plant Sci. 9 , 1397. 10.3389/fpls.2018.01397 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jain D., Sharma J., Kaur G., Bhojiya A. A., Chauhan S., Sharma V., et al.. (2021). Phenetic and molecular diversity of nitrogen-fixing plant growth-promoting Azotobacter isolated from semiarid regions of India . Hindawi BioMed. Res. Intern. 2021 :6686283. 10.1155/2021/6686283 [ CrossRef ] [ Google Scholar ]
- Janati W., Benmrid B., Elhaissoufi W., Zeroual Y., Nasielski J., Bargaz A. (2021). Will phosphate bio-solubilization stimulate biological nitrogen fixation in grain legumes? Front. Agron . 3 :637196. 10.3389/fagro.2021.637196 [ CrossRef ] [ Google Scholar ]
- Jiang W., Pan R., Wu C., Xu L., Abdelaziz M. E., Oelmüller R., et al.. (2020). Piriformospora indica enhances freezing tolerance and post-thaw recovery in Arabidopsi s by stimulating the expression of CBF genes . Plant Signal. Behav . 15 :1745472. 10.1080/15592324.2020.1745472 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jimenez-Jimenez S., Santana O., Lara-Rojas F., Arthikala M. K., Armada E., Hashimoto K., et al.. (2019). Differential tetraspanin genes expression and subcellular localization during mutualistic interactions in Phaseolus vulgaris . PLoS ONE 14 , e0219765. 10.1371/journal.pone.0219765 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jiménez-Mejía R., Medina-Estrada R. I., Carballar-Hernández S., Orozco-Mosqueda M., d,.C, Santoyo G., et al.. (2022). Teamwork to survive in hostile soils: use of plant growth-promoting bacteria to ameliorate soil salinity stress in crops . Microorganisms 10 :150. 10.3390/microorganisms10010150 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jing X., Cui Q., Li X., Yin J., Ravichandran V., Pan D., et al.. (2020). Engineering Pseudomonas protegens Pf-5 to improve its antifungal activity and nitrogen fixation . Microb. Biotechnol . 13 , 118–133. 10.1111/1751-7915.13335 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kandel S. L., Joubert P. M., Doty S. L. (2017). Bacterial endophyte colonization and distribution within plants . Microorganisms 5 :77. 10.3390/microorganisms5040077 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kang S.-M., Radhakrishnan R., Khan A. L., Kim M.-J., Park J.-M., Kim B.-R. (2014b). Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions . Plant Physiol. Biochem . 84 , 115–124. 10.1016/j.plaphy.2014.09.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kang S.-M., Shahzad R., Khan M. A., Hasnain Z., Lee K.-E., Park H.-S., et al.. (2021). Ameliorative effect of indole-3-acetic acid- and siderophore-producing Leclercia adecarboxylata MO1 on cucumber plants under zinc stress . J. Plant Inter . 16 , 30–41. 10.1080/17429145.2020.1864039 [ CrossRef ] [ Google Scholar ]
- Kang S. M., Radhakrishnan R., You Y. H., Joo G. J., Lee I. J., Lee K. E., et al.. (2014a). Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth . Ind. J. Microbiol. 54 , 427–433. 10.1007/s12088-014-0476-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaur S., Samota M. K., Choudhary M., Choudhary M., Pandey A. K., Sharma A., et al.. (2022). How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions . Physiol. Mol. Biol. Plants . 28 , 485–504. 10.1007/s12298-022-01146-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kenneth O. C., Nwadibe E. C., Kalu A. U., Unah U. V. (2019). Plant growth promoting rhizobacteria (PGPR): a novel agent for sustainable food production . Am. J. Agric. Biol. Sci. 14 , 35–54. 10.3844/ajabssp.2019.35.54 [ CrossRef ] [ Google Scholar ]
- Khan M. A., Asaf S., Khan A. L., Jan R., Kang S.-M., Kim K.-M. (2020). Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application . PLoS ONE 15 , e0232228. 10.1371/journal.pone.0232228 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khan N., Bano A., Zandi P. (2018). Effects of exogenously applied plant growth regulators in combination with PGPR on the physiology and root growth of chickpea ( Cicer arietinum ) and their role in drought tolerance . J. Plant Interact . 13 , 239–247. 10.1080/17429145.2018.1471527 [ CrossRef ] [ Google Scholar ]
- Khanna K., Jamwal V. L., Sharma A., Gandhi S. G., Ohri P., Bhardwaj R., et al.. (2019). Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites . Chemosphere . 230 , 628–639. 10.1016/j.chemosphere.2019.05.072 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khare E., Mishra J., Arora N. K. (2018). Multifaceted interactions between endophytes and plant: developments and prospects . Front. Microbiol . 9, 2732. 10.3389/fmicb.2018.02732 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khati P., Parul, Bhatt P., Nisha, Kumar R., Sharma A. (2018). Effect of nanozeolite and plant growth promoting rhizobacteria on maize . 3Biotech 8 :141. 10.1007/s13205-018-1142-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kirui C. K., Njeru E. M., Runo S. (2022). Diversity and phosphate solubilization efficiency of phosphate solubilizing bacteria isolated from semi-arid agroecosystems of Eastern Kenya . Microbiol. Insights 17 :15. 10.1177/11786361221088991 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Köhl J., Kolnaar R., Ravensberg W. J. (2019). Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy . Front. Plant Sci. 10 , 845. 10.3389/fpls.2019.00845 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kousar B., Bano A., Khan N. (2020). PGPR Modulation of secondary metabolites in tomato infested with Spodoptera litura . Agronomy 10 :778. 10.3390/agronomy10060778 [ CrossRef ] [ Google Scholar ]
- Kukreti B., Sharma A., Chaudhary P., Agri U., Maithani D. (2020). Influence of nanosilicon dioxide along with bioinoculants on Zea mays and its rhizospheric soil . 3Biotech 10 :345. 10.1007/s13205-020-02329-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar A., Maurya B. M., Raghuwanshi R. (2021a). The microbial consortium of indigenous rhizobacteria improving plant health, yield and nutrient content in wheat ( Triticum aestivum ). J. Plant Nutr . 44 , 1942–1956. 10.1080/01904167.2021.1884706 [ CrossRef ] [ Google Scholar ]
- Kumar A., Singh S. K., Kant C., Verma H., Kumar D., Singh P. P., et al.. (2021b). Microbial biosurfactant: a new frontier for sustainable agriculture and pharmaceutical industries . Antioxidants 10 :1472. 10.3390/antiox10091472 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar P., Dubey R. C., Maheshwari D. K. (2012). Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens . Microbiological Res. 16 , 493–499. 10.1016/j.micres.2012.05.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar S., Kumar S., Mohapatra T. (2021c). Interaction between macro- and micro-nutrients in plants . Front. Plant Sci. 12 , 665583. 10.3389/fpls.2021.665583 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kusale S. P., Attar Y. C., Sayyed R. Z., Malek R. A., Ilyas N., Suriani N. L., et al.. (2021). Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress . Molecules 26 :1894. 10.3390/molecules26071894 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lahlali R., Ezrari S., Radouane N., Kenfaoui J., Esmaeel Q., El Hamss H., et al.. (2022). Biological control of plant pathogens: a global perspective . Microorganisms 10 :596. 10.3390/microorganisms10030596 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lata R., Chowdhury S., Gond S. K., White J.r, J.F. (2018). Induction of abiotic stress tolerance in plants by endophytic microbes . Lett. Appl. Microbiol . 66 , 268–276. 10.1111/lam.12855 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lee L. H., Wu T. Y., Shak K. P. Y., Lim S. L., Ng K. Y., Nguyen M. N., et al.. (2018). Sustainable approach to biotransform industrial sludge into organic fertilizer via vermicomposting: A mini-review . J. Chem. Technol. Biotechnol. 93 , 925–935. 10.1002/jctb.5490 [ CrossRef ] [ Google Scholar ]
- Liu H., Carvalhais L. C., Crawford M., Singh E., Dennis P. G., Pieterse C. M., et al.. (2017a). Inner plant values: diversity, colonization and benefits from endophytic bacteria . Front. Microbiol . 8, 2552. 10.3389/fmicb.2017.02552 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu S., Tian Y., Jia M., Lu X., Yue L., Zhao X., et al.. (2020). Induction of salt tolerance in Arabidopsis thaliana by volatiles from Bacillus amyloliquefaciens FZB42 via the jasmonic acid signaling patway . Front. Microbiol. 11 , 562934. 10.3389/fmicb.2020.562934 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu Z., Rong Q., Zhou W., Liang G. (2017b). Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil . PLoS ONE 12 , e0172767. 10.1371/journal.pone.0172767 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lopes M. J. S., Dias-Filho M. B., Gurgel E. S. C. (2021). Successful plant growth-promoting microbes: inoculation methods and abiotic factors . Front. Sustain. Food Syst . 5, 606454. 10.3389/fsufs.2021.606454 [ CrossRef ] [ Google Scholar ]
- Lou X., Zhao J., Lou X., Xia X., Feng Y., Li H. (2022). The biodegradation of soil organic matter in soil-dwelling humivorous fauna . Front. Bioeng. Biotechnol . 9 :808075. 10.3389/fbioe.2021.808075 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Luo J., Zhang Z., Hou Y., Diao F., Hao B., Bao Z., et al.. (2021). Exploring microbial resource of different rhizocompartments of dominant plants along the salinity gradient around the hypersaline lake Ejinur . Front. Microbiol . 12, 698479. 10.3389/fmicb.2021.698479 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lurthy T., Cantat C., Jeudy C., Declerck P., Gallardo K., Barraud C., et al.. (2020). Impact of bacterial siderophores on iron status and ionome in pea . Front. Plant Sci . 11, 730. 10.3389/fpls.2020.00730 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ma X., Liu Y., Shen W., Kuzyakov Y. (2021). Phosphatase activity and acidification in lupine and maize rhizosphere depend on phosphorus availability and root properties: coupling zymography with planar optodes . Agric. Ecosyst. Environ. Appl. Soil Ecol . 167 :104029. 10.1016/j.apsoil.2021.104029 [ CrossRef ] [ Google Scholar ]
- Mahanty T., Bhattacharjee S., Goswami M., Bhattacharyya P., Das B., Ghosh A., et al.. (2017). Biofertilizers: a potential approach for sustainable agriculture development . Environ. Sci. Pollut. Res. 24 , 3315–3335. 10.1007/s11356-016-8104-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mahmud A. A., Upadhyay S. K., Srivastava A. K., Bhojiya A. A. (2021). Biofertilizers: A Nexus between soil fertility and crop productivity under abiotic stress . Curr. Res. Environ. Sustain. 3 :100063. 10.1016/j.crsust.2021.100063 [ CrossRef ] [ Google Scholar ]
- Malik K. M., Khan K. S., Billah M., Akhtar M. S., Rukh S., Alam S., et al.. (2021). Organic amendments and elemental sulfur stimulate microbial biomass and sulfur oxidation in alkaline subtropical soils . Agronomy 11 :2514. 10.3390/agronomy11122514 [ CrossRef ] [ Google Scholar ]
- Malusa E., Vassilev N. (2014). A contribution to set a legal framework for biofertilisers . Appl. Microbiol. Biotechnol. 98 , 6599–6607. 10.1007/s00253-014-5828-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marulanda A., Azcón R., Chaumont F., Ruiz-Lozano J. M., Aroca R. (2010). Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize ( Zea mays L.) plants under unstressed and salt-stressed conditions . Planta 232 , 533–543. 10.1007/s00425-010-1196-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mazid M., Khan T. A. (2015). Future of bio-fertilizers in Indian agriculture: an overview . Int. J. Agri. Food Res. 3 :132. 10.24102/ijafr.v3i3.132 [ CrossRef ] [ Google Scholar ]
- Meddich A., Ait M. M., Bourzik W., Mitsui T., Baslam M., Hafidi M. (2018). Optimizing Growth and Tolerance of Date Palm (Phoenix dactylifera L.) to Drought, Salinity, and Vascular Fusarium-Induced Wilt (Fusarium oxysporum) by Application of Arbuscular Mycorrhizal Fungi (AMF) . Cham: Springer. 10.1007/978-3-319-75910-4_9 [ CrossRef ] [ Google Scholar ]
- Meena K. K., Bitla U. M., Sorty A. M., Singh D. P., Gupta V. K., Wakchaure G. C., et al.. (2020). Mitigation of salinity stress in wheat seedlings due to the application of phytohormone-rich culture filtrate extract of methylotrophic Actinobacterium Nocardioides sp. NIMMe6 . Front. Microbiol . 11, 2091. 10.3389/fmicb.2020.02091 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mesa-Marín J., Pérez-Romero J. A., Redondo-Gómez S., Pajuelo E., Rodríguez-Llorente I. D., Mateos-Naranjo E. (2020). Impact of plant growth promoting bacteria on Salicornia ramosissima ecophysiology and heavy metal phytoremediation capacity in estuarine soils . Front. Microbiol . 11, 553018. 10.3389/fmicb.2020.553018 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mishra S., Arora N. K. (2012). Evaluation of rhizospheric Pseudomonas and Bacillus as biocontrol tool for Xanthomonas campestris pv campestris . World J. Microbiol. Biotechnol. 28 , 693–702. 10.1007/s11274-011-0865-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mitter E. K., Tosi M., Obregón D., Dunfield K. E., Germida J. J. (2021). Rethinking crop nutrition in times of modern microbiology: innovative biofertilizer technologies . Front. Sustain. Food Syst . 5, 606815. 10.3389/fsufs.2021.606815 [ CrossRef ] [ Google Scholar ]
- Mokabel S., Olama Z., Ali S., El-Dakak R. (2022). The role of plant growth promoting rhizosphere microbiome as alternative biofertilizer in boosting Solanum melongena L. Adaptation to salinity stress . Plants 11 :659. 10.3390/plants11050659 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mondal M., Skalicky M., Garai S., Hossain A., Sarkar S. H., et al.. (2020). Supplementing nitrogen in combination with Rhizobium inoculation and soil mulch in peanut ( Arachis hypogaea L.) production system: Part II. Effect on phenology, growth, yield attributes, pod quality, profitability and nitrogen use efficiency . Agronomy 10 :1513. 10.3390/agronomy10101513 [ CrossRef ] [ Google Scholar ]
- Morelli M., Bahar O., Papadopoulou K. K., Hopkins D. L., Obradovi,ć A. (2020). Role of endophytes in plant health and defense against pathogens . Front. Plant Sci . 11, 1312. 10.3389/fpls.2020.01312 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morsy M., Blake C., Hayden A.-M. (2020). Fungal endophytes promote tomato growth and enhance drought and salt tolerance . Plants 9 :7877. 10.3390/plants9070877 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moustafa-Farag M., Almoneafy A., Mahmoud A., Elkelish A., Arnao M. B., Li L., et al.. (2019). Melatonin and its protective role against biotic stress impacts on plants . Biomolecules 10 :54. 10.3390/biom10010054 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mukherjee A., Gaurav A. K., Singh S., Yadav S., Bhowmick S., Abeysinghe S., et al.. (2022). The bioactive potential of phytohormones: A review . Biotechnol. Rep. 8 :e00748. 10.1016/j.btre.2022.e00748 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mukhtar S., Zareen M., Khaliq Z., Mehnaz S., Malik K. A. (2020a). Phylogenetic analysis of halophyte-associated rhizobacteria and effect of halotolerant and halophilic phosphate-solubilizing biofertilizers on maize growth under salinity stress conditions . J. Appl. Microbiol. 128 , 556–573. 10.1111/jam.14497 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mukhtar T., Shafiqur R., Smith D., Sultan T., Seleiman M. F., Alsadon A. A., et al.. (2020b). Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling . Sustainability 12 :2159. 10.3390/su12062159 [ CrossRef ] [ Google Scholar ]
- Nacoon S., Jogloy S., Riddech N., Mongkolthanaruk W., Kuyper T., Boonlue S. (2020). Interaction between phosphate solubilizing bacteria and arbuscular mycorrhizal fungi on growth promotion and tuber inulin content of Helianthus tuberosus L . Sci. Rep . 10 :4916. 10.1038/s41598-020-61846-x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nandini B., Puttaswamy H., Saini R. K., Prakash H. S., Geetha N. (2021). Trichovariability in rhizosphere soil samples and their biocontrol potential against downy mildew pathogen in pearl millet . Sci. Rep . 11 :9517. 10.1038/s41598-021-89061-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nassal D., Spohn M., Eltlbany N., Jacquiod S., Smalla K., Marhan S., et al.. (2018). Effects of phosphorus-mobilizing bacteria on tomato growth and soil microbial activity . Plant Soil. 427 , 17–37. 10.1007/s11104-017-3528-y [ CrossRef ] [ Google Scholar ]
- Nie P., Chen C., Yin Q., Jiang C., Guo J., Zhao H., et al.. (2019). Function of miR825 and miR825 * as negative regulators in Bacillus cereus AR156-elicited systemic resistance to Botrytis cinerea in Arabidopsis thaliana . Int. J. Mol. Sci . 20 :5032. 10.3390/ijms20205032 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nitu R., Rajinder K., Sukhminderjit K. (2020). Zinc solubilizing bacteria to augment soil fertility—A comprehensive review . Int. J. Agri. Sci. Vet. Med. 8 , 38–44. [ Google Scholar ]
- Niu J., Li X. (2022). Effects of microbial inoculation with different indigenous bacillus species on physicochemical characteristics and bacterial succession during short-term composting . Fermentation 8 :152. 10.3390/fermentation8040152 [ CrossRef ] [ Google Scholar ]
- Numan M., Bashir S., Khan Y. (2018). Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review . Microbiol Res . 209 , 21–32. 10.1016/j.micres.2018.02.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Odoh C. K., Sam K., Zabbey N., Eze C. N., Nwankwegu A. S., Laku C., et al.. (2020). Microbial Consortium as Biofertilizers for Crops Growing Under the Extreme Habitats. Plant Microbiomes for Sustainable Agriculture . Cham: Springer. 10.1007/978-3-030-38453-1_13 [ CrossRef ] [ Google Scholar ]
- Okamoto T., Shinjo R., Nishihara A., Uesaka K., Tanaka A., Sugiura D., et al.. (2021). Genotypic variation of endophytic nitrogen-fixing activity and bacterial flora in rice stem based on sugar content . Front. Plant Sci . 2021, 1610. 10.3389/fpls.2021.719259 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Okur N. (2018). A review-bio-fertilizers-power of beneficial microorganisms in soils . Biomed. J. Sci. Tech. Res. 4 , 4028–4029. 10.26717/BJSTR.2018.04.0001076 [ CrossRef ] [ Google Scholar ]
- Oubohssaine M., Sbabou L., Aurag J. (2022). Native heavy metal-tolerant plant growth promoting rhizobacteria improves Sulla spinosissima (L.) growth in post-mining contaminated soils . Microorganisms 10 :838. 10.3390/microorganisms10050838 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oukala N., Aissat K., Pastor V. (2021). Bacterial endophytes: The hidden actor in plant immune responses against biotic stress . Plants 10 :1012. 10.3390/plants10051012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pandey J., Singh A. (2012). Opportunities and constraints in organic farming: an Indian perspective . J. Sci. Res. 56 , 47–72. [ Google Scholar ]
- Pang Z., Chen J., Wang T., Gao C., Li Z., Guo L., et al.. (2021). Linking plant secondary metabolites and plant microbiomes: a review . Front. Plant Sci. 12 , 621276. 10.3389/fpls.2021.621276 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paredes-Páliz K., Rodríguez-Vázquez R., Duarte B., Caviedes M. A., Mateos-Naranjo E., Redondo-Gómez S., et al.. (2018). Investigating the mechanisms underlying phytoprotection by plant growth-promoting Rhizobacteria in Spartina densiflora under Metal Stress . Plant Biol . 20 , 497–506 10.1111/plb.12693 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patel M., Patel K., Al-Keridis L. A., Alshammari N., Badraoui R., Elasbali A. M., et al.. (2022). Cadmium-tolerant plant growth-promoting bacteria Curtobacterium oceanosedimentum improves growth attributes and strengthens antioxidant system in chili ( Capsicum frutescens ) . Sustainability 14 :4335. 10.3390/su14074335 [ CrossRef ] [ Google Scholar ]
- Patel S. H., Viradiya M. B., Prajapati B. J. (2021). Effect of potassium and potassium mobilizing bacteria (KMB) with and without FYM on yield of wheat ( Triticum aestivum L.) . Pharmac. Phytochem. 10 , 1615–1620. [ Google Scholar ]
- Popp J., Pet,o K., Nagy J. (2013). Pesticide productivity and food security. A review . Agronomy Sustain. Dev . 33 , 243–255. 10.1007/s13593-012-0105-x [ CrossRef ] [ Google Scholar ]
- Portieles R., Xu H., Yue Q., Zhao L., Zhang D., Du L., et al.. (2021). Heat-killed endophytic bacterium induces robust plant defense responses against important pathogens . Sci. Rep . 11 :12182. 10.1038/s41598-021-91837-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Qiang X., Ding J., Lin W., Li Q., Xu C., Zheng Q., et al.. (2019). Alleviation of the detrimental effect of water deficit on wheat ( Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus . Plant Soil. 439 , 373–391. 10.1007/s11104-019-04028-7 [ CrossRef ] [ Google Scholar ]
- Qurashi A. W., Sabri A. N. (2012). Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress . Braz. J. Microbiol . 43 , 1183–1191. 10.1590/S1517-83822012000300046 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rashid M. H. O., Khan A., Hossain M. T., Chung Y. R. (2017). Induction of systemic resistance against aphids by endophytic Bacillus velezensis YC7010 via expressing PHYTOALEXIN DEFICIENT4 in Arabidopsis . Front. Plant Sci . 8, 211. 10.3389/fpls.2017.00211 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raza A., Razzaq A., Mehmood S. S., Zou X., Zhang X., Lv Y., et al.. (2019). Impact of climate change on crops adaptation and strategies to tackle its outcome: A review . Plants 8 , 34. 10.3390/plants8020034 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reghmit A., Benzina-tihar F., Escudero F. J. L., Halouane-Sahir F., Oukali Z., Bensmail S., et al.. (2021). Trichoderma spp. isolates from the rhizosphere of healthy olive trees in northern Algeria and their biocontrol potentials against the olive wilt pathogen, Verticillium dahliae . Org. Agric. 11 , 639–657. 10.1007/s13165-021-00371-1 [ CrossRef ] [ Google Scholar ]
- Rodriguez M. V., Tano J., Ansaldi N., Carrau A., Srebot M. S., Ferreira V., et al.. (2019). Anatomical and biochemical changes induced by Gluconacetobacter diazotrophicus stand up for Arabidopsis thaliana seedlings from Ralstonia solanacearum infection . Front. Plant Sci. 10 , 1618. 10.3389/fpls.2019.01618 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Romera F. J., García M. J., Lucena C., Martínez-Medina A., Aparicio M. A., Ramos J., et al.. (2019). Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants . Front. Plant Sci . 10, 287. 10.3389/fpls.2019.00287 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roy S., Liu W., Nandety R. S., Crook A., Mysore K. S., Pislariu C. I., et al.. (2020). Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation . Plant Cell. 32 , 15–41. 10.1105/tpc.19.00279 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rybakova D., Rack-Wetzlinger U., Cernava T., Schaefer A., Schmuck M., Berg G. (2017). Aerial warfare: A volatile dialogue between the plant pathogen Verticillium longisporum and its antagonist Paenibacillus polymyxa . Front. Plant Sci . 8, 1294. 10.3389/fpls.2017.01294 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Safdarian M., Askari H., Shariati J. V., Nematzadeh G. (2019). Transcriptional responses of wheat roots inoculated with Arthrobacter nitroguajacolicus to salt stress . Sci. Rep . 9 :1792. 10.1038/s41598-018-38398-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Salem M., Al-Amri (2021). Application of bio-fertilizers for enhancing growth and yield of common bean plants grown under water stress conditions . Saudi J. Biol. Sci . 28 , 3901–3908. 10.1016/j.sjbs.2021.03.064 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Salomon M. V., Bottini R., de Souza Filho G. A., Cohen A. C., Moreno D., Gil M. (2014). Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, abscisic acid accumulation and synthesis of defence-related terpenes in in vitro cultured grapevine . Physiol. Plant. 151 , 359–374. 10.1111/ppl.12117 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Samain E., Aussenac T., Selim S. (2019). The effect of plant genotype, growth stage, and Mycosphaerella graminicola strains on the efficiency and durability of wheat-induced resistance by Paenibacillus sp. Strain B2 . Front. Plant Sci . 10, 587. 10.3389/fpls.2019.00587 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Samaras A., Roumeliotis E., Ntasiou P., Karaoglanidis G. (2021). Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens . Plants 10 :1113. 10.3390/plants10061113 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sánchez-Montesinos B., Santos M., Moreno-Gavíra A., Marín-Rodulfo T., Gea F. J., Diánez F. (2021). Biological control of fungal diseases by T richoderma aggressivum f. europaeum and its compatibility with fungicides . J. Fungi. 24 :7. 10.3390/jof7080598 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sandhu N., Sethi M., Kumar A., Dang D., Singh J., Chhuneja P. (2021). Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: a review . Front. Plant Sci . 12, 657629. 10.3389/fpls.2021.657629 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sandhya V., Ali S. Z., Grover M., Reddy G., Venkateswarlu B. (2010). Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress . Plant Growth Regul . 62 , 21–30. 10.1007/s10725-010-9479-4 [ CrossRef ] [ Google Scholar ]
- Santosh S., Velmourougane K., Idapuganti R. G., Manikandan A., Blaise D. (2022). Potassium solubilizing potential of native bacterial isolates from cotton rhizosphere of rainfed vertisols . Natl. Acad. Sci. Lett. 2022 , 1–4. 10.1007/s40009-022-01113-x [ CrossRef ] [ Google Scholar ]
- Sarkar J., Chakraborty B., Chakraborty U. (2018). Plant growth promoting rhizobacteria protect wheat plants against temperature stress through antioxidant signalling and reducing chloroplast and membrane injury . J. Plant Growth Regul . 37 , 1396–1412. 10.1007/s00344-018-9789-8 [ CrossRef ] [ Google Scholar ]
- Sarwar S., Khaliq A., Yousra M., Sultan T., Ahmad N., Khan M. Z. (2020). Screening of siderophore-producing PGPRs isolated from groundnut ( Arachis hypogaea L.) rhizosphere and their influence on iron release in soil . Commun. Soil Sci. Plant Anal. 51 , 1680–1692. 10.1080/00103624.2020.1791159 [ CrossRef ] [ Google Scholar ]
- Sattar A., Naveed M., Ali M., Zahir Z. A., Nadeem S. M., Yaseen M., et al.. (2019). Perspectives of potassium solubilizing microbes in sustainable food production system: A review . Agric,. Ecosyst. Environ,. Appl. Soil Ecol. 133 , 146–159. 10.1016/j.apsoil.2018.09.012 [ CrossRef ] [ Google Scholar ]
- Sdiri Y., Lopes T., Rodrigues N., Silva K., Rodrigues I., Pereira J. A., et al.. (2022). Biocontrol ability and production of volatile organic compounds as a potential mechanism of action of olive endophytes against Colletotrichum acutatum . Microorganisms 10 :571. 10.3390/microorganisms10030571 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sendi Y., Pfeiffer T., Koch E. (2020). Potential of common bean ( Phaseolus vulgaris L.) root microbiome in the biocontrol of root rot disease and traits of performance . J. Plant Dis. Prot. 127 , 453–462 10.1007/s41348-020-00338-6 [ CrossRef ] [ Google Scholar ]
- Sharma N., Khanna K., Manhas R. K., Bhardwaj R., Ohri P., Alkahtani J., et al.. (2020). Insights into the role of Streptomyces hydrogenans as the plant growth promoter, photosynthetic pigment enhancer and biocontrol agent against Meloidogyne incognita in Solanum lycopersicum Seedlings . Plants 9 :1109. 10.3390/plants9091109 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sheteiwy M. S. Abd Elgawad H. Xiong Y.-C. Macovei A. Brestic M. Skalicky M. (2021) Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress improve seed yield quality of soybean plant. Physiologia Plant. 1–17. 10.1111/ppl.13454 [ PubMed ] [ Google Scholar ]
- Shukla P. S., Agarwal P. K., Jha B. I. (2012). Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria . J. Plant Growth Regul . 31 , 195–206. 10.1007/s00344-011-9231-y [ CrossRef ] [ Google Scholar ]
- Shukla V., Kumar S., Tripathi Y. N., Upadhyay R. S. (2022). Bacillus subtilis - and Pseudomonas fluorescens -mediated systemic resistance in tomato against sclerotium rolfsii and study of physio-chemical alterations . Front. Fungal Biol . 3 :851002. 10.3389/ffunb.2022.851002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Singh P., Arif Y., Miszczuk E., Bajguz A., Hayat S. (2022a). Specific roles of lipoxygenases in development and responses to stress in plants . Plants 11 :979. 10.3390/plants11070979 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Singh S. K., Wu X., Shao C., Zhang H. (2022b). Microbial enhancement of plant nutrient acquisition . Stress Biol . 2 :3. 10.1007/s44154-021-00027-w [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Soumare A., Diedhiou A. G., Thuita M., Hafidi M., Ouhdouch Y., Gopalakrishnan S., et al.. (2020). Exploiting biological nitrogen fixation: a route towards a sustainable agriculture . Plants 9 :1011. 10.3390/plants9081011 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Srivastava S., Verma P. C., Chaudhry V., Singh N., Abhilash P. C., Kumar K. V., et al.. (2013). Influence of inoculation of arsenic-resistant Staphylococcus arlettae on growth and arsenic uptake in Brassica juncea (L.) Czern. Var. R-46 . J. Hazard Mater . 15 , 1039–1047. 10.1016/j.jhazmat.2012.08.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sujayanand G. K., Akram M., Konda A., Nigam A., Bhat S., Dubey J., et al.. (2021). Distribution and toxicity of Bacillus thuringiensis (Berliner) strains from different crop rhizosphere in Indo-Gangetic plains against polyphagous lepidopteran pests . Int. J. Trop. Insect Sci . 41 , 2713–2731. 10.1007/s42690-021-00451-5 [ CrossRef ] [ Google Scholar ]
- Sultana S., Paul S. C., Parveen S., Alam S., Rahman N., Jannat B., et al.. (2020). Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress . Can. J. Microbiol . 66 , 144–160. 10.1139/cjm-2019-0323 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sumbul A., Ansari R. A., Rizvi R., Mahmood I. (2020). Azotobacter : A potential bio-fertilizer for soil and plant health management . Saudi J. Biol. Sci . 27 , 3634–3640. 10.1016/j.sjbs.2020.08.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sundaramoorthy S., Balabaskar P. (2013). Evaluation of combined efficacy of Pseudomonas fluorescens and Bacillus subtilis in managing tomato wilt caused by Fusarium oxysporum f. sp. lycopersici (FOL) . Plant Pathol. J. 12 , 154–161. 10.3923/ppj.2013.154.161 [ CrossRef ] [ Google Scholar ]
- Suzaki T., Takeda N., Nishida H., Hoshino M., Ito M., Misawa F., et al.. (2019). Lack of symbiont accommodation controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus . PLoS Genet . 15, e1007865. 10.1371/journal.pgen.1007865 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taha R. S., Seleiman M. F., Shami A., Alhammad B. A., Mahdi A. H. A. (2021). Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed soil . Plants. 10, 1040. 10.3390/plants10061040 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tufail M. A., Touceda-González M., Pertot I., Ehlers R. U. (2021). Gluconacetobacter diazotrophicus Pal5 enhances plant robustness status under the combination of moderate drought and low nitrogen stress in Zea mays L . Microorganisms 9 :870. 10.3390/microorganisms9040870 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Umair Hassan M., Aamer M., Umer Chattha M., Haiying T., Shahzad B., Barbanti L., et al.. (2020). The critical role of zinc in plants facing the drought stress . Agriculture 10 :396. 10.3390/agriculture10090396 [ CrossRef ] [ Google Scholar ]
- Upadhyay S. K., Saxena A. K., Singh J. S., Singh D. P. (2019). Impact of native ST-PGPR ( Bacillus pumilus ; EU927414) on PGP traits, antioxidants activities, wheat plant growth and yield under salinity . Clim. Change Environ. Sustain . 7 , 157–168. 10.5958/2320-642X.2019.00021.8 [ CrossRef ] [ Google Scholar ]
- Usman M., Balsalobre-Lorente D., Jahanger A., Ahmad P. (2022). Pollution concern during globalization mode in financially resource-rich countries: Do financial development, natural resources, and renewable energy consumption matter? Renewable Ener. 183 , 90–102. 10.1016/j.renene.2021.10.067 [ CrossRef ] [ Google Scholar ]
- Vandana U. K., Rajkumari J., Singha L. P., Satish L., Alavilli H., Sudheer P. D. V. N., et al.. (2021). The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion . Biology 10 :101. 10.3390/biology10020101 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Varga T., Hixson K. K., Ahkami A. H., Sher A. W., Barnes M. E., Chu R. K., et al.. (2020). Endophyte-promoted phosphorus solubilization in populus . Front. Plant Sci . 11, 567918. 10.3389/fpls.2020.567918 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vishwakarma K., Kumar N., Shandilya C., Mohapatra S., Bhayana S., Varma A. (2020). Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review . Front. Microbiol. 11 , 560406. 10.3389/fmicb.2020.560406 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang H., Wang Y., Jiang D., Xiang Z., Wang S., Kang C., et al.. (2022a). Soil microbe inoculation alters the bacterial communities and promotes root growth of Atractylodes lancea under heat stress. Plant Soil . 10.1007/s11104-022-05369-6 [ CrossRef ] [ Google Scholar ]
- Wang R., Lin J.-Q., Liu X.-M., Pang X., Zhang C.-J., Yang C.-L., et al.. (2019). Sulfur oxidation in the acidophilic autotrophic Acidithiobacillus spp . Front. Microbiol . 9, 3290. 10.3389/fmicb.2018.03290 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang X., Cai D., Ji M., Chen Z., Yao L., Han H. (2022b). Isolation of heavy metal-immobilizing and plant growth-promoting bacteria and their potential in reducing Cd and Pb uptake in water spinach . Sci. Total Environ . 819 :153242. 10.1016/j.scitotenv.2022.153242 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Y., Yang Z., Kong Y., Li X., Li W., Du H., et al.. (2020). GmPAP12 is required for nodule development and nitrogen fixation under phosphorus starvation in soybean . Front. Plant Sci . 11, 450. 10.3389/fpls.2020.00450 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wani P. A., Khan M. S. (2013). Nickel detoxification and plant growth promotion by multi metal resistant plant growth promoting Rhizobium species RL9 . Bull. Environ. Contam. Toxicol. 91 , 117–124. 10.1007/s00128-013-1002-y [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Waqas M., Khan A. L., Kamran M., Hamayun M., Kang S. M., Kim Y. H., et al.. (2012). Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress . Molecules 17 , 10754–10773. 10.3390/molecules170910754 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Win T. T., Bo B., Malec P., Khan S., Fu P. (2021). Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens . J. Plant Pathol . 103 , 549–561. 10.1007/s42161-021-00780-x [ CrossRef ] [ Google Scholar ]
- Wu W., Du K., Kang X., Wei H. (2021a). The diverse roles of cytokinins in regulating leaf development . Hortic Res . 8 :118. 10.1038/s41438-021-00558-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu Y., Huang W., Tian Q., Liu J., Xia X., Yang X., et al.. (2021b). Comparative transcriptomic analysis reveals the cold acclimation during chilling stress in sensitive and resistant passion fruit ( Passiflora edulis ) cultivars . Peer J. 9 :e10977. 10.7717/peerj.10977 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xia Y., Liu J., Chen C., Mo X., Tan Q., He Y., et al.. (2022). The multifunctions and future prospects of endophytes and their metabolites in plant disease management . Microorganisms 10 :1072. 10.3390/microorganisms10051072 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yadav A. N., Verma P., Kumar S., Kumar V., Kumar M., Kumari Sugitha T. C. (2018). Actinobacteria From Rhizosphere in New and Future Developments in Microbial Biotechnology and Bioengineering . 10.1016/B978-0-444-63994-3.00002-3 [ CrossRef ] [ Google Scholar ]
- Yang Y., Chen Y., Cai J., Liu X., Huang G. (2021). Antifungal activity of volatile compounds generated by endophytic fungis Sarocladium brachiariae HND5 against Fusarium oxysporum f. sp. cubense . PLoS ONE 16 , e0260747. 10.1371/journal.pone.0260747 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yasmin H., Naeem S., Bakhtawar M., Jabeen Z., Nosheen A., Naz R. (2020). Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean ( Glycine max L.) against salinity stress . PLoS ONE . 15, e0231348. 10.1371/journal.pone.0231348 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yasuda M., Dastogeer K. M. G., Sarkodee-Addo E., Tokiwa C., Isawa T., Shinozaki S., et al.. (2022). Impact of Azospirillum sp. B510 on the rhizosphere microbiome of rice under field conditions . Agronomy 12 :1367. 10.3390/agronomy12061367 [ CrossRef ] [ Google Scholar ]
- You J. M., Xiong K., Mu S., Guo J., Guo X. L., Duan Y. Y., et al.. (2018). Identification of endophytic bacteria Bzjn1 and research on biological control of root rot of Atractylodes Macrocephala . Zhongguo Zhong Yao Za Zhi . 3 , 478–483. 10.19540/j.cnki.cjcmm.20180105.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yu C., Fan L., Gao J., Wang M., Wu Q., Tang J., et al.. (2015). The platelet-activating factor acetyl hydrolase gene derived from Trichoderma harzianum induces maize resistance to Curvularia lunata through the jasmonic acid signaling pathway . J. Environ. Sci. Health B. 50 , 708–717. 10.1080/03601234.2015.1048104 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yu Y., Gui Y., Li Z., Jiang C., Guo J., Niu D. (2022). Induced systemic resistance for improving plant immunity by beneficial microbes . Plants 11 :386. 10.3390/plants11030386 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yu Z., Wang Z., Zhang Y., Wang Y., Liu Z. (2021). Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil . Microbiol. Res . 242 :e126596. 10.1016/j.micres.2020.126596 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zain M., Yasmin S., Hafeez F. Y. (2019). Isolation and characterization of plant growth promoting antagonistic bacteria from cotton and sugarcane plants for suppression of phytopathogenic Fusarium species . Iran. J. Biotechnol . 17 :e1974. 10.21859/ijb.1974 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang L., Xu M., Liu Y., Zhang F., Hodge A., Feng G. (2016). Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium . New Phytol. 210 , 1022–1032. 10.1111/nph.13838 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang T., Hu F., Ma L. (2019). Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth . Open. Life Sci . 14 , 246–254. 10.1515/biol-2019-0028 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang X., Liu Z., Wei G., Yang F., Liu X. (2018). In silico genome-wide analysis reveals the potential links between core genome of Acidithiobacillus thiooxidans and its autotrophic lifestyle . Front. Microbiol . 9, 1255. 10.3389/fmicb.2018.01255 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou C., Zhu L., Xie Y., Li F., Xiao X., Ma Z., et al.. (2017). Bacillus licheniformis SA03 confers increased saline–alkaline tolerance in Chrysanthemum plants by induction of abscisic acid accumulation . Front. Plant Sci . 8, 1143. 10.3389/fpls.2017.01143 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou X. R., Dai L., Xu G. F., Wang H. S. (2021). A strain of Phoma species improves drought tolerance of Pinus tabulaeformis . Sci. Rep . 11 :7637. 10.1038/s41598-021-87105-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhu L., Wang Y., Lu J., Liu S., Min Y., Liu X., et al.. (2021). Complete genome sequence of Bacillus badius NBPM-293, a plant growth-promoting strain isolated from rhizosphere soil . Am. Soc. Microbiol . 10 , e00977–e00921. 10.1128/MRA.00977-21 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zulfikar S. A., Sandhya V., Grover M., Linga V. R., Bandi V. (2011). Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress , J. Plant Inter. 6 , 239–246. 10.1080/17429145.2010.545147 [ CrossRef ] [ Google Scholar ]

IMAGES
COMMENTS
Research Paper. Biofertilizer for crop production and soil fertility. Accepted 30 th August, 2018. ABSTRACT. Modern agricultur e involves usage of pesticides and chemical fertilizers with an ...
Biofertilizers comprise of living or latent cells, which are applied either to soil, seed or seedlings for improving nutrients availability and uptake from soil (Fasusi et al., 2021). Use of biofertilizers has currently emerged as cost effective and ecofriendly alternative than chemical-based fertilizers. ... Busby P.E. Research priorities for ...
Interestingly, the scientific research papers present a very broad interpretation of this term, representing everything from green manures, through animal manures, to plant extracts (Vessey, 2003). The concept of biofertilizer has changed along with the state of knowledge about associations occurring between the soil microorganisms and plants.
Co -inoculant of microbial specie a llow wide range of biofertilizer. efficiency and reliability for the fixation of nitrog en, phosphate solubilization and siderophore production and. balanced ...
Biofertilizers are now an effective way to increase crop yield and soil health in organic farming. Because of their numerous advantages, such as their capacity for nitrogen fixation, solubilizing ...
2.1. Types of Biofertilizers. Biofertilizers are divided into groups based on their functions and mechanisms of action. The most commonly used biofertilizers are nitrogen-fixers (N-fixers), potassium solubilizers (K solubilizers), phosphorus solubilizers (P solubilizer), and plant growth-promoting rhizobacteria (PGPR) [].One gram of rich soil can contain up to 10 10 cfu bacteria, with a live ...
This review paper elucidates the recent updates of potential-biofertilizers in crop production due to an increasingly diverse biological activity for soil enrichment, and yields increased per unit area, including abiotic stress conditions. ... (2018) and Lamaoui et al. (2018), based on their research compiled that, several beneficial bacteria ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Research on biofertilizers ...
Biofertilizer is an eco-friendly alternative to chemical fertilizer for a sustainable agriculture. The microbial inoculants help in growth directly or indirectly by fixing nitrogen (N), by solubilization of phosphorus (P) and potassium (K), by secreting siderophores, antibiotics, enzymes, antifungal, and antibacterial substances, and by ...
Summary. Biofertilizer, the microbial inoculant is an eco-friendly alternative to chemical fertilizer, protects lithosphere, improves biosphere by protecting air, water, soil pollution, and eutrophication and enhances yields of agriculture produce. It helps to enrich the soil with macro- and micro-nutrient and also by releasing plant growth ...
The application of biofertilizers not only improves plant heath parameters but also enhances the crop productivity, soil health and protects from stress environment. More research has been focused on physiological and molecular aspects under different conditions with different crops using biofertilizers under field conditions. Author contributions
Agriculture is facing multiple challenges as it has to produce more food to feed the growing global population. There is still an adequate bridgeable gap in the production which is yet to be exploited. The potentially huge eco-clean market will contribute to the overall development in sustainable agriculture mainly for more efficient production and environment-friendly approaches ...
Extensive works on biofertilizers have revealed their capability of providing required nutrients to the crop in sufficient amounts that resulted in the enhancement of crop yield. ... Extensive research on developing efficient, temperature-tolerant strains is a crucial step to achieve prolonged success in this emerging field. ... Sci Res Essays ...
Biofertilizers comprise of living or latent cells, which are applied either to soil, seed or seedlings for improving nutrients availability and uptake from soil (Fasusi et al., 2021). Use of biofertilizers has currently emerged as cost effective and ecofriendly alternative than chemical-based fertilizers.
Potential use of soil microbes in sustainable crop production. The beneficial soil micro-organisms sustain crop production either as biofertilizers [] or symbiont [].They perform nutrient solubilisation which facilitate nutrient availability and thereby uptake [20, 21].It improves the plant growth by advancing the root architecture [].Their activity provides several useful traits to plants ...
The important contribution of this paper is two-fold: 1. the paper helps to identify the global trends in research on biofertilizer which gives important insights into the thrust areas and future direction on which the research would focus on; 2. the paper will be useful for all the early-stage researchers by highlighting the research areas and ...
The network cooccurrence analysis suggested that the biofertilizers research can be separated into three stages. The first stage (1980-2005) focused on nitrogen fixation. ... The Indian Council of Agricultural Research published the most papers (595), followed by the Egyptian Knowledge Bank (446) and the Nanjing Agricultural University from ...
Biofertilizers are microorganisms containing formulations used to supply nutrients to the plants in an eco-friendly manner. N 2 fixers, phosphate solubilizers, phosphate mobilizers, and plant growth promoters are the types of biofertilizers widely used by farmers for the enhancement of soil fertility and agricultural production (Baby 2002; Jayaraj et al. 2004).
Biofertilizers are classified as N-fixing, phosphate solubilizing, phosphate mobilizing, potassium solubilizing, potassium mobilizing, and sulfur oxidizing. The first commercial outbreak of ...
Figure 2.Representation of the present and future of PGPR-based biofertilizer development. (A) Biofertilizers based on a single bacterial strain.(B) Biofertilizers containing multiple strains (two or more) which are selected by considering their ability in enhancing plant uptake of soil nutrients.(C) Biofertilizers produced by farmers in their own farms by using rudimentary bio-factories ...
words, biofertilizers are natural fertilizes which are livin g microbial inoculants o f bacteria, algae, fungi. alone or in combination and they augment the availability of nutrients to the plants ...
The microbiome: potential significance of beneficial microbes in sustainable agriculture. The rhizosphere, which is the narrow zone of soil surrounding plant roots, can comprise up to 10 11 microbial cells per gram of root [] and above 30,000 prokaryotic species [] that in general, improve plant productivity [].The collective genome of rhizosphere microbial community enveloping plant roots is ...
Reducing fertilizer use and increasing its efficiency will improve the quality of farmland and resource conservation. These are necessary steps to achieving green development in agriculture. Nevertheless, fertilizer-reduction and efficiency-increasing technologies (FREITs) remain limited. To improve the situation, 538 farmers in Jiangsu and Hubei Provinces were surveyed with the goal of ...
Abstract. This paper provides a mini review of liquid biofertilizers, which have been proven to perform better than the other forms in lasting for longer periods of time, improving crop quality, and requiring less amounts for application. The production of liquid biofertilizers, types of liquid inoculants, and their effect on plant growth are ...
Biofertilizers. In India, biofertilizer refers to the use of microorganisms to meet nutritional needs, whereas in other countries, the term microbial bioinoculant is used (Mitter et al., 2021).Biofertilizers are bio-based organic fertilizers that either could be from plant or animal sources or from living or dormant microbial cells that have the potential to improve the bioavailability and ...