 Home > AACR Cancer Progress Report > AACR Cancer Progress Report 2023: Contents > Advancing the Frontiers of Cancer Science and Medicine - Advancing the Frontiers of Cancer Science and Medicine
In this section, you will learn: - Researchers are harnessing the knowledge of the cellular and molecular underpinnings of cancer initiation and progression to develop safer and more effective treatments for cancer.
- Advances in novel and innovative approaches to surgery, radiotherapy, chemotherapy, molecularly targeted therapy, and immunotherapy—the five pillars of cancer treatment—are saving and improving lives.
- From August 1, 2022, to July 31, 2023, FDA has approved 14 new anticancer therapeutics and two new imaging agents and has expanded the use of 12 previously approved anticancer therapeutics to treat additional cancer types.
- Included in the FDA approvals are the first antibody–drug conjugate for the treatment of ovarian cancer, several new molecularly targeted therapeutics and immunotherapeutics to treat rare cancers including blood cancers, two new immune checkpoint inhibitors, and a first of a kind gene therapy to treat bladder cancer.
- While these exciting new advances have the potential to transform patient care, much work is needed to ensure equitable access to these treatments for all populations.
Clinical ResearchProgress across the clinical cancer care continuum, advances in cancer treatment with surgery, improvements in radiation-based approaches to cancer care, advances in treatment with cytotoxic chemotherapy, advances in treatment with molecularly targeted therapy. 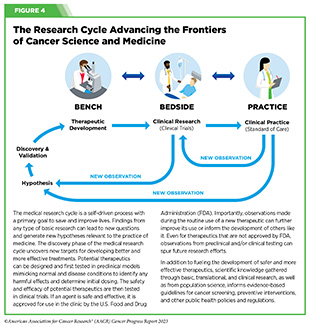 Progress across the continuum of cancer research and patient care improves survival and quality of life for people around the world. In the United States, the annual decline in overall cancer death rate among men, women, children, and adolescents and young adults has accelerated over the past two decades (see Cancer in 2023 ) ( 325 ) Cronin KA, et al. (2022) Cancer, 128: 4251. [LINK NOT AVAILABLE] . This progress is driven by the dedicated efforts of individuals working throughout the cycle of medical research (see Figure 4 and Sidebar 25 ). The rapid pace of progress against cancer is attributable in part to the new and effective treatments that are available today, thanks to the discoveries made through decades of research in basic and translational sciences. These discoveries have deepened our understanding of the cellular and molecular underpinnings of cancer initiation and progression and led to the identification of a range of molecular targets that drive cancer (see Understanding the Path to Cancer Development ). After a potential therapeutic target is identified, it takes many more years of preclinical research before a candidate therapeutic is developed and ready for testing in clinical trials (see Sidebar 26 ). 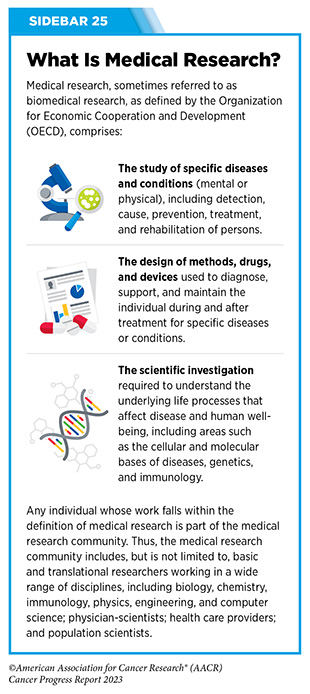 Clinical trials evaluate the safety and efficacy of candidate agents before a preventive intervention or therapeutic can be approved by FDA and used as part of patient care. All clinical trials are critically reviewed and approved by institutional review boards before they can begin and are monitored throughout their duration. There are several types of cancer clinical trials, including prevention trials, screening trials, treatment trials, and supportive or palliative care trials, each designed to answer different research questions (see Sidebar 27 ). Clinical studies in which participants are randomly assigned to receive experimental treatment or standard of care treatment are called randomized clinical trials and are considered the most rigorous. Clinical trials that test candidate therapeutics for patients with cancer have traditionally been done in three successive phases (see Figure 13 ). Observations made during the real-world use of a drug after it is approved by FDA can also be utilized to further enhance the use of that drug. The multiphase clinical testing process requires many patients, takes years to complete, and has a high rate of failure, making it extremely costly and one of the main barriers to rapid translation of scientific knowledge into clinical advances ( 326 ) Arfe A, et al. (2023) J Natl Cancer Inst, 115:917 [LINK NOT AVAILABLE] ( 327 ) Shadbolt C, et al. (2023) JAMA Netw Open, 6: e2250996. [LINK NOT AVAILABLE] . Identifying and implementing more efficient clinical development strategies are an area of extensive investigation for all stakeholders in medical research (see Sidebar 1 ). 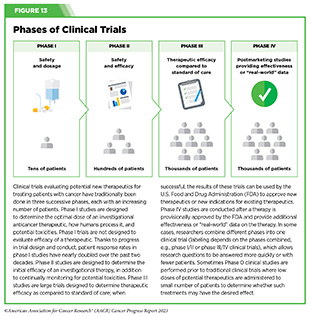 Advances in the understanding of cancer biology have enabled researchers from academia and the pharmaceutical industry to develop new approaches to designing and conducting clinical trials. Among the new concepts and designs for clinical trials that have emerged in recent years are the adaptive, main protocol, and platform trials designs ( 329 ) Li A, et al. (2020) Cancer, 126: 4838. [LINK NOT AVAILABLE] . These designs allow researchers to modify aspects of the trial design, if needed, by leveraging the accumulating data, thereby increasing the efficiency of the clinical research process. Main protocol, also known as master protocol design, and platform design streamline clinical development and allow the evaluation of multiple new agents by matching the right therapeutics with the right patients earlier, reducing the number of patients who need to be enrolled in the trial, and decreasing the length of time it takes for a new anticancer therapeutic to be tested and made available to patients. 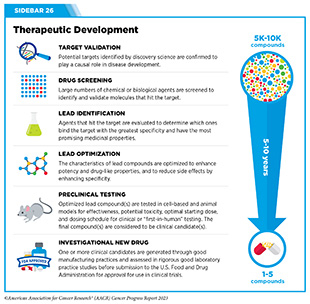 Master protocol can answer multiple clinical questions within a single trial ( 329 ) Li A, et al. (2020) Cancer, 126: 4838. [LINK NOT AVAILABLE] . The emergence of this clinical trial design has largely been driven by accumulating knowledge of the genetic mutations that underpin cancer initiation and growth. As one example, I-SPY 2 is one of the longest-running clinical trials that uses a master protocol which provides the regulatory framework to study multiple treatments for breast cancer within a single study ( 330 ) Quantum Leap Healthcare Collaborative. The I-SPY Trials. Accessed: July 31, 2023. . The platform design of the I-SPY 2 trial allows new treatments to enter and leave the study with a greater efficiency than traditional clinical trials. The study has led to the FDA approval of several breast cancer treatments, including the molecularly targeted therapeutic neratinib (Nerlynx) ( 331 ) Wang H, et al. (2019) Curr Breast Cancer Rep, 11: 303. [LINK NOT AVAILABLE] .  Basket trials are another example of genetic mutation–based master protocol design in clinical trials (see Figure 14 ). These trials allow researchers to test one anticancer therapeutic on a group of patients who all have the same type of genetic mutation, regardless of the anatomic site of the original cancer. As one example, the combination of molecularly targeted therapeutics dabrafenib and trametinib was shown to work against an array of cancer types characterized by a specific genetic feature, or biomarker, called the BRAF V600E mutation, in two recent basket trials including the NCI MATCH study (see Sidebar 9 ) ( 109 ) O’Dwyer PJ, et al. (2023) Nat Med, 29: 1349. [LINK NOT AVAILABLE] . Based on the data from these trials, the combination treatment received FDA approval in June 2022 and is now benefiting many patients with cancer ( 1 ) American Association for Cancer Research. AACR Cancer Progress Report 2022. Accessed: July 5, 2023. . Based on a recent analysis, the use of novel trial designs in clinical cancer research has more than tripled, worldwide, over the past decade ( 332 ) IQVIA. Global Oncology Trends 2023. Accessed: July 5, 2023. . 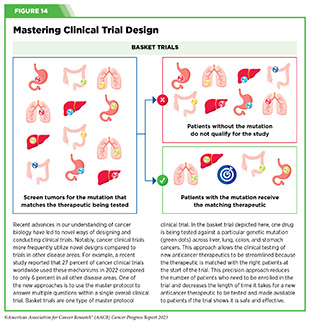 As our understanding of cancer biology continues to evolve and we uncover some of the most elusive questions in cancer medicine (see C ancer Development: Integrating Knowledge ) clinical trial designs will need to evolve as well. Additionally, the design and conduct of clinical cancer research need to keep pace with the new wave of technological advances. Novel designs that integrate emerging approaches such as comprehensive tumor profiling (e.g., of genome, transcriptome, proteome, microbiome, and metabolome, among others), artificial intelligence and machine learning, real-world evidence and data, and leverage inputs from patient advocacy communities and social media platforms will be pivotal to advancing the frontier of cancer clinical trials ( 333 ) Subbiah V (2023) Nat Med, 29: 49. [LINK NOT AVAILABLE] . 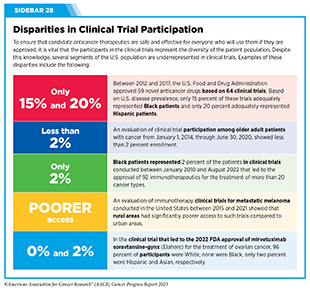 Two of the most pressing challenges that need to be overcome urgently are low participation in cancer clinical trials and a lack of sociodemographic diversity among those who do participate (see Sidebar 28 ). Low participation in clinical trials means that many trials fail to enroll enough participants to draw meaningful conclusions about the effectiveness of the anticancer therapeutic being tested. Lack of diversity in clinical studies means that the trial participant population does not match the actual demographics of the cancer burden under study ( 334 ) In: Bibbins-Domingo K, Helman A, editors. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Washington (DC)2022. [LINK NOT AVAILABLE] . Underrepresentation in clinical trials compromises the applicability of such research findings to the entire U.S. patient population. Understanding and eliminating barriers to clinical trial participation for all segments of the population is vital if we are to accelerate the pace of progress against cancer for everyone. Numerous studies have investigated the existing barriers that limit participation of racial and ethnic minorities and other medically underserved populations in cancer clinical trials. These studies have identified a range of factors such as lack of awareness of clinical trials, financial challenges, limited health literacy, inadequate or complete lack of insurance, medical distrust, implicit biases among health care providers, lack of trial availability, and narrow eligibility criteria, among others ( 13 ) American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023. . These barriers operate at individual, systemic, and societal levels ( 340 ) Kahn JM, et al. (2022) Cancer, 128: 216. [LINK NOT AVAILABLE] . 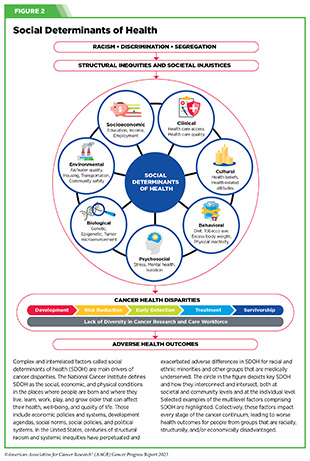 Increased knowledge of the barriers to clinical trial accrual is helping researchers, regulators, and policymakers design and implement evidence-based adaptations that can broaden participant access and promote accrual to clinical research. Such interventions focus on addressing social determinants of health (see Figure 2 ), and include decentralizing many of the trial activities to ease patient participation, expanding eligibility criteria, improving the efficiency of data collection, including patient reported outcomes (PRO), and enhancing community outreach and patient navigation efforts to raise awareness of trials. One critical area of focus for all stakeholders in medical research is fostering greater diversity, equity, and inclusion within the clinical research workforce so the workforce will resemble the patient populations it serves. U.S. lawmakers and FDA are working on legislation and guidelines intended to increase the diversity of clinical trial participants (see D iversifying and Decentralizing Trials ). These include a diversity action plan which would require researchers and funders of clinical trials to submit concrete goals and needed steps for enrolling specific demographic groups in pivotal studies of new drugs ( 341 ) U.S. Food and Drug Administration. Diversity Plans to Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry; Availability. Accessed: July 5, 2023. . COVID-19, despite its adverse effects on all aspects of cancer research and patient care, enabled researchers to decentralize clinical trial designs, so that lifesaving therapeutics could be brought quickly to as many patients as possible ( 9 ) American Association for Cancer Research. AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care. Accessed: June 30, 2022. . Adaptations implemented by NCI and FDA during the pandemic, including consenting patients remotely, permitting telehealth for routine clinical assessments (see Sidebar 29 ), delivering experimental drugs to patients, and allowing the use of local laboratory or imaging facilities accessible to patients have offered a blueprint of success to further revise and reform clinical trials and the drug approval process for the benefit of all patients with cancer. 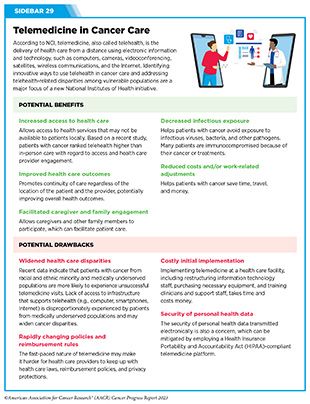 Research discoveries made as a result of innovative cancer science are continually being translated into new medical products for cancer prevention, detection, diagnosis, treatment, and survivorship. The approval of new medical products, including new anticancer treatments, is not the end of a linear research process. Rather, it is an integral part of the medical research cycle (see Figure 4 ) because observations made during the routine use of new medical products can be used to accelerate the pace at which similar products are developed and to stimulate the development of new, more effective products. New FDA-approved medical products are traditionally utilized alongside treatments already in use, including surgery, radiotherapy, and cytotoxic chemotherapy, which continue to be the mainstays of clinical cancer care (see Figure 15 ). Researchers are also evaluating new ways to refine the use of surgery, radiotherapy, and existing cytotoxic chemotherapeutics to improve survival and quality of life for patients. As one example, a recent clinical trial showed that for patients with early-stage prostate cancer, active monitoring of their disease is a safe alternative to receiving immediate surgery or radiotherapy ( 346 ) Hamdy FC, et al. (2023) N Engl J Med, 388: 1547. [LINK NOT AVAILABLE] . In most cases, prostate cancer grows slowly. Therefore, the study directly compared the long-term outcomes of the three approaches, prostate removal surgery, radiotherapy, or active monitoring and found that there was no difference in prostate cancer mortality at the 15-year follow-up between the three groups. These data provide hope for patients with prostate cancer who opt for active monitoring to avoid treatment-related adverse effects, such as sexual and incontinence problems. The following sections focus on the recent advances across the five pillars of cancer treatment including the 14 new anticancer therapeutics approved by the FDA in the 12 months spanning this report, August 1, 2022, to July 31, 2023 (see Table 3 and Supplemental Table 2 ). Also highlighted are the 12 previously approved anticancer therapeutics that received FDA approval for treating additional types of cancer in that period. 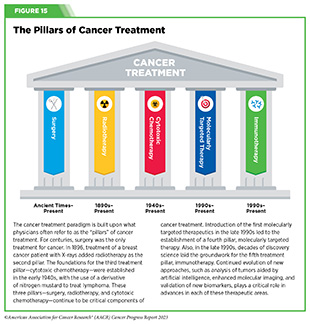 Not discussed are FDA approvals that expand the use of an anticancer therapeutic previously approved for a given cancer type to include treatment with that therapeutic at different timepoints during the course of clinical care or treatment of a different subtype of the same cancer. For example, the August 2022 FDA approval expanded the use of fam-trastuzumab-deruxtecan-nxki (Enhertu), for the treatment of patients with metastatic HER2-low breast cancer that is not removable by surgery. This expansion occurred nearly three years after the molecularly targeted therapeutic was first approved for treating metastatic HER2-positive breast cancer and was based on results from a phase III clinical trial. The study showed that patients with metastatic breast cancer who were treated with fam-trastuzumab-deruxtecan-nxki lived nearly twice as long without their cancer progressing and lived six months longer overall than those treated with standard chemotherapy ( 352 ) Modi S, et al. (2022) N Engl J Med, 387: 9. [LINK NOT AVAILABLE] . Fam-trastuzumab-deruxtecan-nxki is the first treatment approved for patients with HER2-low breast cancer subtype, a newly defined subset of HER2-negative breast cancer. 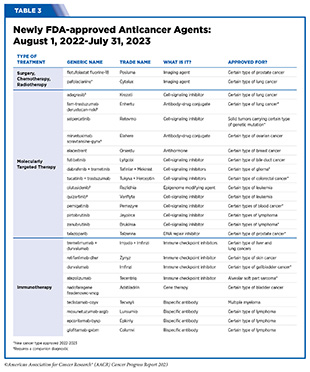 New medical products used across the continuum of clinical cancer care transform lives by improving survival and quality of life. However, not all patients receive the standard of care recommended for the type of cancer with which they have been diagnosed and the stage of cancer at the time of diagnosis (see Sidebar 30 ). Thus, it is imperative that all stakeholders committed to driving progress against cancer work together to address the challenge of disparities in cancer treatment because these can be associated with adverse differences in survival. Recent studies have shown that disparities in survival for prostate cancer or multiple myeloma between Black patients and White patients can be eliminated when both population groups have equivalent access to care and to standard treatments ( 13 ) American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023. . 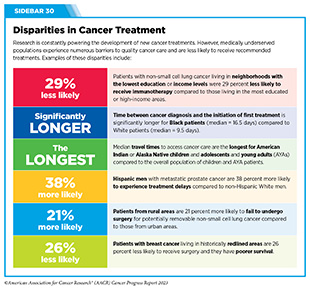 For many years, surgery was the only pillar of cancer treatment (see Figure 15 ). Today, it remains the foundation of curative treatment for many patients. Surgery is used in several ways during the care of a patient with cancer (see Sidebar 31 ). Sometimes, additional therapy is given before, after, or around the time of surgery based on specifics of a patient’s situation (see Sidebar 32 ). Researchers have found that this approach not only improves the surgeon’s ability to remove the tumor (for example by shrinking the tumor when given before the surgery), but also increases the patient’s overall survival and/or quality of life ( 359 ) Burotto M, et al. (2019) Semin Oncol, 46: 83. [LINK NOT AVAILABLE] . Improving Quality of Life After a Cancer SurgeryDespite the immense benefits of surgery for the treatment of cancer, complications are common and can negatively affect patient quality of life. Enhanced recovery after surgery (ERAS) programs are emerging as one approach to address this issue. These comprehensive programs focus on optimizing patient care before, during, and after surgery using strategies that ensure the patient is as physically and emotionally fit for surgery as possible; alleviate the stress of surgery; promote recovery; and reduce the time before patients with cancer can begin adjuvant treatment. Providing patients with an individualized plan that includes exercise, nutrition, stress reduction, and smoking cessation to optimize their physical fitness before surgery is one strategy included in some ERAS programs ( 360 ) Gustafsson UO, et al. (2019) World J Surg, 43: 659. [LINK NOT AVAILABLE] ( 361 ) Santa Mina D, et al. (2017) PM R, 9: S305. [LINK NOT AVAILABLE] . The components of ERAS programs can vary depending on the type of surgery being performed and the center at which the surgery is being performed, but overall, these programs have been promising. 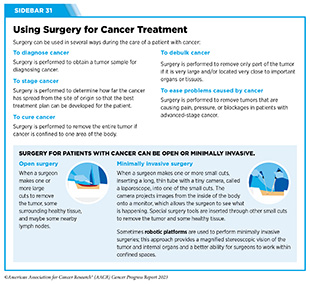 One study found that among patients undergoing surgery for tumors that have metastasized to the spine, those who participated in an ERAS program had reduced blood loss, shorter hospitalization, and significant reduction in opioid pain reliever utilization compared to those who did not participate ( 362 ) Chakravarthy VB, et al. (2022) Cancer, 128: 4109. [LINK NOT AVAILABLE] . Another study showed that among patients undergoing surgery for colorectal cancer, those who participated in an individualized plan that included exercise, nutritional intervention, and psychological support had fewer medical complications and better recovery postsurgery than those who did not participate in such programs ( 363 ) Molenaar CJL, et al. (2023) JAMA Surg, 158: 572. [LINK NOT AVAILABLE] . 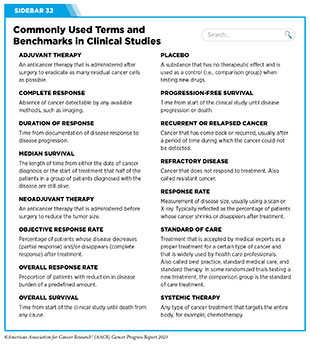 Other approaches to reducing the complications during and after surgery and improving quality of life postprocedure are to perform less extensive and minimally invasive surgeries, such as robotic surgeries or to identify a subset of patients who could skip surgery altogether. As one example, data from a recent clinical trial showed that for certain patients with early-stage non–small cell lung cancer (NSCLC), surgical removal of only part of the affected lobe of lung is an effective treatment option ( 364 ) Altorki N, et al. (2023) N Engl J Med, 388: 489. [LINK NOT AVAILABLE] . The study, which compared the outcomes of patients who had their entire lobes removed to those who had only the tumor-affected regions removed, showed that the 5-year overall survival was similar in the two groups. While the study participants represent only a select subgroup of patients with lung cancer, these data are important considering that removal of less lung tissue can preserve lung function, especially for older adults and those with compromised lung capacity, such as patients with a prior lung cancer. Studies have shown that less invasive surgeries may benefit patients since they can minimize postprocedural complications without compromising and sometimes improving long-term outcomes ( 365 ) Topal H, et al. (2022) JAMA Netw Open, 5: e2248147. [LINK NOT AVAILABLE] ( 366 ) Son SY, et al. (2022) JAMA Surg, 157: 879. [LINK NOT AVAILABLE] ( 367 ) Di Benedetto F, et al. (2023) JAMA Surg, 158: 46. [LINK NOT AVAILABLE] . As one example, in a recent clinical trial, patients with locally advanced stomach cancer who underwent a minimally invasive procedure had significantly lower long-term complications after surgery, but similar 5-year overall and relapse-free survival rates compared to those who had open surgeries ( 366 ) Son SY, et al. (2022) JAMA Surg, 157: 879. [LINK NOT AVAILABLE] . Additionally, two retrospective analyses showed improved disease-free and overall survival for patients with pancreatic cancer and reduced morbidity during surgery for patients with liver cancer who underwent minimally invasive surgeries compared to those who received open surgeries ( 365 ) Topal H, et al. (2022) JAMA Netw Open, 5: e2248147. [LINK NOT AVAILABLE] ( 367 ) Di Benedetto F, et al. (2023) JAMA Surg, 158: 46. [LINK NOT AVAILABLE] . Yet another report from an early-stage clinical trial showed that a selected subset of patients with breast cancer who responded remarkably well to neoadjuvant chemotherapy could potentially forgo surgery without risking tumor recurrence ( 368 ) Kuerer HM, et al. (2022) Lancet Oncol, 23: 1517. [LINK NOT AVAILABLE] . Recent studies have also identified subsets of patients who could skip surgery altogether without compromising outcomes. In a clinical trial, women who had early-stage ( 368 ) Kuerer HM, et al. (2022) Lancet Oncol, 23: 1517. [LINK NOT AVAILABLE] reast cancer with defined clinical characteristics had equally good overall survival whether they received radiotherapy delivered to the lymph nodes in their underarms (axillary radiotherapy), or an invasive surgical procedure to remove these lymph nodes (axillary lymph node dissection) ( 369 ) Bartels SAL, et al. (2023) J Clin Oncol, 41: 2159. [LINK NOT AVAILABLE] . Notably, axillary lymph node dissection is associated with a significantly higher rate of morbidity, particularly lymphedema, which causes swelling in the arms that can cause pain and problems in functioning. These risks are drastically reduced if radiotherapy is given instead and suggests radiation rather than surgery should be the preferred approach in these patients. While less invasive approaches to surgery such as those described above are promising, before they can become standard of care, it is vital that they are shown in rigorous, well-designed, larger clinical trials to have no adverse effect on long-term patient survival. Visualizing Lung Cancers More Precisely During SurgeryLung cancer is the leading cause of cancer deaths in the United States with an estimated 127,070 deaths predicted in 2023 ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . While surgery is the standard treatment and provides the best chance to cure early-stage lung cancer, up to 55 percent of people with lung cancer who undergo surgery with curative intent have a recurrence ( 370 ) Uramoto H, et al. (2014) Transl Lung Cancer Res, 3: 242. [LINK NOT AVAILABLE] . Therefore, it is vital that the entire tumor is removed during surgery. Surgeons rely on either imaging tumors before surgery, visually inspecting tumors under normal white light during surgery, or examining tumors by touch to identify cancerous tissue. Unfortunately, some lung lesions can be difficult to visualize, particularly if they are small, beneath the surface of the lung, or a type of lesion characterized by increased opacity of the lung called ground glass opacity, which is being increasingly diagnosed as the rates of lung cancer screenings rise ( 371 ) Migliore M, et al. (2018) Ann Transl Med, 6: 90. [LINK NOT AVAILABLE] ( 372 ) Huang C, et al. (2019) J Cancer, 10: 6888. [LINK NOT AVAILABLE] . In December 2022, the FDA approved pafolacianine (Cytalux), a folate receptor–targeted fluorescent agent, as the first and only targeted molecular imaging agent that illuminates lung cancers and enhances surgeons’ ability to see cancer in real time as they operate. Molecular imaging using pafolacianine during surgery enables the detection of lung lesions that may have otherwise been missed. Pafolacianine was previously approved to assist surgeons in visualizing hard to detect lesions in adult patients with ovarian cancer during surgery ( 1 ) American Association for Cancer Research. AACR Cancer Progress Report 2022. Accessed: July 5, 2023. . Pafolacianine binds to folate receptors, a protein that is commonly found on the surface of many cancers and illuminates tumor cells under near-infrared light. The agent is administered via intravenous infusion within 24 hours before surgery and assists surgeons in visually identifying additional malignant tissue to be removed during the procedure. The approval in lung cancer was based on a clinical trial that evaluated the utility of pafolacianine in visualizing tumors in the lungs that may otherwise be undetected with conventional visualization under white light ( 373 ) Sarkaria IS, et al. (2023) J Thorac Cardiovasc Surg. [LINK NOT AVAILABLE] . Molecular imaging using pafolacianine during surgery identified in 19 percent of patients primary lung nodules that surgeons could not find using white light and palpation; additionally, pafolacianine revealed in eight percent of patients additional lesions that were completely missed using white light. The expanded approval of pafolacianine represents a significant advancement in the treatment of lung cancer by enhancing detection of lung tumors during surgery, improving the ability to remove them completely, and reducing the probability of leaving behind cancerous tissue. Radiotherapy is the use of high-energy rays (e.g., gamma rays and X-rays) or particles (e.g., electrons, protons, and carbon nuclei) to control or eradicate cancer. Discovery of X-rays in 1895 allowed visualization of internal organs at low doses, and the effective use of X-rays at high doses to treat a breast cancer patient a year later established radiotherapy as the second pillar of cancer treatment (see Figure 15 ). Radiotherapy plays a central role in the management of cancer and works primarily by damaging DNA, leading to cancer cell death. The use of radiotherapy in treatment and management of cancer continues to increase, as indicated by a 16.4 percent increase in radiation facilities across the United States between 2005 and 2020 ( 374 ) Maroongroge S, et al. (2022) Int J Radiat Oncol Biol Phys, 112: 600. [LINK NOT AVAILABLE] .  There are many types and uses of radiotherapy (see Sidebar 33 ). However, it is important to note that radiotherapy may also have harmful side effects, partly because of the radiation-induced damage to healthy cells surrounding the tumor tissue ( 375 ) Wang K, et al. (2021) CA Cancer J Clin, 71: 437. [LINK NOT AVAILABLE] . Researchers are continuously working on making radiotherapy safer and more effective and identifying when radiotherapy can be avoided without affecting the chances of survival for patients. As one example, a recent clinical trial showed that older adult patients with small, early-stage breast cancer may forgo radiation after breast conserving surgery without compromising their overall survival ( 376 ) Kunkler IH, et al. (2023) N Engl J Med, 388: 585. [LINK NOT AVAILABLE] . Traditionally, in these patients, surgery has been followed with radiotherapy to reduce the risk of cancer recurrence. However, radiotherapy can lead to a range of potential side effects including pain, minor risks of organ damage and secondary cancer, as well as time and financial losses. Adverse effects are especially challenging for older adults, many of whom have other comorbidities. The new evidence provides these patients with the option for a less aggressive course of action. Another clinical trial showed that radiation therapy before initial surgery may not be needed for patients with locally advanced rectal cancer that has spread locally within the rectum but not to other organs ( 377 ) Schrag D, et al. (2023) N Engl J Med, 389: 322. [LINK NOT AVAILABLE] . Traditionally, these patients receive radiation combined with chemotherapy, also known as chemoradiotherapy, before surgical removal of their tumors. Chemoradiotherapy shrinks the tumor making it easier to remove and helping to prevent recurrence. Data from the recent clinical trial showed that chemotherapy alone before surgery was just as effective as chemoradiotherapy at keeping the cancer at bay ( 377 ) Schrag D, et al. (2023) N Engl J Med, 389: 322. [LINK NOT AVAILABLE] . Researchers are also designing novel radiotherapeutics, to be used alone or in combination with other treatments, to target more cancer types and benefit more patients. Additionally, technological innovations, such as the development of advanced imaging and sophisticated computer analytic programs assisted by AI, are helping optimize the delivery of the radiation to the tumor while minimizing exposure to normal tissues ( 378 ) Santoro M, et al. (2022) Applied Sciences, 12: 3223. [LINK NOT AVAILABLE] . As one example, Magnetic Resonance Imaging (MRI)-guided radiotherapy (MRgRT) is a novel technology with the potential to transform radiotherapy for many patients including those with prostate cancer ( 379 ) Ng J, et al. (2023) Front Oncol, 13: 1117874. [LINK NOT AVAILABLE] . MRgRT provides the ability to image tumors and internal organs with MRI and adapt the radiotherapy plan in real-time while the patient is undergoing the procedure. Unlike traditional radiotherapy, MRgRT allows monitoring of changes in tumor size and positional changes of internal organs during each treatment to achieve a more accurate delivery of the radiation dose. This is particularly critical for rapidly changing tumors and body regions, such as the prostate, where there could be dramatic changes in organ position during each treatment. Imaging Prostate Cancer More ClearlyProstate cancer is the most common type of cancer in men in the United States. In 2023, an estimated 288,300 new cases will be diagnosed and 34,700 men will die from the disease. Prostate cancer that is confined to the prostate is usually treated with surgery or radiation therapy. Unfortunately, many patients with primary prostate cancer have detectable metastases in their pelvic lymph nodes, which are correlated with a risk for cancer recurrence. Surgical procedures known as pelvic lymph node dissection or pelvic lymphadenectomy are used to detect pelvic node lesions, but their use is imprecise and limited to a planned surgical area. An ideal detection method for metastatic prostate cancer would locate tumors in pelvic nodes as well as more distant sites. The more precise a patient’s diagnosis, the easier it is for a health care provider to tailor the treatment to ensure that it is as effective and safe as possible. Notably, despite surgery or radiotherapy many patients with prostate cancer have local or distal recurrences within 10 years. Among the tools physicians use to make cancer diagnoses is positron emission tomography–computed tomography (PET–CT or PET), a form of imaging that can help physicians precisely locate the position of a patient’s cancer within the body and determine the extent to which the cancer may have spread. Before a PET scan, patients are injected with a radioactive imaging agent. The PET scan detects cancer by identifying where in the body the radioactive agent accumulates. In May 2023, FDA approved flotufolastat fluorine-18 (Posluma) for PET imaging of PSMA-positive lesions in patients with prostate cancer with suspected metastasis or with suspected recurrence based on elevated serum PSA level. PSA is a secreted biochemical marker that is used to screen individuals for prostate cancer and for predicted recurrence of the disease among patients who have received treatment. PSMA is a protein that is present in abundance on the surface of more than 90 percent of primary and metastatic prostate cancer cells. Flotufolastat F-18 contains a short peptide sequence that binds to PSMA and is internalized by cells that express PSMA. Flotufolastat F-18 also contains the radioisotope fluorine-18 which enables PET imaging of the prostate and other areas of the body where prostate cancer may have spread. Clinicians can use this information to decide which patient should receive treatment and spare others from unnecessary procedures. Findings from two clinical trials that FDA used to approve flotufolastat F-18 indicate that detection of prostate cancers using this approach may help physicians make the best treatment decisions for patients ( 380 ) The ASCO Post Staff. FDA Approves Flotufolastat Fluorine-18 Injection, First Radiohybrid PSMA-Targeted PET Imaging Agent for Prostate Cancer. Accessed: July 5, 2023. . One study demonstrated a higher specificity of flotufolastat F-18 for the detection of pelvic lymph node metastasis, compared to standard histopathology, in patients with PSMA-positive lesions. Flotufolastat F-18 provided valuable information that would likely result in changes in clinical management for these patients. In the second study, flotufolastat F-18 demonstrated high prostate cancer recurrence detection rates in patients who had suspected disease recurrence based on elevated PSA levels. Cytotoxic chemotherapy—use of chemicals to kill cancer cells—was first introduced as a pillar of cancer treatment in the early to mid-20th century ( 349 ) DeVita VT, Jr., et al. (2008) Cancer Res, 68: 8643. [LINK NOT AVAILABLE] . Chemotherapy remains a backbone of cancer treatment and its use is continually evolving to minimize potential harms to patients with cancer, while maximizing its benefits. As with surgery, chemotherapy is more commonly used to treat cancer in combination with one or more additional types of treatments. Furthermore, FDA continues to grant approvals to newer and more effective chemotherapeutics. FDA also routinely expands the use of previously approved chemotherapeutics for additional cancer types through review of new clinical trials as well as by monitoring of current real-world use of such agents. The FDA Project Renewal leverages expertise of clinical researchers to review existing published literature on drug utilization and maintain updated labeling of older, commonly prescribed anticancer therapeutics. As one example of this approach, in December 2022, FDA approved updated labeling for the chemotherapeutic capecitabine (Xeloda) which included new indications and dosing regimens for capecitabine tablets. Treatment with cytotoxic chemotherapeutics can have adverse effects on patients. These effects can occur during treatment and continue in the long term, or they can appear months or even years later. Health care providers and researchers are investigating different approaches to make chemotherapeutics safer for patients. Areas of ongoing investigation include designing modifiable chemotherapeutics, e.g., with “on” and “off ” switches, that are selectively delivered to tumors while sparing healthy tissue; evaluating less aggressive chemotherapy regimens which can allow patients the chance of an improved quality of life without compromising survival; and identifying biomarkers such as circulating tumor DNA to correctly predict which patients will or will not benefit from chemotherapy, among other approaches ( 381 ) East P, et al. (2022) Nat Commun, 13: 5632. [LINK NOT AVAILABLE] ( 382 ) Rais R, et al. (2022) Sci Adv, 8: eabq5925. [LINK NOT AVAILABLE] ( 383 ) Kotani D, et al. (2023) Nat Med, 29: 127. [LINK NOT AVAILABLE] . Notably, due to complex reasons, the United States is amid a significant chemotherapeutic shortage. The situation is affecting many patients and disrupting clinical research nationwide. It is imperative that all stakeholders in health care come together and identify ways to address these shortages at the earliest possible time (see Addressing Cancer Drug Shortages ). Remarkable advances in our understanding of the biology of cancer, including the identification of numerous genetic mutations that fuel tumor growth, set the stage for a new era of precision medicine, an era in which the standard of care for many patients is changing from a one-size-fits-all approach to one in which greater understanding of the individual patient and the characteristics of his or her cancer dictates the best treatment option for the patient (see Understanding the Path to Cancer Development ). Therapeutics directed to molecules influencing cancer cell multiplication and survival target tumor cells more precisely than cytotoxic chemotherapeutics, which generally target all rapidly dividing cells, and thereby limit damage to healthy tissues. The greater precision of these molecularly targeted therapeutics tends to make them more effective and less toxic than cytotoxic chemotherapeutics. As a result, they are not only saving the lives of patients with cancer, but also allowing these individuals to have a higher quality of life. Unfortunately, because of multilevel barriers to health care, there are disparities in the utilization of molecularly targeted treatments among patients from racial and ethnic minorities and other medically underserved populations ( 13 ) American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023. . It is vital that ongoing research and future public health policies are aimed to ensure equitable access to precision cancer medicine including tumor genetic testing and the receipt of molecularly targeted therapeutics for all patients. In the 12 months spanning August 1, 2022, to July 31, 2023, FDA approved seven new molecularly targeted anticancer therapeutics (see Table 3 ). During this period, FDA also approved nine previously approved molecularly targeted anticancer therapeutics for treating additional types of cancer. Expanding Treatment Options for Patients with Lung Cancer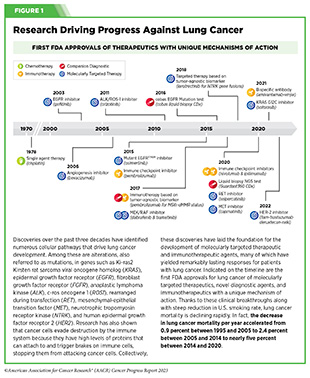 Lung cancer is the second most diagnosed cancer in both men and women and the most common cause of cancer death. More than 127,000 deaths are estimated to occur from the disease in 2023 in the United States ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Decades of basic and translational research have significantly increased our understanding of the genetic changes that drive lung cancer growth and have fueled the development of therapeutics that target these changes (see Figure 1 ) ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Two recent FDA decisions have the potential to drive more progress against lung cancer because they have provided new molecularly targeted therapeutic options for certain patients with the disease. About 81 percent of lung cancers diagnosed in the United States are classified as non–small cell lung cancers (NSCLC) and approximately 25 percent of patients with NSCLC carry mutations in the gene that is responsible for producing KRAS, an essential protein needed for growth and survival of normal lung cells, but which can contribute to cancer if mutated ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. ( 384 ) Janne PA, et al. (2022) N Engl J Med, 387: 120. [LINK NOT AVAILABLE] . Mutated KRAS represents one of the most common genetic alterations responsible for the development and progression of human cancers. Patients with NSCLC harboring KRAS mutations often develop resistance to standard treatments such as chemotherapy, radiation therapy, and immunotherapy, and only 25 percent of these patients live five years or more after diagnosis ( 438 ) Voruganti T, et al. (2023) JAMA Oncol, 9: 334. [LINK NOT AVAILABLE] . The most common KRAS mutation in patients with NSCLC is known as KRAS G12C, an alteration that is more frequently found in individuals who smoke currently or have smoked previously. The G12C mutation causes KRAS protein to prefer an “on” or “active” state, leading to uncontrollable cell growth that can form tumors. 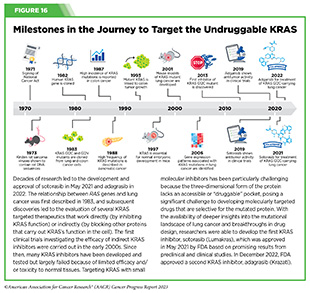 Historically, KRAS has been considered an undruggable target because of the difficulties in designing a therapeutic that could selectively bind and inhibit KRAS in cancers. Despite major breakthroughs in selective targeting of a range of other genetic drivers of NSCLC, no effective treatment options were available for patients with KRAS G12C until two years ago. Thanks to enhanced understanding of KRAS biology and unprecedented progress in structural biology and drug development, in May 2021, sotorasib (Lumakras) became the first ever molecularly targeted therapeutic approved by the FDA for the treatment of patients with NSCLC with the KRAS G12C mutation (see Figure 16 ) ( 4 ) American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2023. . 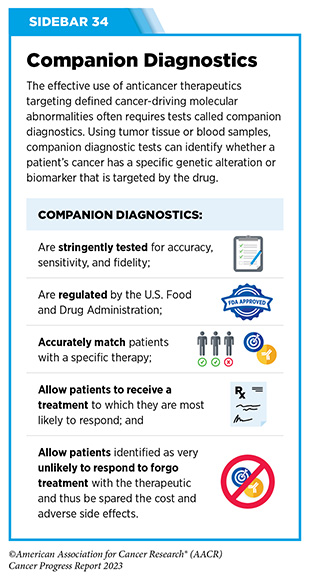 In December 2022, FDA approved a new molecularly targeted therapeutic, adagrasib (Krazati), for adult patients with locally advanced or metastatic NSCLC that has the KRAS G12C mutation, as determined by an FDA-approved test, and who have received at least one prior systemic treatment such as chemotherapy or immunotherapy. The FDA also approved companion diagnostics (see Sidebar 34 ), QIAGEN therascreen KRAS RGQ PCR kit (tumor tissue-based) and Agilent Resolution ctDx FIRST Assay (blood-based) to help identify patients with NSCLC carrying the KRAS G12C mutation. Both sotorasib and adagrasib bind to KRAS G12C protein and lock it in an inactive state thus blocking tumor growth. The FDA approval of adagrasib was granted after it was shown in a phase II clinical trial that 43 percent of the patients who received the targeted therapeutic had complete or partial tumor shrinkage and continued to respond for a median of 8.5 months without their cancer progressing ( 384 ) Janne PA, et al. (2022) N Engl J Med, 387: 120. [LINK NOT AVAILABLE] . A critical finding from the clinical trial was that adagrasib was able to reach the brains of patients with NSCLC and shrink tumors that had metastasized to the brain ( 385 ) Kotecha R, et al. (2022) N Engl J Med, 387: 1238. [LINK NOT AVAILABLE] . While additional research is needed to confirm therapeutic activity in the brain, these data are extremely encouraging considering recent findings that 27 to 42 percent of patients with NSCLC whose tumors harbor the KRAS G12C mutation may have central nervous system (CNS) metastases at diagnosis, and such metastases are associated with a poor prognosis ( 384 ) Janne PA, et al. (2022) N Engl J Med, 387: 120. [LINK NOT AVAILABLE] . Another major advance against lung cancer during the 12 months covered in this report is the FDA approval of fam-trastuzumab deruxtecan-nxki (Enhertu) for adult patients with surgically unremovable or metastatic NSCLC whose tumors have a type of mutation in the human epidermal growth factor receptor 2 (HER2) gene, called an activating mutation, as detected by an FDA-approved test, and who have received a prior systemic therapy. The FDA also approved Oncomine Dx Target Test (tissue-based) and Guardant360 CDx (blood-based) as companion diagnostics to test patients for activating HER2 mutations. HER2-mutated NSCLC, which accounts for three percent of all NSCLC cases, is associated with female sex, never-smoking history, and a poor prognosis. Furthermore, this type of NSCLC has a higher incidence of brain metastases than NSCLC without HER2 mutations or with other mutations ( 386 ) Li BT, et al. (2022) N Engl J Med, 386: 241. [LINK NOT AVAILABLE] ( 387 ) Riudavets M, et al. (2021) ESMO Open, 6: 100260. [LINK NOT AVAILABLE] . Fam-trastuzumab deruxtecan-nxki is a type of molecularly targeted therapeutic called an antibody–drug conjugate. It comprises a cytotoxic agent, deruxtecan, attached to the HER2-targeted antibody, trastuzumab (Herceptin), by a linker. When the antibody attaches to HER2 protein on the surface of lung cancer cells, the antibody–drug conjugate is internalized by the cells. This leads to deruxtecan being released from the linker and the antibody. Once free, the deruxtecan is toxic to the cancer cells, which ultimately die. The approval of fam-trastuzumab deruxtecan-nxki was primarily based on the results of a phase II clinical trial in which treatment with the HER2-targeted therapeutic shrank tumors in nearly 60 percent of the study participants ( 388 ) National Cancer Institute. Enhertu Approved for Lung Cancer. Accessed: July 5, 2023. . Among patients whose tumors shrank, the treatment kept their lung cancer at bay for nearly 9 months. While fam-trastuzumab deruxtecan-nxki has previously been approved for the treatment of patients with HER2-driven breast and gastric cancers (4,389), this was the first approval of a HER2-targeted therapeutic for NSCLC and provides new hope for patients with NSCLC who carry an activating HER2 mutation. Like most cancer treatments, fam-trastuzumab deruxtecan-nxki can have adverse effects, some of which can be severe. One of the most concerning and, in the case of NSCLC, life threatening, is interstitial lung disease which causes stiffness in the lungs, making it difficult to breathe and get oxygen to the bloodstream. Therefore, FDA approved fam-trastuzumab deruxtecan-nxki with a warning for interstitial lung disease and recommends that patients being treated with the molecularly targeted therapeutic be monitored for signs and symptoms of interstitial lung disease, including cough, dyspnea (difficult or labored breathing), fever and other new or worsening respiratory symptoms. If interstitial lung disease is suspected, further testing and intervention must be considered. While FDA approvals of sotorasib, adagrasib and fam-trastuzumab deruxtecan-nxki are significant advances for patients with NSCLC, all stakeholders in public health need to work together to ensure that every patient has access to and benefits from the latest developments in precision cancer medicine. Patients with lung cancer who receive molecularly targeted therapies have better survival compared to those who do not receive targeted therapies ( 390 ) Goulart BHL, et al. (2021) Clin Lung Cancer, 22: e723. [LINK NOT AVAILABLE] ( 391 ) Lemmon CA, et al. (2023) JCO Precis Oncol, 7: e2200294. [LINK NOT AVAILABLE] . Unfortunately, according to recent data, many patients with advanced NSCLC do not receive appropriate molecular tests or the appropriate molecularly targeted treatments due to gaps in the delivery of clinical care ( 392 ) Osazuwa-Peters OL, et al. (2023) Clin Lung Cancer, 24: 305. [LINK NOT AVAILABLE] ( 393 ) Sadik H, et al. (2022) JCO Precis Oncol, 6: e2200246. [LINK NOT AVAILABLE] . Targeting Cancers Based on a Common Genetic Feature, Not Tissue of Origin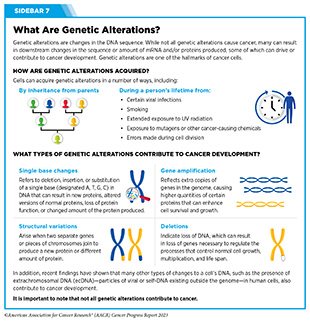 Chromosomal translocations that involve the RET gene and lead to the production of activating RET fusion proteins (see Sidebar 7 ) are rare alterations observed mostly in patients with certain types of thyroid cancer and lung cancer ( 394 ) Duke ES, et al. (2023) Clin Cancer Res: OF1. [LINK NOT AVAILABLE] . In 2020, FDA approved the RET-targeted therapeutic, selpercatinib (Retevmo), for treating patients with metastatic NSCLC and certain thyroid cancers that test positive for chromosomal translocations involving the RET gene ( 389 ) Sengupta R, et al. (2020) Clin Cancer Res, 26: 5055. [LINK NOT AVAILABLE] . In solid tumors other than lung cancer and thyroid cancer, RET gene fusions are rarer, observed in less than one percent of patients ( 394 ) Duke ES, et al. (2023) Clin Cancer Res: OF1. [LINK NOT AVAILABLE] . However, this is a distinct patient population since RET gene fusions are mutually exclusive of other genetic alterations and provide a unique opportunity for therapeutic intervention ( 394 ) Duke ES, et al. (2023) Clin Cancer Res: OF1. [LINK NOT AVAILABLE] . A recent decision from FDA offers a new treatment option for these patients who until this approval had no molecularly targeted therapeutics available for their cancer. In September 2022, FDA expanded the approval of selpercatinib for the treatment of adult patients with locally advanced or metastatic solid tumors with a RET gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options. The approval of selpercatinib was based on the results of a phase I/II basket clinical trial (see Figure 14 ) in which treatment with the RET-targeted therapeutic shrank tumors in nearly 44 percent of the study participants ( 395 ) Subbiah V, et al. (2022) Lancet Oncol, 23: 1261. [LINK NOT AVAILABLE] . Patients with a range of cancer types including pancreatic adenocarcinoma, colorectal, salivary gland, unknown primary, breast, soft tissue sarcoma, bronchial carcinoid, ovarian, small intestine, and cholangiocarcinoma responded to selpercatinib, emphasizing the importance of basket clinical trial designs in driving progress in precision medicine. Delivering a Cytotoxic Drug Precisely to Ovarian Cancer CellsIn 2023, an estimated 19,710 new cases of ovarian cancer will be diagnosed in the United States, and 13,270 women will die from the disease ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Many patients with ovarian cancer are diagnosed at an advanced stage. Patients with advanced disease are usually treated with platinum-based chemotherapeutics. Although most patients respond initially to platinum-based treatment, nearly 80 percent will experience relapse. Unfortunately, patients with recurrent ovarian cancer are resistant to platinum-based treatments and have a poor prognosis. Folate receptor alpha (FRα) is a cell surface protein that binds to and transports folate (also known as vitamin B9) into cells. Research has shown that FRα is expressed at much higher levels in advanced ovarian cancer cells, compared to healthy adult tissues ( 396 ) Dilawari A, et al. (2023) Clin Cancer Res, OF1. [LINK NOT AVAILABLE] . There is also emerging evidence, including clinical data, that elevated FRα expression may be associated with lack of response to standard chemotherapy in ovarian cancer ( 397 ) Matulonis UA, et al. (2023) J Clin Oncol, 41: 2436. [LINK NOT AVAILABLE] . These attributes make FRα a promising target for therapeutic intervention in ovarian cancer. 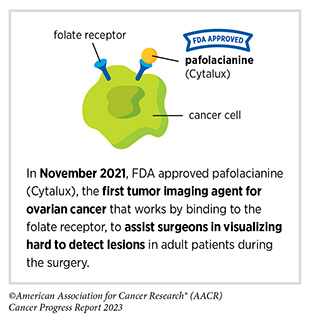 The molecularly targeted therapeutic mirvetuximab soravtansine-gynx (Elahere) targets FRα and, in November 2022, received FDA approval for adult patients with FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have received one to three prior systemic treatment regimens. FDA also approved the companion diagnostic VENTANA FOLR1 RxDx Assay to identify patients eligible for the therapy. Mirvetuximab soravtansine-gynx is an antibody–drug conjugate designed to deliver a cytotoxic drug to cells that express FRα. It is the first antibody–drug conjugate to be approved by FDA to treat platinum-resistant ovarian cancer and marks the first FDA approval since 2014 for platinum chemotherapy-resistant ovarian cancer, which is associated with a poor prognosis. The approval was based on results from a phase III clinical trial that enrolled 106 patients. Nearly 32 percent of patients responded to mirvetuximab soravtansine-gynx, with a median duration of response of 6.9 months ( 396 ) Dilawari A, et al. (2023) Clin Cancer Res, OF1. [LINK NOT AVAILABLE] ( 397 ) Matulonis UA, et al. (2023) J Clin Oncol, 41: 2436. [LINK NOT AVAILABLE] . The approval of mirvetuximab soravtansine-gynx is great news for patients, such as Jacly n (Jackie) VanRaaphorst . There is preliminary evidence that mirvetuximab soravtansine-gynx also improves overall survival for this FRα-positive ovarian cancer patient population ( 398 ) Angelergues A, et al. (2023) Journal of Clinical Oncology, 41: LBA5507. [LINK NOT AVAILABLE] . A common adverse effect of mirvetuximab soravtansine-gynx is ocular toxicity—changes that affect the structure or function of the eye. Therefore, FDA approved mirvetuximab soravtansine-gynx with a warning that patients being treated with the molecularly targeted therapeutic be monitored and treated for signs and symptoms of vision impairment and corneal disorders. Improving Outcomes for Patients with Metastatic Breast CancerDespite major advances in the treatment of breast cancer, it remains the second leading cause of cancer-related death for women in the United States ( ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Recent FDA decisions have the potential to power more progress against breast cancer because they have provided new therapeutic options for certain patients with the disease. For patients with breast cancer, one factor determining what treatment options should be considered is the presence or absence of three tumor biomarkers, estrogen and progesterone hormone receptors, which drive tumor growth upon engagement with their respective hormones, and the protein HER2. About 70 percent of breast cancers diagnosed in the United States are characterized as hormone receptor–positive and HER2-negative ( 3 ) Giaquinto AN, et al. (2022) CA Cancer J Clin, 72: 524. [LINK NOT AVAILABLE] . Potential treatment options for these patients include the combination of an antihormone therapeutic such as tamoxifen, which works by preventing the hormone estrogen from attaching to its receptor; or letrozole, which works by lowering the level of estrogen in the body; or fulvestrant, which works by destroying estrogen receptors (ER) with a cyclin-dependent kinase 4/6 inhibitor. Treatment with antihormone therapeutics is also called endocrine therapy. 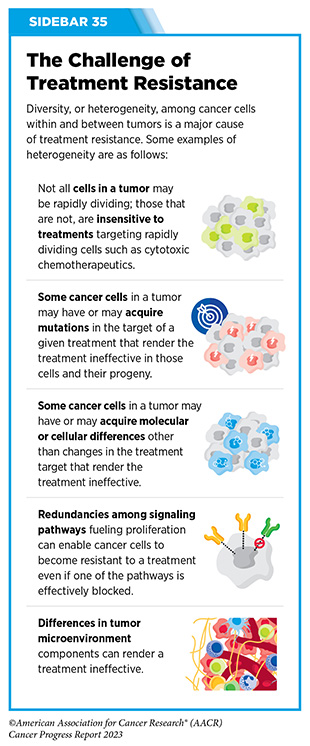 Unfortunately, most advanced, hormone receptor-positive breast cancers that initially respond to endocrine therapy eventually become treatment resistant (see Sidebar 35 ). Resistance to fulvestrant commonly develops due to mutations in ESR1, the gene that encodes the ER protein. Until recently, fulvestrant was the only available FDA-approved treatment that worked by destroying ER. Therefore, patients whose tumors become resistant to it were left with limited treatment options. The FDA approval of elacestrant (Orserdu) in January 2023 brings new hope to these patients. Elacestrant, which also works by destroying the ER, was approved for postmenopausal women or adult men with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy. Unlike fulvestrant, which is delivered through intramuscular injections, elacestrant is administered orally, making it more convenient for patients to receive the treatment. The approval was based on results from a phase III, randomized clinical trial showing that among patients with ESR1 mutations, those treated with elacestrant had a 45 percent lower risk of death or disease progression than those treated with other endocrine therapies ( 399 ) Bidard FC, et al. (2022) J Clin Oncol, 40: 3246. [LINK NOT AVAILABLE] . Personalizing Treatment for Patients with a Rare Solid TumorRare cancer is defined by the National Cancer Institute as cancer that occurs in fewer than 15 out of 100,000 people each year. Rare cancers can be challenging for researchers to study and for physicians to treat (see Sidebar 36 ). During the 12 months covered by this report, August 1, 2022, to July 31, 2023, the FDA approved molecularly targeted therapeutics and immunotherapeutics for treating several rare cancers, bringing the promise of precision medicine to patients, such as Isabella (Bella) Snow Fraser, p. 110, and Alexis Browning, p. 112, who often have few treatment options.  Bile duct cancer, also known as cholangiocarcinoma, is a rare but aggressive disease in which cancer arises from cells in the bile ducts. Cholangiocarcinoma is often diagnosed at an advanced stage. There are two types of bile duct cancer: intrahepatic, where cancer forms in the bile ducts inside the liver; and extrahepatic, where cancer forms in the bile ducts outside the liver. Less than 8,000 new cases of bile duct cancer are estimated to be diagnosed in the United States in 2023 and only a small number of bile duct cancers are intrahepatic ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . While rare, the incidence of intrahepatic cholangiocarcinoma is increasing worldwide ( 400 ) Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE] . Surgery is the main curative treatment option for patients with intrahepatic cholangiocarcinoma. However, up to two thirds of patients have disease recurrence and patients with intrahepatic cholangiocarcinoma have a 5-year overall survival rate of less than eight percent ( 400 ) Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE] . Alterations in fibroblast growth factor receptor 2 (FGFR2), a protein involved in many cellular processes including multiplication, migration, and survival, are associated with several cancers including bile duct cancers. Nearly 14 percent of patients with intrahepatic cholangiocarcinoma have fusions or rearrangements in the FGFR2 gene (see Sidebar 7 ) ( 400 ) Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE] . FDA had previously approved two molecularly targeted therapeutics, pemigatinib (Pemazyre) and infigratinib (Truseltiq), which block the function of FGFR2 proteins, for the treatment of patients with cholangiocarcinoma with confirmed FGFR2 fusions or rearrangements ( 389 ) Sengupta R, et al. (2020) Clin Cancer Res, 26: 5055. [LINK NOT AVAILABLE] ( 401 ) Sengupta R, et al. (2021) Clin Cancer Res, 27: 5757. [LINK NOT AVAILABLE] . While these agents are benefiting many patients with bile duct cancer, their efficacy has been somewhat limited due to the development of treatment resistance ( 400 ) Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE] . In September 2022, the FDA granted approval to a third FGFR2-targeted therapeutic, futibatinib (Lytgobi) for adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma that tests positive for FGFR2 fusions or other rearrangements. The approval was based on the results of a phase I/II clinical trial that showed that futibatinib shrank tumors in 42 percent of patients ( 400 ) Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE] . The median duration of response was 9.7 months. Futibatinib works differently than pemigatinib and infigratinib and preliminary data indicate that it may mitigate the challenge of treatment resistance since patients who had disease progression after prior FGFR-targeted therapy with other inhibitors maintained sustained clinical benefit with futibatinib ( 400 ) Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE] . Combining Molecularly Targeted Therapeutics to Block Tumor GrowthThe BRAF enzyme has a critical role in controlling cell growth. The BRAF gene is altered in approximately six percent of all human cancers, including melanoma and colorectal cancer ( 402 ) Dankner M, et al. (2018) Oncogene, 37: 3183. [LINK NOT AVAILABLE] . Most cancer-related changes in the BRAF gene cause the protein to continuously stay active, thus helping cancer cells grow faster than normal cells. One of the most common cancer-related changes is the BRAF gene is called the BRAF V600E mutation. Presence of the BRAF V600E mutation is associated with poor outcomes for patients with certain types of cancer. The first time FDA approved the use of two molecularly targeted therapeutics as a combination treatment for cancer was in January 2014 ( 67 ) Arteaga CL, et al. (2014) Clin Cancer Res, 20: S1. [LINK NOT AVAILABLE] . The approval was for the use of dabrafenib (Tafinlar) and trametinib (Mekinist) for treating patients with metastatic melanoma that tests positive for activating BRAF V600E and BRAF V600K mutations. The two therapeutics target different components of the BRAF signaling pathway. Dabrafenib targets altered BRAF proteins containing V600 mutations, while trametinib targets MEK1 and MEK2, which are two proteins that mediate the function of BRAF. By blocking both BRAF and MEK, the combination therapy can more completely and effectively shut down the signaling pathway. The combination was approved after it was shown to almost double the length of time before disease progression compared with dabrafenib alone ( 403 ) Flaherty KT, et al. (2012) N Engl J Med, 367: 1694. [LINK NOT AVAILABLE] . In March 2023, the same combination of molecularly targeted therapeutics was approved for pediatric patients one year of age and older with low-grade glioma with a BRAF V600E mutation who require systemic therapy. The FDA also approved new oral formulations of dabrafenib and trametinib for pediatric patients who are unable to swallow pills. Brain and other nervous system tumors are the second most diagnosed cancers in children. Low-grade glioma is the most common type of pediatric brain cancer. Research has demonstrated that BRAF signaling pathway activation is common in pediatric low-grade gliomas. Therefore, the March approval of dabrafenib and trametinib combination therapy brings hope to many parents and families whose children are diagnosed with the disease. FDA approved the combination therapy based on data from a clinical trial showing that a significantly higher percentage of patients who received dabrafenib and trametinib had their tumors shrink compared to those who received the standard of care chemotherapy (47 percent vs. 11 percent, respectively) ( 404 ) Hargrave DR, et al. (2022) J Clin Oncol, 40: 2009. [LINK NOT AVAILABLE] . Patients treated with dabrafenib and trametinib also had a 69 percent lower risk of disease progression, with a progression-free survival of 20 months, compared to seven months among patients receiving chemotherapy ( 404 ) Hargrave DR, et al. (2022) J Clin Oncol, 40: 2009. [LINK NOT AVAILABLE] . The FDA approval of a second combination therapy during the 12 months covered in the report provides a new and first of a kind treatment option for certain patients with colorectal cancer. The combination of tucatinib (Tukysa) and trastuzumab (Herceptin), both HER2-targeted therapeutics, was approved by FDA in January 2023 for patients with HER2-positive unresectable or metastatic colorectal cancer that has progressed following at least two standard treatments, including chemotherapy. To be eligible to receive the new combination, patients’ tumors must also not have driver mutations in the RAS group of genes. Colorectal cancer is the second most common cause of cancer death in the United States. An estimated 153,020 people are expected to be diagnosed with colorectal cancer in the United States in 2023 ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Excessive production of the HER2 protein which leads to tumor cell multiplication, invasion, and metastasis is found in approximately three to five percent of patients with metastatic colorectal cancers ( 405 ) Ahcene Djaballah S, et al. (2022) Am Soc Clin Oncol Educ Book, 42: 1. [LINK NOT AVAILABLE] such as that of B rian Beck . The approval of the tucatinib and trastuzumab combination for this patient population was based on a phase II clinical trial which showed that 38 percent of patients who received the drug combination had their tumors shrink or disappear ( 406 ) Strickler JH, et al. (2023) Lancet Oncol, 24: 496. [LINK NOT AVAILABLE] . Considering that prior treatment options for patients with HER2-positive colorectal cancer that has returned or started growing again after receiving standard treatments were not very effective, the approval of tucatinib and trastuzumab represents a significant breakthrough for this subset of patients with metastatic colorectal cancer. Ongoing studies are evaluating whether addition of tucatinib and trastuzumab to standard treatment regimens could be used earlier on as the initial treatment for metastatic HER2-positive colorectal cancer. Adding Precision to the Treatment of Blood Cancers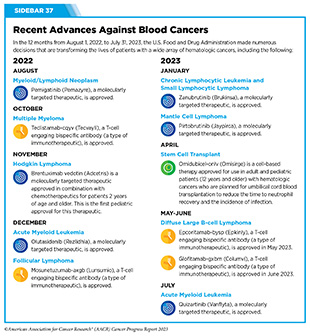 Cancers that arise in blood-forming tissues, such as the bone marrow, or in cells of the immune system, are called blood cancers, or hematologic cancers. In the 12 months covered by this report, FDA has made numerous decisions that are transforming the lives of patients with a wide array of hematologic cancers (see Sidebar 37 ). Acute myeloid leukemia (AML) is the most commonly diagnosed leukemia in the United States, with 20,380 new cases anticipated in 2023 ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . AML has only 32 percent overall 5-year relative survival rate, the lowest among leukemias ( 5 ) Surveillance, Epidemiology, and End Results (SEER) Program. Accessed: July 5, 2023. . Research has substantially increased our understanding of the biology of AML, in particular the different types of genetic mutations that promote AML development. This knowledge is fueling the emergence of molecularly targeted therapeutics for defined groups of patients with the disease. One of the genes known to be mutated in about seven to 14 percent of AML cases is IDH1 ( 407 ) de Botton S, et al. (2023) Blood Adv, 7: 3117. [LINK NOT AVAILABLE] . Mutation in IDHI gene results in an altered IDH1 protein, which can drive cancer formation by interfering with normal cellular maturation and promote uncontrolled cell multiplication. This knowledge led to the development of ivosidenib (Tibsovo), the first therapeutic to target IDH1, which was approved by FDA in 2018. In December 2022, FDA approved a second IDH1-targeted agent, olutasidenib (Rezlidhia), for adult patients with AML that has not responded to or has relapsed after other treatments, and that harbors an IDH1 mutation as detected by an FDA-approved test. At the same time that the molecularly targeted therapeutic was approved, FDA also approved the companion diagnostic, Abbott RealTime IDH1 Assay, to identify patients with AML with an IDH1 mutation. Olutasidenib was approved for the treatment of AML after it was shown in a phase I/II clinical trial that 32 percent of patients treated with the molecularly targeted therapeutic had complete remission, meaning that there was no evidence of disease and full recovery of blood counts ( 407 ) de Botton S, et al. (2023) Blood Adv, 7: 3117. [LINK NOT AVAILABLE] . Not only does the approval of olutasidenib increase treatment options for patients with IDH1-mutated AML, but there is also preliminary evidence that patients may respond longer to olutasidenib compared to the other IDHI-targeted therapy ( 407 ) de Botton S, et al. (2023) Blood Adv, 7: 3117. [LINK NOT AVAILABLE] . A potential side effect observed among patients treated with olutasidenib is differentiation syndrome. The condition is caused by a large, rapid release of immune molecules called cytokines from leukemia cells and can lead to fever, cough, troubled breathing, build-up of excess fluid around the heart and lungs, low blood pressure, and kidney failure, but is generally readily treated with full resolution. The FDA approval is accompanied by a warning highlighting the risk of this potentially fatal adverse effect. In July 2023, the FDA approved a second new molecularly targeted therapeutic, quizartinib (Vanflyta), for the treatment of AML. Quizartinib was approved for treating adults who have newly diagnosed AML that tests positive for a mutated FLT3 gene known as FLT3 internal tandem duplication (ITD). Mutations in the FLT3 gene promote the multiplication and survival of AML cells in 25 to 30 percent of cases, and patients with this type of AML have particularly poor outcomes ( 408 ) Yanada M, et al. (2005) Leukemia, 19: 1345. [LINK NOT AVAILABLE] . The approval was based on results from a phase III clinical trial showing that patients who received quizartinib had a 22 percent reduced risk of death compared to those who received standard chemotherapy during the course of the clinical study ( 409 ) Erba HP, et al. (2023) Lancet, 401: 1571. [LINK NOT AVAILABLE] . Quizartinib can cause several cardiac adverse effects and is therefore available only through a restricted program. At the same time that the FDA made the decision about quizartinib, it expanded the use of the LeukoStrat CDx FLT3 Mutation Assay as a companion diagnostic to identify patients with FLT3 ITD mutation–positive AML who are eligible for treatment with the new molecularly targeted therapeutic. Myeloid/lymphoid neoplasm (MLN) with fibroblast growth factor receptor 1 (FGFR1) rearrangement is a rare, aggressive disease characterized by higher-than-normal levels of certain white blood cells. MLNs do not respond well to standard chemotherapy and can rapidly progress to AML ( 410 ) Verstovsek S, et al. (2018) Ann Oncol, 29: 1880. [LINK NOT AVAILABLE] . FGFR1 is a cell surface protein that stimulates cellular proliferation upon binding with specific extracellular molecules. In rare instances, the FGFR1 gene fuses with another gene (an alteration known as a genetic rearrangement) resulting in a fusion protein that drives the development of MLNs. Pemigatinib (Pemazyre) inhibits the function of FGFR1 to suppress the growth of FGFR1-driven cancers ( 410 ) Verstovsek S, et al. (2018) Ann Oncol, 29: 1880. [LINK NOT AVAILABLE] and in August 2022, it was approved by FDA for adults with MLNs with FGFR1 rearrangement who have not responded to or have relapsed after other treatments. The approval was based on results from a phase II clinical trial that showed that 79 percent of patients had a complete response to pemigatinib. Therefore, the FDA approval of pemigatinib for adult patients with relapsed or refractory MLNs with FGFR1 alteration is a major milestone for the treatment of patients who are diagnosed with the disease. Non-Hodgkin lymphoma (NHL) is the most commonly diagnosed blood cancer in the United States. In 2023, 77,240 people in the United States are expected to be newly diagnosed with the disease ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Notably, NHL encompasses many different types of cancer, most of which arise in immune cells called B cells. Two molecularly targeted therapeutics, recently approved by FDA for treating different subtypes of NHL arising in B cells— pirtobrutinib (Jaypirca) and zanubrutinib (Brukinsa)—target a protein called Bruton tyrosine kinase (BTK). BTK was first identified in 1993. Since its discovery, the role of BTK has been studied extensively in blood cancers and inflammatory diseases. Researchers have found that BTK is a key component of a signaling pathway that promotes the survival and expansion of NHL B cells. Consequently, BTK inhibitors have revolutionized the treatment of NHL arising in B-cells, particularly chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL) as well as certain inflammatory diseases ( 411 ) Alu A, et al. (2022) J Hematol Oncol, 15: 138. [LINK NOT AVAILABLE] . 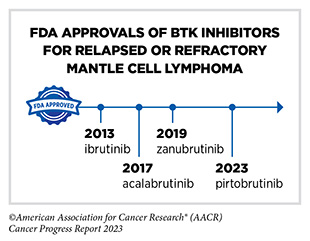 Ibrutinib was the first BTK inhibitor approved by FDA. The approval, in 2013, was for the treatment of patients with relapsed and refractory mantle cell lymphoma (MCL). While the approval of ibrutinib was a major milestone in personalized treatment for B-cell cancers, researchers soon discovered that in patients on continuous treatment with BTK-targeted therapy, cancer cells can acquire mutations in the BTK gene that render the therapeutic ineffective. Since then, newer and more sophisticated BTK inhibitors with improved specificities and thus reduced toxicities have been developed to mitigate the challenge of acquired resistance (see Sidebar 35 ). Pirtobrutinib (Jaypirca) is a new BTK targeted therapeutic that FDA approved in January 2023 for treating MCL. The agent was approved for patients with relapsed or refractory MCL that has not responded to or has relapsed after another treatment, including a BTK inhibitor. Pirtobrutinib has a unique mechanism of action that makes it effective even against mutated forms of BTK that are resistant to other BTK-targeted therapeutics ( 412 ) Zhang J, et al. (2022) Biomark Res, 10: 17. [LINK NOT AVAILABLE] . The approval of pirtobrutinib was based on results from a phase I/II clinical trial, which showed that 50 percent of MCL patients treated with the molecularly targeted therapeutic had tumor shrinkage, with 13 percent having their tumors disappear. Zanubrutinib, another BTK-targeted therapeutic, was approved for treating patients with MCL in November 2019 ( 413 ) Sengupta R, et al. (2020) Cancer Epidemiol Biomarkers Prev, 29: 1843. [LINK NOT AVAILABLE] . In January 2023, FDA approved zanubrutinib for treating adults who have CLL or SLL, which are slow-growing types of NHL. CLL and SLL are essentially the same disease but have different names depending on where in the body the NHL cells accumulate. CLL cells are found mostly in the blood and bone marrow, whereas SLL cells are found mostly in the lymph nodes. The approval of zanubrutinib to treat CLL and SLL was based on results from two phase III clinical trials. The first trial which evaluated the efficacy of zanubrutinib in previously untreated patients with CLL/SLL showed that patients who received zanubrutinib lived a longer time without their cancer worsening compared with patients who received standard treatments. In the second trial, which compared zanubrutinib to ibrutinib in CLL/SLL patients whose disease did not respond to or came back after prior treatments, a greater percentage of patients receiving zanubrutinib were alive during the course of the study with no growth of their cancer, compared to patients taking ibrutinib ( 414 ) Brown JR, et al. (2023) N Engl J Med, 388: 319. [LINK NOT AVAILABLE] . Blocking Progression of Metastatic Prostate CancersProstate cancer is the most commonly diagnosed cancer among men living in the United States. In 2023 alone, more than 288,000 men are expected to be diagnosed with the disease ( 28 ) American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Research has shown that up to 30 percent of prostate cancers have mutations in genes that influence the homologous recombination repair (HRR) pathway (e.g., BRCA, ATM), a cellular process in which a group of proteins work together to repair DNA damage ( 415 ) de Bono J, et al. (2020) N Engl J Med, 382: 2091. [LINK NOT AVAILABLE] . Changes in the HRR pathway may result in the inability to repair DNA and lead to accumulation of mutations and cancer. Poly-ADP ribose polymerase (PARP) proteins are central to a second type of DNA repair pathway called base excision repair. Researchers have found that breast, ovarian, pancreatic, and prostate cancers with genetic mutations that lead to HRR deficiency are responsive to PARP-targeted therapeutics because disruption of these two DNA repair pathways leads to pervasive DNA damage that kills cancer cells. In July 2023, FDA approved a PARP-targeted therapeutic, talazoparib (Talzenna) for treating certain groups of men with metastatic prostate cancer carrying mutations in genes that influence the homologous recombination DNA repair pathway. Men, such as Colbert English, p. 96, who are diagnosed with metastatic prostate cancer are often treated initially with therapeutics that target androgens, the hormones that fuel prostate cancer growth. When the cancer stops responding to these treatments, it is referred to as castration-resistant prostate cancer. Talazoparib was approved in combination with the androgen-targeted therapeutic enzalutamide (Xtandi) for patients with HRR gene-mutated metastatic castration-resistant prostate cancer. Mutations in HRR genes such as BRCA1, BRCA2, and ATM were assessed prospectively using tumor tissue and/or blood-based DNA sequencing assays. The approval was based on results from a phase III clinical trial that showed that treatment with talazoparib significantly improved progression-free survival compared with treatment with enzalutamide alone ( 416 ) Agarwal N, et al. (2023) Lancet, 402: 291. [LINK NOT AVAILABLE] . - A Message from AACR
- Executive Summary
- A Snapshot of a Year in Progress
- Cancer in 2023
- Understanding the Path to Cancer Development
- Reducing the Risk of Cancer Development
- Screening for Early Detection
- Spotlight on Immunotherapy: Pushing the Frontier of Cancer Medicine
- Perspective: Looking to the Future of Immunology
- Supporting Cancer Patients and Survivors
- Envisioning the Future of Cancer Science and Medicine
- Advancing the Future of Cancer Research and Care Through Evidence-based Policies
- AACR Call to Action
- AACR President’s Vision: Future of Cancer Research and Care
- AACR Cancer Progress Report 2023: Steering Committee
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer.  Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off. Cancer Research Catalyst The Official Blog of the American Association for Cancer Research Home > Cancer Research Catalyst > Experts Forecast Cancer Research and Treatment Advances in 2022 Experts Forecast Cancer Research and Treatment Advances in 2022The year 2021 defied our expectations in a variety of ways. The delta and omicron COVID-19 variants imposed unprecedented challenges on the health care system and threatened our hopes of an end to the pandemic, but widespread vaccine distribution provided protection, preventing an estimated 36 million cases and 1 million deaths in the United States. As omicron called into question the efficacy of existing vaccines, tests, and treatments, the U.S. Food and Drug Administration (FDA) provided new options, in the form of emergency use authorizations for the first two oral COVID-19 drugs, nirmatrelvir/ritonavir (Paxlovid) and molnupiravir (Lagevrio). Aside from the pandemic, supply chain delays and worker shortages sparked frustration, but the national unemployment rate gradually fell to its lowest percentage since February 2020. Through a year of harsh weather conditions ranging from ice storms to wildfires to hurricanes and tornadoes, the United States doubled down on initiatives to battle climate change . In spite of the year’s setbacks, the field of cancer research also made progress. The FDA approved 16 new oncology drugs —including two to treat genetic conditions that cause high rates of tumor formation—as well as two cancer detection agents that help physicians better identify certain tumors during imaging or surgery. We celebrated the 50th anniversary of the National Cancer Act , saw marked progress in many areas of cancer research , and helped provide cancer patients with reliable information about their COVID-19 risks and vaccine efficacy . As in previous years , we have asked a panel of experts to reflect on the progress made in 2021 and forecast their predictions for cancer research in the year 2022. We spoke with AACR President-Elect Lisa Coussens, PhD, FAACR , about basic research; AACR board member and co-editor-in-chief of Cancer Discovery Luis Diaz Jr., MD , about precision immunotherapy; co-editor-in-chief of Cancer Prevention Research Michael Pollak, MD , and deputy editor of Cancer Prevention Research Avrum Spira, MD , about cancer prevention; and AACR board member and former Annual Meeting Program Chair John Carpten, PhD, FAACR , about cancer disparities. Priorities for Basic Research in 2022 “There isn’t a drug on the market that doesn’t have its origins in a basic science discovery,” said Lisa Coussens, PhD, FAACR , chair of the department of Cell Development and Cancer Biology at Oregon Health and Science University, when asked about the ways that laboratory science has shaped the landscape of cancer care. “We can’t lose sight of the importance of basic research at any step in the pipeline toward advancing cancer medicine and improving outcomes for our patients.” Basic science—fundamental research about the way cells and molecules function and interact—spans applications from protein chemistry to cell genomics to animal models. Such discoveries help researchers determine, for example, which proteins can be targeted with drugs to fight a disease, or which biomarkers might help determine a patient’s prognosis or course of treatment. An important priority for improving our knowledge of cancer cell biology, Coussens explained, is to better understand how cells shift between different states, especially in response to a disease or therapy. “We need to understand nuances between different tissue states within our body, and how they respond to changes in their environment,” Coussens said, noting that this is true in healthy organs as well as in evolving tumors, where single cell types typically steer disease processes but are dependent on cues from the multiple cell types surrounding them. “Understanding those nuances will lead to bigger discoveries about how to target cell state changes so we can return cells back to normal control mechanisms,” she continued. Tumor cells are not the only cells that might change their patterns of gene expression and metabolism during the course of cancer progression and treatment, however. Other cells that surround and interact with the tumor, such as fibroblasts and immune cells, play a vital role in determining how the tumor behaves.  “A full understanding of tumor ecosystems includes the neoplastic cells—the ‘bad guys’ with mutations—as well as the normal host cells that are recruited or co-opted to help tumor cells survive and disseminate,” Coussens said. Emerging classes of therapies, such as immune checkpoint inhibitors, leverage elements of the tumor microenvironment to kill cancer cells. In order to develop more drugs targeting these cancer support systems, researchers need to learn more about how tumors interact with their surroundings. “I think the next years will bring a major focus on understanding communication networks between all the different types of cells in tumor ecosystems,” Coussens said, adding that a basic understanding of cell communications could produce benefits beyond the scope of cancer. “Basic discoveries about tumor ecosystems can have far-reaching impacts on autoimmune diseases, chronic inflammatory diseases, and how individuals respond to therapies that are designed to treat Alzheimer’s, for example,” she explained. Coussens believes that many of these discoveries will be driven by the expanded use of technology and data science. Since the turn of the century, rapid advances in genomics, proteomics, and metabolomics have created an abundance of biological data from patients, animal models, and cell lines. Designing computational programs capable of integrating these data and determining how to analyze them in meaningful ways has been a constant source of innovation over the past 20 years. Coussens emphasized that continued progress in this area could significantly shape basic research in the coming years. “The biggest impact we’re seeing right now is with the emergence of technology development and computational data sciences,” Coussens said. “I think the greatest advances we will see over the next several years will be emerging out of team science embracing technology, data science, and biology.” As technological advances spur more integration between different disciplines, Coussens predicts that collaboration will become more crucial than ever. “Science has changed—we no longer do science in isolation,” she said. “The best science today, I think, comes out of multidisciplinary team science. I’m a biologist, but I now need to be able to communicate with data scientists, epidemiologists, and chemists.” Coussens expressed that young investigators entering the field should consider this new paradigm when planning their training. “The more you can round out your education in a multidisciplinary way, the better. You need to be able to communicate your science with people who don’t necessarily speak your field’s language.” Part of her advice hinged on trainees finding strong mentors who can help guide them toward these opportunities, especially as they recover from lost time and funding resulting from the COVID-19 pandemic. “Invest your time and energy in identifying mentors who care about who you are and the trajectory of your career. Find mentors who you will grow to respect and love,” she said. Overall, Coussens was optimistic about the state of basic research moving forward. “The basic science discoveries we’re going to see in the next five years will reshape the medical landscape for years to come,” she said. PRIORITIES FOR Precision Immunotherapy IN 2022 The art of deciding which cancer therapies to give a patient, based on their individual tumor characteristics, has evolved over the past several decades, according to Luis Diaz Jr., MD , head of Solid Tumor Oncology at Memorial Sloan Kettering Cancer Center and a member of the National Cancer Advisory Board. Such decisions were first made based on protein markers expressed by the tumor, then by genetic changes in the tumor’s DNA. Now, Diaz said, a precise understanding of tumor characteristics can predict which patients may benefit most from immunotherapy. “One example has been PD-L1 overexpression, either on the tumors themselves or on the surrounding cells,” Diaz said. “Another is mismatch repair deficiency, which seems to prime cells to become very sensitive to immunotherapy.” This is just one of the ways that the fields of precision medicine and immunotherapy have grown to complement each other in recent years. As Diaz noted, antibodies targeting PD-1 or PD-L1 have become an effective therapy for patients whose tumors express these immunosuppressive markers. The treatment of patients with CAR T cells—immune cells which are harvested from a patient’s body, engineered to target tumors, and returned to the patient’s bloodstream—represents an even more patient-specific approach to immunotherapy. But these therapies are not appropriate for all cancer types, and many patients who receive these therapies eventually relapse, creating a need for the expansion of immunotherapy types and indications.  Diaz believes researchers can improve the efficacy of immunotherapy by offering it earlier in a patient’s course of treatment. “In many cases, we’re testing new therapies on patients for whom all standard therapies have already failed,” he said. “As we move forward, we need to begin to treat earlier in the diagnosis.” Diaz emphasized that treating advanced cancer poses far more challenges than intervening in early-stage disease or preventing tumor formation altogether. “If we can begin to bring targeted therapy and immunotherapy into the prevention space, I think we’ll see a profound impact,” he said. A different approach to improving immunotherapy efficiency is to reach more patients by making cell-based immunotherapies, such as CAR T, effective against a broader range of tumor types, including solid tumors. To overcome these hurdles, Diaz said, “The priority needs to be in maximizing specificity and minimizing toxicity.” Solid tumors, Diaz explained, are often heterogeneous. An immune response against a single target may kill some of the tumor, but cancer cells that don’t express the target may continue to grow and evade the immune system. Researchers have designed CAR T cells that target multiple tumor cell markers, but more targets also increase the likelihood of harmful side effects. “It’s a mathematical problem we can’t solve very easily,” Diaz said. “We need some clever new ideas.” Boosting the number of people who receive immunotherapy also involves addressing accessibility issues, especially for patients in rural or underresourced communities. Diaz speculated that the increase in remote care options resulting from the COVID-19 pandemic might provide a blueprint for the decentralization of clinical trials, paving the way for large cancer centers to collaborate with community hubs. He emphasized that one way to promote decentralization is to encourage more clinical trial ownership from clinicians rather than pharmaceutical companies. “I’d like to see our investigators becoming the initiators of more trials to be run at large cancer centers and elsewhere,” Diaz said. He noted that clinical trial decentralization will pose some challenges, such as standardizing procedures and supplies and ensuring that quality does not suffer. However, he was optimistic that it would eventually improve care. “I think it will make clinical development move faster than it ever has before,” he said. Targeting new populations and tumor types with immunotherapy, however, will only benefit patients whose tumors mount an immune response. Some tumors—deemed immunologically “cold”—expertly evade the immune system, and the mechanisms underlying that process are complex. “We need a better understanding of what makes tumors immunogenic so we can harness that knowledge to make cancers more immunogenic,” Diaz said. He noted that research into the interface between immune cells and cancer cells has done a great job of producing the therapies on the market today, but that advancing precision immunotherapy will require those efforts to continue. “As exciting as everything is that we’re doing, we need to do so much more,” Diaz said. “What’s popular right now is probably only the tip of the iceberg.” Priorities for Cancer Prevention in 2022 “The most transformative impact we could have on cancer care would be to prevent cancer from happening in the first place,” said Avrum Spira, MD , a professor of medicine, pathology and laboratory medicine, and bioinformatics at the Boston University School of Medicine and global head of the Lung Cancer Initiative at Johnson & Johnson. Spira and his colleagues study how physicians can better detect early-stage lung cancer or signs of precancerous changes in the lungs. He also studies how to intervene in these early stages to prevent disease progression. “Researchers have found molecular alterations in late-stage cancer and used that information to develop new targeted therapies and immunotherapies that are transforming the treatment of advanced-stage disease,” Spira said. “It’s absolutely critical to move that fundamental molecular understanding to early-stage and even premalignant disease.” Understanding what drives benign cells into a tumorigenic state is an important component of this process, Spira emphasized. Drawing on the success of large-scale programs such as The Cancer Genome Atlas , the Human Cell Atlas , and the Human Tumor Atlas Network , dedicated to fully characterizing the blueprints of the human body, researchers have embarked on the development of a Pre-cancer Atlas . “Within the Human Tumor Atlas Network, researchers are forming large coalitions for multiple different cancer types to develop a temporal and spatial atlas of the cellular and molecular changes associated with the transition of a premalignant lesion to a fully-blown invasive cancer,” Spira said. “I think, in 2022, we’re going to see a proliferation of those types of studies, generating a vast amount of cellular and molecular data from premalignant tissue across many cancer types.” Spira believes such an atlas will benefit patients in two key ways: the development of biomarkers that can help predict which precancerous lesions will advance to cancer, and the identification of drug targets to stop the progression.  “For most cancer types, we don’t know what those early events are, and therefore, we have no effective way to intercept the disease process,” he said. “I think in 2022, we will begin to understand these events and gain unprecedented insight into targeted approaches aimed at intercepting premalignancy.” Spira elaborated more on the need for biomarkers, which may not only identify patients at an elevated cancer risk but may also determine which patients with abnormal imaging results may need a biopsy. The most effective biomarkers, he stressed, would be the ones detectable via noninvasive tests. “I’m excited about the future of blood-based tests looking at nucleic acids,” Spira said. “The technologies are evolving very rapidly to the point where they can now detect very small amounts of DNA or epigenetic changes that are circulating in the blood, and they can screen people across multiple cancer types.” While blood-based liquid biopsies have attracted a great deal of attention in recent years, Spira also drew attention to other emerging noninvasive tests with the potential to have a significant impact on early cancer detection, such as urine markers of urologic cancer, stool markers of colon cancer, and nasal brushings to assess lung cancer risk. Spira hopes these noninvasive tests can be integrated with each other and with imaging results to give the best possible assessment of a patient’s risk. “That’s a complicated space, but I think this convergent approach is one that will advance significantly in 2022,” he said. Even noninvasive tests, however, can only benefit patients who are able to access them. Spira pointed out a few ways the field adapted during the COVID-19 pandemic that could continue to be leveraged moving forward. “We need to find ways to get screening to patients as opposed to them having to come to the hospital,” Spira said. He highlighted advances such as remote clinical trial management, as well as mobile CT and radiology units, set up in large vans or trucks that can drive to various neighborhoods to perform screening. Used during COVID-19 to promote social distancing and minimize virus exposure, such units could be used in the future to help people catch up on screenings missed during the pandemic, especially in areas with poor health care access. Spira also noted that the pandemic placed a spotlight on behavioral risk factors that increased COVID-19 susceptibility and the risk for severe disease, such as smoking, alcohol consumption, obesity, and physical inactivity. He pointed out that, often, these same behaviors contribute to cancer risk. “This has become a teachable moment,” Spira said. “I think we can encourage the public to alter some cancer-causing behaviors that are also related to virus susceptibility.” Michael Pollak, MD , a professor of oncology and medicine at McGill University in Montreal, Canada, who studies cancer prevention through the lens of reducing risk, also emphasized addressing lifestyle behaviors that affect multiple health conditions. “An important trend for 2022 may be the concept of healthy lifestyle behaviors integrated across diseases,” Pollak said. “We have to recognize that some of the activities and lifestyles and approaches to cancer risk just contribute to overall good health.” While many behavioral factors are known to broadly increase risk of several cancers, Pollak noted that risks vary in unique ways among different individuals. “Oncologists are used to personalization of treatments,” he said. “We try to find out what treatment would be particularly useful for one patient as compared to their neighbor. In prevention, we may discover an analogy to that customization.” He explained that an individual assessment of risk may make the message of behavioral intervention more personal. “If you hear your doctor saying that, in your particular case, the way your body is put together, your weight especially increases your risk for cancer, it may help motivate some people.” Pollak believes risk assessment can be further personalized beyond the level of the individual, down to the level of discrete cell types. “We’re used to thinking of a person’s cancer risk as if the person was homogeneous, but carcinogenesis takes place at the cellular level,” he said. “We need to know what’s going on differently in the different cells that might determine risk on a per-cell basis.” Pollak mentioned the Pre-cancer Atlas as an important vehicle for realizing this goal. “With the Pre-cancer Atlas, we’ll learn more about the cellular composition and subcellular features that lead to carcinogenesis,” he said, noting that such a granular understanding of tumor formation could pave the way for improved therapies. “We really won’t be able to prevent every cancer, but even if we confine our goals to preventing the subset of cancers that are preventable, that’s estimated to be about half of all cancers,” Pollak concluded. “Even acknowledging the limitations, the potential gains are absolutely enormous.” Priorities for Cancer Disparities in 2022 The past two years have presented health care challenges beyond COVID-19, encompassing financial and access-related struggles that affected many facets of medicine, including cancer care. Many individuals have had to delay routine cancer screenings, alter the course of treatment, or miss follow-up appointments as a result of the pandemic. Such problems were more pronounced in some communities than others. “The pandemic has definitely impacted our opportunities to move forward toward eliminating disparities in all areas of cancer research,” said John Carpten, PhD, FAACR , chair of the department of Translational Genomics at the University of Southern California Keck School of Medicine and chair of the National Cancer Advisory Board. “As we consider gaps in cancer screening and cancer diagnosis, many challenges were further exacerbated in underrepresented minority communities during the pandemic.” Carpten also pointed out the disproportionate challenges minority cancer researchers faced during COVID-19. “Many underrepresented minority investigators, who may have already had challenges in terms of access to funding, were also impacted severely by the pandemic,” he said. “This is especially true for early-stage investigators and postdoctoral fellows who were unable to be in their laboratories to perform research.” Although the issue of lost time and funding due to the pandemic may be difficult to solve, Carpten believes that other initiatives to support underrepresented minority researchers—especially trainees and early-career investigators—will positively influence health disparities research in 2022. Carpten specifically listed diversifying the biomedical workforce as a key priority for tackling health disparities. “Increasing underrepresented minority faculty members will increase the number of mentors who will then be able to train more underrepresented minorities and fellows,” he said.  He mentioned the National Institutes of Health (NIH) FIRST program , a funding opportunity provided to institutions to promote the hiring of early-career investigator cohorts from diverse backgrounds in support of their career development. Providing a supportive environment and sufficient resources to these investigators, Carpten said, can make significant strides toward ensuring a successful career trajectory in academic research. “We believe that this is going to be a huge component in the growth of underrepresented minorities in the area of biomedical research, specifically cancer research,” he said. Encouraging diversity of researchers, however, is only one step where meaningful interventions can occur. Another is the broader inclusion of diverse patients and samples in cancer research, especially of patients recruited into clinical trials. “We need to understand the broader impact of new therapies for all people, preferably prior to approvals, to ensure that we have the most accurate picture relative to effectiveness and toxicity profiles across representative groups of patients,” Carpten said. Diversity in preclinical studies, including patient-derived samples, genetic data, and model systems, is also key to understanding the biological basis of cancer health disparities. “Whether it’s understanding the influence of genetic factors on cancer risk or understanding how collections of mutations that occur in cancer cells differ across individuals from different groups, it will be very important for us to continue increasing representation of the reagents, models, and data that we use,” Carpten said. “Ensuring that we understand how biological changes impact cancer initiation, progression, and growth across an array of models will provide additional information so that we can really capture the full complexity of cancer,” he added. Carpten also encourages working to address the cultural, social, and access-related issues underlying cancer health disparities by striving harder to engage with the community. “We need to advance our relationships with various stakeholders, especially in terms of community engagement, outreach, and involvement,” Carpten said. “If we don’t build better relationships with the community, get their feedback, understand their issues, and work together to address them, I think we’ll continue to have challenges.” As observed during the pandemic , improving community engagement can help health care providers build trust with their patients, bring care to broader geographic areas, and better understand the needs of the populations disparities researchers are working to serve. “I really look forward to working with my colleagues in academia, industry, and the government, but most importantly, with our colleagues in the community,” Carpten concluded. “Their voice really needs to be heard and will be key in achieving cancer health equity.” - About This Blog
- Blog Policies
- Tips for Contributors
 Cervical Cancer: Advances in Prevention, Screening, and Treatment  Immunotherapy Conference: “Translating Science Into Survival”  What are the Side Effects of Immunotherapies and How Do They Arise? Cancel replyYour email address will not be published. Required fields are marked * Join the Discussion (max: 750 characters)... This site uses Akismet to reduce spam. Learn how your comment data is processed .  Thank you so much for sharing such useful information over here with us. This is really a great blog have you written. I really enjoyed reading your article. I will be looking forward to reading your next post. https://atulkulkarnidotblog.wordpress.com/ https://atulkulkarnidotblog.blogspot.com/ https://atulblog123.tumblr.com/  I was distraught when I found out that my grandmother was diagnosed with cancer. I appreciate that this post emphasized that it is important to get the right kind of treatment, to target the cells precisely. I think I will advise my mother to seek the right kind of treatment by speaking with professional oncologists.  This is very informative blog about Cancer research forecast, I will definitely share this content with others. - Experts Forecast Cancer Research and Treatment...
- Executive Summary
- Experts Forecast 2024, Part 1: Advances in...
Explore CRI’s 2023 Cancer Research Impact Immune to Cancer: The CRI Blog  How Focused Ultrasound and Immunotherapy Could Transform Cancer TreatmentRecent advancements in cancer treatment are reshaping patient care in multiple ways. Focused ultrasound and cancer immunotherapy are leading the charge to develop safer and more efficient combination therapies. These cutting-edge technologies are proving to be pivotal and offer new hope for improved outcomes. Recently, CRI’s CEO and Director of Scientific Affairs, Dr. Jill O’Donnell-Tormey, joined the Focused Ultrasound Foundation’s podcast, Curing with Sound, to explore the future of cancer immunotherapy and its integration with focused ultrasound. Precision and Safety: The Power of Focused Ultrasound Focused ultrasound delivers targeted treatments with pinpoint accuracy, disrupting and killing cancer cells. When combined with immunotherapies, it opens new doors in cancer treatment, enhancing effectiveness and offering a fresh ray of hope. Just as cancer immunotherapy has transformed treatment paradigms, focused ultrasound is driving new strategies in combating cancer and other diseases. Cancer Immunotherapy: A Revolutionary Approach Cancer immunotherapy harnesses the body’s immune system to identify and destroy cancer cells. Key forms include checkpoint blockades and CAR T cell therapy. Checkpoint blockades, which ’release the brakes’ on the immune system, have revolutionized treatment for melanoma and non-small cell lung cancer, and are now approved for over 29 cancers. However, their benefits are limited for some patients, indicating the need for further advancements. Synergizing Focused Ultrasound with Immunotherapy Like the immune system, focused ultrasound is complex, with various applications that take time to fully understand. Its notable safety and precision make it an ideal candidate for combining with immunotherapies. This synergy has the potential to minimize adverse events and maximize treatment effectiveness. The integration of focused ultrasound with immunotherapy represents a promising frontier that could revolutionize treatment and help create a world immune to cancer. To listen to Dr. O’Donnell-Tormey’s full discussion, click here. Let's spread the word about Immunotherapy! Click to share this page with your community.This website uses tracking technologies, such as cookies, to provide a better user experience. If you continue to use this site, then you acknowledge our use of tracking technologies. For additional information, review our Privacy Policy . Cancer patients often do better with less intensive treatment, research shows Scaling back treatment for three kinds of cancer can make life easier for patients without compromising outcomes, doctors reported at the world’s largest cancer conference . It’s part of a long-term trend toward studying whether doing less — less surgery, less chemotherapy or less radiation — can help patients live longer and feel better. The latest studies involved ovarian and esophageal cancer and Hodgkin lymphoma. Thirty years ago, cancer research was about doing more, not less. In one sobering example, women with advanced breast cancer were pushed to the brink of death with massive doses of chemotherapy and bone marrow transplants. The approach didn’t work any better than chemotherapy and patients suffered. Now, in a quest to optimize cancer care, researchers are asking: “Do we need all that treatment that we have used in the past?” It’s a question, “that should be asked over and over again,” said Dr. Tatjana Kolevska, medical director for the Kaiser Permanente National Cancer Excellence Program, who was not involved in the new research. Often, doing less works because of improved drugs. “The good news is that cancer treatment is not only becoming more effective, it’s becoming easier to tolerate and associated with less short-term and long-term complications,” said Dr. William G. Nelson of Johns Hopkins School of Medicine, who was also not involved in the new research. Latest news on cancer treatment- Cancer-fighting antibodies inject chemo directly into tumor cells, upping effectiveness.
- Long-term study shows 'remarkable' treatment helps patients with deadly nonsmoking-related lung cancer.
- FDA approves groundbreaking treatment for advanced melanoma.
Studies demonstrating the trend were discussed over the weekend at an American Society of Clinical Oncology conference in Chicago. Here are the highlights: Ovarian cancerFrench researchers found that it’s safe to avoid removing lymph nodes that appear healthy during surgery for advanced ovarian cancer. The study compared the results for 379 patients — half had their lymph nodes removed and half did not. After nine years, there was no difference in how long the patients lived and those with less-extreme surgery had fewer complications, such as the need for blood transfusions. The research was funded by the National Institute of Cancer in France. Esophageal cancerThis German study looked at 438 people with a type of cancer of the esophagus that can be treated with surgery. Half received a common treatment plan that included chemotherapy and surgery on the esophagus, the tube that carries food from the throat to the stomach. Half got another approach that includes radiation too. Both techniques are considered standard. Which one patients get can depend on where they get treatment. After three years, 57% of those who got chemo and surgery were alive, compared to 51% of those who got chemo, surgery and radiation. The German Research Foundation funded the study. Hodgkin lymphomaA comparison of two chemotherapy regimens for advanced Hodgkin lymphoma found the less intensive treatment was more effective for the blood cancer and caused fewer side effects. After four years, the less harsh chemo kept the disease in check in 94% of people, compared to 91% of those who had the more intense treatment. The trial included 1,482 people in nine countries — Germany, Austria, Switzerland, the Netherlands, Denmark, Sweden, Norway, Australia and New Zealand — and was funded by Takeda Oncology, the maker of one of the drugs used in the gentler chemo that was studied.  The Associated Press Featured TopicsFeatured series. A series of random questions answered by Harvard experts. Explore the GazetteRead the latest.  Faster ‘in a dish’ model may speed up treatment for Parkinson’s If it feels too hot to run, maybe it is Does your brain reflect your sex? Taha Rakhshandehroo (left) and Mohammad Rashidian in their lab. Niles Singer/Harvard Staff Photographer Fixing key flaw in revolutionary cancer treatmentResearchers devise way to boost CAR T-cell therapy to potentially ensure it doesn’t fade prematurely Alvin Powell Harvard Staff Writer Researchers from Harvard Medical School and the Dana-Farber Cancer Institute have figured out how to give a timely boost to a revolutionary treatment that enlists immune cells in the anticancer fight, potentially bypassing a flaw that allows the therapy to fade before all diseased cells are gone. The researchers, Mohammad Rashidian , assistant professor of cancer immunology at Dana-Farber and radiology at HMS, postdoctoral fellow Taha Rakhshandehroo, and their team created an enhancer protein that selectively energizes the anticancer cell therapy called CAR T-cells. This protein not only boosts the cells’ anticancer activity, it also promotes the development of memory CAR T-cells, which provide long-term immune protection against cancer, similar to the immune response after chicken pox infection or vaccination. The cancer treatment, CAR-T cell therapy, was approved by federal regulators in 2017. It works by extracting immune T-cells from patients and reprograming them with a “chimeric antigen receptor” (CAR) on the cell surface. The receptor works like a lock and key for a protein marker on the surface of the cancer cells. That allows the CAR T-cells to recognize and attack cancer cells once they’re infused back into the body. In recent years, CAR T-cell therapy has made headlines by working where conventional treatments failed, in some cases completely clearing cancer cells from the sickest patients. But once the CAR T-cells have cleared most of the cancer, their numbers fade over time, allowing any remaining diseased cells to proliferate. For example, in multiple myeloma, a cancer of white blood cells, CAR T-cell therapy increases patients’ survival over the short term, but half of patients relapse within one to two years. Within three years, most patients see their cancers recur. The advance, which has yet to be tested in humans, was developed using models of multiple myeloma in preclinical mice studies in work sponsored by Dana-Farber’s Innovation Research Fund Award, the Parker Institute for Cancer Immunotherapy, and a Blavatnik Therapeutics Challenge Award. Results of the study were published recently in the journal Nature Biotechnology. The researchers said the procedure should be useful against other cancers, and have studies underway testing it against leukemia and lymphoma. CAR T-cell therapy is one of a suite of relatively recent cancer treatments that have revolutionized care. Unlike traditional surgery, chemotherapy, and radiation therapy, the therapy turns the body’s own immune system into a powerful weapon to fight the disease. The enhancer protein devised by Rashidian and Rakhshandehroo selectively targets CAR T-cells, enhancing their activity and persistence. The pair began the work two years ago with the goal of developing a procedure that could quickly translate to clinical care. Other researchers have been addressing the CAR T-cell longevity problem for a decade by focusing on re-engineering them to extend their lifespan in the body, a strategy that largely fell short. Instead, Rashidian and Rahkshandehroo focused on stimulating the CAR-T cells post-infusion and at a desired time, rather than altering them. To do so, they designed a protein that targets and stimulates CAR T-cells. The CAR receptor is the engineered part of the CAR T-cell that allows it to recognize cancer cells by detecting a specific marker on them. The enhancer protein fuses the cancer marker to a molecule called IL-2, which enhances T-cell activity and persistence. The IL-2 is engineered to be weak so it does not affect normal T-cells, thus avoiding toxicities. However, because the enhancer protein targets CAR T-cells, the weak IL-2 enhances their activity through proximity. “Sometimes in science, you see marginal differences here and there, and then you do the statistics, and you find out the significance,” Rakhshandehroo said. “For us, it was like night and day. Once we saw it, we knew there was something very robust happening here.” Rakhshandehroo said subsequent experiments were aimed at illuminating specific questions about the process, but what impressed them was the persistence of the response despite shifting variables. “We’ve tuned our experiments to be more specific, trying to answer specific questions, but what we’ve seen has always been very robust,” Rahkshandehroo said. “The door has been opened, and everyone can come and take advantage of the system and use it to understand the biology behind the enhancer’s impact on T-cells and their persistence as well as finding new therapies.” To avoid overstimulating the CAR T-cells, Rashidian said they’ve tuned the enhancer protein to have a short circulatory half-life of just two hours before it clears from the body. That will give it time to stimulate the CAR T-cells without overstimulating them. That also allows closer control of dosing once human trials begin — Rashidian has started the search for funding for a Phase 1 trial to gauge efficacy and safety. With such a short enhancer circulatory lifetime, Rashidian said, researchers can more easily adjust doses according to how patients respond in the trial. “I’m very excited about it,” Rashidian said. “It works beyond what we have expected. It’s incredibly robust. I’m very hopeful that it will save patients’ lives.” Share this articleYou might like. Could result in personalized models to test diagnostic and treatment strategies  Experts who have seen health consequences close-up offer guidelines for summer athletes  Precision medicine is just one field where the answer matters Garber to serve as president through 2026-27 academic yearSearch for successor will launch in 2026 Finding right mix on campus speech policiesLegal, political scholars discuss balancing personal safety, constitutional rights, academic freedom amid roiling protests, cultural shifts Good genes are nice, but joy is betterHarvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier For the best browsing experience please enable JavaScript. Instructions for Microsoft Edge and Internet Explorer , other browsers  - About cancer
- Get involved
- Our research
- Funding for researchers
- Cancer types
- Cancer in general
- Causes of cancer
- Coping with cancer
- Health professionals
- Do your own fundraising
- By cancer type
- By cancer subject
- Our funding schemes
- Applying for funding
- Managing your research grant
- How we deliver our research
- Find a shop
- Shop online
- Our eBay shop
- Our organisation
- Current jobs
- Cancer news
 Treatment for cancerYour treatment depends on where your cancer is, how big it is, whether it has spread, and your general health. There are different types of treatment you might have. Understanding your treatment and the side effects can help you to cope. - Go to our information about different types of cancer for details about their treatment
Preparing for treatment and life afterwards (prehabilitation)There are things you can do to help you feel more in control of your physical and mental health when preparing for treatment. In the hospital, preparing for treatment is also called prehabilitation or prehab. Treatment by cancer typeWe have specific treatment information for each cancer type. Choose the cancer type you want to find out about the treatment from this A-Z list of treatments by cancer type. Cancer drugs A to Z listThere are many cancer drugs, cancer drug combinations and they have individual side effects. ChemotherapyChemotherapy is a standard treatment for some types of cancer. It uses anti cancer drugs to destroy cancer cells. Cancer drugsSurgery for cancer. Surgery is one of the main treatments for many types of cancer. Find out about when and why you might have it and what to expect before and after your operation. RadiotherapyFind out about cancer treatment with radiotherapy, including external radiotherapy, internal radiotherapy, side effects, radiotherapy for symptoms and follow up after treatment. Hormone therapyHormone therapy blocks or lowers the amount of hormones in the body to stop or slow down the growth of cancer. Stem cell and bone marrow transplantsStem cell or bone marrow transplants are treatments for some types of cancer including leukaemia, lymphoma and myeloma. You have them with high dose chemotherapy and sometimes radiotherapy. Targeted cancer drugsTargeted cancer drugs work by ‘targeting’ those differences that help a cancer cell to survive and grow. Find out more about what they are and the different types. ImmunotherapyImmunotherapy uses our immune system to fight cancer. It's a standard treatment for some types of cancer and is in clinical trials for other types of cancer. Find out more about it. Radioisotope therapyRadioisotope therapy uses radioactive medicines to treat some types of cancer. It is also known as radionuclide therapy. You have the radioisotope as a drink, capsule or injection. What is personalised medicine?Personalised medicine involves using information about a person’s cancer to help diagnose, treat and find out about how well treatment is working. BisphosphonatesBisphosphonates are drugs that can help prevent or treat bone loss and reduce the risk of fractures. There are several different types of bisphosphonates, and they each work slightly differently. Watch and waitYou might not need treatment straight away, or never need it. Doctors monitor you with regular check ups and tests. They call this 'watch and wait', 'active monitoring', or 'active surveillance'. Other treatmentsThese are cancer treatments using medical technologies (interventional treatments) including laser treatment, photodynamic therapy and cryotherapy.  Complementary and alternative therapiesThe phrases complementary therapy and alternative therapy are often used as if they mean the same thing. They may also be combined into one phrase – complementary and alternative therapies (CAMs). Palliative treatmentIn advanced cancer, palliative treatment might help someone to live longer and more comfortably, even if they cannot be cured. The palliative care team can support people with any stage of cancer and help with symptoms or side effects of treatment. Access to treatmentThere are several decisions to be made about a cancer treatment before you can have it on the NHS or HSC.  It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day. Visit the Cancer Chat forum  About Cancer generously supported by Dangoor Education since 2010.  Find a clinical trialSearch our clinical trials database for all cancer trials and studies recruiting in the UK  Cancer Chat forumTalk to other people affected by cancer  Nurse helpline 0808 800 4040Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us - Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Immunotherapy for Cancer: What Are My Options?- How It's Used
- Side Effects
- List of Drugs
- Where to Get Treated
Immunotherapy is a relatively new approach to cancer treatment. It improves the body's immune system's ability to find and fight cancer. Since 2010, immunotherapy drugs have been developed, tested, and deployed as treatments for some cancer types and stages. They're not a silver bullet, but for some people and types of cancer, immunotherapy drugs have transformed the treatment landscape. Immunotherapy approved for cancer that has come back or has spread may not help with early-stage cancers. This article will review what cancer types and stages may benefit from immunotherapy, as well as information about immunotherapy's successes and the side effects of treatment. It will review how immunotherapy works, how much immunotherapy costs, and when immunotherapy for cancer would and wouldn't be used. Karl Tapales / Getty Images How Immunotherapy Treats Cancer Immunotherapy drugs work by helping the body’s immune system fight back against cancer. They may remove blockers that cancers hide behind to escape detection, or they may train immune systems to detect the cancer and attack it. In many cases, immunotherapy ramps up the immune system. Each immunotherapy treatment is tested and approved for different cancers one at a time. They’re approved for specific stages and types of cancer, so they’re not used across the board. Their use depends on your cancer stage and your healthcare provider's determination of the best treatment plan based on your health and goals. New treatment combinations are being tested all the time. Immunotherapies may be used alone or combined with other immunotherapies, with chemotherapy, radiation, surgery, targeted treatments, and other therapies and medications. Certain cancers have immunotherapies approved by the Food and Drug Administration (FDA). Immunotherapies have been FDA-approved to treat these types of cancer: - Bladder cancer
- Brain cancer
- Breast cancer
- Cervical cancer
- Colorectal cancer
- Esophageal cancer
- Head and neck cancer
- Kidney or renal cell cancer
- Liver cancer
- Lung cancer
- Lymphoma (Hodgkin's and non-Hodgkin's)
- Multiple myeloma
- Ovarian cancer
- Pancreatic cancer
- Prostate cancer
- Skin cancer
- Stomach cancer
- Uterine (endometrial) cancer
Immunotherapy is also in clinical trials for many other types and stages of cancer. The appropriate stage for cancer treatment with immunotherapy depends on the cancer and which drugs have been approved for that type of cancer. Success of Treatment: Does Immunotherapy Work?Immunotherapies are typically approved first for more advanced cancers, especially those that cannot be removed with surgery. In these cases, they can extend someone’s life or keep their cancer from worsening. More recently, immunotherapies are being used to treat early cancers, even as a first-line treatment, alone or with other therapies, including chemotherapy, radiation, and surgery. Sometimes, people can skip other treatments like chemotherapy if immune therapies work well. Immunotherapy vs. ChemotherapySome people wonder if they should ask their healthcare providers for immunotherapy or chemotherapy. The treatment plans for cancer are rarely so simple. Both treatments can have side effects, sometimes severe. Both can be effective when used in ways that research has shown to benefit people. There’s not any way to compare the strength of immunotherapies and chemotherapy. Both treatment types encompass multiple drugs and drug types and are used in different cancer types and stages for different reasons. The same is true for radiation. Some cancers only need radiation, while others respond better to chemotherapy plus radiation or other treatments. One cancer treatment is not necessarily “better” than the others. Often, immunotherapies seem to have fewer or more manageable side effects. However, people can still have severe adverse reactions to the infusion or develop autoimmune reactions that can require stopping the drug. In some cases, immunotherapy alone or immunotherapy or plus surgery may be the only treatment needed to cure early-stage and locally advanced cancers. Treatment with immunotherapy before surgery can shrink the tumors and make surgery easier. Some people on immunotherapy who previously would have had only months to live with standard treatments have experienced no progression in their cancer for many years with immunotherapy. Not all cancers react to immunotherapy. Even someone with the “right” type and stage of cancer may not respond as well as the next person for various reasons. These may have to do with the genetics of their immune system or the genetics and characteristics of their cancer. Scientists are still studying ways of finding out who is most likely to respond to immunotherapy and who isn’t. Types of ImmunotherapyImmunotherapy drugs treat cancer by increasing the immune system’s ability to find and fight cancer cells. Several types of immunotherapy are used to treat cancer with different approaches. These include: - Immune checkpoint inhibitors turn down the body’s natural inhibitory mechanisms, called checkpoints, that stop the immune cells from attacking healthy cells. Some cancer cells use these checkpoints to hide from the immune system. Taking the brakes off these checkpoints with inhibitors can let the immune system find and fight cancer cells.
- Cancer vaccines activate the immune system. Many vaccines prevent disease by priming the immune system against viruses or bacteria. These are preventive vaccines, some of which fight cancer-causing viruses. Other vaccines are treatment vaccines used to activate the immune system against cancer.
- Immune cell therapies remove immune cells from the person with cancer and train them to fight cancer. The cells are modified in the lab to boost their ability to fight cancer and are then reinfused into the person. It uses a special virus that helps the T cells (a type of immune cell) attach to the tumor cells and kill them.
- Immunomodulators are drugs that generally boost the immune system. They can help fight certain types of cancer. Cytokines are one type of immunomodulator. These small proteins carry messages between cells, stimulating the immune cells to attack cancer.
Monoclonal AntibodiesAnother type of cancer treatment is targeted treatment using monoclonal antibodies. These antibodies are immune system proteins that are made outside of the body. They’re created to see and tag either cancer-related proteins or proteins of the cancer or tumor. Targeted antibodies are sometimes classified as immunotherapy, but most don’t directly activate the immune system. They are typically classified as targeted therapies because they attack cancer directly, delivering drugs or immune cells to the tumor. Some monoclonal antibodies mark cancer cells so the immune system can find and destroy them. Such monoclonal antibodies are a type of immunotherapy. Potential Side Effects of ImmunotherapySide effects vary by the type of immunotherapy used. Immune checkpoint inhibitors, for example, may cause side effects, including rash or other skin problems, digestive issues like nausea and diarrhea, and fatigue or fever. More dangerous reactions to checkpoint inhibitors include infusion reactions, autoimmune reactions, and widespread inflammation. Immune system-modulating drugs can cause flu-like symptoms, including fever and chills, weakness and dizziness, nausea or vomiting, muscle or joint aches, fatigue, and headache. Cytokines, specifically, can lead to serious side effects. These include: - Breathing problems.
- Blood pressure issues (low or high)
- Allergic reactions
- Decreased blood counts, leading to infections and bleeding
- Blood clots
- Mood, behavioral, thinking, and memory issues
- Rash, burning, or ulcers on the skin
- Organ damage
Cell therapies are complex procedures that can cause severe reactions. One side effect of T-cell therapy is cytokine release syndrome, in which the procedure triggers the release of high levels of cytokines into the blood. It can cause flu-like symptoms, a rapid heartbeat, low blood pressure, and trouble breathing. It can be mild or severe and life-threatening. Manipulated T cells can also attack normal cells. It can cause a range of issues, including organ damage. Another cell therapy–related issue is capillary leak syndrome, when fluid and proteins leak out of blood vessels and into surrounding tissues. It causes low blood pressure and can lead to organ failure and shock. Most preventative vaccines have very mild side effects. Cancer treatment vaccines can cause flu-like symptoms. Some vaccine components can cause a severe allergic reaction. Specific cancer treatment vaccines have their risks, including stroke, tumor lysis syndrome , and herpesvirus infection. List of Immunotherapy Drugs for Cancer Numerous immunotherapies have been approved to treat various cancers. Many more are in clinical trials. The lists below include immunotherapies approved by the FDA and the type of cancer they’re authorized to treat. Immune Checkpoint InhibitorsImmune checkpoint inhibitors include: - Bavencio (avelumab) for Merkel cell carcinoma, bladder and urinary tract (urothelial cell) cancer , and kidney cancer
- Imfinzi (durvalumab) for non-small cell lung cancer , small cell lung cancer , biliary tract cancer , bladder cancer, and liver cancer
- Imjudo (tremelimumab-actl) for non-small cell lung cancer and liver cancer
- Jemperli (dostarlimab-gxly) for endometrial cancer
- Keytruda (pembrolizumab) for melanoma , non-small cell lung cancer, Hodgkin lymphoma , Merkel cell carcinoma, B-cell lymphoma , stomach cancer , cervical cancer , head and neck squamous cell cancer , bladder and urinary tract cancer, esophageal cancer, liver cancer, kidney cancer, endometrial cancer, squamous cell skin cancer , breast cancer
- Libtayo (cemiplimab-rwlc) for squamous cell and basal cell skin cancer and non-small cell lung cancer
- Opdivo (nivolumab) for melanoma, lung cancer, mesothelioma , kidney cancer, Hodgkin's lymphoma, head and neck squamous cell cancer, bladder and urinary tract cancer, colorectal cancer , liver cancer, esophageal cancer, and stomach cancer
- Opdualag (nivolumab and relatlimab-rmbw) for melanoma
- Sylvant (siltuximab) for Castleman's disease
- Tecentriq (atezolizumab) for bladder cancer, non-small cell lung cancer, small cell lung cancer, liver cancer, melanoma, and sarcomas
- Yervoy (ipilimumab) for melanoma, kidney cancer, colorectal cancer, liver cancer, non-small cell lung cancer, mesothelioma, and esophageal cancer
- Zynyz (retifanlimab-dlwr) for Merkel cell cancer
Cancer VaccinesCancer vaccines include: - Vaccines can prevent viral infections that can lead to cancers, like the human papillomavirus vaccines for cervical cancer and throat cancer and the hepatitis B vaccine for liver cancer.
- Bacillus Calmette-Guerin (BCG) therapy uses a type of bacteria that doesn’t typically cause disease to trigger an immune response against bladder cancer.
- Oncolytic virus therapy for melanoma with Imlygic (talimogene laherparepvec, or T-VEC) uses a modified virus that breaks apart cancer cells and triggers a local and systemic immune response, including tumor-specific T cells.
Cell TherapiesImmune cell therapies include: - Abecma (idecabtagene vicleucel) for multiple myeloma
- Breyanzi (lisocabtagene maraleucel) for B-cell lymphoma
- Carvykti (ciltacabtagene autoleucel) for multiple myeloma
- Kymriah (tisagenlecleucel) for acute lymphoblastic leukemia , B-cell acute lymphoblastic leukemia, B-cell lymphoma, and follicular lymphoma
- Provenge (sipuleucel-T) for prostate cancer (sometimes called a cancer treatment vaccine)
- Tecartus (brexucabtagene autoleucel) for Mantle cell lymphoma and acute lymphoblastic leukemia
- Yescarta (axicabtagene ciloleucel) for B-cell lymphoma and follicular lymphoma
ImmunomodulatorsImmunomodulators include: - Adstiladrin (nadofaragene firadenovec-vncg) for bladder cancer
- Aldara (imiquimod) for skin cancers
- Elzonris (tagraxofusp-erzs) for blood cancer (blastic plasmacytoid dendritic cell neoplasm)
- Multiferon (interferon alpha) for hairy cell leukemia , Kaposi's sarcoma , lymphoma, chronic myeloid leukemia , and melanoma
- Pomalyst (pomalidomide) for multiple myeloma and Kaposi's sarcoma
- Proleukin (interleukin-2) for melanoma and kidney cancer
- Revlimid (lenalidomide) for multiple myeloma and mantle cell lymphoma
- Sylatron (peginterferon alfa-2b) for melanoma
- Thalomid (thalidomide) for multiple myeloma
Where to Get Immunotherapy Treatment Talk to your oncologist (cancer specialist) about immunotherapy options and clinical trials for your type and stage of cancer. Where you get immunotherapy depends on the kind of treatment and your cancer. FDA-approved immunotherapies should be available through your oncologist at the hospital or cancer center. Immunotherapies in clinical trials would only be available if you’re enrolled and at participating hospitals. Because there is a lot of variation in the drugs classified as immunotherapies, they range in cost. Cancer immunotherapy can cost more than $100,000 per person. A drug taken as a pill and easily manufactured may be much less expensive than an infusion with a hard-to-manufacture monoclonal antibody. Infusions of monoclonal antibodies can cost thousands of dollars per visit every few weeks for years. Even more costly are personalized cell therapies, which require cells to be manipulated and grown in the lab, which may cost hundreds of thousands of dollars. Some of these costs may be covered by Medicaid, Medicare, or private health insurance, but it varies by type of cancer and the drug. Immunotherapy manufacturers and other organizations may offer financial assistance programs. Many immunotherapies are given as infusions. These require visiting a medical setting, like a hospital, doctor’s office, or infusion clinic. They’re usually given every two to six weeks, sometimes in cycles or as a maintenance therapy, for as long as they’re effective. Other immunotherapies are more complex procedures that would be done at the hospital, typically as an outpatient. Immunotherapy is a relatively new approach to cancer treatment. It uses the body's immune system to fight cancer. FDA-approved immunotherapies treat a variety of cancers, including brain, colorectal, skin, and lung cancers, among many others. How oncologists use immunotherapy depends on the type and stage of cancer. They may use immunotherapy alone or in combination with other cancer treatments. Other treatments include chemotherapy, radiation therapy, targeted therapy, and surgery. Types of immunotherapy include immune checkpoint inhibitors, cancer vaccines, cell therapies, and immunomodulators. They work in different ways. The success of immunotherapy depends on the type of cancer and the stage of the cancer. Immunotherapies can have side effects. These include rash, diarrhea, fatigue, and fever. More severe side effects include infusion reactions, immune overreactions, and autoimmune reactions. These can cause organ damage. Immunotherapy is a promising new treatment for cancer, but it is still under development. It is not always effective, and it is not suitable for everyone with cancer. The cost of immunotherapy can be high. Medicare, Medicaid, or commercial health insurance may cover some or all of the cost. There are also financial assistance programs available. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer ? BMC Med . 2016;14:73. doi:10.1186/s12916-016-0623-5 National Cancer Institute. Immunotherapy for cancer . Cancer Research Institute. Immunotherapy by cancer type . Cancer Research Institute. Immunotherapy in depth . National Cancer Institute. Immunotherapy and… nothing else? Studies test potential paradigm shift in cancer treatment . American Cancer Society. How immunotherapy is used to treat cancer . National Cancer Institute. Immune checkpoint inhibitors . National Cancer Institute. Immune system modulators to treat cancer . National Cancer Institute. T-cell transfer therapy - immunotherapy . National Cancer Institute. Cancer treatment vaccines - immunotherapy . Understanding Cancer Immunotherapy Research. Immunotherapy drugs . American Cancer Society. Intravesical therapy for bladder cancer . Ferrucci PF, Pala L, Conforti F, Cocorocchio E. Talimogene laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma . Cancers (Basel) . 2021;13(6):1383. doi:10.3390/cancers13061383 By Jennifer Welsh Welsh is a Connecticut-based freelance science and health writer with a graduate certificate in science communication from UCSC. - Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this! August 8, 2024 This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility: fact-checked trusted source Lifestyle habits can alleviate the effects of cancer treatment in childrenby Béatrice St-Cyr-Leroux, University of Montreal  Over the past 30 years, the success rate of pediatric cancer treatments has improved dramatically. It is now above 80%—and even higher for some cancers. While the reduction in mortality is heartening, there's a downside: about two-thirds of children who survive cancer will later suffer adverse effects from the aggressive treatments they received at a young age. Cancer treatments can cause damage to growing bodies, including neurocognitive, endocrine and cardiometabolic complications such as dyslipidemia, hypertension and prediabetes. To alleviate these effects, several years ago a multidisciplinary research team at Sainte-Justine children's hospital in Montreal—including Valérie Marcil, a professor in the Department of Nutrition at Université de Montréal—launched an initiative called Projet VIE. The project has proven successful and is being extended this summer to all university hospitals in Quebec that treat children with cancer: CHU de Québec-Université Laval, CHU de Sherbrooke and the Montreal Children's Hospital. Intervening as soon as possibleProjet VIE and its Quebec-wide version, Projet VIE-Québec, promote healthy lifestyle habits (nutrition, physical activity and mental health ) to mitigate the effects of cancer during and after treatment. "The aim is to promote the well-being of children and teens with cancer," Marcil said. "We intervene as soon as possible after diagnosis because the treatments quickly cause changes" such as digestive complications and the ability to properly taste food. Projet VIE has nutritionists who provide personalized follow-up and lead cooking workshops to encourage children and their families to eat a balanced diet , help them discover new foods and make eating an enjoyable experience again. Patients and their families also receive psychological counseling and participate in physical activities, since exercise has protective effects on many of the body's systems. 'They've regained their confidence'Young people who take part in Projet VIE along with their families appreciate the support provided by the program, Marcil said. "Many children with cancer feel a loss of control over their bodies and develop a negative self-image. With this more comprehensive approach, they tell us they've regained the confidence to be physically active and rediscovered the joy of eating." Marcil believes promoting a healthy lifestyle will also yield long-term benefits, particularly for cardiometabolic health, and she'll be studying this hypothesis in the coming years. Explore further Feedback to editors  US health regulator rejects MDMA treatment for PTSD, for now22 hours ago  Study finds baked potatoes can improve heart health for diabeticsAug 9, 2024  National study shows how internal medicine chief residency has changed over 20 years Vegan diet better than Mediterranean, finds new research Memory problems in old age linked to a key enzyme, study in mice finds Key factor found in drug-context links, relapse Researchers outline promises, challenges of understanding AI for biological discovery The dengue vaccine is effective and safe: Confirmation from the first global meta-analysis 'PTNM' system provides new classification for Peyronie's disease and penile curvature Researchers crack a key celiac mystery: Where the gluten reaction beginsRelated stories, precision medicine for pediatric cancers: new hope for children and adolescents. Apr 29, 2019  When do Quebec doctors recommend exercise?Jun 14, 2024  Report: Canada should ban all unhealthy food marketing that may be seen by childrenMar 20, 2024  Study: Preventing type 2 diabetes in young people is possible without medicationMar 14, 2023  Rebooting healthy eating habits in child cancer survivorsJul 13, 2020  How play can help break the cycle of violenceFeb 7, 2024 Recommended for you Epigenetic change to DNA associated with cancer risk in 'multi-omics' study Feedback loop promotes cancer cells' adaption to molecular stress Unmasking hidden potential: LMO4 enhances T cell cancer-fighting abilities Cannabis use tied to head and neck cancerAug 8, 2024  Drug shows promise for treating brain tumors resulting from breast cancer, trial reports Computer simulations clarify how breast cancer spreadsLet us know if there is a problem with our content. Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ). Please select the most appropriate category to facilitate processing of your request Thank you for taking time to provide your feedback to the editors. Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages. E-mail the storyYour email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form. Newsletter sign upGet weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties. More information Privacy policy Donate and enjoy an ad-free experienceWe keep our content available to everyone. Consider supporting Science X's mission by getting a premium account. E-mail newsletterThere’s a powerful story behind every headline at Ohio State Health & Discovery. As one of the largest academic health centers and health sciences campuses in the nation, we are uniquely positioned with renowned experts covering all aspects of health, wellness, science, research and education. Ohio State Health & Discovery brings this expertise together to deliver today’s most important health news and the deeper story behind the most powerful topics that affect the health of people, animals, society and the world. Like the science and discovery news you find here? You can support more innovations fueling advances across medicine, science, health and wellness by giving today. BROUGHT TO YOU BY- The Ohio State University
- College of Dentistry
- College of Medicine
- College of Nursing
- College of Optometry
- College of Pharmacy
- College of Public Health
- College of Veterinary Medicine
- Ohio State Wexner Medical Center
- Ohio State's Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
Subscribe. The latest from Ohio State Health & Discovery delivered right to your inbox. Reversing the side effects of immunotherapy cancer treatmentWhen immunotherapy triggers autoimmune and other side effects, a unique clinic is giving patients relief. Contributing Writer Ohio State Wexner Medical Center - Share on Facebook
- Share on Linkedin
- Share via Email
- Share this page
After months of breast cancer treatment, Julie Wullkotte was accustomed to enduring difficult side effects. But knowing her cancer cells were being destroyed was enough to keep her from complaining. The burning sensation in her mouth was different. Her mouth felt on fire whenever she ate anything other than the blandest foods. The slightest addition of garlic, onion or black pepper was like inhaling ghost peppers. When she lost 15 pounds and complained the pain was affecting her quality of life, Wullkotte was referred to the Immunotherapy Management Clinic at The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute ( OSUCCC – James ). The clinic is among the first of its kind in the United States, with a team of experts in rheumatology managing side effects of immunotherapy . Wullkotte was diagnosed with a Sjögren’s-like syndrome, an autoimmune disease that’s a side effect of her immunotherapy drug. The same treatment that kept her cancer-free was also causing her immune system to attack itself. With the help of Alexa Meara, MD , a rheumatologist at the OSUCCC – James, Wullkotte got better. “She knew exactly what it was and created a treatment plan to reverse the problem,” Wullkotte says. Today, she hardly has symptoms. “I can eat Mexican food again, put pepper on my eggs in the morning and brush my teeth without screaming.”  Understanding immunotherapy and related side effectsImmunotherapy for cancer is a treatment that uses the body’s own natural defense systems to target and destroy cancer cells. Immunotherapy has quickly become the first line in treating many cancers. For these patients, cancer becomes more like a chronic disease that waxes and wanes. A side effect of immunotherapy is that the therapy can cause a patient’s immune system to go haywire. Immunotherapy’s side effects can occur at the time of treatment or even months or years later. Despite these adverse events, immunotherapy’s benefits outweigh the risks. “You have a subset of patients with a purposely disordered immune system to keep the cancer at bay. However, all you need is some sort of other environmental stimulus, and now you have a new autoimmune disease,” Dr. Meara says. The more common immunotherapy treatment becomes, the more Dr. Meara sees patients experiencing side effects.  Improving quality of life for cancer patients and survivorsDr. Meara and her team achieve results people dream about when deciding to become a doctor, such as: - A patient confined to a wheelchair from severe joint pain can walk again.
- A man with aggressive mouth sores can finally speak and swallow food and liquid again.
- A patient covered in skin rashes heals and regains quality of life.
Inflammation is a common side effect for patients with cancer treated by immunotherapy . As the treatment boosts the immune system to attack cancer cells, this heightened immune response can also target healthy tissues, causing inflammation . Rheumatologists like Dr. Meara are trained to treat inflammation. “Most people think of rheumatic diseases as joint pain and rheumatoid arthritis, but there’s a whole world of autoimmune diseases that have nothing to do with joints,” Dr. Meara says. For cancer patients who suffer from severe side effects of the immunotherapy drugs that kill cancer, Dr. Meara’s treatment can seem like a miracle. To Dr. Meara, it’s a matter of solving a puzzle. “I developed a reputation by figuring out the rarest of the rare diagnoses. And what’s more complicated than a patient with cancer and then weird autoimmune symptoms?” she says. Giving immunotherapy patients their lives backOn any given day at the Immunotherapy Management Clinic, Dr. Meara and her team see everything from patients with joints so swollen they can’t walk to those experiencing skin rashes and sores. With the constraint of keeping the patient on the cancer medication, Dr. Meara’s goal is to determine what medication or lifestyle modification will reduce or cure a patient’s symptoms. Each case typically takes some trial and error, but the results can feel like a miracle to patients. Kara Corps, DVM, PhD, an assistant professor in the Department of Veterinary Biosciences, received immunotherapy for her triple-negative breast cancer and developed rare side effects from the treatment. “I was declared cancer-free in December 2022, but my care is ongoing in the immunotherapy clinic to manage the side effects of the treatment that saved my life. I’m receiving extraordinary care that maintains my quality of life,” she says.  In the year since Mary Caldwell, APRN-CNP, joined the clinic as a nurse practitioner, she says it’s not unusual for patients to get back to feeling like their old selves after first coming to the clinic in wheelchairs due to pain or severe fatigue. “They tell us they wish they would have found us earlier, but they’re so thankful we’re able to bring them back to their normal level of function,” Caldwell says. Amanda Logsdon, RN, says Dr. Meara goes above and beyond to figure out the complexities of patients’ symptoms. “They often come here as a last-ditch effort and with significant depression because no one can help them,” Logsdon says. With the clinic’s help, many patients’ lives are turned around. “It makes me feel so proud to be at her side to help accomplish these goals for the patient,” Logsdon says. Opening doors to chronic disease research and careAutoimmune diseases in general can be difficult to diagnose. The advent of immunotherapy means they’re showing up in cancer patients in new ways.  The challenge of treating rheumatology-oncology patients also presents opportunities. For example, Dr. Meara sees patients develop new disorders, like type 1 diabetes, nearly overnight. There are case reports of using some of the drugs designed for rheumatoid arthritis to reverse that. “If we can open the door to inflammation and type 1 diabetes, that could be a game changer,” Dr. Meara says. As immunotherapy continues to cure more patients’ cancer, there’s still much to learn about how the therapy affects patients’ immune systems, says Dr. Meara. “I think oncology is changing the face of rheumatology and autoimmune diseases in a way that is fundamentally changing the immune system. There’s a whole new world out there, and I think that’s really exciting.” Specialized treatment for immunotherapy side effectsThe James Immunotherapy Management Clinic can help.  - Autoimmune Disease ,
- Cancer Care ,
- Immuno-Oncology ,
- Immunosuppressive ,
- Immunotherapy ,
- Immunotherapy Side Effects ,
- Immunotherapy Treatment ,
- OSUCCC – James
Related websites- Ohio State's Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
Articles on Health Hitting the gravel to fuel cancer research at Pelotonia’s Gravel DayBy Steve Wartenberg A gravel bike ride is raising money for cancer research at Ohio State as part of Pelotonia.  How to organize your medical recordsBy George Xanthopoulos, RHIA When faced with a complex medical problem, organized medical records can help you stay focused on getting better. An Ohio State expert has tips. Is menopause related to dementia?By Jonathan Schaffir, MD When are hiccups serious?By J. Chad Hoyle, MD Going bald too early?By Kelly Tyler, MD Get articles and stories about health, wellness, medicine, science and education delivered right to your inbox from the experts at Ohio State. Required fields By clicking "Subscribe" you agree to our Terms of Use . Learn more about how we use your information by reading our Privacy Policy . Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. - View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS FEATURE
- 14 February 2024
- Correction 15 February 2024
The future of precision cancer therapy might be to try everythingElie Dolgin is a science journalist in Somerville, Massachusetts. You can also search for this author in PubMed Google Scholar Illustration: Shira Inbar The blood cancer had returned, and Kevin Sander was running out of treatment options. A stem-cell transplant would offer the best chance for long-term survival, but to qualify for the procedure he would first need to reduce the extent of his tumour — a seemingly insurmountable goal, because successive treatments had all failed to keep the disease in check. Access optionsAccess Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription 24,99 € / 30 days cancel any time Subscribe to this journal Receive 51 print issues and online access 185,98 € per year only 3,65 € per issue Rent or buy this article Prices vary by article type Prices may be subject to local taxes which are calculated during checkout Nature 626 , 470-473 (2024) doi: https://doi.org/10.1038/d41586-024-00392-2 Updates & CorrectionsCorrection 15 February 2024 : An earlier version of this feature stated that Philipp Staber’s lab uses a 386-well plate. Kornauth, C. et al. Cancer Discov. 12 , 372–387 (2022). Article PubMed Google Scholar Snijder, B. et al. Lancet Haematol. 4 , e595–e606 (2017). O’Dwyer, P. J. et al. Nature Med. 29 , 1349–1357 (2023). Malani, D. et al. Cancer Discov. 12 , 388–401 (2022). Ranjan, T. et al. Cell Rep. Med. 4 , 101025 (2023). Ranjan, T. et al. Neurooncol Adv. 5 , vdad055 (2023). PubMed Google Scholar Manzano-Muñoz, A. et al. npj Precis. Oncol. 6 , 90 (2022). Mayoh, C. et al. Cancer Res. 83 , 2716–2732 (2023). Al-Aloosi, M. et al. Front. Oncol. 13 , 1267650 (2024). Gray, H. J. et al. npj Precis. Oncol. 7 , 45 (2023). Bangi, E. et al. iScience 24 , 102212 (2021). Bangi, E. et al. Sci. Adv. 5 , eaav6528 (2019). Tsai, L. L. et al. Ann. Surg. 277 , e1143–e1149 (2023). Peruzzi, P. et al. Sci. Transl. Med. 15 , eadi0069 (2023). Download references Reprints and permissions Related Articles  Neuronal substance P drives metastasis through an extracellular RNA–TLR7 axis Article 07 AUG 24 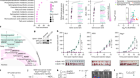 Glycosphingolipid synthesis mediates immune evasion in KRAS-driven cancer 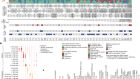 The genomic landscape of 2,023 colorectal cancers  Engineered brain parasite ferries useful proteins into neurons Research Highlight 07 AUG 24  Blood tests could soon predict your risk of Alzheimer’s News Feature 07 AUG 24  Breast-cancer cells enlist nerves to spread throughout the body News 07 AUG 24 Recruitment of Talent Positions at Shengjing Hospital of China Medical UniversityCall for top experts and scholars in the field of science and technology. Shenyang, Liaoning, China Shengjing Hospital of China Medical University  The Recruitment of Fuyao University of Science and TechnologyThis recruitment of Fuyao University Technologyof Science andUcovers 7 departments including the 6 Schools and the Faculty of Fundamental Disciplines. Fuzhou, Fujian (CN) Fuzhou FuYao Institute for Advanced Study  Educational ConsultantYou will build and maintain strong relationships with local representatives, key distributors, schools, Ministries of Education, etc. Riyadh - hybrid working model Springer Nature Ltd  Senior Marketing Manager – Journal AwarenessJob Title: Senior Marketing Manager – Journal Awareness Location(s): London, UK - Hybrid Working Model Closing date: 25th August 2024 A... London (Central), London (Greater) (GB)  Faculty Positions& Postdoctoral Research Fellow, School of Optical and Electronic Information, HUSTJob Opportunities: Leading talents, young talents, overseas outstanding young scholars, postdoctoral researchers. Wuhan, Hubei, China School of Optical and Electronic Information, Huazhong University of Science and Technology  Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Quick links- Explore articles by subject
- Guide to authors
- Editorial policies
 An official website of the United States government Here’s how you know Official websites use .gov A .gov website belongs to an official government organization in the United States. Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites. Biden-Harris Administration Awards Nearly $9 Million to Improve Access to Cancer Screening and Connections to Follow-up Treatment in Underserved Communities to Deliver on Biden Cancer Moonshot GoalsFunding will leverage outreach specialists and patient navigators to conduct engagement in underserved communities to promote early cancer detection, connect people to screening services, and provide assistance accessing cancer care and treatment. Today, to mark National Health Center Week, the U.S. Department of Health and Human Services (HHS), through the Health Resources and Services Administration (HRSA), awarded nearly $9 million to 18 HRSA-funded health centers to improve access to life-saving cancer screenings in underserved communities. Health centers will partner directly with National Cancer Institute-Designated Cancer Centers to expedite patient access to cancer care and treatment. These awards advance the Biden Cancer Moonshot mission to prevent 4 million cancer deaths by 2047 and end cancer as we know it. This effort builds on work supported by the 21st Century Cures Act to expand use of proven cancer prevention and early detection strategies to reduce cancer risk in all populations. “HHS supports efforts to help people live longer, healthier lives. That’s why we are doing all we can to make cancer prevention and screening services accessible to all Americans,” said HHS Secretary Xavier Becerra. “The funding for health centers announced today is another step towards reducing health disparities across races, ethnicities, genders, and incomes—which is essential to realizing the President’s goal of ending cancer as we know it.” Two years ago, President Biden and First Lady Jill Biden reignited the Cancer Moonshot and set two national goals: To decrease the cancer death rate by at least 50% over 25 years and to improve the experience of people who are touched by cancer. The Biden-Harris Administration placed a strong emphasis on cancer screening, since Americans missed more than 10 million cancer screenings during the early days of the COVID-19 pandemic and patient outcomes are drastically improved with early detection. “No matter where you live or what resources you have, everyone should be able to benefit from the tools we have to detect, diagnose and treat cancer before it’s too late,” said HRSA Administrator Carole Johnson. “HRSA is proud to increase our investment in partnerships between our health centers and cancer centers to improve access to live-saving cancer prevention in communities that have been underserved for too long.” Cancer is the second-leading cause of death in the United States, with approximately 600,000 deaths annually. Appropriate screening and timely follow-up care help to detect cancer early and improve outcomes for patients. However, significant disparities in cancer screening and follow-up care persist, particularly among individuals of different income levels, insurance statuses, and racial or ethnic backgrounds. Today’s awards build on HRSA’s previous investment of $11 million in 2023 and $5 million in 2022 announced as part of the Biden Cancer Moonshot. HRSA’s Health Center Program is a cornerstone of our country’s health care system, especially for individuals and families who are uninsured, enrolled in Medicaid, living in rural or underserved areas, struggling to afford co-pays, experiencing homelessness, residing in public housing, or having difficulty finding a doctor or paying for care. To locate a HRSA-supported health center, visit: https://findahealthcenter.hrsa.gov . See the table below for a full list of the Fiscal Year 2024 Accelerating Cancer Screening awardees announced today: | Health Center | City | State | Award Amount |
|---|
| Operation Samahan, Inc. | National City | CA | $500,000 | | WellSpace Health | Sacramento | CA | $500,000 | | Centro de Salud de la Comunidad de San Ysidro, Inc. | San Diego | CA | $500,000 | | Denver Health and Hospitals Authority | Denver | CO | $500,000 | | Healthlinc, Inc. | Valparaiso | IN | $500,000 | | Bronx Community Health Network, Inc. | Bronx | NY | $500,000 | | Sunset Park Health Council, Inc. | Brooklyn | NY | $500,000 | | The Institute for Family Health | New York | NY | $500,000 | | Lower Lights Christian Health Center, Inc. | Columbus | OH | $500,000 | | Comanche County Hospital Authority | Lawton | OK | $500,000 | | Resources for Human Development, Inc. | Philadelphia | PA | $491,693 | | Stephen F. Austin Community Health Center, Inc. | Alvin | TX | $500,000 | | Lone Star Community Health | Conroe | TX | $499,393 | | Gulf Coast Health Center, Inc. | Port Arthur | TX | $500,000 | | Midtown Community Health Center, Inc. | Ogden | UT | $499,973 | | Neighborhood Health | Alexandria | VA | $500,000 | | Peninsula Community Health Services | Bremerton | WA | $500,000 | | Sea-Mar Community Health Center | Seattle | WA | $500,000 |
Sign Up for Email UpdatesReceive the latest updates from the Secretary, Blogs, and News Releases Subscribe to RSSReceive latest updates  Related News ReleasesBiden-harris administration announces more than $68 million to improve access to hiv care for women, infants, children and youth, hhs, treasury, and interior departments and the executive office of the president aim to reduce administrative burden for tribal grant recipients, biden-harris administration invests over $200 million to help primary care doctors, nurses, and other health care providers improve care for older adults, related blog posts.  Upholding Research Integrity at HHSMedia inquiries. For general media inquiries, please contact [email protected] . Disclaimer Policy: Links with this icon ( ) mean that you are leaving the HHS website.- The Department of Health and Human Services (HHS) cannot guarantee the accuracy of a non-federal website.
- Linking to a non-federal website does not mean that HHS or its employees endorse the sponsors, information, or products presented on the website. HHS links outside of itself to provide you with further information.
- You will be bound by the destination website's privacy policy and/or terms of service when you follow the link.
- HHS is not responsible for Section 508 compliance (accessibility) on private websites.
For more information on HHS's web notification policies, see Website Disclaimers . How CRISPR Is Changing Cancer Research and TreatmentJuly 27, 2020 , by NCI Staff  CRISPR is a highly precise gene editing tool that is changing cancer research and treatment. Ever since scientists realized that changes in DNA cause cancer , they have been searching for an easy way to correct those changes by manipulating DNA . Although several methods of gene editing have been developed over the years, none has really fit the bill for a quick, easy, and cheap technology. But a game-changer occurred in 2013, when several researchers showed that a gene-editing tool called CRISPR could alter the DNA of human cells like a very precise and easy-to-use pair of scissors. The new tool has taken the research world by storm, markedly shifting the line between possible and impossible. As soon as CRISPR made its way onto the shelves and freezers of labs around the world, cancer researchers jumped at the chance to use it. “CRISPR is becoming a mainstream methodology used in many cancer biology studies because of the convenience of the technique,” said Jerry Li, M.D., Ph.D., of NCI’s Division of Cancer Biology . Now CRISPR is moving out of lab dishes and into trials of people with cancer. In a small study, for example, researchers tested a cancer treatment involving immune cells that were CRISPR-edited to better hunt down and attack cancer. Despite all the excitement, scientists have been proceeding cautiously, feeling out the tool’s strengths and pitfalls, setting best practices, and debating the social and ethical consequences of gene editing in humans. How Does CRISPR Work?Like many other advances in science and medicine, CRISPR was inspired by nature. In this case, the idea was borrowed from a simple defense mechanism found in some microbes, such as bacteria. To protect themselves against invaders like viruses, these microbes capture snippets of the intruder’s DNA and store them away as segments called CRISPRs, or clustered regularly interspersed short palindromic repeats. If the same germ tries to attack again, those DNA segments (turned into short pieces of RNA ) help an enzyme called Cas find and slice up the invader’s DNA. After this defense system was discovered, scientists realized that it had the makings of a versatile gene-editing tool. Within a handful of years, multiple groups had successfully adapted the system to edit virtually any section of DNA, first in the cells of other microbes, and then eventually in human cells.  CRISPR consists of a guide RNA (RNA-targeting device, purple) and the Cas enzyme (blue). When the guide RNA matches up with the target DNA (orange), Cas cuts the DNA. A new segment of DNA (green) can then be added. In the laboratory, the CRISPR tool consists of two main actors: a guide RNA and a DNA-cutting enzyme, most commonly one called Cas9. Scientists design the guide RNA to mirror the DNA of the gene to be edited (called the target). The guide RNA partners with Cas and—true to its name—leads Cas to the target. When the guide RNA matches up with the target gene's DNA, Cas cuts the DNA. What happens next depends on the type of CRISPR tool that’s being used. In some cases, the target gene's DNA is scrambled while it's repaired, and the gene is inactivated . With other versions of CRISPR, scientists can manipulate genes in more precise ways such as adding a new segment of DNA or editing single DNA letters . Scientists have also used CRISPR to detect specific targets, such as DNA from cancer-causing viruses and RNA from cancer cells . Most recently, CRISPR has been put to use as an experimental test to detect the novel coronavirus . Why Is CRISPR a Big Deal?Scientists consider CRISPR to be a game-changer for a number of reasons. Perhaps the biggest is that CRISPR is easy to use, especially compared with older gene-editing tools. “Before, only a handful of labs in the world could make the proper tools [for gene editing]. Now, even a high school student can make a change in a complex genome ” using CRISPR, said Alejandro Chavez, M.D., Ph.D., an assistant professor at Columbia University who has developed several novel CRISPR tools. CRISPR is also completely customizable. It can edit virtually any segment of DNA within the 3 billion letters of the human genome, and it’s more precise than other DNA-editing tools. And gene editing with CRISPR is a lot faster. With older methods, “it usually [took] a year or two to generate a genetically engineered mouse model , if you’re lucky,” said Dr. Li. But now with CRISPR, a scientist can create a complex mouse model within a few months, he said. Another plus is that CRISPR can be easily scaled up. Researchers can use hundreds of guide RNAs to manipulate and evaluate hundreds or thousands of genes at a time. Cancer researchers often use this type of experiment to pick out genes that might make good drug targets . And as an added bonus, “it’s certainly cheaper than previous methods,” Dr. Chavez noted. What Are CRISPR’s Limitations?With all of its advantages over other gene-editing tools, CRISPR has become a go-to for scientists studying cancer. There’s also hope that it will have a place in treating cancer, too. But CRISPR isn’t perfect, and its downsides have made many scientists cautious about its use in people. A major pitfall is that CRISPR sometimes cuts DNA outside of the target gene—what’s known as “off-target” editing. Scientists are worried that such unintended edits could be harmful and could even turn cells cancerous , as occurred in a 2002 study of a gene therapy . “If [CRISPR] starts breaking random parts of the genome, the cell can start stitching things together in really weird ways, and there’s some concern about that becoming cancer,” Dr. Chavez explained. But by tweaking the structures of Cas and the guide RNA, scientists have improved CRISPR’s ability to cut only the intended target, he added. Another potential roadblock is getting CRISPR components into cells. The most common way to do this is to co-opt a virus to do the job. Instead of ferrying genes that cause disease, the virus is modified to carry genes for the guide RNA and Cas. Slipping CRISPR into lab-grown cells is one thing; but getting it into cells in a person's body is another story. Some viruses used to carry CRISPR can infect multiple types of cells, so, for instance, they may end up editing muscle cells when the goal was to edit liver cells. Researchers are exploring different ways to fine-tune the delivery of CRISPR to specific organs or cells in the human body. Some are testing viruses that infect only one organ, like the liver or brain. Others have created tiny structures called nanocapsules that are designed to deliver CRISPR components to specific cells. Because CRISPR is just beginning to be tested in humans, there are also concerns about how the body—in particular, the immune system —will react to viruses carrying CRISPR or to the CRISPR components themselves. Some wonder whether the immune system could attack Cas (a bacterial enzyme that is foreign to human bodies) and destroy CRISPR-edited cells. Twenty years ago, a patient died after his immune system launched a massive attack against the viruses carrying a gene therapy he had received. However, newer CRISPR-based approaches rely on viruses that appear to be safer than those used for older gene therapies. Another major concern is that editing cells inside the body could accidentally make changes to sperm or egg cells that can be passed on to future generations. But for almost all ongoing human studies involving CRISPR, patients’ cells are removed and edited outside of their bodies. This “ ex vivo ” approach is considered safer because it is more controlled than trying to edit cells inside the body, Dr. Chavez said. However, one ongoing study is testing CRISPR gene editing directly in the eyes of people with a genetic disease that causes blindness, called Leber congenital amaurosis. The First Clinical Trial of CRISPR for CancerThe first trial in the United States to test a CRISPR-made cancer therapy was launched in 2019 at the University of Pennsylvania. The study, funded in part by NCI, is testing a type of immunotherapy in which patients’ own immune cells are genetically modified to better “see” and kill their cancer. The therapy involves making four genetic modifications to T cells , immune cells that can kill cancer. First, the addition of a synthetic gene gives the T cells a claw-like protein (called a receptor ) that “sees” NY-ESO-1, a molecule on some cancer cells. Then CRISPR is used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities. The finished product, dubbed NYCE T cells, were grown in large numbers and then infused into patients.  The first trial of CRISPR for patients with cancer tested T cells that were modified to better "see" and kill cancer. CRISPR was used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities. “We had done a prior study of NY-ESO-1–directed T cells and saw some evidence of improved response and low toxicity ,” said the trial’s leader, Edward Stadtmauer, M.D., of the University of Pennsylvania. He and his colleagues wanted to see if removing the three genes with CRISPR would make the T cells work even better, he said. The goal of this study was to first find out if the CRISPR-made treatment was safe. It was tested in two patients with advanced multiple myeloma and one with metastatic sarcoma . All three had tumors that contained NY-ESO-1, the target of the T-cell therapy. Initial findings suggest that the treatment is safe . Some side effects did occur, but they were likely caused by the chemotherapy patients received before the infusion of NYCE cells, the researchers reported. There was no evidence of an immune reaction to the CRISPR-edited cells. Only about 10% of the T cells used for the therapy had all four of the desired genetic edits. And off-target edits were found in the modified cells of all three patients. However, none of the cells with off-target edits grew in a way that suggested they had become cancer, Dr. Stadtmauer noted. The treatment had a small effect on the patients’ cancers. The tumors of two patients (one with multiple myeloma and one with sarcoma) stopped growing for a while but resumed growing later. The treatment didn't work at all for the third patient. It's exciting that the treatment initially worked for the sarcoma patient because “ solid tumors have been a much more difficult nut to crack with cellular therapy," Dr. Stadtmauer said. "Perhaps [CRISPR] techniques will enhance our ability to treat solid tumors with cell therapies.” Although the trial shows that CRISPR-edited cell therapy is possible, the long-term effects still need to be monitored, Dr. Stadtmauer continued. The NYCE cells are “safe for as long as we’ve been watching [the study participants]. Our plan is to keep monitoring them for years, if not decades,” he said. More Studies of CRISPR Treatments to Come While the study of NYCE T cells marked the first trial of a CRISPR-based cancer treatment, there are likely more to come. “This [trial] was really a proof-of-principle, feasibility, and safety thing that now opens up the whole world of CRISPR editing and other techniques of [gene] editing to hopefully make the next generation of therapies,” Dr. Stadtmauer said. Other clinical studies of CRISPR-made cancer treatments are already underway. A few trials are testing CRISPR-engineered CAR T-cell therapies , another type of immunotherapy. For example, one company is testing CRISPR-engineered CAR T cells in people with B cell cancers and people with multiple myeloma . There are still a lot of questions about all the ways that CRISPR might be put to use in cancer research and treatment. But one thing is for certain: The field is moving incredibly fast and new applications of the technology are constantly popping up. “People are still improving CRISPR methods,” Dr. Li said. “It’s quite an active area of research and development. I’m sure that CRISPR will have even broader applications in the future.” Featured PostsJune 5, 2024, by Linda Wang May 3, 2024, by Carmen Phillips May 1, 2024, by Edward Winstead - Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
Advertisement Supported by Study Puts a $43 Billion Yearly Price Tag on Cancer ScreeningThe estimate focused on five cancers for which there is medically recommended screening — breast, cervical, colorectal, lung and prostate — and found that colonoscopies accounted for most of the costs.  By Gina Kolata The United States spent $43 billion annually on screening to prevent five cancers, according to one of the most comprehensive estimates of medically recommended cancer testing ever produced. The analysis, published on Monday in The Annals of Internal Medicine and based on data for the year 2021, shows that cancer screening makes up a substantial proportion of what is spent every year on cancer in the United States, which most likely exceeds $250 billion. The researchers focused their estimate on breast, cervical, colorectal, lung and prostate cancers, and found that more than 88 percent of screening was paid for by private insurance and the rest mostly by government programs. Dr. Michael Halpern, the lead author of the estimate and a medical officer in the federally funded National Cancer Institute’s health care delivery research program, said his team was surprised by the high cost, and noted that it was likely to be an underestimate because of the limits of the analysis. For Karen E. Knudsen, the chief executive of the American Cancer Society, the value of screening for the cancers is clear. “We are talking about people’s lives,” she said. “Early detection allows a better chance of survival. Full stop. It’s the right thing to do for individuals.” “We screen for cancer because it works,” Dr. Knudsen added. “The cost is small compared to the cost of being diagnosed with late-stage disease.” Other researchers say the finding supports their contentions that screening is overused, adding that there is a weak link between early detection and cancer survival and that the money invested in cancer testing is not being well spent. We are having trouble retrieving the article content. Please enable JavaScript in your browser settings. Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times. Thank you for your patience while we verify access. Already a subscriber? Log in . Want all of The Times? Subscribe .  | 
































































































IMAGES
VIDEO
COMMENTS
Treatment Research. A new cellular immunotherapy approach shrank tumors in 3 of 7 patients with metastatic colon cancer, in a small NCI clinical trial. Normal white blood cells from each patient were genetically engineered to produce receptors that recognize and attack their specific cancer cells.
The earliest evidence of cancer treatment can be traced back to an ancient Egyptian medical text, ... These advances reflect the focus placed on cancer research and oncology by governments ...
Cancer is a global health problem responsible for one in six deaths worldwide. Treating cancer has been a highly complex process. Conventional treatment approaches, such as surgery, chemotherapy, and radiotherapy, have been in use, while significant advances are being made in recent times, including stem cell therapy, targeted therapy, ablation therapy, nanoparticles, natural antioxidants ...
Selected NCI Activities in Cancer Treatment Research. For more than 50 years, NCI has played an active role in cancer drug development—from conducting preclinical studies in the laboratory to testing potential therapies in humans. NCI researchers conduct clinical trials to test cancer treatments at the National Institutes of Health in ...
The American Cancer Society (ACS) has helped make possible almost every major cancer breakthrough since 1946. Since then, we've invested more than $5 billion in cancer research, making us the largest nonprofit funder of cancer research in the United States, outside of the federal government. We remain committed to finding more - and better ...
Nevertheless, after years of painstaking research, CAR T-cell therapies have entered the mainstream of cancer treatment, said Steven Rosenberg, M.D., Ph.D., chief of the Surgery Branch in NCI's Center for Cancer Research (CCR), an immunotherapy and CAR T-cell therapy pioneer. " [CAR T cells] are now widely available in the United States and ...
Cancer therapy describes the treatment of cancer in a patient, often with surgery, chemotherapy and/or radiotherapy. Targeted therapies are also available for some cancer types.
New treatments and technologies offer exciting prospects for cancer research and care, but their global impact rests on widespread implementation and accessibility. Cancer care has advanced at an ...
The MIT team began by treating cancer cells with several different chemotherapy drugs, at different doses. Twenty-four hours after the treatment, the researchers added dendritic cells to each dish, followed 24 hours later by T cells. Then, they measured how well the T cells were able to kill the cancer cells.
In recent years, research into cancer medicine has taken remarkable steps towards more effective, precise and less invasive cancer treatments (Figure 1). While nanomedicine, combined with targeted therapy, helped improving the biodistribution of new or already tested chemotherapeutic agents around the specific tissue to be treated, other ...
Cancer Research and Treatment: Search: Author Index . ORIGINAL ARTICLE 2024 January 18 Phase 1/2a Study of Rivoceranib, a Selective VEGFR-2 Angiogenesis Inhibitor, in Patients with Advanced Solid Tumors: Purpose This study aimed to report the results from an early-phase study of rivoceranib, an oral tyrosine kinase inhibitor highly selective ...
Thanks to Lifesaving Cancer Research18,000,000cancer survivors in the United States are living with, through, and beyond their disease. Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer. Donate Now. 615 Chestnut St., 17th Floor.
Research discoveries made as a result of innovative cancer science are continually being translated into new medical products for cancer prevention, detection, diagnosis, treatment, and survivorship. The approval of new medical products, including new anticancer treatments, is not the end of a linear research process.
The year 2021 defied our expectations in a variety of ways. The delta and omicron COVID-19 variants imposed unprecedented challenges on the health care system and threatened our hopes of an end to the pandemic, but widespread vaccine distribution provided protection, preventing an estimated 36 million cases and 1 million deaths in the United States. As omicron called into question the efficacy ...
Cancer immunotherapy harnesses the body's immune system to identify and destroy cancer cells. Key forms include checkpoint blockades and CAR T cell therapy. Checkpoint blockades, which 'release the brakes' on the immune system, have revolutionized treatment for melanoma and non-small cell lung cancer, and are now approved for over 29 cancers.
Some people with cancer will have only one treatment. But most people have a combination of treatments, such as surgerywith chemotherapyand/or radiation therapy. You may also have immunotherapy, targeted therapy, or hormone therapy. Clinical trialsmight also be an option for you. Clinical trials are research studiesthat involve people.
The research was funded by the National Institute of Cancer in France. Esophageal cancer This German study looked at 438 people with a type of cancer of the esophagus that can be treated with surgery.
Researchers from Harvard Medical School and the Dana-Farber Cancer Institute have figured out how to give a timely boost to a revolutionary treatment that enlists immune cells in the anticancer fight, potentially bypassing a flaw that allows the therapy to fade before all diseased cells are gone.. The researchers, Mohammad Rashidian, assistant professor of cancer immunology at Dana-Farber and ...
Overall, CAR T cells have been successful for the treatment of B cell malignancies and it will be exciting to continue research on this new treatment modality for intractable types of cancer ...
Cancer treatments include surgery, radiotherapy and drug treatments (such as chemotherapy, hormone therapy or targeted cancer drugs). Find out about treatments and side effects. ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee.
Both treatment types encompass multiple drugs and drug types and are used in different cancer types and stages for different reasons. The same is true for radiation. Some cancers only need radiation, while others respond better to chemotherapy plus radiation or other treatments. One cancer treatment is not necessarily "better" than the others.
Over the past 30 years, the success rate of pediatric cancer treatments has improved dramatically. It is now above 80%—and even higher for some cancers. ... Daily science news on research ...
NCI is the nation's leader in cancer research. Learn about key research areas, initiatives, progress, and research tools, including specimens and data. ... Explore the many ways you can participate in cancer research, including treatment and non-treatment studies. Learn More Frederick National Laboratory for Cancer Research: A Decade as a ...
Immunotherapy for cancer is a treatment that uses the body's own natural defense systems to target and destroy cancer cells. Immunotherapy has quickly become the first line in treating many cancers. For these patients, cancer becomes more like a chronic disease that waxes and wanes.
Brain cancer research by a Duke scientist who's spent more than six decades at the university helped a drug receive FDA approval this week.. Why it matters: The approval is the first major advancement in low-grade brain cancer treatment in more than two decades, according to the news outlet Fierce Pharma. Driving the news: The drug, called Voranigo, is the result of collaborative research by ...
The blood cancer had returned, and Kevin Sander was running out of treatment options. A stem-cell transplant would offer the best chance for long-term survival, but to qualify for the procedure he ...
Health centers will partner directly with National Cancer Institute-Designated Cancer Centers to expedite patient access to cancer care and treatment. These awards advance the Biden Cancer Moonshot mission to prevent 4 million cancer deaths by 2047 and end cancer as we know it.
Research has found an association between colorectal cancer and consumption of red meat and processed meat, as well as with low dietary fiber and calcium. Low fruit and vegetable consumption has ...
July 27, 2020 , by NCI Staff. CRISPR is a highly precise gene editing tool that is changing cancer research and treatment. Credit: Ernesto del Aguila III, National Human Genome Research Institute. Ever since scientists realized that changes in DNA cause cancer, they have been searching for an easy way to correct those changes by manipulating DNA.
Dr. Michael Halpern, the lead author of the estimate and a medical officer in the federally funded National Cancer Institute's health care delivery research program, said his team was surprised ...