Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 October 2021

Collaboration in the time of COVID: a scientometric analysis of multidisciplinary SARS-CoV-2 research
- Eoghan Cunningham ORCID: orcid.org/0000-0002-0435-1962 1 , 2 ,
- Barry Smyth ORCID: orcid.org/0000-0003-0962-3362 1 , 2 &
- Derek Greene ORCID: orcid.org/0000-0001-8065-5418 1 , 2
Humanities and Social Sciences Communications volume 8 , Article number: 240 ( 2021 ) Cite this article
3919 Accesses
21 Citations
12 Altmetric
Metrics details
- Complex networks
- Science, technology and society
A Correction to this article was published on 08 November 2021
This article has been updated
The novel coronavirus SARS-CoV-2 and the COVID-19 illness it causes have inspired unprecedented levels of multidisciplinary research in an effort to address a generational public health challenge. In this work we conduct a scientometric analysis of COVID-19 research, paying particular attention to the nature of collaboration that this pandemic has fostered among different disciplines. Increased multidisciplinary collaboration has been shown to produce greater scientific impact, albeit with higher co-ordination costs. As such, we consider a collection of over 166,000 COVID-19-related articles to assess the scale and diversity of collaboration in COVID-19 research, which we compare to non-COVID-19 controls before and during the pandemic. We show that COVID-19 research teams are not only significantly smaller than their non-COVID-19 counterparts, but they are also more diverse. Furthermore, we find that COVID-19 research has increased the multidisciplinarity of authors across most scientific fields of study, indicating that COVID-19 has helped to remove some of the barriers that usually exist between disparate disciplines. Finally, we highlight a number of interesting areas of multidisciplinary research during COVID-19, and propose methodologies for visualising the nature of multidisciplinary collaboration, which may have application beyond this pandemic.
Similar content being viewed by others
Grand challenges and emergent modes of convergence science
Interdisciplinary research attracts greater attention from policy documents: evidence from COVID-19

Interdisciplinarity revisited: evidence for research impact and dynamism
Introduction.
The scientific response to the SARS-CoV-2 pandemic has been unprecedented with researchers from several surprising fields—e.g. artificial intelligence (Nguyen et al., 2021 ), economics (Nicola et al., 2020 ), and particle physics (Lustig et al., 2020 )—contributing to solving the many and varied clinical and societal challenges arising from the pandemic. As a result, by January 2021, The Allen Institute for AI (Allen Institute, 2021 ) and the World Health Organisation (WHO, 2021 ) had identified over 166,000 research papers relating to SARS-CoV-2 and the COVID-19 illness it causes, highlighting an unprecedented period of scientific productivity. In this study we analyse this body of work to better understand the scale and nature of the collaboration and fields of study that have defined this research.
The benefits of collaboration during scientific research are well documented and widely accepted and in recent years there has been steady growth in research team size across all scientific disciplines (Leahey, 2016 ; Youngblood and Lahti, 2018 ), which has been shown to correlate positively with research impact (Lariviere et al., 2014 ; Porter and Rafols, 2008 ; Wuchty et al., 2007 ). Moreover, multidisciplinary science, which brings together researchers from many disparate subject areas has been shown to be among the most successful scientific endeavours (Lariviere et al., 2015 ; Okamura, 2019 ). Indeed, multidisciplinary research has been highlighted as a key enabler when it comes to addressing some of the most complex challenges facing the world today (Leahey, 2016 ). Not surprisingly then, there have been numerous attempts to encourage and promote collaboration and cooperation in the fight against COVID-19: the World Health Organisation maintains a COVID-19 global research database; scientific journals have published explicit calls for teamwork and cooperation (Budd et al., 2020 ; Chakraborty et al., 2020 ); in many cases COVID-19-related research has been made freely available to the public and the scientific community; comprehensive datasets have been created and shared; and reports from the International Chamber of Commerce (ICC) and the Organisation for Economic Co-operation and Development (OECD) have argued for international and multidisciplinary collaboration in the response to the pandemic.
Although early studies have found that the pandemic has generated a significant degree of novel collaboration (Liu et al.; 2020 , Porter and Hook, 2020 ) other research has suggested that COVID-19 research have been less internationally collaborative than expected, compared with recent research from the years immediately prior to the pandemic (Fry et al., 2020 ; Porter and Hook, 2020 ). There is also some evidence that COVID-19 teams have been smaller than their pre-2020 counterparts (Cai et al., 2021 , Fry et al., 2020 ). Thus, despite calls for greater collaboration, the evidence points to less collaboration in COVID-19 related research, perhaps due to the startup and coordination costs associated with multidisciplinary research (Cai et al., 2021 ; Fry et al., 2020 ; Porter and Hook, 2020 ) combined with the urgency of the response to the pandemic.
In this study we evaluate the scale and nature of collaboration in COVID-19 research during 2020, using scientometric analysis techniques to analyse COVID and non-COVID publications before (non-COVID) and during (COVID and non-COVID) the pandemic. We determine the nature of collaboration in these datasets using three different collaboration measures: (i) the Collaboration Index (CI) (Youngblood and Lahti, 2018 ), to estimate the degree of collaboration in a body of research; (ii) author multidisciplinarity to estimate the rate at which authors publish in different disciplines; and (iii) team multidisciplinarity to estimate subject diversity across research teams. We find a lower CI for COVID-related research teams, despite an increasing CI trend for non-COVID work, before and during the pandemic, but COVID-related research is associated with higher author multidisciplinarity and more diverse research teams. This research can help us to better understand the nature of the research that has been conducted under pandemic conditions, which may be useful when it comes to coordinating similar large-scale initiatives in the future. Moreover, we develop a number of techniques for exploring the nature of collaborative research, which we believe will be of general interest to academics, research institutions, and funding agencies.
In this section we describe our methods for evaluating scientific collaboration in COVID-19 research. We describe the data that we use throughout our analysis, and we outline three approaches used to evaluate collaboration activity.
The COVID-19 Open Research Dataset (CORD-19) (Lu Wang et al., 2020 ) comprises more than 400,000 scholarly articles, including over 150,000 with full text, all related to COVID-19, SARS-CoV-2, and similar coronaviruses. CORD-19 papers are sourced from PubMed, PubMed Central, bioRxiv, medRxiv, arXiv, and the World Health Organisation’s COVID-19 database. We generate a set COVID-19-related research by excluding articles dated prior to 2020 and the resulting dataset contains CORD-19 metadata for 166,356 research papers containing the terms "COVID", "COVID-19", "Coronavirus", "Corona virus", "2019-nCoV", "SARS-CoV", "MERS-CoV", "Severe Acute Respiratory Syndrome" or "Middle East Respiratory Syndrome". We supplement this metadata with bibliographic information from the Microsoft Academic Graph (MAG) (Sinha et al., 2015 ).
Notably, we use the MAG fields of study (FoS) to categorise research papers. The MAG uses hierarchical topic modelling to identify and assign research topics to individual papers, each of which represents a specific field of study. To date, this approach has identified a hierarchy of over 700,000 topics within the Microsoft Academic Knowledge corpus. In our dataset of 166,356 COVID-19 research articles, the average paper is associated with 9 FoS from different levels in this hierarchy and in total, 65,427 unique fields are represented. To produce a more useful categorisation of articles, we first reduce the number of topics by replacing each field with its parent and then consider topics at two levels in the FoS hierarchy: (i) the 19 FoS at level 0, which we refer to as ’disciplines’ , and (ii) the 292 FoS at level 1, which we refer to as ‘ sub-disciplines ’. In this way, each article is associated with a set of disciplines (e.g. ’Medicine’, ’Physics’, ’Engineering’) and sub-disciplines (e.g. ’Virology’, ’Particle Physics’, ’Electronic Engineering’), which are identified by traversing the FoS hierarchy from the fields originally assigned to the paper.
We further extend this dataset with any additional research published by the authors in the COVID-related dataset. Thus, for each author, we include MAG metadata from any available articles dated after 2015. The final dataset consists of metadata for 5,389,445 research papers, which we divide into three distinct groups as follows; see Table 1 with further detail provided in the Supplementary materials that accompany this article (Supplementary Tables 1 – 3 ).
2020-COVID-related research: the 166,356 COVID-related articles published during the pandemic (2020);
Pre-2020 research: 4,017,655 non-COVID-related articles published before the pandemic, that is during 2016–2019, inclusive;
2020-non-COVID research: 1,205,434 non-COVID-related articles published during the pandemic period and which are not in the CORD dataset.
Collaboration Index
The Annual Collaboration Index (CI) is defined, for a body of work, as the ratio of the number of authors of co-authored articles to the total number of co-authored articles (Youngblood and Lahti, 2018 ). Since larger (more collaborative) teams have been shown to be more successful than smaller teams (Klug and Bagrow, 2014 ; Lariviere et al., 2014 ; Leahey, 2016 ), we can use CI to compare COVID-related research to non-COVID baselines. However, CI is sensitive to the total number of articles in the corpus. Therefore, to address this bias and facilitate comparison across our COVID and non-COVID baselines, we generate a CI distribution for each dataset by re-sampling 50,000 papers 1000 times, without replacement, from each year, and we calculate the sample distribution for these CI values for each year in our dataset.
Author multidisciplinarity
To evaluate the multidisciplinarity of individual authors, we consider the extent to which they publish across multiple disciplines, based on a network representation of their publications. An un-weighted bipartite network, populated by research fields and authors, links researchers to subjects (that is, based on the subjects of their publications). A projection of this network produces a dense graph of the 292 sub-disciplines at level 1 in the MAG FoS hierarchy, in which two sub-disciplines/fields are linked if an author has published work in both. We refer to this projection as a field of study network . In such a network, the edges between fields are weighted according to the number of authors publishing in both fields. Due to the large number of researchers, and the relatively small number of sub-disciplines, the resulting graph is almost fully connected. Thus, the edge weights are an important way to distinguish between edges. Using the MAG FoS hierarchy, we divide the network nodes into 19 overlapping "communities”, based on their assignment to level 0 fields of study. This facilitates the characterisation of the edges in the graph: an edge within a community represents an author publishing in two sub-disciplines within the same parent discipline, while an edge between communities represents an author publishing in two sub-disciplines from different parent disciplines. For example, if an author publishes research in ’Machine Learning’ and ’Databases’, the resulting edge is considered to be within the community/discipline of ’Computer Science’. Conversely, if an author publishes in ’Machine Learning’ and ’Radiography’, the resulting edge is considered to be between the ’Medicine’ and ’Computer Science’ communities. An edge between disciplines may represent either a single piece of interdisciplinary research or an author publishing separate pieces of research in two different disciplines. To evaluate the effect of COVID-19 on author multidisciplinarity, we produce a field of study network for each year in our dataset and calculate the proportion of the total edge weights that exist between communities. In the special case of 2020 we also explore this proportion for non-COVID research, (i.e., after we remove COVID-19 research from the graph).
Research team disciplinary diversity
In addition to author multidisciplinarity, we also consider the multidisciplinarity of the research teams, by calculating their disciplinary diversity . To do this we compare the research backgrounds of different authors using publication vectors based on the proportions of a researcher’s work published across different fields (Feng and Kirkley, 2020 ). Specifically, we construct publication vectors for authors in our dataset using the 19 MAG disciplines. Thus, an author’s publication vector is a 19-dimensional vector, with each value indicating the proportion of the author’s research published in the corresponding domain. For example, an author who has 50 publications classified under ’Computer Science’, 30 publications classified under ’Mathematics’, and 20 publications classified under ’Biology’ would have a publication vector with values {0.5,0.3,0.2} for the entries corresponding to these disciplines respectively, and zeros elsewhere. By using publication vectors to represent an individual’s research profile, we can quantify the disciplinary diversity of a research team using Eq. ( 1 ) from (Feng and Kirkley, 2020 ).
Note, in Eq. ( 1 ) ∣ p ∣ refers to the size of the research team and S i j is the cosine similarity of the publication vectors for authors i and j . The team research similarity score for an article is a normalised sum of the pairwise cosine similarities for all authors of the article. In cases where we find no available research for a particular author, that author is excluded from the disciplinary similarity calculation. That is, they contribute no publication vector and the disciplinary similarity score is normalised according to an updated team size which excludes that author.
To evaluate research team disciplinary diversity, we compute the teams’ disciplinary similarity based on publication vectors from pre-2020 research, and we report 1− S team as the teams’ diversity. The year of the paper is excluded from the publication vector to avoid reducing team diversity with the common publication. As such, team disciplinary diversities for COVID-related research (and non-COVID research from 2020) are calculated from publication vectors which exclude work from 2020. We compare these scores with disciplinary diversity scores for research in 2019 when, similarly, the publication vectors exclude work from 2019 and 2020. As the potential for disciplinary diversity in research teams is limited by the number of team members, we compare diversity by team size.
Case studies of multidisciplinarity in COVID-19 research
The field of study network structure used to calculate author multidisciplinarity encodes relationships between fields of study, with respect to the authors who publish in them. Since these relationships are altered in COVID-related research, we propose a modified network structure to explore the changes to these relationships visually, and to highlight interesting case studies of multidisciplinary research in the COVID-19 literature. In this modified network structure, COVID-related research articles contribute directed edges (SD A , SD B ) to the graph, for all sub-disciplines SD A in which the authors publish in their pre-2020 work, and all sub-disciplines SD B which relate to the article. For example, an edge between the pair of sub-disciplines ’Machine Learning’ and ’Radiology’ represents an author who published in the field of ’Machine Learning’ in their pre-2020 work (2016–2019), publishing COVID-19 research in the field of ’Radiology’. We produce networks of this structure from different subsets of COVID-related research articles, which we will visualise using flow diagrams, where the pre-2020 sub-disciplines are on the left and the COVID-related disciplines are on the right.
Research team size and Collaboration Index
Figure 1 reports the mean Collaboration Index for the samples of 50,000 research papers taken from each year in the dataset. Mean values for samples of COVID-19 research articles are also included. The Collaboration Index increases year-on-year, indicating a move towards larger research teams. This trend has been noted across many disciplines of academic research (Lariviere et al., 2014 ; Leahey, 2016 ; Porter and Rafols, 2008 ).
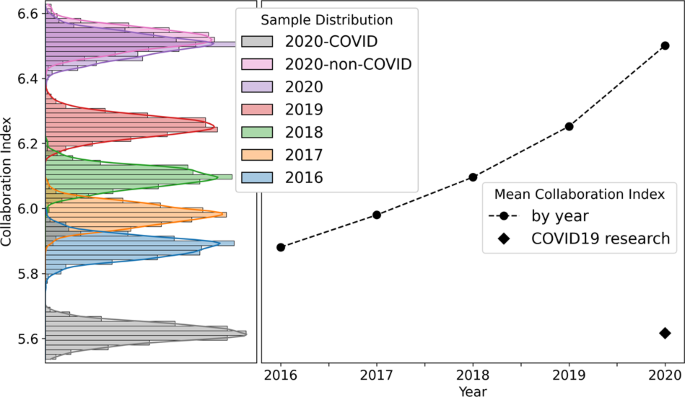
1000 samples are taken from each year (2016–2020). Collaboration index increases annually, r 2 = 0.94, and the CI for COVID-19 articles is significantly less that the CI associated with non-COVID 2020 research; in fact the mean COVID-19 CI is 25 standard deviations below the mean of of non-COVID samples taken from 2020. Thus, research teams publishing COVID-19 research are significantly smaller than expected for research teams in 2020 containing the same authors.
COVID-19 research presents with a very different CI (~5.6), however, indicating that COVID-19 research teams are significantly smaller than expected for research conducted by the same authors in 2020. This result is robust with respect to re-sampling size and in Supplementary materials that accompany this article (see Supplementary Fig. 1 ) we report comparable results using sample sizes n = 10,000 and n = 100,000.
Author multidisciplinary publication
We quantify author multidisciplinarity in a year of research by measuring the proportion of the total number of edges in an author-FoS network that are between communities (i.e., disciplines). We find that this proportion is increasing slowly over time when we produce FoS networks for each year in our data. Figure 2 reports the odds ratio effect size when the proportion of the edges that are between communities in a given year is compared with that of the previous year. These scores are reported for each community and for the entire network. The proportion of external edges in the entire network is shown to increase increase significantly each year, with the greatest increase coming in 2020. In the case of 2020 we also report the odds ratio achieved when we compare 2019 with 2020-non-COVID research i.e., after we remove COVID-19 research from the graph. Figure 2 shows a significant increase in multidisciplinary publication in 2020 across almost all disciplines. The increase in author multidisciplinarity is much greater when we include COVID-19 research in the graph. Despite representing <20% of the work published in 2020, COVID-19 research contributes greatly to the proportion inter-disciplinary edges in the FoS network.
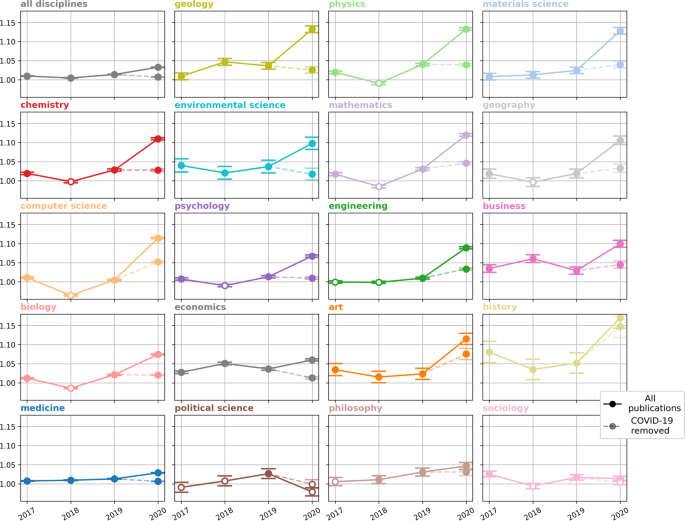
A score of 1 indicates that authors are no more likely to publish in other disciplines than they were in the previous year. Error bars are used to plot a 95% confidence interval and solid points indicate a statistically significant increase in interdisciplinary publication ( p < 0.05) according to Fisher’s Exact test.
When we compare authors by their publication backgrounds, encoded as publication vectors, we find COVID-19 research teams to be more diverse than equivalently-sized research teams who published before 2020. Figure 3 presents the relative increase in mean research team disciplinary diversity for different team sizes, when research teams from 2020 are compared with teams from 2019. We divide 2020 research into two sets: (i) 2020-COVID-related; (ii) 2020-non-COVID research and report relative increases in team diversity for each set. Independent t tests show COVID-19 research teams to be significantly more diverse than both pre-2020 and 2020-non-COVID research teams of the same size ( p < 0.01, see Supplementary Table 5 ).
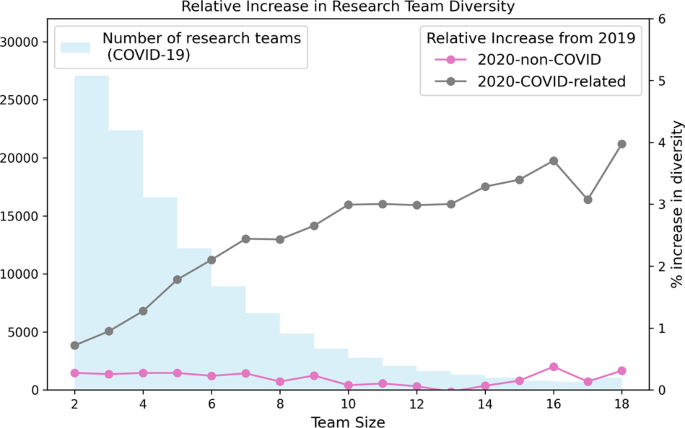
The distribution of research team sizes is also shown.
Despite the recent trend towards larger, more collaborative research teams (Feng and Kirkley, 2020 ; Lariviere et al., 2014 ; Leahey, 2016 ; Porter and Rafols, 2008 ), COVID-19 research appears to have significantly fewer authors than other publications by the same researchers, during 2020. This may be a concerning finding amid evidence that larger teams produce more impactful scientific research (Lariviere et al., 2014 ): it may have limited the value of the research produced, notwithstanding the incredible achievements that have been made, or it may be a reality of working under the constraints of a global pandemic. We do see some examples of larger teams and their greater potential for research impact in our analysis: 20% of COVID-19 research papers have more than 8 listed authors and this portion of the dataset accounts for over 60 of the 100 most cited publications relating to the coronavirus. Yet, the majority of COVID-19 research papers (53%) have 4 or fewer authors. We find no evidence that the reduced Collaboration Index of COVID-19 research is due to working conditions and restrictions during the pandemic. Despite a global shift towards remote work, research in 2020 continues the recent trend of increasing collaboration. The preference for smaller research teams appears to be specific to COVID-19 research and not simply a factor of research during COVID-19.
The prevalence of smaller research teams is important to understand about COVID-19 research. Smaller teams have been shown to play a different role to larger teams in both research and technology (Wu et al., 2019 ). In an analysis of research collaborations, Wu et al. show that small research teams can disrupt science and technology by exploring and amplifying promising ideas from older and less-popular work, while large teams develop on recent successes by solving acknowledged problems (Wu et al., 2019 ). The definition by Wu et al. of disruptive articles relates closely to the metric of betweenness centrality for citation networks. That is, disruptive papers can connect otherwise separate communities in a research network. We find some evidence that COVID-19 research may be increasing the connectivity between disciplines, as authors are more likely to publish across multiple fields and research teams are more diverse. A trend towards greater levels of multidisciplinary collaboration has been identified in many scientific disciplines (Porter and Rafols, 2008 ). This trend is evident in the non-COVID-19 portions of our dataset. Research teams of fewer than 10 members publishing in 2020 exhibit greater disciplinary diversity than similarly-sized teams publishing in 2019, for example. Likewise, the number of authors publishing in multiple disciplines is increasing steadily year-on-year. In COVID-19 research, the increase in multidisciplinarity (of both teams and individuals) exceeds the established trend. This may be evidence of the disruptive nature of COVID-related research. Below, we use flow diagrams to explore author multidisciplinarity in specific topics in the COVID-related research dataset.
Figures 4 – 7 present four selected case studies of author multidisciplinarity in COVID-related research in 2020. To provide a clear visualisation of the strongest trends that exist, each FoS network shows only the 50 edges with the greatest weights. We choose Virology as a case study because it is largest subset in COVID-related research, while Computer Science and Materials Science were chosen to show considerable increases in author multidisciplinarity in 2020 (see Fig. 2 ), and Development Economics presents with a very diverse set of contributing disciplines. For example, Figure 4 shows the intersection between Medicine, Biology and Chemistry in COVID-19 research relating to Virology. Sub-disciplines Molecular Biology, Biochemistry, Immunology, and Virology all appear closely related in this graph. They are strongly interconnected, indicating many instances of authors publishing between disciplines and each acts as both a source and as a destination in the network, as authors who publish in any of these sub-disciplines prior to COVID-19 are likely to publish in the others during COVID-19. Figure 5 illustrates the multidisciplinary nature of Computer Science research in COVID-19. Unlike the Virology graph in Fig. 4 , there are only two destinations in this network: Computer Science and Medicine. Computer Science research in the COVID-19 dataset is primarily focused on Machine Learning solutions to automating COVID-19 detection from medical images (Nguyen et al., 2021 ) (see Supplementary Table 7 (a)). This effort is evident in the graph, as Computer Science research in COVID-19 is most commonly characterised within the sub-disciplines Machine Learning, Artificial Intelligence, Pathology, Surgery and Algorithm. Also evident is the multidisciplinary nature of the effort, as researchers with backgrounds in many of the STEM fields are shown to contribute. Figure 6 reports the FoS network for COVID-19 research relating to Materials Science. The graphs illustrates an intersection between the fields of Physics, Chemistry, Engineering and Materials Science as researchers from each of these disciplines contributes to coronavirus research. Many of the most cited articles in this subset relate to airborne particles and the efficacy of face masks (Lustig et al., 2020 ), along with the use of electrochemical biosensors for pathogen detection (Cesewski and Johnson, 2020 ) (see Supplementary Table 7 (a)). Figure 7 presents the FoS network for the COVID-19-related research papers in the field of Development Economics. Some of the most cited articles in this subset concern studies of the socio-economic implications and effects of the pandemic globally (Nicola et al., 2020 ; Walker et al., 2020 ), and of health inequity in low- and middle-income countries (Patel et al., 2020 ; Wang and Tang, 2020 ) (see Supplementary Table 8 (a)). Research in this subset is characterised by the diverse set of sub-disciplines shown on the left of the figure, as authors with backgrounds in social science, social psychology, medicine, statistics, economics, and biology are all found to contribute.
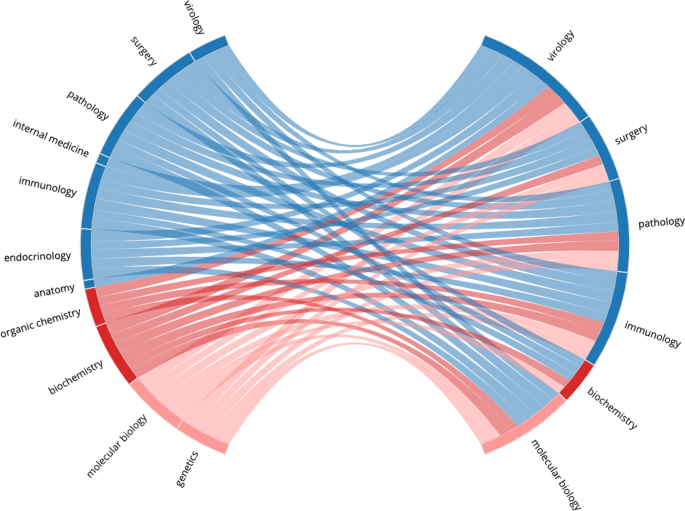
The graph relates an author’s research background to the fields they publish in COVID-related articles. This network is produced from 22,561 COVID-related research papers which were assigned the MAG field `Virology'. Pre-COVID sub-disciplines (common in the research backgrounds of the authors) are shown on the left and COVID-related sub-disciplines (common in the article subset) are shown on the right. Sub-disciplines are coloured by their parent disciplines and edges are assigned the colour of the pre-2020 node. Edges are weighted by the numbers of authors who published in both of the corresponding sub-disciplines. The bi-gram terms which occurred most frequently in the titles of these papers were: COVID-19 pandemic, coronavirus disease, SARS-CoV-2 infection and novel coronavirus . (see Supplementary Table 6 ).
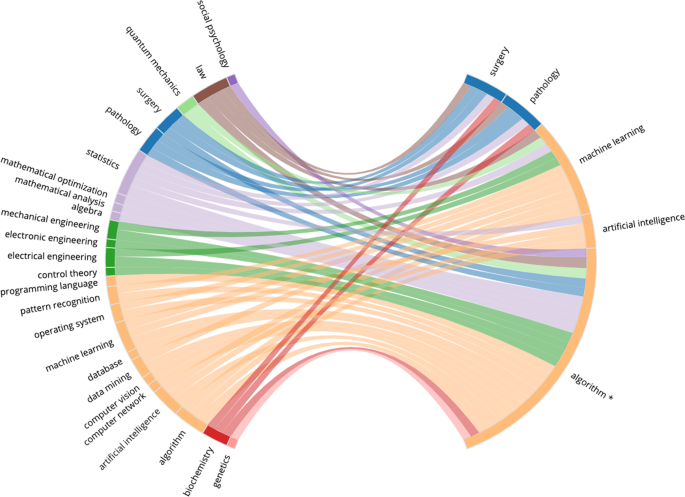
This network is produced from 9004 COVID-related research papers which were attributed the MAG field `Computer Science'. The bi-gram terms which occurred most frequently in the titles of these papers were: COVID-19 pandemic, deep learning, neural network, machine learning, contact tracing and chest x-ray . *The MAG sub-discipline 'Algorithm' is a level 1 parent for any algorithms identified in the fields of study. The most frequently occurring children of the Algorithm field in this subset are 'artificial neural network', 'cluster analysis', 'inference', and ’support vector machine' (see Supplementary Table 7 ).
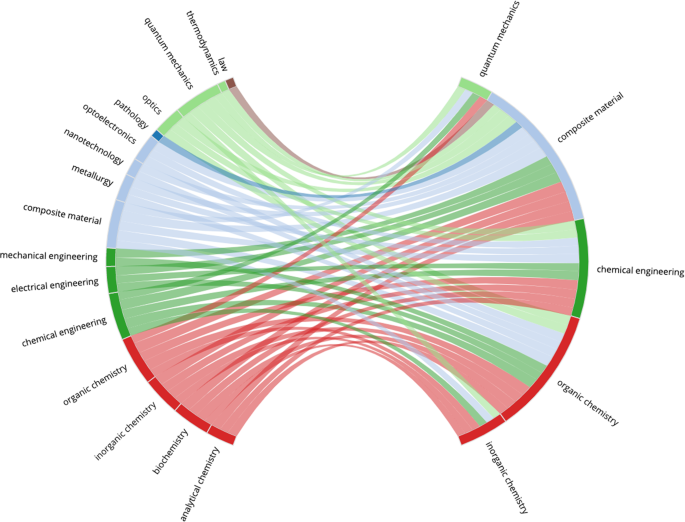
This network is produced from 1229 COVID-related research papers which were attributed the MAG field `Materials science'. The bi-gram terms which occurred most frequently in the titles of these papers were: filtration efficiency, additive manufacturing , and face mask (see Supplementary Table 8 ).

The graph relates an author’s research background to the fields they publish in COVID-related articles. This network is produced from 1564 COVID-related research papers which were attributed the MAG field 'Development Economics'. The most cited articles in this subset concern studies of the socio-economic implications and effects of the pandemic globally (Nicola et al., 2020 ; Walker et al., 2020 ), and of health inequity in low- and middle-income countries (Patel et al., 2020 ; Wang and Tang, 2020 ) (see Supplementary Table 9 ).
The methods outlined in this work could be applied in future scientometric analyses to assess and visualise multidisciplinarity in a body of research. This may be of interest to researchers seeking to understand the evolution of their own field of study, or to funding agencies who recognise the established benefits of multidisciplinary collaboration. In the case of this work, we show COVID-19 research teams to be smaller yet more multidisciplinary than non-COVID-19 teams. It is suggested in early work that authors publishing COVID-19 research favoured smaller, less international collaborations in order to reduce co-ordination costs and contribute to the public health effort sooner (Fry et al., 2020 ). We would like to elaborate on this characterisation of collaboration in COVID-19 research; adding that authors sought to minimise the limitations of working in smaller teams by collaborating with scientists from diverse research backgrounds. That is to say, in the urgency of the pandemic, scientists favour smaller, more multidisciplinary research teams in order to collaborate more efficiently.
Data availability
The data used in our study can be reproduced from the set of Microsoft Academic Graph article IDs available at https://doi.org/10.7910/DVN/ACSGKS .
Change history
08 november 2021.
A Correction to this paper has been published: https://doi.org/10.1057/s41599-021-00957-w
Allen Institute (2021) Allen Institute for A.I. https://allenai.org . Accessed 16 Sep 2021
Budd J et al. (2020) Communication, collaboration and cooperation can stop the 2019 coronavirus. Nat Med 26(2):151–151
Article Google Scholar
Cai X, Fry CV, Wagner CS (2021) International collaboration during the COVID-19 crisis: Autumn 2020 developments. Scientometrics 126(4):3683–3692
Article CAS Google Scholar
Cesewski E, Johnson BN (2020) Electrochemical biosensors for pathogen detection. Biosens Bioelectron 159:112214
Chakraborty C et al. (2020) Extensive partnership, collaboration, and teamwork is required to stop the COVID-19 outbreak. Arch Med Res 51(7):728–730
Feng S, Kirkley A(2020) Mixing patterns in interdisciplinary co-authorship networks at multiple scales. Sci Rep 10(1):1–11
Google Scholar
Fry CV et al. (2020) Consolidation in a crisis: patterns of international collaboration in early COVID-19 research. PLoS ONE 15(7):e0236307–e0236307
Klug M, Bagrow JP(2016) Understanding the group dynamics and success of teams. Royal Soc Open Sci 3(4):160007
Article ADS Google Scholar
Larivière V, Haustein S, Börner K (2015) Long-distance interdisciplinarity leads to higher scientific impact. PLoS ONE 10(3):e0122565–e0122565
Larivière V et al.(2015) Team size matters: Collaboration and scientific impact since 1900. J Assoc Info Sci Technol 66(7):1323–1332
Leahey E (2016) From sole investigator to team scientist: trends in the practice and study of research collaboration. Annu Rev Sociol 42(1):81–100
Liu M et al. (2020) Can pandemics transform scientific novelty? Evidence from COVID-19. CoRR https://arxiv.org/abs/2009.12500
Lu Wang L et al. (2020) CORD-19: the Covid-19 open research dataset. Preprint at https://arxiv.org/abs/200410706v2 https://pubmed.ncbi.nlm.nih.gov/32510522
Lustig S et al. (2020) Effectiveness of common fabrics to block aqueous aerosols of COVID virus-like nanoparticles. ACS Nano 14(6):7651–7658
Nguyen T T et al. (2021) Artificial intelligence in the battle against coronavirus (COVID-19): a survey and future research directions. Preprint at https://arxiv.org/abs/200807343
Nicola M et al. (2020) The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 78:185–193
Okamura K (2019) Interdisciplinarity revisited: evidence for research impact and dynamism. Palgrave Commun 5(1):141
Patel JA et al. (2020) Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health 183:110–111
Porter A, Ràfols I (2008) Is science becoming more interdisciplinary? Measuring and mapping six research fields over time. Scientometrics 81:719–745
Porter SJ, Hook DW (2020) How COVID-19 is changing research culture. Digital Science, London
Sinha A et al. (2015) An overview of Microsoft Academic Service (MAS) and applications. In: (ed Gangemi A) Proceedings of the 24th international conference on World Wide Web. Association for Computing Machinery, New York, pp. 243–246, https://books.google.ie/books/about/Proceedings_of_the_24th_International_Co.html?id=xvFxAQAACAAJ&redir_esc=y
Walker PGT et al. (2020) The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science 369(6502):413–422
Article ADS MathSciNet CAS Google Scholar
Wang Z, Tang K (2020) Combating COVID-19: health equity matters. Nat Med 26(4):458–458
WHO (2021) World Health Organization. https://who.int . Accessed 16 Sep 2021
Wu L, Wang D, Evans JA (2019) Large teams develop and small teams disrupt science and technology. Nature 566(7744):378–382
Article ADS CAS Google Scholar
Wuchty S, Jones B, Uzzi B (2007) The increasing dominance of teams in production of knowledge. Science 316:1036–9
Youngblood M, Lahti D (2018) A bibliometric analysis of the interdisciplinary field of cultural evolution. Palgrave Commun 4:1–9
Download references
Acknowledgements
This research was supported by Science Foundation Ireland (SFI) under Grant Number SFI/12/RC/2289_P2.
Author information
Authors and affiliations.
Insight SFI Research Centre for Data Analytics, University College, Dublin, Ireland
Eoghan Cunningham, Barry Smyth & Derek Greene
School of Computer Science, University College, Dublin, Ireland
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Eoghan Cunningham .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementals, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cunningham, E., Smyth, B. & Greene, D. Collaboration in the time of COVID: a scientometric analysis of multidisciplinary SARS-CoV-2 research. Humanit Soc Sci Commun 8 , 240 (2021). https://doi.org/10.1057/s41599-021-00922-7
Download citation
Received : 05 July 2021
Accepted : 27 September 2021
Published : 19 October 2021
DOI : https://doi.org/10.1057/s41599-021-00922-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Artificial intelligence centric scientific research on covid-19: an analysis based on scientometrics data.
- Amit K. Shukla
- Taniya Seth
- Pranab K. Muhuri
Multimedia Tools and Applications (2023)
Leadership and international collaboration on COVID-19 research: reducing the North–South divide?
- Danilo Silva Carvalho
- Lucas Lopes Felipe
- Bruna de Paula Fonseca
Scientometrics (2023)
How the Covid-19 crisis shaped research collaboration behaviour
- Giovanni Abramo
- Ciriaco Andrea D’Angelo
- Flavia Di Costa
Scientometrics (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Role of intelligent computing in COVID-19 prognosis: A state-of-the-art review
Affiliations.
- 1 Department of Information Technology, Veer Surendra Sai University of Technology (VSSUT), Burla, Sambalpur-768018, Odisha, India.
- 2 Department of Computer Science and Engineering, Aditya Institute of Technology and Management (AITAM), Tekkali, Andhra Pradesh 532201, India.
- 3 Department of Computer Application, Veer Surendra Sai University of Technology (VSSUT), Burla, Sambalpur-768018, Odisha, India.
- PMID: 32836916
- PMCID: PMC7256553
- DOI: 10.1016/j.chaos.2020.109947
The World Health Organization (WHO) declared novel coronavirus 2019 (COVID-19), an infectious epidemic caused by SARS-CoV-2, as Pandemic in March 2020. It has affected more than 40 million people in 216 countries. Almost in all the affected countries, the number of infected and deceased patients has been enhancing at a distressing rate. As the early prediction can reduce the spread of the virus, it is highly desirable to have intelligent prediction and diagnosis tools. The inculcation of efficient forecasting and prediction models may assist the government in implementing better design strategies to prevent the spread of virus. In this paper, a state-of-the-art analysis of the ongoing machine learning (ML) and deep learning (DL) methods in the diagnosis and prediction of COVID-19 has been done. Moreover, a comparative analysis on the impact of machine learning and other competitive approaches like mathematical and statistical models on COVID-19 problem has been conducted. In this study, some factors such as type of methods(machine learning, deep learning, statistical & mathematical) and the impact of COVID research on the nature of data used for the forecasting and prediction of pandemic using computing approaches has been presented. Finally some important research directions for further research on COVID-19 are highlighted which may facilitate the researchers and technocrats to develop competent intelligent models for the prediction and forecasting of COVID-19 real time data.
Keywords: COVID-19; Deep learning; Machine learning; SARS-CoV-2; Statistical methods.
© 2020 Elsevier Ltd. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare that this manuscript has no conflict of interest with any other published source and has not been published previously (partly or in full). No data have been fabricated or manipulated to support our conclusions.
Transmission of Corona viruses from…
Transmission of Corona viruses from animals to Human.
Interpretation of the SARS-CoV-2 virion…
Interpretation of the SARS-CoV-2 virion .
No of positive cases up…
No of positive cases up to May, 3, 2020.
Daily no of deaths.
Week wise Analysis of publications…
Week wise Analysis of publications on COVID-19.
Articles published on COVID-19 using…
Articles published on COVID-19 using different approaches.
Distribution of articles by Journals.
Country wise distribution of articles…
Country wise distribution of articles on diagnosis and prognosis of COVID-19.
Articles published on COVID-19 Prediction,…
Articles published on COVID-19 Prediction, Classification and Forecasting.
Similar articles
- Fusion of intelligent learning for COVID-19: A state-of-the-art review and analysis on real medical data. Ding W, Nayak J, Swapnarekha H, Abraham A, Naik B, Pelusi D. Ding W, et al. Neurocomputing (Amst). 2021 Oct 7;457:40-66. doi: 10.1016/j.neucom.2021.06.024. Epub 2021 Jun 16. Neurocomputing (Amst). 2021. PMID: 34149184 Free PMC article.
- Machine Learning, Deep Learning, and Mathematical Models to Analyze Forecasting and Epidemiology of COVID-19: A Systematic Literature Review. Saleem F, Al-Ghamdi ASA, Alassafi MO, AlGhamdi SA. Saleem F, et al. Int J Environ Res Public Health. 2022 Apr 22;19(9):5099. doi: 10.3390/ijerph19095099. Int J Environ Res Public Health. 2022. PMID: 35564493 Free PMC article. Review.
- A COVID-19 Pandemic Artificial Intelligence-Based System With Deep Learning Forecasting and Automatic Statistical Data Acquisition: Development and Implementation Study. Yu CS, Chang SS, Chang TH, Wu JL, Lin YJ, Chien HF, Chen RJ. Yu CS, et al. J Med Internet Res. 2021 May 20;23(5):e27806. doi: 10.2196/27806. J Med Internet Res. 2021. PMID: 33900932 Free PMC article.
- Intelligent system for COVID-19 prognosis: a state-of-the-art survey. Nayak J, Naik B, Dinesh P, Vakula K, Rao BK, Ding W, Pelusi D. Nayak J, et al. Appl Intell (Dordr). 2021;51(5):2908-2938. doi: 10.1007/s10489-020-02102-7. Epub 2021 Jan 6. Appl Intell (Dordr). 2021. PMID: 34764577 Free PMC article.
- Machine Learning Approaches for Tackling Novel Coronavirus (COVID-19) Pandemic. Rahman MM, Islam MM, Manik MMH, Islam MR, Al-Rakhami MS. Rahman MM, et al. SN Comput Sci. 2021;2(5):384. doi: 10.1007/s42979-021-00774-7. Epub 2021 Jul 19. SN Comput Sci. 2021. PMID: 34308367 Free PMC article. Review.
- Algorithms for predicting COVID outcome using ready-to-use laboratorial and clinical data. Lourenço AA, Amaral PHR, Paim AAO, Marques GF, Gomes-de-Pontes L, da Mata CPSM, da Fonseca FG, Pérez JCG, Coelho-Dos-Reis JGA. Lourenço AA, et al. Front Public Health. 2024 May 14;12:1347334. doi: 10.3389/fpubh.2024.1347334. eCollection 2024. Front Public Health. 2024. PMID: 38807995 Free PMC article.
- COVID-19 Hierarchical Classification Using a Deep Learning Multi-Modal. Althenayan AS, AlSalamah SA, Aly S, Nouh T, Mahboub B, Salameh L, Alkubeyyer M, Mirza A. Althenayan AS, et al. Sensors (Basel). 2024 Apr 20;24(8):2641. doi: 10.3390/s24082641. Sensors (Basel). 2024. PMID: 38676257 Free PMC article.
- Analysis of computational intelligence approaches for predicting disease severity in humans: Challenges and research guidelines. Narasimhan G, Victor A. Narasimhan G, et al. J Educ Health Promot. 2023 Sep 29;12:334. doi: 10.4103/jehp.jehp_298_23. eCollection 2023. J Educ Health Promot. 2023. PMID: 38023081 Free PMC article. Review.
- A novel bidirectional LSTM deep learning approach for COVID-19 forecasting. Aung NN, Pang J, Chua MCH, Tan HX. Aung NN, et al. Sci Rep. 2023 Oct 20;13(1):17953. doi: 10.1038/s41598-023-44924-8. Sci Rep. 2023. PMID: 37863921 Free PMC article.
- Automatic diagnosis of COVID-19 from CT images using CycleGAN and transfer learning. Ghassemi N, Shoeibi A, Khodatars M, Heras J, Rahimi A, Zare A, Zhang YD, Pachori RB, Gorriz JM. Ghassemi N, et al. Appl Soft Comput. 2023 Sep;144:110511. doi: 10.1016/j.asoc.2023.110511. Epub 2023 Jun 13. Appl Soft Comput. 2023. PMID: 37346824 Free PMC article.
- Orbann Carolyn. Defining epidemics in computer simulation models: How do definitions influence conclusions? Epidemics. 2017;19:24–32. - PubMed
- Centers for Disease Control and Prevention. "Principles of epidemiology in public health practice: an introduction to applied epidemiology and biostatistics." (2006).
- Morse Stephen S. Plagues and politics. Palgrave Macmillan; London: 2001. Factors in the emergence of infectious diseases; pp. 8–26.
- Henderson Donald Ainslie. Vol. 237. Prometheus Books; Amherst, NY: 2009. (Smallpox: the death of a disease).
- Spreeuwenberg Peter, Kroneman Madelon, Paget John. "Reassessing the global mortality burden of the 1918 influenza pandemic. Am J Epidemiol. 2018;187(12):2561–2567. - PMC - PubMed
Related information
Linkout - more resources, full text sources.
- Elsevier Science
- Europe PubMed Central
- PubMed Central
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
Microsoft Academic
Impact of covid-19 on the computer science research community, share this page.
By March 10 th , 2020 the novel coronavirus (COVID-19) has infected ~117k and been responsible for the deaths of over 4k people worldwide. The World Health Organization (WHO) has not yet classified the COVID-19 outbreak as a pandemic, however, COVID-19 has resulted in a significant impact on individual lives and global economics. Following the Microsoft Academic team’s goal to “help researchers stay on top of their game”, we are providing an analysis of the COVID-19 impact on the computer science (CS) research community to help enable conference organizers and institutions to respond accordingly and manage the impact.
Our analysis shows that:
- There is increasing author participation in both CS and AI conferences from the COVID-19 impacted regions in the past ten years, from about 6% to over 20% in 2019.
- The disease could impact one-fifth of conference attendees in both AI and CS (21.3%).
- May to early September is the period most CS conferences occur, controlling COVID-19 by May will help to reduce the impact in CS research to a minimum.
- As of this article date, the US Center for Disease Control and Prevention (CDC) has issued a travel warning and alert (opens in new tab) recommending travelers avoid or postpone nonessential travel to areas including: China, South Korea, Iran, Italy and Japan. A handful of airlines have reduced or suspended flights to these areas. Also, some cities have imposed lockdowns to contain the virus.
- On the other hand, CS conference publications rely heavily on authors’ physical presentations during the conference. Since the travel interruption could potentially prevent researchers from attending conferences and thus affecting the publications, this analysis considers the areas above as COVID-19 impacted.
- In computer science, conference publication is often preferred over other publications for its higher visibility, greater impact, and faster turnaround time. The COVID-19 impact is most likely to affect the CS research community through conference publications, hence our focus.
- We have picked the 105 most impactful CS conferences for this analysis. Among these 105 CS conferences, 32 are related to artificial intelligence (AI) and used to analyze the COVID-19 impact on AI research
- 2020 conferences being hosting in the impacted areas
- Authors located in the impacted area. Headquarter locations for each authors’ last known affiliation are used to determine their location
- The number of publications for each CS conferences in 2019 (2018 if it’s biennial)
- The share of publications from COVID-19 affected areas for the past 10 years
- A publication is considered from the COVID-19 affected areas if the headquarter of the first author’s affiliation is located in those areas. Please find the discussion of this approach in section “How to Determine a Publication is Impacted by COVID-19” below
- All publication data is sourced from the Microsoft Academic Graph (opens in new tab)
Analysis and Discussion
1. cs and ai conference publication statistics.
The graph below (Figure 1a) shows the total number of CS and AI conference publications in 2019. Only the top 20 regions are shown here. The US followed by the EU, China, Japan and Canada had the highest volume of published papers among the 105 selected CS conferences as well as in the 32 AI conferences.
The table below shows the number of 2019 publications and percentages for the CDC warning/alert areas. Regions are categorized according to the US CDC travel risk assessment, please refer to the CDC (opens in new tab) for the description of each level. China, Iran, South Korea, Italy, Japan and Hong Kong together contributed 21.25% and 21.33% to CS and AI publications in 2019. This could be the rate of authors who couldn’t attend the conferences in 2020 due to the travel interruption by COVID-19 in these areas.
To further confirm the rate of publications from impacted areas, we gathered data between 2000 and 2019. As shown in Figure 1b, there is clearly an increase in publications from the COVID-19 impacted areas. The impacted publication rate for both CS and AI conferences are above 21% in 2019 and possibly higher in 2020.
2. 2020 CS Conference Publications Impact – by Conference Location
The graph below (Figure 2) shows the estimated impact on 2020 CS conferences hosting in COVID-19 impacted areas. The number of publications from 2019 is used to estimate the number of publications in 2020. The solid blue line shows the accumulated number of impacted publications over time.
3. 2020 CS and AI Conference Publications Impact – by Author Location
The graph below (Figure 3) shows 2020 CS and AI conferences which are hosting outside COVID-19 impacted areas. The numbers of publications for each impacted conference are estimated by the number of publications in 2019 (2018 if it’s biennial) from COVID-19 impacted areas. According to our analysis, among the CS conferences scheduled in the coming four months, ICC, IMTC, ICDE and ISCAS have the most publications contributed from the COVID-19 impacted areas (each above 30%). We listed the conferences in the next four months with the 2019 publication statistics in Appendix 1 at the end. A majority of the AI conferences for the next four months have 10% to 20% impact rate based on 2019 data (Appendix 2).
4. 2020 CS and AI Conference Publications Impact – Total
The graph below (Figure 4) shows the COVID-19 impact estimates in CS and AI conferences combining the impact from both conference location and author location. The impact has a similar pattern in CS and AI conferences. Starting from May 2019, the impact increases considerably as many conferences occur during the summer months (northern meteorological). If COVID-19 can be contained and the travel interruption is lifted before May 2020, the impact on CS conferences should be minimal. On the contrary, if the outbreak situation cannot be improved by September, there could be significant impact to the CS research community.
5. How to Determine if a Publication is Impacted by COVID-19
As mentioned earlier, we consider a publication to be impacted by COVID-19 if the headquarters of the first author’s affiliation is in one of the affected areas.
We choose the first author’s affiliation location instead of all authors because 1) first author normally is the presenter of the paper and 2) it simplifies our analysis while not significantly impacting the result. A previous paper (opens in new tab) pointed out there are 25-fold increases in international collaborations for scientific development. For the CS publications we analyzed, the cross-region collaboration increases from 7.8% to 23.9% in the past 20 years. Although the cross-region rate is high, only 4% of publications have authors from non-impacted regions while first author is located in impacted regions. Therefore, we believe the first author’s location is a good representation of the publication’s locations.
In the case that the first author is associated with multiple affiliations and one affiliation is in affected areas, we count the publication as affected. Only 0.17% of CS publications have first authors associated with multiple affiliations.
Some affiliations could have multiple locations, such as Microsoft. The headquarter location is used in this scenario. And we estimate there are less than 2% such cases.
All the above estimates are based on the the most current information we could obtain using MAG. If the current situation continues, the data shows the potential for significant impact on CS conferences unless conference organizers take actions to mitigate the impact.
Some conference organizers have already taken actions, such as:
- Postpone and change location . INFOCOM 2020, which was originally planned to be in Beijing China in late April, is moving to Toronto Canada in July.
- Create backup plans . The ACM SIGIR Executive Committee is preparing a backup plan for SIGIR 2020 for potential worst case scenarios, e.g. moving from Xi’an China to Toronto Canada if the WHO extend the “Public Health Emergency of International Concern” by the end of April.
- Extend deadlines . The Web Conference 2020 is extending the early bird deadline by four weeks to give attendees more flexibility to plan their trip.
- Enable remote and video presentations . AAAI 2020 enabled authors to present remotely using teleconferencing or by submitting a video presentation.
Additional CS Conference Updates regarding COVID-19
In an effort to help the CS community we will continue to monitor CS conference announcements regarding COVID-19 and provide updates below:
- 2020-03-10: ICLR 2020 (opens in new tab) is working through potential methods to hold a remote conference this year.
Stay healthy and research on!
2020 March to June, Non-AI CS Conferences.
2020 March to June, AI Conferences.
- Follow on X
- Like on Facebook
- Follow on LinkedIn
- Subscribe on Youtube
- Follow on Instagram
- Subscribe to our RSS feed
Share this page:
- Share on Facebook
- Share on LinkedIn
- Share on Reddit
Machine Learning Models for Early Prediction of COVID-19 Infections Based on Clinical Signs
- Original Research
- Published: 06 January 2024
- Volume 5 , article number 158 , ( 2024 )
Cite this article

- Boulbaba Ben Ammar 1 ,
- Ali Salem 1 ,
- Mouna Ben Said 2 , 3 &
- Mohamed Ben Aouicha 1
106 Accesses
Explore all metrics
Nowadays, the appearance of common symptoms, such as cough, fever, and loss of smell and taste, is the starting point of a battle against the coronavirus. The first standard method of COVID-19 infection assertion has become the RT-PCR test, which is however an uncomfortable solution for both patients and medical staff due to its high cost, timeliness, and false-negative result issue. This has raised the need for reliable automatic detection systems that aid in the early prediction of the COVID-19 infections with a lower cost. In this work, we aim at profiting from the Machine Learning (ML) advances to provide a reliable and low-cost COVID-19 prediction system. This system is based on the disease starting point, which is the patients’ clinical symptoms, that are still under-explored. We developed seven predictive models using traditional ML classification algorithms using a public dataset of obvious high-risk factors from patients’ clinical signs. The dataset has first undergone a pre-processing phase consisting of feature engineering and dataset resampling to deal with imbalanced dataset issue. Our best classification model is able to detect true positives and true negatives and weed out false positive and false negatives with an accuracy of 93%.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Machine Learning Approach for Analyzing Symptoms Associated with COVID-19 Risk Factors

A Technical Review on Machine Learning-Based Prediction on COVID-19 Diagnosis

A Case Study of Using Machine Learning Techniques for COVID-19 Diagnosis
Data availability statement.
Publicly available datasets were used in this study. The executable, source code and data are available at: https://github.com/boulbaba1981/COVID-19 .
Coronavirus Disease 2019 Clinical Data Repository. Accessed from https://covidclinicaldata.org/.
Where TP is the true positive, TN is the true negative, FP is the false positive and FN is the false-negative.
Perc M, Miksić NG, Slavinec M, Stožer A. Forecasting covid-19. Front Phys. 2020;8:127.
Article Google Scholar
Neji N, Boulbaba BA, Habib MK. Prediction of COVID-19 active cases using polynomial regression and arima models. In International Conference on Intelligent Systems Design and Applications, 2021;1–12. Springer.
Momtazmanesh S, Ochs HD, Uddin LQ, Perc M, Routes JM, Vieira DN, Al-Herz W, Baris S, Prando C, Rosivall L, Latiff AHA, Ulrichs T, Roudenok V, Becerra JCA, Salunke DB, Goudouris E, Condino-Neto A, Stashchak A, Kryvenko O, Stashchak M, Bondarenko A, Rezaei N. All together to fight COVID-19. Am J Trop Med Hyg. 2020;102(6):1181–3.
Kumar A, Gupta PK, Srivastava A. A review of modern technologies for tackling COVID-19 pandemic. Diabetes & Metabolic Syndrome. Clin Res Rev. 2020;14(4):569–73.
Google Scholar
Mohamadou Y, Halidou A, Kapen PT. A review of mathematical modeling, artificial intelligence and datasets used in the study, prediction and management of COVID-19. Appl Intellig. 2020;50(11):3913–25.
Xiaowei Xu, Jiang Xiangao, Ma Chunlian, Peng Du, Li Xukun, Lv Shuangzhi, Liang Yu, Ni Qin, Chen Yanfei, Junwei Su, et al. A deep learning system to screen novel coronavirus disease 2019 pneumonia. Engineering. 2020;6(10):1122–9.
Wang L, Lin ZQ, Wong A. COVID-net: A tailored deep convolutional neural network design for detection of COVID-19 cases from chest x-ray images. Scient Rep. 2020;10(1):1–12.
Vaid Shashank, Kalantar Reza, Bhandari Mohit. Deep learning covid-19 detection bias: accuracy through artificial intelligence. Int Orthop. 2020;44:1539–42.
Narin A, Kaya C, Pamuk Z. Automatic detection of coronavirus disease (COVID-19) using x-ray images and deep convolutional neural networks. Pattern Analy Appl. 2021;24:1–14.
Ingle VA, Ambad PM. Cvdeep-COVID-19 detection model. SN Computer Sci. 2021;2(3):1–16.
Umer MJ, Amin J, Sharif M, Anjum MA, Azam F, Shah JH. An integrated framework for COVID-19 classification based on classical and quantum transfer learning from a chest radiograph. Concurren Comput Pract Exp. 2021;34.
Chen Nanshan, Zhou Min, Dong Xuan, Jieming Qu, Gong Fengyun, Han Yang, Qiu Yang, Wang Jingli, Liu Ying, Wei Yuan, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–13.
Brinati Davide, Campagner Andrea, Ferrari Davide, Locatelli Massimo, Banfi Giuseppe, Cabitza Federico. Detection of COVID-19 infection from routine blood exams with machine learning: a feasibility study. J Med Syst. 2020;44(8):1–12.
Chowdhury MEH, Rahman T, Khandakar A, Al-Madeed S, Zughaier SM, Doi SAR, Hassen H, Islam MT. An early warning tool for predicting mortality risk of COVID-19 patients using machine learning. Cognit Computat. 2021;1–16.
Assaf Dan, Gutman Ya’ara, Neuman Yair, Segal Gad, Amit Sharon, Gefen-Halevi Shiraz, Shilo Noya, Epstein Avi, Mor-Cohen Ronit, Biber Asaf, et al. Utilization of machine-learning models to accurately predict the risk for critical COVID-19. Int Emerg Med. 2020;15(8):1435–43.
Arpaci I, Huang S, Al-Emran M, Al-Kabi MN, Peng M. Predicting the COVID-19 infection with fourteen clinical features using machine learning classification algorithms. Multimedia Tools Appl. 2021;80(8):11943–57.
Nan SN, Ya Y, Ling TL, Nv GH, Ying PH, Bin J, et al. A prediction model based on machine learning for diagnosing the early COVID-19 patients. medRxiv, 2020.
Muhammad LJ, Algehyne EA, Usman SS, Ahmad A, Chakraborty C, Mohammed IA. Supervised machine learning models for prediction of COVID-19 infection using epidemiology dataset. SN Comput Sci. 2021;2(1):1–13.
Watson J, Whiting P. Coronavirus: how accurate are coronavirus tests. The Conversation, 2020.
Kaur H, Pannu HS, Malhi AK. A systematic review on imbalanced data challenges in machine learning: applications and solutions. ACM Computing Surveys (CSUR). 2019;52(4):1–36.
Rubaidi Z, Ammar BB, Aouicha MB. Fraud detection using large-scale imbalance dataset. Int J Artif Intellig Tools. 09 2022.
Suthaharan Shan. Machine learning models and algorithms for big data classification. Integr Ser Inf Syst. 2016;36:1–12.
MathSciNet Google Scholar
Chen Xiaofeng, Tang Yanyan, Mo Yongkang, Li Shengkai, Lin Daiying, Yang Zhijian, Yang Zhiqi, Sun Hongfu, Qiu Jinming, Liao Yuting, et al. A diagnostic model for coronavirus disease 2019 (COVID-19) based on radiological semantic and clinical features: a multi-center study. Eur Radiol. 2020;30(9):4893–902.
Burian E, Jungmann F, Kaissis GA, Lohöfer FK, Spinner CD, Lahmer T, Treiber M, Dommasch M, Schneider G, Geisler F, et al. Intensive care risk estimation in COVID-19 pneumonia based on clinical and imaging parameters: experiences from the munich cohort. J Clin Med. 2020;9(5):1514.
Villavicencio CN, Jeng J-H, Hsieh J-G. Support vector machine modelling for covid-19 prediction based on symptoms using r programming language. In 2021 The 4th International Conference on Machine Learning and Machine Intelligence, 2021;65–70.
Villavicencio CN, Macrohon JJE, Inbaraj XA, Jeng J-H, Hsieh J-G. COVID-19 prediction applying supervised machine learning algorithms with comparative analysis using Weka. Algorithms. 2021;14(7):201.
Villavicencio CN, Macrohon JJ, Inbaraj XA, Jeng J-H, Hsieh J-G. Development of a machine learning based web application for early diagnosis of COVID-19 based on symptoms. Diagnostics. 2022;12(4):821.
Sun Z, Ding R, Zhou X. Machine learning applications in forecasting of covid-19 based on patients’ individual symptoms. In 2021 the 3rd International Conference On Intelligent Science And Technology (ICIST), 2021;39–44.
Zoabi Y, Deri-Rozov S, Shomron N. Machine learning-based prediction of COVID-19 diagnosis based on symptoms. NPJ Digital Med. 2021;4(1):1–5.
Download references
This work was supported by the Ministry of Higher Education and Scientific Research in Tunisia (MoHESR) as part of the Federated Research Project PRFCOV19-D1-P1.
Author information
Authors and affiliations.
Data Engineering and Semantics Research Unit, Faculty of Science, University of Sfax, Sfax, Tunisia
Boulbaba Ben Ammar, Ali Salem & Mohamed Ben Aouicha
Digital Research Center of Sfax, Ministry of Higher Education and Scientific Research, Sfax, Tunisia
Mouna Ben Said
Signals systeMs aRtificial intelligence and neTworkS Laboratory (SM@RTS), Digital Research Center of Sfax, Sfax, Tunisia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Boulbaba Ben Ammar .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Ethical Considerations and Consent to Participate
In this study, we used a publicly available dataset of clinical characteristics of patients who have taken a COVID-19 test https://covidclinicaldata.org/ .
Consent for Publication
All authors have given their consent for publication.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Ben Ammar, B., Salem, A., Ben Said, M. et al. Machine Learning Models for Early Prediction of COVID-19 Infections Based on Clinical Signs. SN COMPUT. SCI. 5 , 158 (2024). https://doi.org/10.1007/s42979-023-02489-3
Download citation
Received : 29 August 2021
Accepted : 13 November 2023
Published : 06 January 2024
DOI : https://doi.org/10.1007/s42979-023-02489-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Machine learning
- Early diagnosis
- Clinical signs
- Find a journal
- Publish with us
- Track your research
How computer science can help fight COVID-19
Uchicago researchers launch projects exploring health disparities, machine learning.
The COVID-19 pandemic has mobilized the world’s scientific community like no other recent crisis, including many researchers using the most modern data science and artificial intelligence approaches. At the University of Chicago, public health experts, computer scientists, economists and policy analysts have launched projects using computational tools to better detect, diagnose, treat and prevent the spread of the deadly virus.
This summer, three of these projects received seed funding from the C3.ai Digital Transformation Institute (DTI) , a new partnership of technology companies and universities committed to accelerating the benefits of artificial intelligence for business, government and society. The research attacks the pandemic from several angles: helping policymakers control disease spread by identifying and addressing key social factors, physicians detect the disease at earlier stages, and hospitals decide which patients require admission. A fourth project, a collaboration led by UChicago Medicine’s Maryellen Giger , was funded by the organization in spring.
The awards were part of $5.4 million in funding distributed by DTI, after their inaugural call for proposals in March. The group also provides AI software tools and a “data lake” of COVID-19 datasets to aid researchers studying the pandemic.
“The enthusiastic response among scientists and researchers coupled with the diverse, high-quality and compelling proposals we’ve received suggests that we have the potential to alter the course of this global pandemic,” said Thomas M. Siebel, CEO of C3.ai. “In the face of this crisis, the Institute is proud to bring together the best and brightest minds and provide direction and leadership to support objective analysis and AI-based, data-driven science to mitigate COVID-19.”
Modeling health disparities
The early toll of the COVID-19 pandemic revealed severe health inequities in who catches the disease and who suffers death and morbidity. Latin and African Americans are more than three times as likely to catch the virus and twice as likely to die as white Americans, according to CDC data . Many experts believe this disparity goes beyond medical comorbidities, to social determinants such as housing, jobs and neighborhood features.
Anna Hotton , a research assistant professor at UChicago Medicine, previously studied the relationship between social factors and viral spread in the context of other infectious diseases. With her DTI grant, she’s working with fellow UChicago researchers Aditya Khanna, Harold Pollack and John Schneider to adapt that work to COVID-19, with help from agent-based modeling experts Jonathan Ozik and Charles Macal at Argonne National Laboratory.
“A lot of my substantive work focuses around understanding social and structural factors as they impact HIV transmission,” Hotton said. “With COVID-19, there are a lot of similarities in terms of the social factors that shape people’s vulnerability to infection, and I’m motivated to shed light on some of these social issues and help guide work around reducing health inequities.”
Agent-based modeling is a powerful form of computer simulation for studying complex systems, from molecular interactions to traffic congestion. Over the last decade, Argonne researchers Ozik and Macal have gradually assembled a computer model for the entire city of Chicago and its population, using it to observe and predict the spread of diseases both real (MRSA, influenza) and imagined ( a zombie outbreak ). Recently, the team has focused their ChiSIM model on the spread of COVID -19, looking for types of buildings and areas of the city where people gather and disease transmission risk is high.
With Hotton and her collaborators, Ozik and Macal are working on adding new data to their synthetic Chicago population of 2.7 million “agents,” including information on housing, occupations and other social determinants that likely influence virus spread. The team will also use machine learning to identify the data elements that are most important to include in the model from a long list of options, such as time spent on public transit, ability to work from home, number of family members in a household, and many other details.
Once enriched with this data, the researchers will be able to better simulate various scenarios of disease spread and virtually test how different public health or social policy strategies can help mitigate the disease. Their results will be shared with partners in the Chicago and Illinois Departments of Public Health, advising these agencies on how best to deploy testing, reopening of businesses and schools, and, eventually, vaccination.
“Agent-based modeling allows us to explore intervention approaches in a virtual environment before rolling out interventions in real life, in addition to making predictions about trends in incidence and mortality,” Hotton said. “Later, when vaccines are available, we’ll need to figure out how to deploy them most efficiently to the populations with greatest need.”
Admit or release?
One of the toughest decisions physicians face during the pandemic is deciding which COVID-19 patients to keep in the hospital, and which are safe to recover at home. In the face of overwhelmed hospital capacity and a brand-new disease with little data-based evidence for diagnosis and treatment, old rubrics for deciding which patients to admit have proven ineffective. But machine learning could help make the right decision earlier, saving lives and lowering health care costs.
A team led by Prof. Sendhil Mullainathan of Chicago Booth will work with a large northwest U.S. hospital network on creating a new model for predicting acute respiratory distress syndrome (ARDS), the most severe symptom and primary cause of death for COVID-19 patients. Using over 4 million chest X-rays, the team—which also includes Aleksander Madry of Massachusetts Institute of Technology and Ziad Obermeyer from University of California, Berkeley—will build a new machine learning model that predicts the likelihood of this pulmonary collapse.
To work around the issue of limited COVID-19 data early in the pandemic, the team will feed their model with X-rays from other conditions that affect the lungs, such as influenza and pneumonia.
“No one has enough data on COVID yet to apply the modern machine learning toolkit,” said Obermeyer. “But in a pulmonary infection such as COVID, the lungs actually have a very limited physiological playbook. When the lungs are attacked by a virus or bacterium, they basically only react in one way. Our hypothesis is that we can learn about deterioration in COVID by looking at deterioration in other conditions.”
Once validated, their AI model will be made open source and available to other health systems around the world. The project also allows Mullainathan and Obermeyer an opportunity to develop a medical decision-making algorithm that controls for the bias they identified in other health care software in previous research .
“Even if you’re using objective biological data like X-rays, your outcomes are biased because they’re produced by a health system that is biased,” Obermeyer said. “The optimistic view of our prior work on racial bias is that once you’re aware of those biases, you can make algorithms that take them into account.”
Early detection: Treating a pandemic like engine failure
In the early stages of a disease outbreak, detecting cases is critical to prevent population spread, but also very difficult—a proverbial “needle in the haystack” data problem. But computer scientists have already developed artificial intelligence systems for such challenges in other contexts, such as detecting mechanical faults in jet engines or anomalous and potentially fraudulent financial transactions. Models built for these applications must be able to accurately and reliably find rare occurrences in a flood of data—nobody wants to discover airplane engine failure too late.
In previous work at Caltech, UChicago computer scientist Yuxin Chen built these early detection systems for mechanical engineers and other domain experts. With DTI funding, he’ll work with researchers from UC Berkeley and UCSF on transferring these approaches to detecting infection from COVID and other diseases using medical and public health surveillance data. The team will adapt solutions for common challenges such as training models on sparse data, combining data from different sources and collection techniques, and minimizing false negatives that could have dire consequences if infected patients are missed.
Chen’s portion of the project focuses on his primary research interest: interactive machine learning . As opposed to the passive, “black box” of most AI models, these systems actively work with human experts, suggesting new data sources that should be gathered to improve predictions, or asking for help when a particular diagnosis is unclear.
“If the model is not very confident about the predictive results for a certain medical diagnosis that we have data on, it will flag these data and ask experts to verify or correct the predictive results,” said Chen, an assistant professor. “We also care about interpretable recommendations; we're training our AI system to effectively communicate with the human users to collaboratively make detection and diagnosis decisions. So we need to build an interpretable interface that sits between the system and medical professionals in order to make the collaboration seamless.”
—This story was first published by the Department of Computer Science.
Recommended Stories

Supercomputers, giant accelerators lend a hand in the fight against…

UChicago joins consortium to accelerate AI innovation
Get more with UChicago News delivered to your inbox.
Related Topics
Latest news, chen lab: new ways to grow cells to protect our lungs from disease.

Olympics 2024
Scholar reflects on the Olympics' enduring appeal: 'The ritual is central'
Startup to build massive quantum campus on Chicago’s South Side

faculty honors
Prof. Shadi Bartsch-Zimmer elected to the British Academy

Go 'Inside the Lab' at UChicago
Explore labs through videos and Q&As with UChicago faculty, staff and students

Ancient color
How did people use color in the ancient world?

NASA’s Webb telescope peers into the boundary between day and night on a distant world
Around uchicago.

Recommended Reading

Eight books to add to your summer 2024 reading list
Quantrell and PhD Teaching Awards
UChicago announces 2024 winners of Quantrell and PhD Teaching Awards
Campus News
Project to improve accessibility, sustainability of Main Quadrangles
National Academy of Sciences
Five UChicago faculty elected to National Academy of Sciences in 2024

UChicago alum named 2024 Knight-Hennessy Scholar

University of Chicago Law School
Coase-Sandor Institute for Law and Economics celebrates decade of impact
Biological Sciences Division
“You have to be open minded, planning to reinvent yourself every five to seven years.”

Meet A UChicagoan
Organist pulls out all the stops to bring Bach to UChicago
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
The impact of the COVID-19 pandemic on scientific research in the life sciences
Roles Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing
Affiliation AXES, IMT School for Advanced Studies Lucca, Lucca, Italy
Roles Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Chair of Systems Design D-MTEC, ETH Zürich, Zurich, Switzerland
- Massimo Riccaboni,
- Luca Verginer

- Published: February 9, 2022
- https://doi.org/10.1371/journal.pone.0263001
- Reader Comments
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on world scientific production in the life sciences and find indications that the usage of medical subject headings (MeSH) has changed following the outbreak. We estimate through a difference-in-differences approach the impact of the start of the COVID-19 pandemic on scientific production using the PubMed database (3.6 Million research papers). We find that COVID-19-related MeSH terms have experienced a 6.5 fold increase in output on average, while publications on unrelated MeSH terms dropped by 10 to 12%. The publication weighted impact has an even more pronounced negative effect (-16% to -19%). Moreover, COVID-19 has displaced clinical trial publications (-24%) and diverted grants from research areas not closely related to COVID-19. Note that since COVID-19 publications may have been fast-tracked, the sudden surge in COVID-19 publications might be driven by editorial policy.
Citation: Riccaboni M, Verginer L (2022) The impact of the COVID-19 pandemic on scientific research in the life sciences. PLoS ONE 17(2): e0263001. https://doi.org/10.1371/journal.pone.0263001
Editor: Florian Naudet, University of Rennes 1, FRANCE
Received: April 28, 2021; Accepted: January 10, 2022; Published: February 9, 2022
Copyright: © 2022 Riccaboni, Verginer. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The processed data, instructions on how to process the raw PubMed dataset as well as all code are available via Zenodo at https://doi.org/10.5281/zenodo.5121216 .
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The COVID-19 pandemic has mobilized the world scientific community in 2020, especially in the life sciences [ 1 , 2 ]. In the first three months after the pandemic, the number of scientific papers about COVID-19 was fivefold the number of articles on H1N1 swine influenza [ 3 ]. Similarly, the number of clinical trials related to COVID-19 prophylaxis and treatments skyrocketed [ 4 ]. Thanks to the rapid mobilization of the world scientific community, COVID-19 vaccines have been developed in record time. Despite this undeniable success, there is a rising concern about the negative consequences of COVID-19 on clinical trial research, with many projects being postponed [ 5 – 7 ]. According to Evaluate Pharma, clinical trials were one of the pandemic’s first casualties, with a record number of 160 studies suspended for reasons related to COVID-19 in April 2020 [ 8 , 9 ] reporting a total of 1,200 trials suspended as of July 2020. As a consequence, clinical researchers have been impaired by reduced access to healthcare research infrastructures. Particularly, the COVID-19 outbreak took a tall on women and early-career scientists [ 10 – 13 ]. On a different ground, Shan and colleagues found that non-COVID-19-related articles decreased as COVID-19-related articles increased in top clinical research journals [ 14 ]. Fraser and coworker found that COVID-19 preprints received more attention and citations than non-COVID-19 preprints [ 1 ]. More recently, Hook and Porter have found some early evidence of ‘covidisation’ of academic research, with research grants and output diverted to COVID-19 research in 2020 [ 15 ]. How much should scientists switch their efforts toward SARS-CoV-2 prevention, treatment, or mitigation? There is a growing consensus that the current level of ‘covidisation’ of research can be wasteful [ 4 , 5 , 16 ].
Against this background, in this paper, we investigate if the COVID-19 pandemic has induced a shift in biomedical publications toward COVID-19-related scientific production. The objective of the study is to show that scientific articles listing covid-related Medical Subject Headings (MeSH) when compared against covid-unrelated MeSH have been partially displaced. Specifically, we look at several indicators of scientific production in the life sciences before and after the start of the COVID-19 pandemic: (1) number of papers published, (2) impact factor weighted number of papers, (3) opens access, (4) number of publications related to clinical trials, (5) number of papers listing grants, (6) number of papers listing grants existing before the pandemic. Through a natural experiment approach, we analyze the impact of the pandemic on scientific production in the life sciences. We consider COVID-19 an unexpected and unprecedented exogenous source of variation with heterogeneous effects across biomedical research fields (i.e., MeSH terms).
Based on the difference in difference results, we document the displacement effect that the pandemic has had on several aspects of scientific publishing. The overall picture that emerges from this analysis is that there has been a profound realignment of priorities and research efforts. This shift has displaced biomedical research in fields not related to COVID-19.
The rest of the paper is structured as follows. First, we describe the data and our measure of relatedness to COVID-19. Next, we illustrate the difference-in-differences specification we rely on to identify the impact of the pandemic on scientific output. In the results section, we present the results of the difference-in-differences and network analyses. We document the sudden shift in publications, grants and trials towards COVID-19-related MeSH terms. Finally, we discuss the findings and highlight several policy implications.
Materials and methods
The present analysis is based primarily on PubMed and the Medical Subject Headings (MeSH) terminology. This data is used to estimate the effect of the start of the COVID 19 pandemic via a difference in difference approach. This section is structured as follows. We first introduce the data and then the econometric methodology. This analysis is not based on a pre-registered protocol.
Selection of biomedical publications.
We rely on PubMed, a repository with more than 34 million biomedical citations, for the analysis. Specifically, we analyze the daily updated files up to 31/06/2021, extracting all publications of type ‘Journal Article’. For the principal analysis, we consider 3,638,584 papers published from January 2019 to December 2020. We also analyze 11,122,017 papers published from 2010 onwards to identify the earliest usage of a grant and infer if it was new in 2020. We use the SCImago journal ranking statistics to compute the impact factor weighted number (IFWN) of papers in a given field of research. To assign the publication date, we use the ‘electronically published’ dates and, if missing, the ‘print published’ dates.
Medical subject headings.
We rely on the Medical Subject Headings (MeSH) terminology to approximate narrowly defined biomedical research fields. This terminology is a curated medical vocabulary, which is manually added to papers in the PubMed corpus. The fact that MeSH terms are manually annotated makes this terminology ideal for classification purposes. However, there is a delay between publication and annotation, on the order of several months. To address this delay and have the most recent classification, we search for all 28 425 MeSH terms using PubMed’s ESearch utility and classify paper by the results. The specific API endpoint is https://eutils.ncbi.nlm.nih.gov/entrez/eutils/esearch.fcgi , the relevant scripts are available with the code. For example, we assign the term ‘Ageusia’ (MeSH ID D000370) to all papers listed in the results of the ESearch API. We apply this method to the whole period (January 2019—December 2020) and obtain a mapping from papers to the MeSH terms. For every MeSH term, we keep track of the year they have been established. For instance, COVID-19 terms were established in 2020 (see Table 1 ): in January 2020, the WHO recommended 2019-nCoV and 2019-nCoV acute respiratory disease as provisional names for the virus and disease. The WHO issued the official terms COVID-19 and SARS-CoV-2 at the beginning of February 2020. By manually annotating publications, all publications referring to COVID-19 and SARS-CoV-2 since January 2020 have been labelled with the related MeSH terms. Other MeSH terms related to COVID-19, such as coronavirus, for instance, have been established years before the pandemic (see Table 2 ). We proxy MeSH term usage via search terms using the PubMed EUtilities API; this means that we are not using the hand-labelled MeSH terms but rather the PubMed search results. This means that the accuracy of the MeSH term we assign to a given paper is not perfect. In practice, this means that we have assigned more MeSH terms to a given term than a human annotator would have.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0263001.t001
The list contains only terms with at least 100 publications in 2020.
https://doi.org/10.1371/journal.pone.0263001.t002
Clinical trials and publication types.
We classify publications using PubMed’s ‘PublicationType’ field in the XML baseline files (There are 187 publication types, see https://www.nlm.nih.gov/mesh/pubtypes.html ). We consider a publication to be related to a clinical trial if it lists any of the following descriptors:
- D016430: Clinical Trial
- D017426: Clinical Trial, Phase I
- D017427: Clinical Trial, Phase II
- D017428: Clinical Trial, Phase III
- D017429: Clinical Trial, Phase IV
- D018848: Controlled Clinical Trial
- D065007: Pragmatic Clinical Trial
- D000076362: Adaptive Clinical Trial
- D000077522: Clinical Trial, Veterinary
In our analysis of the impact of COVID-19 on publications related to clinical trials, we only consider MeSH terms that are associated at least once with a clinical trial publication over the two years. We apply this restriction to filter out MeSH terms that are very unlikely to be relevant for clinical trial types of research.
Open access.
We proxy the availability of a journal article to the public, i.e., open access, if it is available from PubMed Central. PubMed Central archives full-text journal articles and provides free access to the public. Note that the copyright license may vary across participating publishers. However, the text of the paper is for all effects and purposes freely available without requiring subscriptions or special affiliation.
We infer if a publication has been funded by checking if it lists any grants. We classify grants as either ‘old’, i.e. existed before 2019, or ‘new’, i.e. first observed afterwards. To do so, we collect all grant IDs for 11,122,017 papers from 2010 on-wards and record their first appearance. This procedure is an indirect inference of the year the grant has been granted. The basic assumption is that if a grant number has not been listed in any publication since 2010, it is very likely a new grant. Specifically, an old grant is a grant listed since 2019 observed at least once from 2010 to 2018.
Note that this procedure is only approximate and has a few shortcomings. Mistyped grant numbers (e.g. ‘1234-M JPN’ and ‘1234-M-JPN’) could appear as new grants, even though they existed before, or new grants might be classified as old grants if they have a common ID (e.g. ‘Grant 1’). Unfortunately, there is no central repository of grant numbers and the associated metadata; however, there are plans to assign DOI numbers to grants to alleviate this problem (See https://gitlab.com/crossref/open_funder_registry for the project).
Impact factor weighted publication numbers (IFWN).
In our analysis, we consider two measures of scientific output. First, we simply count the number of publications by MeSH term. However, since journals vary considerably in terms of impact factor, we also weigh the number of publications by the impact factor of the venue (e.g., journal) where it was published. Specifically, we use the SCImago journal ranking statistics to weigh a paper by the impact factor of the journal it appears in. We use the ‘citation per document in the past two years’ for 45,230 ISSNs. Note that a journal may and often has more than one ISSN, i.e., one for the printed edition and one for the online edition. SCImago applies the same score for a venue across linked ISSNs.
For the impact factor weighted number (IFWN) of publication per MeSH terms, this means that all publications are replaced by the impact score of the journal they appear in and summed up.
COVID-19-relatedness.
To measure how closely related to COVID-19 is a MeSH term, we introduce an index of relatedness to COVID-19. First, we identify the focal COVID-19 terms, which appeared in the literature in 2020 (see Table 1 ). Next, for all other pre-existing MeSH terms, we measure how closely related to COVID-19 they end up being.
Our aim is to show that MeSH terms that existed before and are related have experienced a sudden increase in the number of (impact factor weighted) papers.
Intuitively we can read this measure as: what is the probability in 2020 that a COVID-19 MeSH term is present given that we chose a paper with MeSH term i ? For example, given that in 2020 we choose a paper dealing with “Ageusia” (i.e., Complete or severe loss of the subjective sense of taste), there is a 96% probability that this paper also lists COVID-19, see Table 1 .
Note that a paper listing a related MeSH term does not imply that that paper is doing COVID-19 research, but it implies that one of the MeSH terms listed is often used in COVID-19 research.
In sum, in our analysis, we use the following variables:
- Papers: Number of papers by MeSH term;
- Impact: Impact factor weighted number of papers by MeSH term;
- PMC: Papers listed in PubMed central by MeSH term, as a measure of Open Access publications;
- Trials: number of publications of type “Clinical Trial” by MeSH term;
- Grants: number of papers with at least one grant by MeSH term;
- Old Grants: number of papers listing a grant that has been observed between 2010 and 2018, by MeSH term;
Difference-in-differences
The difference-in-differences (DiD) method is an econometric technique to imitate an experimental research design from observation data, sometimes referred to as a quasi-experimental setup. In a randomized controlled trial, subjects are randomly assigned either to the treated or the control group. Analogously, in this natural experiment, we assume that medical subject headings (MeSH) have been randomly assigned to be either treated (related) or not treated (unrelated) by the pandemic crisis.
Before the COVID, for a future health crisis, the set of potentially impacted medical knowledge was not predictable since it depended on the specifics of the emergency. For instance, ageusia (loss of taste), a medical concept existing since 1991, became known to be a specific symptom of COVID-19 only after the pandemic.
Specifically, we exploit the COVID-19 as an unpredictable and exogenous shock that has deeply affected the publication priorities for biomedical scientific production, as compared to the situation before the pandemic. In this setting, COVID-19 is the treatment, and the identification of this new human coronavirus is the event. We claim that treated MeSH terms, i.e., MeSH terms related to COVID-19, have experienced a sudden increase in terms of scientific production and attention. In contrast, research on untreated MeSH terms, i.e., MeSH terms not related to COVID-19, has been displaced by COVID-19. Our analysis compares the scientific output of COVID-19 related and unrelated MeSH terms before and after January 2020.
In our case, some of the terms turn out to be related to COVID-19 in 2020, whereas most of the MeSH terms are not closely related to COVID-19.
Thus β 1 identifies the overall effect on the control group after the event, β 2 the difference across treated and control groups before the event (i.e. the first difference in DiD) and finally the effect on the treated group after the event, net of the first difference, β 3 . This last parameter identifies the treatment effect on the treated group netting out the pre-treatment difference.
For the DiD to have a causal interpretation, it must be noted that pre-event, the trends of the two groups should be parallel, i.e., the common trend assumption (CTA) must be satisfied. We will show that the CTA holds in the results section.
To specify the DiD model, we need to define a period before and after the event and assign a treatment status or level of exposure to each term.
Before and after.
The pre-treatment period is defined as January 2019 to December 2019. The post-treatment period is defined as the months from January 2020 to December 2020. We argue that the state of biomedical research was similar in those two years, apart from the effect of the pandemic.
Treatment status and exposure.
The treatment is determined by the COVID-19 relatedness index σ i introduced earlier. Specifically, this number indicates the likelihood that COVID-19 will be a listed MeSH term, given that we observe the focal MeSH term i . To show that the effect becomes even stronger the closer related the subject is, and for ease of interpretation, we also discretize the relatedness value into three levels of treatment. Namely, we group MeSH terms with a σ between, 0% to 20%, 20% to 80% and 80% to 100%. The choice of alternative grouping strategies does not significantly affect our results. Results for alternative thresholds of relatedness can be computed using the available source code. We complement the dichotomized analysis by using the treatment intensity (relatedness measure σ ) to show that the result persists.
Panel regression.
In this work, we estimate a random effects panel regression where the units of analysis are 28 318 biomedical research fields (i.e. MeSH terms) observed over time before and after the COVID-19 pandemic. The time resolution is at the monthly level, meaning that for each MeSH term, we have 24 observations from January 2019 to December 2020.
The outcome variable Y it identifies the outcome at time t (i.e., month), for MeSH term i . As before, P t identifies the period with P t = 0 if the month is before January 2020 and P t = 1 if it is on or after this date. In (3) , the treatment level is measure by the relatedness to COVID-19 ( σ i ), where again the γ 1 identifies pre-trend (constant) differences and δ 1 the overall effect.
In total, we estimate six coefficients. As before, the δ l coefficient identifies the DiD effect.
Verifying the Common Trend Assumption (CTA).
We show that the CTA holds for this model by comparing the pre-event trends of the control group to the treated groups (COVID-19 related MeSH terms). Namely, we show that the pre-event trends of the control group are the same as the pre-event trends of the treated group.
Co-occurrence analysis
To investigate if the pandemic has caused a reconfiguration of research priorities, we look at the MeSH term co-occurrence network. Precisely, we extract the co-occurrence network of all 28,318 MeSH terms as they appear in the 3.3 million papers. We considered the co-occurrence networks of 2018, 2019 and 2020. Each node represents a MeSH term in these networks, and a link between them indicates that they have been observed at least once together. The weight of the edge between the MeSH terms is given by the number of times those terms have been jointly observed in the same publications.
Medical language is hugely complicated, and this simple representation does not capture the intricacies, subtle nuances and, in fact, meaning of the terms. Therefore, we do not claim that we can identify how the actual usage of MeSH terms has changed from this object, but rather that it has. Nevertheless, the co-occurrence graph captures rudimentary relations between concepts. We argue that absent a shock to the system, their basic usage patterns, change in importance (within the network) would essentially be the same from year to year. However, if we find that the importance of terms changes more than expected in 2020, it stands to reason that there have been some significant changes.
To show that that MeSH usage has been affected, we compute for each term in the years 2018, 2019 and 2020 their PageRank centrality [ 17 ]. The PageRank centrality tells us how likely a random walker traversing a network would be found at a given node if she follows the weights of the empirical edges (i.e., co-usage probability). Specifically, for the case of the MeSH co-occurrence network, this number represents how often an annotator at the National Library of Medicine would assign that MeSH term following the observed general usage patterns. It is a simplistic measure to capture the complexities of biomedical research. Nevertheless, it captures far-reaching interdependence across MeSH terms as the measure uses the whole network to determine the centrality of every MeSH term. A sudden change in the rankings and thus the position of MeSH terms in this network suggests that a given research subject has risen as it is used more often with other important MeSH terms (or vice versa).
We then compare the growth for each MeSH i term in g i (2019), i.e. before the the COVID-19 pandemic, with the growth after the event ( g i (2020)).
Publication growth
Changes in output and COVID-19 relatedness
Before we show the regression results, we provide descriptive evidence that publications from 2019 to 2020 have drastically increased. By showing that this growth correlates strongly with a MeSH term’s COVID-19 relatedness ( σ ), we demonstrate that (1) σ captures an essential aspect of the growth dynamics and (2) highlight the meteoric rise of highly related terms.
We look at the year over year growth in the number of the impact weighted number of publications per MeSH term from 2018 to 2019 and 2019 to 2020 as defined in the methods section.
Fig 1 shows the yearly growth of the impact weighted number of publications per MeSH term. By comparing the growth of the number of publications from the years 2018, 2019 and 2020, we find that the impact factor weighted number of publications has increased by up to a factor of 100 compared to the previous year for Betacoronavirus, one of the most closely related to COVID-19 MeSH term.
Each dot represents, a MeSH term. The y axis (growth) is in symmetric log scale. The x axis shows the COVID-19 relatedness, σ . Note that the position of the dots on the x-axis is the same in the two plots. Below: MeSH term importance gain (PageRank) and their COVID-19 relatedness.
https://doi.org/10.1371/journal.pone.0263001.g001
Fig 1 , first row, reveals how strongly correlated the growth in the IFWN of publication is to the term’s COVID-19 relatedness. For instance, we see that the term ‘Betacoronavirus’ skyrocketed from 2019 to 2020, which is expected given that SARS-CoV-2 is a species of the genus. Conversely, the term ‘Alphacoronavirus’ has not experienced any growth given that it is twin a genus of the Coronaviridae family, but SARS-CoV-2 is not one of its species. Note also the fast growth in the number of publications dealing with ‘Quarantine’. Moreover, MeSH terms that grew significantly from 2018 to 2019 and were not closely related to COVID-19, like ‘Vaping’, slowed down in 2020. From the graph, the picture emerges that publication growth is correlated with COVID-19 relatedness σ and that the growth for less related terms slowed down.
To show that the usage pattern of MeSH terms has changed following the pandemic, we compute the PageRank centrality using graph-tool [ 18 ] as discussed in the Methods section.
Fig 1 , second row, shows the change in the PageRank centrality of the MeSH terms after the pandemic (2019 to 2020, right plot) and before (2018 to 2019, left plot). If there were no change in the general usage pattern, we would expect the variance in PageRank changes to be narrow across the two periods, see (left plot). However, PageRank scores changed significantly more from 2019 to 2020 than from 2018 to 2019, suggesting that there has been a reconfiguration of the network.
To further support this argument, we carry out a DiD regression analysis.
Common trends assumption
As discussed in the Methods section, we need to show that the CTA assumption holds for the DiD to be defined appropriately. We do this by estimating for each month the number of publications and comparing it across treatment groups. This exercise also serves the purpose of a placebo test. By assuming that each month could have potentially been the event’s timing (i.e., the outbreak), we show that January 2020 is the most likely timing of the event. The regression table, as noted earlier, contains over 70 estimated coefficients, hence for ease of reading, we will only show the predicted outcome per month by group (see Fig 2 ). The full regression table with all coefficients is available in the S1 Table .
The y axis is in log scale. The dashed vertical line identifies January 2020. The dashed horizontal line shows the publications in January 2019 for the 0–20% group before the event. This line highlights that the drop happens after the event. The bands around the lines indicate the 95% confidence interval of the predicted values. The results are the output of the Stata margins command.
https://doi.org/10.1371/journal.pone.0263001.g002
Fig 2 shows the predicted number per outcome variable obtained from the panel regression model. These predictions correspond to the predicted value per relatedness group using the regression parameters estimated via the linear panel regression. The bands around the curves are the 95% confidence intervals.
All outcome measures depict a similar trend per month. Before the event (i.e., January 2020), there is a common trend across all groups. In contrast, after the event, we observe a sudden rise for the outcomes of the COVID-19 related treated groups (green and red lines) and a decline in the outcomes for the unrelated group (blue line). Therefore, we can conclude that the CTA assumption holds.
Regression results
Table 3 shows the DiD regression results (see Eq (3) ) for the selected outcome measures: number of publications (Papers), impact factor weighted number of publications (Impact), open access (OA) publications, clinical trial related publications, and publications with existing grants.
https://doi.org/10.1371/journal.pone.0263001.t003
Table 3 shows results for the discrete treatment level version of the DiD model (see Eq (4) ).
Note that the outcome variable is in natural log scale; hence to get the effect of the independent variable, we need to exponentiate the coefficient. For values close to 0, the effect is well approximated by the percentage change of that magnitude.
In both specifications we see that the least related group, drops in the number of publications between 10% and 13%, respectively (first row of Tables 3 and 4 , exp(−0.102) ≈ 0.87). In line with our expectations, the increase in the number of papers published by MeSH term is positively affected by the relatedness to COVID-19. In the discrete model (row 2), we note that the number of documents with MeSH terms with a COVID-19 relatedness between 20 and 80% grows by 18% and highly related terms by a factor of approximately 6.6 (exp(1.88)). The same general pattern can be observed for the impact weighted publication number, i.e., Model (2). Note, however, that the drop in the impact factor weighted output is more significant, reaching -19% for COVID-19 unrelated publications, and related publications growing by a factor of 8.7. This difference suggests that there might be a bias to publish papers on COVID-19 related subjects in high impact factor journals.
https://doi.org/10.1371/journal.pone.0263001.t004
By looking at the number of open access publications (PMC), we note that the least related group has not been affected negatively by the pandemic. However, the number of COVID-19 related publications has drastically increased for the most COVID-19 related group by a factor of 6.2. Note that the substantial increase in the number of papers available through open access is in large part due to journal and editorial policies to make preferentially COVID research immediately available to the public.
Regarding the number of clinical trial publications, we note that the least related group has been affected negatively, with the number of publications on clinical trials dropping by a staggering 24%. At the same time, publications on clinical trials for COVID-19-related MeSH have increased by a factor of 2.1. Note, however, that the effect on clinical trials is not significant in the continuous regression. The discrepancy across Tables 3 and 4 highlights that, especially for trials, the effect is not linear, where only the publications on clinical trials closely related to COVID-19 experiencing a boost.
It has been reported [ 19 ] that while the number of clinical trials registered to treat or prevent COVID-19 has surged with 179 new registrations in the second week of April 2020 alone. Only a few of these have led to publishable results in the 12 months since [ 20 ]. On the other hand, we find that clinical trial publications, considering related MeSH (but not COVID-19 directly), have had significant growth from the beginning of the pandemic. These results are not contradictory. Indeed counting the number of clinical trial publications listing the exact COVID-19 MeSH term (D000086382), we find 212 publications. While this might seem like a small number, consider that in 2020 only 8,485 publications were classified as clinical trials; thus, targeted trials still made up 2.5% of all clinical trials in 2020 . So while one might doubt the effectiveness of these research efforts, it is still the case that by sheer number, they represent a significant proportion of all publications on clinical trials in 2020. Moreover, COVID-19 specific Clinical trial publications in 2020, being a delayed signal of the actual trials, are a lower bound estimate on the true number of such clinical trials being conducted. This is because COVID-19 studies could only have commenced in 2020, whereas other studies had a head start. Thus our reported estimates are conservative, meaning that the true effect on actual clinical trials is likely larger, not smaller.
Research funding, as proxied by the number of publications with grants, follows a similar pattern, but notably, COVID-19-related MeSH terms list the same proportion of grants established before 2019 as other unrelated MeSH terms, suggesting that grants which were not designated for COVID-19 research have been used to support COVID-19 related research. Overall, the number of publications listing a grant has dropped. Note that this should be because the number of publications overall in the unrelated group has dropped. However, we note that the drop in publications is 10% while the decline in publications with at least one grant is 15%. This difference suggests that publications listing grants, which should have more funding, are disproportionately COVID-19 related papers. To further investigate this aspect, we look at whether the grant was old (pre-2019) or appeared for the first time in or after 2019. It stands to reason that an old grant (pre-2019) would not have been granted for a project dealing with the pandemic. Hence we would expect that COVID-19 related MeSH terms to have a lower proportion of old grants than the unrelated group. In models (6) in Table 4 we show that the number of old grants for the unrelated group drops by 13%. At the same time, the number of papers listing old grants (i.e., pre-2019) among the most related group increased by a factor of 3.1. Overall, these results suggest that COVID-19 related research has been funded largely by pre-existing grants, even though a specific mandate tied to the grants for this use is unlikely.
The scientific community has swiftly reallocated research efforts to cope with the COVID-19 pandemic, mobilizing knowledge across disciplines to find innovative solutions in record time. We document this both in terms of changing trends in the biomedical scientific output and the usage of MeSH terms by the scientific community. The flip side of this sudden and energetic prioritization of effort to fight COVID-19 has been a sudden contraction of scientific production in other relevant research areas. All in all, we find strong support to the hypotheses that the COVID-19 crisis has induced a sudden increase of research output in COVID-19 related areas of biomedical research. Conversely, research in areas not related to COVID-19 has experienced a significant drop in overall publishing rates and funding.
Our paper contributes to the literature on the impact of COVID-19 on scientific research: we corroborate previous findings about the surge of COVID-19 related publications [ 1 – 3 ], partially displacing research in COVID-19 unrelated fields of research [ 4 , 14 ], particularly research related to clinical trials [ 5 – 7 ]. The drop in trial research might have severe consequences for patients affected by life-threatening diseases since it will delay access to new and better treatments. We also confirm the impact of COVID-19 on open access publication output [ 1 ]; also, this is milder than traditional outlets. On top of this, we provide more robust evidence on the impact weighted effect of COVID-19 and grant financed research, highlighting the strong displacement effect of COVID-19 on the allocation of financial resources [ 15 ]. We document a substantial change in the usage patterns of MeSH terms, suggesting that there has been a reconfiguration in the way research terms are being combined. MeSH terms highly related to COVID-19 were peripheral in the MeSH usage networks before the pandemic but have become central since 2020. We conclude that the usage patterns have changed, with COVID-19 related MeSH terms occupying a much more prominent role in 2020 than they did in the previous years.
We also contribute to the literature by estimating the effect of COVID-19 on biomedical research in a natural experiment framework, isolating the specific effects of the COVID-19 pandemic on the biomedical scientific landscape. This is crucial to identify areas of public intervention to sustain areas of biomedical research which have been neglected during the COVID-19 crisis. Moreover, the exploratory analysis on the changes in usage patterns of MeSH terms, points to an increase in the importance of covid-related topics in the broader biomedical research landscape.
Our results provide compelling evidence that research related to COVID-19 has indeed displaced scientific production in other biomedical fields of research not related to COVID-19, with a significant drop in (impact weighted) scientific output related to non-COVID-19 and a marked reduction of financial support for publications not related to COVID-19 [ 4 , 5 , 16 ]. The displacement effect is persistent to the end of 2020. As vaccination progresses, we highlight the urgent need for science policy to re-balance support for research activity that was put on pause because of the COVID-19 pandemic.
We find that COVID-19 dramatically impacted clinical research. Reactivation of clinical trials activities that have been postponed or suspended for reasons related to COVID-19 is a priority that should be considered in the national vaccination plans. Moreover, since grants have been diverted and financial incentives have been targeted to sustain COVID-19 research leading to an excessive entry in COVID-19-related clinical trials and the ‘covidisation’ of research, there is a need to reorient incentives to basic research and otherwise neglected or temporally abandoned areas of biomedical research. Without dedicated support in the recovery plans for neglected research of the COVID-19 era, there is a risk that more medical needs will be unmet in the future, possibly exacerbating the shortage of scientific research for orphan and neglected diseases, which do not belong to COVID-19-related research areas.
Limitations
Our empirical approach has some limits. First, we proxy MeSH term usage via search terms using the PubMed EUtilities API. This means that the accuracy of the MeSH term we assign to a given paper is not fully validated. More time is needed for the completion of manually annotated MeSH terms. Second, the timing of publication is not the moment the research has been carried out. There is a lead time between inception, analysis, write-up, review, revision, and final publication. This delay varies across disciplines. Nevertheless, given that the surge in publications happens around the alleged event date, January 2020, we are confident that the publication date is a reasonable yet imperfect estimate of the timing of the research. Third, several journals have publicly declared to fast-track COVID-19 research. This discrepancy in the speed of publication of COVID-19 related research and other research could affect our results. Specifically, a surge or displacement could be overestimated due to a lag in the publication of COVID-19 unrelated research. We alleviate this bias by estimating the effect considering a considerable time after the event (January 2020 to December 2020). Forth, on the one hand, clinical Trials may lead to multiple publications. Therefore we might overestimate the impact of COVID-19 on the number of clinical trials. On the other hand, COVID-19 publications on clinical trials lag behind, so the number of papers related COVID-19 trials is likely underestimated. Therefore, we note that the focus of this paper is scientific publications on clinical trials rather than on actual clinical trials. Fifth, regarding grants, unfortunately, there is no unique centralized repository mapping grant numbers to years, so we have to proxy old grants with grants that appeared in publications from 2010 to 2018. Besides, grant numbers are free-form entries, meaning that PubMed has no validation step to disambiguate or verify that the grant number has been entered correctly. This has the effect of classifying a grant as new even though it has appeared under a different name. We mitigate this problem by using a long period to collect grant numbers and catch many spellings of the same grant, thereby reducing the likelihood of miss-identifying a grant as new when it existed before. Still, unless unique identifiers are widely used, there is no way to verify this.
So far, there is no conclusive evidence on whether entry into COVID-19 has been excessive. However, there is a growing consensus that COVID-19 has displaced, at least temporally, scientific research in COVID-19 unrelated biomedical research areas. Even though it is certainly expected that more attention will be devoted to the emergency during a pandemic, the displacement of biomedical research in other fields is concerning. Future research is needed to investigate the long-run structural consequences of the COVID-19 crisis on biomedical research.
Supporting information
S1 table. common trend assumption (cta) regression table..
Full regression table with all controls and interactions.
https://doi.org/10.1371/journal.pone.0263001.s001
- View Article
- Google Scholar
- PubMed/NCBI
- 8. Brown A, Edwin E, Fagg J. Evaluate Pharma 2021 Preview; 2020. https://www.evaluate.com/thought-leadership/vantage/evaluate-vantage-2021-preview .
- 15. Hook D, Porter S. The COVID Brain Drain; 2020. https://www.natureindex.com/news-blog/covid-brain-drain-scientific-research .
- 17. Page L, Brin S, Motwani R, Winograd T. The PageRank citation ranking: Bringing order to the web. Stanford InfoLab; 1999.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center
Factors Affecting Computer Science Student's Academic Performance During Covid-19

- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Introduction
- Conclusions
- Article Information
In this platform trial with multiple study drugs, participants were able to choose what agents they were willing to be randomized to receive. Participants were first randomized in a ratio of m :1, where m is the number of study drugs for which the participant was eligible. After randomization to receive an active agent vs placebo, participants were randomized with equal probability among the study drugs for which they were eligible. The consort diagram illustrates a sequential exclusion process, where each step of exclusion was applied to ensure that the reasons for participant removal were mutually exclusive. As a result, each participant was excluded for 1 reason even though multiple exclusions may have been present.
Thick vertical lines denote the estimated mean of the posterior distribution. Density is the relative likelihood of posterior probability distribution. Outcomes with higher density are more likely than outcomes with lower density.
Recovery was defined as the third of 3 consecutive days without symptoms. Fifty-nine participants did not provide any follow-up data beyond day 1 and were immediately censored. Fourty-seven participants were censored after some follow-up. All other participantss were followed up until recovery, death, or the end of short-term 28-day follow-up. Median (IQR) time to recovery was 11 (11-12) days in the ivermectin group and 12 (11-12) days in the placebo group. Shaded regions denote the pointwise 95% CIs.
Trial protocol
Statistical analysis plan
Nonauthor collaborators
Data sharing statement
- Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19 JAMA Original Investigation October 25, 2022 This randomized clinical trial compares the efficacy of ivermectin vs placebo in shortening symptom duration among adult outpatients in the US with symptomatic mild to moderate COVID-19. Susanna Naggie, MD, MHS; David R. Boulware, MD, MPH; Christopher J. Lindsell, PhD; Thomas G. Stewart, PhD; Nina Gentile, MD; Sean Collins, MD, MSci; Matthew William McCarthy, MD; Dushyantha Jayaweera, MD; Mario Castro, MD, MPH; Mark Sulkowski, MD; Kathleen McTigue, MD, MPH, MS; Florence Thicklin; G. Michael Felker, MD, MHS; Adit A. Ginde, MD, MPH; Carolyn T. Bramante, MD, MPH; Alex J. Slandzicki, MD; Ahab Gabriel, MD; Nirav S. Shah, MD, MPH; Leslie A. Lenert, MD, MS; Sarah E. Dunsmore, PhD; Stacey J. Adam, PhD; Allison DeLong, BS; George Hanna, MD; April Remaly, BA; Rhonda Wilder, MS; Sybil Wilson, RN; Elizabeth Shenkman, PhD; Adrian F. Hernandez, MD, MHS; Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators; William (Kelly) Vincent; Raina Vincent; Ray Bianchi; Jen Premas; Diana Cordero-Loperena; Evelyn Rivera; Madhu Gupta ; Greg Karawan; Carey Ziomek; Joseph Arena; Sonaly DeAlmeida; Soroush Ramin; Jaya Nataraj; Michael Paasche-Orlow; Lori Henault; Katie Waite; David Miller; Ginger Brounce; Constance George-Adebayo; Adeolu Adebayo; Jessica Wallan; Alex Slandzicki; Claudia Vogel; Sebastian Munoz; David Kavtaradze; Cassandra Watson; David Singleton; Maria Rivon; Amanda Sevier; Arnold Del Pilar; Amber Spangler; Sohail Rao; Luis Cantu; Arvind Krishna; Kathy Evans; Tylene Falkner; Brandi Kerr; Robert Spees; Mailyn Marta; G. Michael Felker; Amanda Harrington; Rowena Dolor; Madison Frazier; Lorraine Vergara; Jessica Wilson; Valencia Burruss; Terri Hurst; Igho Ofotokun; Laurel Bristow; Rajesh Prabhu; Krystal Klicka; Amber Lightfeather; Vicki James; Marcella Rogers; Pradeep Parihar; De'Ambra Torress; Chukwuemeka Oragwu; Ngozi Oguego; Rajesh Pillai; Mustafa Juma; Ahab Gabriel; Emad Ghaly; Dafer Al-Haddadin; Courtney Ramirez; Gammal Hassanien; Samah Ismail; Andrew Meltzer; Seamus Moran; Scott Brehaut; Angelina Roche; Manisha Mehta; Nicole Koppinger; Jose Baez; Ivone Pagan; Dallal Abdelsayed; Mina Aziz; Philip Robinson; Julie Nguyen; Victoria Pardue; Llisa Hammons; Juan Ruiz-Unger; Susan Gonzalez; Lionel Reyes; John Cienki; Gisselle Jimenez; Jonathan Cohen; Matthew Wong; Ying Yuan; Jeremy Szeto; Mark Sulkowski; Lauren Stelmash; Arch Amon; Daniel Haight; Deryl Lamb; Amron Harper; Nancy Pyram-Bernard; Arlen Quintero; Eftim Adhami; Josette Maria; Diksha Paudel; Oksana Raymond; Jeffrey Summers; Tammy Turner; Leslie Lenert; Sam Gallegos; Elizabeth Ann Szwast; Ahsan Abdulghani; Pravin Vasoya; Conrad Miller; Hawa Wiley; Nirav Shah; Tovah Klein; Julie Castex; Phillip Feliciano; Jacqueline Olivo; Marian Ghaly; Zainub Javed; Alexandra Nawrocki; Anthony Vecchiarelli; Nikki Vigil; Vijaya Cherukuri; Erica Burden; Dawn Linn; Laura Fisher; Vijay Patel; Praksha Patel; Yuti Patel; Leonard Ellison; Jeffrey Harrison; Binod Shah; Sugata Shah; Upinder Singh; Julia Donahue; Yasmin Jazayeri; Anita Gupta ; N Chandrasekar; Beth Moritz; Tabitha Fortt; Anisa Fortt; Ingrid Jones-Ince; Alix McKee; Christy Schattinger; Jason Wilson; Brenda Farlow; Nina Gentile; Lillian Finlaw; Randall Richwine; Tearani Williams; Penny Paizer; Lisa Carson; Edward Michelson; Danielle Austin; Sangeeta Khetpal; Tiffany Cantrell; Drew Franklin; Karissa Marshall; Arvind Mahadevan; Madelyn Rosequist; Martin Gnoni; Crystal Daffner; Carla VandeWeerd; Mitchell Roberts; Mark D'Andrea; Stephen Lim; Wayne Swink; Margaret Powers-Fletcher; Sylvere Mukunzi; Elizabeth Shenkman; Jamie Hensley; Brittney Manning; Carmen Isache; Jennifer Bowman; Angelique Callaghan-Brown; Taylor Scott; Tiffany Schwasinger-Schmidt; Ashlie Cornejo; Dushyantha Jayaweera; Maria Almanzar; Letty Ginsburg; Americo Hajaz; Carolyn Bramante; Matthew Robinson; Michelle Seithel; Akira Sekikawa; Emily Klawson; Luis Ostrosky; Virginia Umana; Thomas Patterson; Robin Tragus; Patrick Jackson; Caroline Hallowell; Heather Haughey; Bhavna Vaidya-Tank; Cameron Gould; Parul Goyal; Carly Gatewood; John Williamson; Hannah Seagle; Matthew McCarthy; Elizabeth Salsgiver; Eddie Armas; Jhonsai Cheng; Priscilla Huerta; Julia Garcia-Diaz; David Aamodt; JaMario Ayers; Jess Collins; John Graves; James Grindstaff; Frank Harrell; Jessica Lai; Itzel Lopez; Jessica Marlin; Alyssa Merkel; Sam Nwosu; Savannah Obregon; Dirk Orozco; Yoli Perez-Torres; Nelson Prato; Colleen Ratcliff; Max Rhode; Russell Rothman; Jana Shirey-Rice; Krista Vermillion; Hsi-Nien Tan; Seibert Tregoning; Meghan Vance; Amber Vongsamphanh; Maria Weir; Nicole Zaleski
- Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19 JAMA Original Investigation January 24, 2023 This randomized, placebo-controlled platform trial compares the use of low-dose fluvoxamine (50 mg twice daily) for 10 days compared with placebo in outpatients with mild to moderate COVID-19. Matthew W. McCarthy, MD; Susanna Naggie, MD, MHS; David R. Boulware, MD, MPH; Christopher J. Lindsell, PhD; Thomas G. Stewart, PhD; G. Michael Felker, MD, MHS; Dushyantha Jayaweera, MD; Mark Sulkowski, MD; Nina Gentile, MD; Carolyn Bramante, MD, MPH; Upinder Singh, MD; Rowena J. Dolor, MD, MHS; Juan Ruiz-Unger, MD; Sybil Wilson, RN; Allison DeLong, BS; April Remaly, BA; Rhonda Wilder, MS; Sean Collins, MD, MSci; Sarah E. Dunsmore, PhD; Stacey J. Adam, PhD; Florence Thicklin; George Hanna, MD; Adit A. Ginde, MD, MPH; Mario Castro, MD, MPH; Kathleen McTigue, MD, MPH, MS; Elizabeth Shenkman, PhD; Adrian F. Hernandez, MD, MHS; Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators; William (Kelly) Vincent; Raina Vincent; Ray Bianchi; Jen Premas; Diana Cordero-Loperena Evelyn Rivera; Madhu Gupta ; Greg Karawan; Carey Ziomek; Joseph Arena; Sonaly DeAlmeida; Soroush Ramin; Jaya Nataraj; Michael Paasche-Orlow; Lori Henault; Katie Waite; David Miller; Ginger Brounce; Constance George-Adebayo; Adeolu Adebayo; Jessica Wallan; Claudia Vogel; Sebastian Munoz; David Kavtaradze; Cassandra Watson; David Singleton; Maria Rivon; Amanda Sevier; Arnold Del Pilar; Amber Spangler; Sohail Rao; Luis Cantu; Arvind Krishna; Kathy Evans; Tylene Falkner; Brandi Kerr; Robert Spees; Mailyn Marta; Amanda Harrington; Madison Frazier; Lorraine Vergara; Jessica Wilson; Valencia Burruss; Terri Hurst; Igho Ofotokun; Laurel Bristow; Rajesh Prabhu; Krystal Klicka; Amber Lightfeather; Vicki James; Marcella Rogers; Pradeep Parihar; De'Ambra Torress; Chukwuemeka Oragwu; Ngozi Oguego; Rajesh Pillai; Mustafa Juma; Emad Ghaly; Dafer Al-Haddadin; Courtney Ramirez; Gammal Hassanien; Samah Ismail; Andrew Meltzer; Seamus Moran; Scott Brehaut; Angelina Roche; Manisha Mehta; Nicole Koppinger; Jose Baez; Ivone Pagan; Dallal Abdelsayed; Mina Aziz; Philip Robinson; Julie Nguyen; Victoria Pardue; Llisa Hammons; Susan Gonzalez; Lionel Reyes; John Cienki; Gisselle Jimenez; Jonathan Cohen; Matthew Wong; Ying Yuan; Jeremy Szeto; Lauren Stelmash; Arch Amon; Daniel Haight; Deryl Lamb; Amron Harper; Nancy Pyram-Bernard; Arlen Quintero; Eftim Adhami; Josette Maria; Diksha Paudel; Oksana Raymond; Jeffrey Summers; Tammy Turner; Sam Gallegos; Elizabeth Ann Szwast; Ahsan Abdulghani; Pravin Vasoya; Conrad Miller; Hawa Wiley; Tovah Klein; Julie Castex; Phillip Feliciano; Jacqueline Olivo; Marian Ghaly; Zainub Javed; Alexandra Nawrocki; Anthony Vecchiarelli; Nikki Vigil; Vijaya Cherukuri; Erica Burden; Dawn Linn; Laura Fisher; Vijay Patel; Praksha Patel; Yuti Patel; Leonard Ellison; Jeffrey Harrison; Binod Shah; Sugata Shah; Upinder Shah; Julia Donahue; Yasmin Jazayeri; Anita Gupta ; N Chandrasekar; Beth Moritz; Tabitha Fortt; Anisa Fortt; Ingrid Jones-Ince; Alix McKee; Christy Schattinger; Jason Wilson; Brenda Farlow; Lillian Finlaw; Randall Richwine; Tearani Williams; Penny Paizer; Lisa Carson; Edward Michelson; Danielle Austin; Sangeeta Khetpal; Tiffany Cantrell; Drew Franklin; Karissa Marshall; Arvind Mahadevan; Madelyn Rosequist; Martin Gnoni; Crystal Daffner; Carla VandeWeerd; Mitchell Roberts; Mark D'Andrea; Stephen Lim; Wayne Swink; Margaret Powers-Fletcher; Sylvere Mukunzi; Jamie Hensley; Brittney Manning; Carmen Isache; Jennifer Bowman; Angelique Callaghan-Brown; Taylor Scott; Tiffany Schwasinger-Schmidt; Ashlie Cornejo; Maria Almanzar; Letty Ginsburg; Americo Hajaz; Matthew Robinson; Michelle Seithel; Akira Sekikawa; Emily Klawson; Luis Ostrosky; Virginia Umana; Thomas Patterson; Robin Tragus; Patrick Jackson; Caroline Hallowell; Heather Haughey; Bhavna Vaidya-Tank; Cameron Gould; Parul Goyal; Carly Gatewood; John Williamson; Hannah Seagle; Elizabeth Salsgiver; Eddie Armas; Jhonsai Cheng; Priscilla Huerta; Julia Garcia-Diaz; David Aamodt; JaMario Ayers; Jess Collins; John Graves; James Grindstaff; Jessica Lai; Itzel Lopez; Jessica Marlin; Alyssa Merkel; Sam Nwosu; Savannah Obregon; Dirk Orozco; Yoli Perez-Torres; Nelson Prato; Colleen Ratcliff; Max Rhode; Russell Rothman; Jana Shirey-Rice; Krista Vermillion; Hsi-Nien Tan; Seibert Tregoning; Meghan Vance; Amber Vongsamphanh; Maria Weir; Nicole Zaleski
- Managing Persistent Uncertainty in the Ethics of Clinical Research JAMA Editorial March 21, 2023 Alex John London, PhD; Christopher W. Seymour, MD, MSc
- Highlights from CROI, the Conference on Retroviruses and Opportunistic Infections JAMA Medical News & Perspectives March 28, 2023 This Medical News Q&A discusses research highlights from the recent Conference on Retroviruses and Opportunistic Infections. Rita Rubin, MA
- Higher-Dose Fluvoxamine and Time to Sustained Recovery in Outpatients With COVID-19 JAMA Original Investigation December 26, 2023 This randomized study examines the effect of higher-dose fluvoxamine on time to sustained recovery from mild to moderate COVID-19 or progression to severe disease in nonhospitalized adults. Thomas G. Stewart, PhD; Paulina A. Rebolledo, MD, MSc; Ahmad Mourad, MD; Christopher J. Lindsell, PhD; David R. Boulware, MD, MPH; Matthew W. McCarthy, MD; Florence Thicklin; Idania T. Garcia del Sol, MD; Carolyn T. Bramante, MD, MPH; Leslie A. Lenert, MD, MS; Stephen Lim, MD; John C. Williamson, PharmD; Orlando Quintero Cardona, MD; Jake Scott, MD; Tiffany Schwasinger-Schmidt, MD, PhD; Adit A. Ginde, MD, MPH; Mario Castro, MD, MPH; Dushyantha Jayaweera, MD; Mark Sulkowski, MD; Nina Gentile, MD; Kathleen McTigue, MD; G. Michael Felker, MD, MHS; Allison DeLong, BS; Rhonda Wilder, MS; Russell L. Rothman, MD, MPP; Sean Collins, MD, MSci; Sarah E. Dunsmore, PhD; Stacey J. Adam, PhD; George J. Hanna, MD; Elizabeth Shenkman, PhD; Adrian F. Hernandez, MD, MHS; Susanna Naggie, MD, MHS; Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators; Ryan Fraser; Mark Ward; Jennifer Gamboa Jackman; M. Patricia McAdams; Julia Vail; Kayla Korzekwinski; Martina Oyelakin; Julie Chopp; Desmon Randle; Samantha Dockery; Rodney Adkins; Mathew Crow; Erin Nowell; Kadie Wells; Alicia Herbert; Allegra Stone; Heather Heavlin; Linley Brown; Tina Harding; Amanda Harrington; Meaghan Beauchaine; Kelly Lindblom; Andrea Burns; David Aamodt; Jess Collins; Sheri Dixon; Yue Gao; John Graves; James Grindstaff; Frank Harrell; Jessica Lai; Vicky Liao; Itzel Lopez; Elizabeth Manis; Kalley Mankowski; Jessica Marlin; Alyssa Merkel; Sam Nwosu; Savannah Obregon; Dirk Orozco; Nelson Prato; Max Rohde; Jana Shirey-Rice; Krista Vermillion; Jacob Smith; Hsi-nien Tan; Meghan Vance; Maria Weir; William (Kelly) Vincent; Raina Vincent; Ray Bianchi; Jen Premas; Diana Cordero-Loperena; Evelyn Rivera; Madhu Gupta; Greg Karawan; Joseph Arena; Sonaly DeAlmeida; Soroush Ramin; Jaya Nataraj; Julien Dedier; Ana Maria Ramirez; Katherine Waite; Jason Okulicz; Joseph Marcus; Alexis Southwell; Genice Jacques; Cedar Sexton; David Miller; Ginger Brounce; Constance George-Adebayo; Adeolu Adebayo; Jose Zapatero; Julie Clement; Theresa Ronan; Ashley Woods; Christopher Gallegos; Tamara Flys; Olivia Sloan; Anthony Olofintuyi; Joshua Samraj; Jackelyn Samraj; Alma Vasbinder; Amaya Averett; Alex Slandzicki; Aaron Milstone; Jessica Wallan; Lindsey Robbs; Claudia Vogel; Sebastian Munoz; David Kavtaradze; Casandra Watson; David Singleton; Marcus Sevier; Maria Rivon; Arnold Del Pilar; Amber Spangler; Sohail Rao; Luis Cantu; Arvind Krishna; Heidi Daugherty; Brandi Kerr; Kathy Evans; Robert Spees; Mailyn Marta; Rowena Dolor; Lorraine Vergara; Jackie Jordan; Valencia Burruss; Terri Hurst; Igho Ofotokun; Paulina A. Rebolledo; Cecilia Zhang; Veronica E. Smith; Rajesh Prabhu; Krystal Klicka; Amber Lightfeather; Vickie James; Marcella Rogers; Pradeep Parihar; De'Ambra Torress; Chukwuemeka Oragwu; Ngozi Oguego; Rajesh Pillai; Mustafa Juma; Ahab Gabriel; Emad Ghaly; Marian Michal; Michelle Vasquez; Angela Mamon; Michelle Sheets; Gammal Hassanien; Samah Ismail; Yehia Samir; Andrew Meltzer; Soroush Shahamatdar; Ryan S. Heidish; Scott Brehaut; Angelina Roche; Manisha Mehta; Nicole Koppinger; Jose Baez; Ivone Pagan; Dallal Abdelsayed; Mina Aziz; Philip Robinson; Grace Lozinski; Julie Nguyen; Alvin Griffin; Michael Morris; Nicole Love; Bonnie Mattox; Raykel Martin; Victoria Pardue; Teddy Rowland; Juan Ruiz-Unger; Lionel Reyes; Yadira Zamora; Navila Bacallao; John Cienki; Jonathan Cohen; Ying Yuan; Jenny Li; Jeremy Szeto; Mark Sulkowski; Lauren Stelmash; Idania Garcia del Sol; Ledular Morales Castillo; Anya Gutierrez; Sabrina Prieto; Arch Amon; Andrew Barbera; Andrew Bugajski; Walter Wills; Kellcee Jacklin; Deryl Lamb; Amron Harper; Elmer Stout; Katherine Weeks; Merischia Griffin; Nancy Pyram-Bernard; Arlen Quintero; Eftim Adhami; Giovanni Carrillo; Josette Maria; Diksha Paudel; Oksana Raymond; Jeffrey Summers; Tammy Turner; Ebony Panaccione; Elizabeth Szwast; Ahsan Abdulghani; Pravin Vasoya; Conrad Miller; Hawa Wiley; Austin Chan; Saadia Khizer; Nirav Shah; Oluwadamilola Adeyemi; Wei Ning Chi; July Chen; Melissa Morton-Jost; Julie Castex; Phillip Feliciano; Jacqueline Olivo; Maria Maldonado; Anthony Vecchiarelli; Diana Gaytan-Alvarez; Vijaya Cherukuri; Santia Lima; Radica Alicic; Allison A. Lambert; Carissa Urbat; Joni Baxter; Ann Cooper; Dawn Linn; Laura Fisher; Vijay Patel; Yuti Patel; Roshan Talati; Priti Patel; Leonard Ellison; Angee Roman; Jeffrey Harrison; James Moy; Dina Naquiallah; Binod Shah; Upinder Singh; Yasmin Jazayeri; Andrew O’Donnell; Orlando Quintero; Divya Pathak; Anita Gupta; N Chandrasekar; Clifford Curtis; Briana White; Martha Dockery; Maya Hicks; Tabitha Fortt; Anisa Fortt; Ingrid Jones-Ince; Alix McKee; Jason Wilson; Brenda Farlow; Nina Gentile; Casey Grady; Randall Richwine; Tearani Williams; Penny Pazier; Edward Michelson; Susan Watts; Diluma Kariyawasam; Leann Rodriguez; Jose Luis Garcia; Ismarys Manresa; Angel Achong; Mari Garcia; Sangeeta Khetpal; Faith Posey; Arvind Mahadevan; Martin Gnoni; Carla VandeWeerd; Erica Sappington; Mitchell Roberts; Jennifer Wang; Melissa Adams; Xinyi Ding; Mark D'Andrea; Stephen Lim; Wayne Swink; Emily Bozant; Margaret Powers-Fletcher; Delia Miller; Sylvere Mukunzi; Brittney Manning; Carmen Isache; Jennifer Bowman; Angelique Callaghan-Brown; Debra Martin; Ashley Ast; Brent Duran; Ashlie Cornejo; Allie Archer; Dushyantha Jayaweera; Maria Almanzar; Vanessa Motel; Neeta Bhat; Daniela Parra; Matthew Pullen; Paula Campora; Matthew Robinson; Michelle Seithel; Akira Sekikawa; Emily Klawson; Jonathan Arnold; Luis Ostrosky-Zeichner; Virginia Umana; Laura Nielsen; Carolyn Z. Grimes; Thomas F. Patterson; Robin Tragus; Bridgette T. Soileau; Patrick E.H. Jackson; Carolina Hallowell; Heather M. Haughey; Bhavna Vaidya-Tank; Cameron Gould; Parul Goyal; Sue Sommers; Haley Pangburn; Carly Jones; John Williamson; Rica Abbott; Hannah Seagle; Mathias DeComarmond; Nicholas Pickell; Unwana Umana; Candace Alleyne; Eddie Armas; Ramon O. Perez Landabur; Michelle De La Cruz; Martha Ballmajo
- Error in the Exclusion of Participants From Analysis in the ACTIV-6 Platform Randomized Clinical Trial JAMA Comment & Response June 4, 2024 Susanna Naggie, MD, MHS
- Errors in Results From Erroneous Exclusion of Participants in Analysis JAMA Correction June 4, 2024
- At a Higher Dose and Longer Duration, Ivermectin Still Not Effective Against COVID-19 JAMA Editor's Note March 21, 2023 Kirsten Bibbins-Domingo, PhD, MD, MAS; Preeti N. Malani, MD, MSJ
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Naggie S , Boulware DR , Lindsell CJ, et al. Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19 : A Randomized Clinical Trial . JAMA. 2023;329(11):888–897. doi:10.1001/jama.2023.1650
Manage citations:
© 2024
- Permissions
Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19 : A Randomized Clinical Trial
- 1 Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina
- 2 Department of Medicine, Duke University School of Medicine, Durham, North Carolina
- 3 Division of Infectious Diseases and International Medicine, University of Minnesota, Minneapolis
- 4 Vanderbilt University Medical Center, Nashville, Tennessee
- 5 School of Data Science, University of Virginia, Charlottesville
- 6 Clinical Trials Center of Middle Tennessee, Franklin
- 7 University Medical Center New Orleans, Louisiana State University Health Sciences Center, New Orleans
- 8 Jadestone Clinical Research, LLC, Silver Spring, Maryland
- 9 David Kavtaradze, Inc, Cordele, Georgia
- 10 Lakeland Regional Medical Center, Lakeland, Florida
- 11 Focus Clinical Research Solutions, Charlotte, North Carolina
- 12 Department of Emergency Medicine, Lewis Katz School of Medicine at Temple University, Philadelphia, Pennsylvania
- 13 Department of Medicine, Miller School of Medicine, University of Miami, Miami, Florida
- 14 Weill Cornell Medicine, New York, New York
- 15 Division of Infectious Diseases, Johns Hopkins University, Baltimore, Maryland
- 16 Veterans Affairs Tennessee Valley Healthcare System, Geriatric Research, Education and Clinical Center (GRECC), Nashville
- 17 National Center for Advancing Translational Sciences, Bethesda, Maryland
- 18 Foundation for the National Institutes of Health, Bethesda, Maryland
- 19 Stakeholder Advisory Committee, Pittsburgh, Pennsylvania
- 20 Biomedical Advanced Research and Development Authority, Washington, DC
- 21 University of Colorado School of Medicine, Aurora
- 22 Division of Pulmonary, Critical Care and Sleep Medicine, University of Missouri-Kansas City School of Medicine, Kansas City
- 23 Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania
- 24 Department of Health Outcomes & Biomedical Informatics, College of Medicine, University of Florida, Gainesville
- Editorial Managing Persistent Uncertainty in the Ethics of Clinical Research Alex John London, PhD; Christopher W. Seymour, MD, MSc JAMA
- Editor's Note At a Higher Dose and Longer Duration, Ivermectin Still Not Effective Against COVID-19 Kirsten Bibbins-Domingo, PhD, MD, MAS; Preeti N. Malani, MD, MSJ JAMA
- Original Investigation Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19 Susanna Naggie, MD, MHS; David R. Boulware, MD, MPH; Christopher J. Lindsell, PhD; Thomas G. Stewart, PhD; Nina Gentile, MD; Sean Collins, MD, MSci; Matthew William McCarthy, MD; Dushyantha Jayaweera, MD; Mario Castro, MD, MPH; Mark Sulkowski, MD; Kathleen McTigue, MD, MPH, MS; Florence Thicklin; G. Michael Felker, MD, MHS; Adit A. Ginde, MD, MPH; Carolyn T. Bramante, MD, MPH; Alex J. Slandzicki, MD; Ahab Gabriel, MD; Nirav S. Shah, MD, MPH; Leslie A. Lenert, MD, MS; Sarah E. Dunsmore, PhD; Stacey J. Adam, PhD; Allison DeLong, BS; George Hanna, MD; April Remaly, BA; Rhonda Wilder, MS; Sybil Wilson, RN; Elizabeth Shenkman, PhD; Adrian F. Hernandez, MD, MHS; Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators; William (Kelly) Vincent; Raina Vincent; Ray Bianchi; Jen Premas; Diana Cordero-Loperena; Evelyn Rivera; Madhu Gupta ; Greg Karawan; Carey Ziomek; Joseph Arena; Sonaly DeAlmeida; Soroush Ramin; Jaya Nataraj; Michael Paasche-Orlow; Lori Henault; Katie Waite; David Miller; Ginger Brounce; Constance George-Adebayo; Adeolu Adebayo; Jessica Wallan; Alex Slandzicki; Claudia Vogel; Sebastian Munoz; David Kavtaradze; Cassandra Watson; David Singleton; Maria Rivon; Amanda Sevier; Arnold Del Pilar; Amber Spangler; Sohail Rao; Luis Cantu; Arvind Krishna; Kathy Evans; Tylene Falkner; Brandi Kerr; Robert Spees; Mailyn Marta; G. Michael Felker; Amanda Harrington; Rowena Dolor; Madison Frazier; Lorraine Vergara; Jessica Wilson; Valencia Burruss; Terri Hurst; Igho Ofotokun; Laurel Bristow; Rajesh Prabhu; Krystal Klicka; Amber Lightfeather; Vicki James; Marcella Rogers; Pradeep Parihar; De'Ambra Torress; Chukwuemeka Oragwu; Ngozi Oguego; Rajesh Pillai; Mustafa Juma; Ahab Gabriel; Emad Ghaly; Dafer Al-Haddadin; Courtney Ramirez; Gammal Hassanien; Samah Ismail; Andrew Meltzer; Seamus Moran; Scott Brehaut; Angelina Roche; Manisha Mehta; Nicole Koppinger; Jose Baez; Ivone Pagan; Dallal Abdelsayed; Mina Aziz; Philip Robinson; Julie Nguyen; Victoria Pardue; Llisa Hammons; Juan Ruiz-Unger; Susan Gonzalez; Lionel Reyes; John Cienki; Gisselle Jimenez; Jonathan Cohen; Matthew Wong; Ying Yuan; Jeremy Szeto; Mark Sulkowski; Lauren Stelmash; Arch Amon; Daniel Haight; Deryl Lamb; Amron Harper; Nancy Pyram-Bernard; Arlen Quintero; Eftim Adhami; Josette Maria; Diksha Paudel; Oksana Raymond; Jeffrey Summers; Tammy Turner; Leslie Lenert; Sam Gallegos; Elizabeth Ann Szwast; Ahsan Abdulghani; Pravin Vasoya; Conrad Miller; Hawa Wiley; Nirav Shah; Tovah Klein; Julie Castex; Phillip Feliciano; Jacqueline Olivo; Marian Ghaly; Zainub Javed; Alexandra Nawrocki; Anthony Vecchiarelli; Nikki Vigil; Vijaya Cherukuri; Erica Burden; Dawn Linn; Laura Fisher; Vijay Patel; Praksha Patel; Yuti Patel; Leonard Ellison; Jeffrey Harrison; Binod Shah; Sugata Shah; Upinder Singh; Julia Donahue; Yasmin Jazayeri; Anita Gupta ; N Chandrasekar; Beth Moritz; Tabitha Fortt; Anisa Fortt; Ingrid Jones-Ince; Alix McKee; Christy Schattinger; Jason Wilson; Brenda Farlow; Nina Gentile; Lillian Finlaw; Randall Richwine; Tearani Williams; Penny Paizer; Lisa Carson; Edward Michelson; Danielle Austin; Sangeeta Khetpal; Tiffany Cantrell; Drew Franklin; Karissa Marshall; Arvind Mahadevan; Madelyn Rosequist; Martin Gnoni; Crystal Daffner; Carla VandeWeerd; Mitchell Roberts; Mark D'Andrea; Stephen Lim; Wayne Swink; Margaret Powers-Fletcher; Sylvere Mukunzi; Elizabeth Shenkman; Jamie Hensley; Brittney Manning; Carmen Isache; Jennifer Bowman; Angelique Callaghan-Brown; Taylor Scott; Tiffany Schwasinger-Schmidt; Ashlie Cornejo; Dushyantha Jayaweera; Maria Almanzar; Letty Ginsburg; Americo Hajaz; Carolyn Bramante; Matthew Robinson; Michelle Seithel; Akira Sekikawa; Emily Klawson; Luis Ostrosky; Virginia Umana; Thomas Patterson; Robin Tragus; Patrick Jackson; Caroline Hallowell; Heather Haughey; Bhavna Vaidya-Tank; Cameron Gould; Parul Goyal; Carly Gatewood; John Williamson; Hannah Seagle; Matthew McCarthy; Elizabeth Salsgiver; Eddie Armas; Jhonsai Cheng; Priscilla Huerta; Julia Garcia-Diaz; David Aamodt; JaMario Ayers; Jess Collins; John Graves; James Grindstaff; Frank Harrell; Jessica Lai; Itzel Lopez; Jessica Marlin; Alyssa Merkel; Sam Nwosu; Savannah Obregon; Dirk Orozco; Yoli Perez-Torres; Nelson Prato; Colleen Ratcliff; Max Rhode; Russell Rothman; Jana Shirey-Rice; Krista Vermillion; Hsi-Nien Tan; Seibert Tregoning; Meghan Vance; Amber Vongsamphanh; Maria Weir; Nicole Zaleski JAMA
- Original Investigation Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19 Matthew W. McCarthy, MD; Susanna Naggie, MD, MHS; David R. Boulware, MD, MPH; Christopher J. Lindsell, PhD; Thomas G. Stewart, PhD; G. Michael Felker, MD, MHS; Dushyantha Jayaweera, MD; Mark Sulkowski, MD; Nina Gentile, MD; Carolyn Bramante, MD, MPH; Upinder Singh, MD; Rowena J. Dolor, MD, MHS; Juan Ruiz-Unger, MD; Sybil Wilson, RN; Allison DeLong, BS; April Remaly, BA; Rhonda Wilder, MS; Sean Collins, MD, MSci; Sarah E. Dunsmore, PhD; Stacey J. Adam, PhD; Florence Thicklin; George Hanna, MD; Adit A. Ginde, MD, MPH; Mario Castro, MD, MPH; Kathleen McTigue, MD, MPH, MS; Elizabeth Shenkman, PhD; Adrian F. Hernandez, MD, MHS; Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators; William (Kelly) Vincent; Raina Vincent; Ray Bianchi; Jen Premas; Diana Cordero-Loperena Evelyn Rivera; Madhu Gupta ; Greg Karawan; Carey Ziomek; Joseph Arena; Sonaly DeAlmeida; Soroush Ramin; Jaya Nataraj; Michael Paasche-Orlow; Lori Henault; Katie Waite; David Miller; Ginger Brounce; Constance George-Adebayo; Adeolu Adebayo; Jessica Wallan; Claudia Vogel; Sebastian Munoz; David Kavtaradze; Cassandra Watson; David Singleton; Maria Rivon; Amanda Sevier; Arnold Del Pilar; Amber Spangler; Sohail Rao; Luis Cantu; Arvind Krishna; Kathy Evans; Tylene Falkner; Brandi Kerr; Robert Spees; Mailyn Marta; Amanda Harrington; Madison Frazier; Lorraine Vergara; Jessica Wilson; Valencia Burruss; Terri Hurst; Igho Ofotokun; Laurel Bristow; Rajesh Prabhu; Krystal Klicka; Amber Lightfeather; Vicki James; Marcella Rogers; Pradeep Parihar; De'Ambra Torress; Chukwuemeka Oragwu; Ngozi Oguego; Rajesh Pillai; Mustafa Juma; Emad Ghaly; Dafer Al-Haddadin; Courtney Ramirez; Gammal Hassanien; Samah Ismail; Andrew Meltzer; Seamus Moran; Scott Brehaut; Angelina Roche; Manisha Mehta; Nicole Koppinger; Jose Baez; Ivone Pagan; Dallal Abdelsayed; Mina Aziz; Philip Robinson; Julie Nguyen; Victoria Pardue; Llisa Hammons; Susan Gonzalez; Lionel Reyes; John Cienki; Gisselle Jimenez; Jonathan Cohen; Matthew Wong; Ying Yuan; Jeremy Szeto; Lauren Stelmash; Arch Amon; Daniel Haight; Deryl Lamb; Amron Harper; Nancy Pyram-Bernard; Arlen Quintero; Eftim Adhami; Josette Maria; Diksha Paudel; Oksana Raymond; Jeffrey Summers; Tammy Turner; Sam Gallegos; Elizabeth Ann Szwast; Ahsan Abdulghani; Pravin Vasoya; Conrad Miller; Hawa Wiley; Tovah Klein; Julie Castex; Phillip Feliciano; Jacqueline Olivo; Marian Ghaly; Zainub Javed; Alexandra Nawrocki; Anthony Vecchiarelli; Nikki Vigil; Vijaya Cherukuri; Erica Burden; Dawn Linn; Laura Fisher; Vijay Patel; Praksha Patel; Yuti Patel; Leonard Ellison; Jeffrey Harrison; Binod Shah; Sugata Shah; Upinder Shah; Julia Donahue; Yasmin Jazayeri; Anita Gupta ; N Chandrasekar; Beth Moritz; Tabitha Fortt; Anisa Fortt; Ingrid Jones-Ince; Alix McKee; Christy Schattinger; Jason Wilson; Brenda Farlow; Lillian Finlaw; Randall Richwine; Tearani Williams; Penny Paizer; Lisa Carson; Edward Michelson; Danielle Austin; Sangeeta Khetpal; Tiffany Cantrell; Drew Franklin; Karissa Marshall; Arvind Mahadevan; Madelyn Rosequist; Martin Gnoni; Crystal Daffner; Carla VandeWeerd; Mitchell Roberts; Mark D'Andrea; Stephen Lim; Wayne Swink; Margaret Powers-Fletcher; Sylvere Mukunzi; Jamie Hensley; Brittney Manning; Carmen Isache; Jennifer Bowman; Angelique Callaghan-Brown; Taylor Scott; Tiffany Schwasinger-Schmidt; Ashlie Cornejo; Maria Almanzar; Letty Ginsburg; Americo Hajaz; Matthew Robinson; Michelle Seithel; Akira Sekikawa; Emily Klawson; Luis Ostrosky; Virginia Umana; Thomas Patterson; Robin Tragus; Patrick Jackson; Caroline Hallowell; Heather Haughey; Bhavna Vaidya-Tank; Cameron Gould; Parul Goyal; Carly Gatewood; John Williamson; Hannah Seagle; Elizabeth Salsgiver; Eddie Armas; Jhonsai Cheng; Priscilla Huerta; Julia Garcia-Diaz; David Aamodt; JaMario Ayers; Jess Collins; John Graves; James Grindstaff; Jessica Lai; Itzel Lopez; Jessica Marlin; Alyssa Merkel; Sam Nwosu; Savannah Obregon; Dirk Orozco; Yoli Perez-Torres; Nelson Prato; Colleen Ratcliff; Max Rhode; Russell Rothman; Jana Shirey-Rice; Krista Vermillion; Hsi-Nien Tan; Seibert Tregoning; Meghan Vance; Amber Vongsamphanh; Maria Weir; Nicole Zaleski JAMA
- Medical News & Perspectives Highlights from CROI, the Conference on Retroviruses and Opportunistic Infections Rita Rubin, MA JAMA
- Original Investigation Higher-Dose Fluvoxamine and Time to Sustained Recovery in Outpatients With COVID-19 Thomas G. Stewart, PhD; Paulina A. Rebolledo, MD, MSc; Ahmad Mourad, MD; Christopher J. Lindsell, PhD; David R. Boulware, MD, MPH; Matthew W. McCarthy, MD; Florence Thicklin; Idania T. Garcia del Sol, MD; Carolyn T. Bramante, MD, MPH; Leslie A. Lenert, MD, MS; Stephen Lim, MD; John C. Williamson, PharmD; Orlando Quintero Cardona, MD; Jake Scott, MD; Tiffany Schwasinger-Schmidt, MD, PhD; Adit A. Ginde, MD, MPH; Mario Castro, MD, MPH; Dushyantha Jayaweera, MD; Mark Sulkowski, MD; Nina Gentile, MD; Kathleen McTigue, MD; G. Michael Felker, MD, MHS; Allison DeLong, BS; Rhonda Wilder, MS; Russell L. Rothman, MD, MPP; Sean Collins, MD, MSci; Sarah E. Dunsmore, PhD; Stacey J. Adam, PhD; George J. Hanna, MD; Elizabeth Shenkman, PhD; Adrian F. Hernandez, MD, MHS; Susanna Naggie, MD, MHS; Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators; Ryan Fraser; Mark Ward; Jennifer Gamboa Jackman; M. Patricia McAdams; Julia Vail; Kayla Korzekwinski; Martina Oyelakin; Julie Chopp; Desmon Randle; Samantha Dockery; Rodney Adkins; Mathew Crow; Erin Nowell; Kadie Wells; Alicia Herbert; Allegra Stone; Heather Heavlin; Linley Brown; Tina Harding; Amanda Harrington; Meaghan Beauchaine; Kelly Lindblom; Andrea Burns; David Aamodt; Jess Collins; Sheri Dixon; Yue Gao; John Graves; James Grindstaff; Frank Harrell; Jessica Lai; Vicky Liao; Itzel Lopez; Elizabeth Manis; Kalley Mankowski; Jessica Marlin; Alyssa Merkel; Sam Nwosu; Savannah Obregon; Dirk Orozco; Nelson Prato; Max Rohde; Jana Shirey-Rice; Krista Vermillion; Jacob Smith; Hsi-nien Tan; Meghan Vance; Maria Weir; William (Kelly) Vincent; Raina Vincent; Ray Bianchi; Jen Premas; Diana Cordero-Loperena; Evelyn Rivera; Madhu Gupta; Greg Karawan; Joseph Arena; Sonaly DeAlmeida; Soroush Ramin; Jaya Nataraj; Julien Dedier; Ana Maria Ramirez; Katherine Waite; Jason Okulicz; Joseph Marcus; Alexis Southwell; Genice Jacques; Cedar Sexton; David Miller; Ginger Brounce; Constance George-Adebayo; Adeolu Adebayo; Jose Zapatero; Julie Clement; Theresa Ronan; Ashley Woods; Christopher Gallegos; Tamara Flys; Olivia Sloan; Anthony Olofintuyi; Joshua Samraj; Jackelyn Samraj; Alma Vasbinder; Amaya Averett; Alex Slandzicki; Aaron Milstone; Jessica Wallan; Lindsey Robbs; Claudia Vogel; Sebastian Munoz; David Kavtaradze; Casandra Watson; David Singleton; Marcus Sevier; Maria Rivon; Arnold Del Pilar; Amber Spangler; Sohail Rao; Luis Cantu; Arvind Krishna; Heidi Daugherty; Brandi Kerr; Kathy Evans; Robert Spees; Mailyn Marta; Rowena Dolor; Lorraine Vergara; Jackie Jordan; Valencia Burruss; Terri Hurst; Igho Ofotokun; Paulina A. Rebolledo; Cecilia Zhang; Veronica E. Smith; Rajesh Prabhu; Krystal Klicka; Amber Lightfeather; Vickie James; Marcella Rogers; Pradeep Parihar; De'Ambra Torress; Chukwuemeka Oragwu; Ngozi Oguego; Rajesh Pillai; Mustafa Juma; Ahab Gabriel; Emad Ghaly; Marian Michal; Michelle Vasquez; Angela Mamon; Michelle Sheets; Gammal Hassanien; Samah Ismail; Yehia Samir; Andrew Meltzer; Soroush Shahamatdar; Ryan S. Heidish; Scott Brehaut; Angelina Roche; Manisha Mehta; Nicole Koppinger; Jose Baez; Ivone Pagan; Dallal Abdelsayed; Mina Aziz; Philip Robinson; Grace Lozinski; Julie Nguyen; Alvin Griffin; Michael Morris; Nicole Love; Bonnie Mattox; Raykel Martin; Victoria Pardue; Teddy Rowland; Juan Ruiz-Unger; Lionel Reyes; Yadira Zamora; Navila Bacallao; John Cienki; Jonathan Cohen; Ying Yuan; Jenny Li; Jeremy Szeto; Mark Sulkowski; Lauren Stelmash; Idania Garcia del Sol; Ledular Morales Castillo; Anya Gutierrez; Sabrina Prieto; Arch Amon; Andrew Barbera; Andrew Bugajski; Walter Wills; Kellcee Jacklin; Deryl Lamb; Amron Harper; Elmer Stout; Katherine Weeks; Merischia Griffin; Nancy Pyram-Bernard; Arlen Quintero; Eftim Adhami; Giovanni Carrillo; Josette Maria; Diksha Paudel; Oksana Raymond; Jeffrey Summers; Tammy Turner; Ebony Panaccione; Elizabeth Szwast; Ahsan Abdulghani; Pravin Vasoya; Conrad Miller; Hawa Wiley; Austin Chan; Saadia Khizer; Nirav Shah; Oluwadamilola Adeyemi; Wei Ning Chi; July Chen; Melissa Morton-Jost; Julie Castex; Phillip Feliciano; Jacqueline Olivo; Maria Maldonado; Anthony Vecchiarelli; Diana Gaytan-Alvarez; Vijaya Cherukuri; Santia Lima; Radica Alicic; Allison A. Lambert; Carissa Urbat; Joni Baxter; Ann Cooper; Dawn Linn; Laura Fisher; Vijay Patel; Yuti Patel; Roshan Talati; Priti Patel; Leonard Ellison; Angee Roman; Jeffrey Harrison; James Moy; Dina Naquiallah; Binod Shah; Upinder Singh; Yasmin Jazayeri; Andrew O’Donnell; Orlando Quintero; Divya Pathak; Anita Gupta; N Chandrasekar; Clifford Curtis; Briana White; Martha Dockery; Maya Hicks; Tabitha Fortt; Anisa Fortt; Ingrid Jones-Ince; Alix McKee; Jason Wilson; Brenda Farlow; Nina Gentile; Casey Grady; Randall Richwine; Tearani Williams; Penny Pazier; Edward Michelson; Susan Watts; Diluma Kariyawasam; Leann Rodriguez; Jose Luis Garcia; Ismarys Manresa; Angel Achong; Mari Garcia; Sangeeta Khetpal; Faith Posey; Arvind Mahadevan; Martin Gnoni; Carla VandeWeerd; Erica Sappington; Mitchell Roberts; Jennifer Wang; Melissa Adams; Xinyi Ding; Mark D'Andrea; Stephen Lim; Wayne Swink; Emily Bozant; Margaret Powers-Fletcher; Delia Miller; Sylvere Mukunzi; Brittney Manning; Carmen Isache; Jennifer Bowman; Angelique Callaghan-Brown; Debra Martin; Ashley Ast; Brent Duran; Ashlie Cornejo; Allie Archer; Dushyantha Jayaweera; Maria Almanzar; Vanessa Motel; Neeta Bhat; Daniela Parra; Matthew Pullen; Paula Campora; Matthew Robinson; Michelle Seithel; Akira Sekikawa; Emily Klawson; Jonathan Arnold; Luis Ostrosky-Zeichner; Virginia Umana; Laura Nielsen; Carolyn Z. Grimes; Thomas F. Patterson; Robin Tragus; Bridgette T. Soileau; Patrick E.H. Jackson; Carolina Hallowell; Heather M. Haughey; Bhavna Vaidya-Tank; Cameron Gould; Parul Goyal; Sue Sommers; Haley Pangburn; Carly Jones; John Williamson; Rica Abbott; Hannah Seagle; Mathias DeComarmond; Nicholas Pickell; Unwana Umana; Candace Alleyne; Eddie Armas; Ramon O. Perez Landabur; Michelle De La Cruz; Martha Ballmajo JAMA
- Comment & Response Error in the Exclusion of Participants From Analysis in the ACTIV-6 Platform Randomized Clinical Trial Susanna Naggie, MD, MHS JAMA
- Correction Errors in Results From Erroneous Exclusion of Participants in Analysis JAMA
Question Does ivermectin, with a maximum targeted dose of 600 μg/kg daily for 6 days, compared with placebo, shorten symptom duration among adult (≥30 years) outpatients with symptomatic mild to moderate COVID-19?
Findings In this double-blind, randomized, placebo-controlled platform trial including 1432 US adults with COVID-19 during February 2022 to July 2022, the median time to sustained recovery was 11 days in the ivermectin group and 12 days in the placebo group. In this largely vaccinated (83%) population, the posterior probability that ivermectin reduced symptom duration by more than 1 day was less than 0.1%.
Meaning These findings do not support the use of ivermectin among outpatients with COVID-19.
Importance It is unknown whether ivermectin, with a maximum targeted dose of 600 μg/kg, shortens symptom duration or prevents hospitalization among outpatients with mild to moderate COVID-19.
Objective To evaluate the effectiveness of ivermectin at a maximum targeted dose of 600 μg/kg daily for 6 days, compared with placebo, for the treatment of early mild to moderate COVID-19.
Design, Setting, and Participants The ongoing Accelerating COVID-19 Therapeutic Interventions and Vaccines 6 (ACTIV-6) platform randomized clinical trial was designed to evaluate repurposed therapies among outpatients with mild to moderate COVID-19. A total of 1432 participants older than 30 years with confirmed COVID-19 experiencing at least 2 symptoms of acute infection for less than or equal to 7 days were enrolled at 93 sites in the US from February 16, 2022, through July 22, 2022, with follow-up data through November 10, 2022.
Interventions Participants were randomly assigned to receive ivermectin, with a maximum targeted dose of 600 μg/kg (n = 708) daily, or placebo (n = 724) for 6 days.
Main Outcomes and Measures The primary outcome was time to sustained recovery, defined as at least 3 consecutive days without symptoms. The 7 secondary outcomes included a composite of hospitalization, death, or urgent/emergent care utilization by day 28.
Results Among 1432 randomized participants who received study medication or placebo, the median (IQR) age was 48 (38-58) years, 854 (59.6%) were women, and 1188 (83.1%) reported receiving at least 2 SARS-CoV-2 vaccine doses. The median (IQR) time to sustained recovery was 11 (11-12) days in the ivermectin group and 12 (11-12) days in the placebo group. The hazard ratio for improvement in time to recovery was 1.02 (95% credible interval, 0.92-1.12; P value for efficacy = .65). Among those receiving ivermectin, 39 (5.5%) were hospitalized, died, or had urgent or emergency care visits compared with 42 (5.8%) receiving placebo (hazard ratio, 0.97 [95% credible interval, 0.60-1.45]; P = .55). In the ivermectin group, 1 participant died and 6 were hospitalized (1.0%); 2 participants (0.3%) were hospitalized in the placebo group and there were no deaths. Adverse events were uncommon in both groups.
Conclusions and Relevance Among outpatients with mild to moderate COVID-19, treatment with ivermectin, with a maximum targeted dose of 600 μg/kg daily for 6 days, compared with placebo did not improve time to sustained recovery. These findings do not support the use of ivermectin in patients with mild to moderate COVID-19.
Trial Registration ClinicalTrials.gov Identifier: NCT04885530
Despite treatment advances for COVID-19, the evolution of SARS-CoV-2 variants and subvariants has shifted therapeutic options, including the recent loss of effectiveness of monoclonal antibodies. Novel oral antivirals have been authorized for high-risk individuals in high-income countries. 1 , 2 However, efficacy of these antivirals in those vaccinated or with prior SARS-CoV-2 infection remains unclear. Interest remains for the potential of repurposed drugs to improve symptoms and clinical outcomes among patients with COVID-19.
Numerous repurposed drugs have been investigated for COVID-19 management, with several large randomized outpatient trials published. 3 - 5 Trial results have been mixed. Trials of some drugs suggest possible benefit by reducing emergency department (ED) visits or hospitalizations, including fluvoxamine dosed at 100 mg twice daily 3 and immediate-release metformin. 6 Others have failed to show a reduction in ED visits or hospitalizations, such as fluvoxamine 50 mg twice daily. 6 , 7 Although recently completed trials benefit from the increasing representation of vaccinated people, which is more relevant to the pandemic’s current state, the results have not affected treatment guidelines largely due to study design limitations, including definitions of outcomes that were of unclear significance in the US health care setting. 8 - 10
Ivermectin, an antiparasitic drug used worldwide for onchocerciasis and strongyloidiasis, emerged in 2020 as a potential repurposed drug for COVID-19 initially informed by an in vitro study suggesting possible antiviral activity. 11 The interest for ivermectin as a therapy for COVID-19 has remained high and, although there have been numerous ivermectin studies, its use has become controversial due to a lack of high-quality adequately powered randomized trials and article retractions of some of the earlier and most positive studies. 12 - 15 Three large randomized outpatient trials of people with symptomatic mild or moderate COVID-19 failed to identify a clinical benefit of ivermectin when dosed at 400 μg/kg daily for 3 days. 16 - 18 One possibility is that the dose and duration studied were too low and too short, missing the therapeutic window for ivermectin. A combination of modeling studies and a proof-of-concept clinical study have suggested doses up to 600 μg/kg daily may achieve system levels sufficient for in vitro antiviral activity. 18 , 19 For this reason we tested ivermectin, with a maximum targeted dose of 600 μg/kg daily, for 6 days from February 16, 2022, through July 22, 2022. This report describes the effectiveness of this dose and duration of ivermectin compared with placebo for the treatment of early mild to moderate COVID-19. The primary outcome was time to sustained recovery, defined as at least 3 consecutive days without symptoms, and secondary outcomes included a composite of hospitalization, death, or urgent/emergent care utilization by day 28.
Accelerating COVID-19 Therapeutic Interventions and Vaccines 6 (ACTIV-6) is an ongoing, fully remote (decentralized), double-blind, randomized placebo-controlled platform trial investigating repurposed drugs for the treatment of mild to moderate COVID-19 in the outpatient setting. The platform protocol is designed to be flexible, allowing enrollment across a wide range of settings within health care systems and the community, as well as virtually. The platform enrolls outpatients with mild to moderate COVID-19 with a confirmed positive SARS-CoV-2 test result. The full trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2 .
The trial protocol was approved by each site’s institutional review board. Participants provided informed consent either via written consent or an electronic consent process. An independent data monitoring committee oversaw participant safety and trial conduct.
Recruitment into the platform trial opened on June 11, 2021, and ivermectin 600 μg/kg was included on the platform beginning on February 16, 2022. Enrollment into the ivermectin 600 μg/kg group was stopped on July 22, 2022, when 1432 participants had received their study drug, identical matched placebo, or contributing placebo. Participants were either identified by sites or self-identified by contacting a central study telephone hotline or website.
Study staff verified eligibility criteria including age of 30 years or older, SARS-CoV-2 infection within 10 days (positive polymerase chain reaction or antigen test result, including home-based tests), and experiencing at least 2 symptoms of acute COVID-19 for no more than 7 days from enrollment. The protocol defined “mild to moderate” as having symptoms as noted above self-reported at the time of enrollment, and symptoms were graded by participants as none, mild, moderate, or severe. Symptoms included fatigue, dyspnea, fever, cough, nausea, vomiting, diarrhea, body aches, chills, headache, sore throat, nasal symptoms, and new loss of sense of taste or smell. Exclusion criteria included hospitalization, ivermectin use within 14 days, and known allergy or contraindication to the study drug ( Supplement 1 ). Vaccination against SARS-CoV-2 was allowable, as was concurrent use of standard therapies for COVID-19 available under US Food and Drug Administration Emergency Use Authorization or approval.
Participants were randomized using a random number generator in a 2-step process ( Figure 1 ). First, participants were randomized to receive an active agent or placebo in a ratio of m :1, where m is the number of study drugs for which the participant was eligible; the other study drug under investigation during this period was fluvoxamine 50 mg twice daily for 10 days. Participants could choose to opt out of specific study drug groups during the consent process if they or the site investigator did not feel there was equipoise or if there was a contraindication to any study drug on the platform. After randomization to receive an active agent vs placebo, participants were randomized with equal probability among the study drugs for which they were eligible. The more study drugs a participant was eligible for, the greater the chance of receiving an active agent. Participants who were eligible to receive both ivermectin and fluvoxamine 50 mg but were randomized to the fluvoxamine-matched placebo group were included in and contributed to the placebo group for ivermectin.
A central pharmacy supplied ivermectin or placebo to participants via direct home delivery. Ivermectin was supplied as a bottle of 7-mg tablets. Participants were instructed to take a prespecified number of tablets for 6 consecutive days based on their weight for a maximum targeted daily dose of approximately 600 μg/kg. The dosing schedule was based on weight ranges as follows: those weighing 35 to 52 kg received a 21-mg daily dose; 53 to 69 kg, 28-mg daily dose; 70 to 89 kg, 42-mg daily dose; 90 to 109 kg, 49-mg daily dose; 110 to 129 kg, 56-mg daily dose; and more than 129 kg, 70-mg daily dose. This schedule resulted in a range of doses from 400 to 600 μg/kg (eFigure 1 in Supplement 3 ) and a median (IQR) dose of 498 (464-532) μg/kg per day. The median daily dose was calculated among participants randomized to receive ivermectin. Packaging for the matched placebo was identical to ivermectin and packaging for the contributing placebos was identical to that of the associated study drug, which in this case was fluvoxamine 50 mg twice daily.
The primary measure of effectiveness was time to sustained recovery, defined as the number of days between study drug receipt and the third of 3 consecutive days without symptoms. This outcome was selected a priori from among the 2 co–primary end points that remain available to other study drugs in the platform ( Supplement 2 ). The key secondary outcome was the composite of hospitalization or death by day 28. Other secondary outcomes included mean time unwell, estimated from a longitudinal ordinal model; COVID-19 Clinical Progression Scale score on days 7, 14, and 28; mortality through day 28; and the composite of urgent or emergency care visits, hospitalizations, or death through day 28. The final secondary outcome, the Patient-Reported Outcomes Measurement Information System 29 profile, was to be assessed through day 90 and is not reported in this article because of the longer follow-up.
The study was designed as a fully remote, or decentralized, trial. Screening and eligibility confirmation were participant-reported and site-confirmed. A positive SARS-CoV-2 polymerase chain reaction or antigen test result was verified prior to randomization via uploading into the participant portal and reviewal by the site. At screening, participant-reported demographic information was collected and included race and ethnicity, eligibility criteria, medical history, concomitant medications, symptom reporting, and quality-of-life questionnaires.
A central investigational pharmacy distributed the study drug (either active or placebo) using a next-day priority shipping service. Delivery was tracked and participants needed to have received the study drug within 7 days of enrollment to be included. Confirmation that the study drug was delivered to the participant’s address was required for the participant to be included in the analysis. Receipt of study drug was defined as study day 1.
Participants were asked to complete daily assessments and report adverse events through day 14. Assessments included symptoms and severity, health care visits, and medications. If symptoms were still ongoing at day 14, daily surveys continued until participants experienced 3 consecutive days without symptoms or until day 28. At days 28 and 90, all participants completed assessments. Supplement 1 presents survey details. Additional details of participant monitoring during follow-up are available in Supplement 3 .
This platform trial was designed to be analyzed accepting the possibility of adding and dropping groups as the trial progressed. The general analytical approach was regression modeling. Proportional hazard regression was used for time-to-event analyses and cumulative probability ordinal regression models were used for ordinal outcomes. In addition, the mean time spent unwell was estimated using a longitudinal ordinal regression model as a quantification of benefit.
The complete statistical analysis plan is provided in Supplement 2 . Briefly, the planned primary end point analysis was a bayesian proportional hazards model for time to sustained recovery. The primary inferential (decision-making) quantity was the posterior distribution for the treatment assignment hazard ratio (HR), with HR greater than 1 indicating faster recovery. Decision thresholds and modeling parameters are as previously described 16 and provided in Supplement 2 . The study design was estimated to have 80% power to detect an HR of 1.2 in the primary end point with approximately 1200 participants. To achieve this sample size in an ongoing platform trial, once 1200 participants had been randomized to the study group or matching placebo and had received the study drug, the study group became unavailable for new participants expressing interest in the platform. Some participants had already consented to participate but had not yet been randomized or received the study drug at the time of group closure, and these participants were allowed to continue as assigned.
The primary end point–adjusted model included the following predictor variables in addition to randomization assignment: age (as restricted cubic spline), sex, duration of symptoms at study drug receipt, calendar time (as restricted cubic spline, surrogate for SARS-CoV-2 variant/subvariant), vaccination status (no vaccination vs ≥1 dose), geographic region (Northeast, Midwest, South, West), call center indicator, and day 1 symptom severity. This adjusted model was prespecified. The proportional hazards assumption of the primary end point was evaluated by generating visual diagnostics, such as the log-log plot and plots of time-dependent regression coefficients for each predictor in the model, a diagnostic that indicates deviations from proportionality if the time-dependent coefficients are not constant in time.
Secondary end points were analyzed with bayesian regression models (either proportional hazards or proportional odds) using noninformative priors for all parameters. Secondary end points were not used for formal decision-making, and no decision threshold was selected. Due to an increased potential for type I error due to multiple comparisons, secondary end points should be interpreted as exploratory. The same covariates used in the primary end point model were used in the adjusted analysis of secondary end points, provided that the end point accrued enough events to be analyzed with covariate adjustment.
As a platform trial, the primary analysis is implemented separately for each study drug, where the placebo group consists of contemporaneously randomized participants who met the eligibility criteria for that study drug; this includes both matched and contributing placebo. For this trial, the modified intention-to-treat analysis set for the primary analyses included all participants who received the study drug, and participants were analyzed as assigned. All available data were used to compare ivermectin vs placebo, regardless of postrandomization study drug treatment adherence. In both the primary and secondary end point analyses, missing data among covariates used for adjustment were addressed with conditional mean imputation because the amount of missing covariate data was minimal (<4%).
A prespecified analysis tested for differential treatment effects as a function of preexisting participant characteristics. Analysis of heterogeneity of treatment effect included age, symptom duration, body mass index (BMI), symptom severity on day 1, calendar time (surrogate for SARS-CoV-2 variant), sex, and vaccination status; continuous variables were modeled as such without creating subgroups.
Analyses were performed with R, version 4.1 (R Foundation for Statistical Computing) with primary packages of rstanarm, rmsb, and survival. 20 Additional details are available in Supplement 3 .
Of the 2213 participants who consented for inclusion in the ivermectin group, 1619 were eligible to receive ivermectin and randomized, of whom 1459 were randomized in the ivermectin platform (718 to receive ivermectin and 741 to receive placebo). In the placebo group, 678 were randomized to receive matching placebo and 63 were randomized to receive a shared placebo from another group on the platform. Ultimately, 708 in the ivermectin group and 724 in the placebo group received their medication by mail within 7 days and were included in the modified intention-to-treat cohort for analysis ( Figure 1 ).
Those randomized to receive the active agent in the ivermectin group received the active study drug with a targeted maximum dose of 600 μg/kg; the median (IQR) dose of ivermectin was 498 (464-532) μg/kg (eFigure 1 in Supplement 3 ).
The median (IQR) age of the participants was 48 (38-58) years and 653 (45.6%) were 50 years or older ( Table 1 ). The population included 854 (59.6%) women and 115 participants (8.0%) identified as Black or African American, 102 (7.1%) identified as Asian, and 306 (21.4%) reported being of Latino/Hispanic ethnicity. Although not required for enrollment, high-risk comorbidities included BMI greater than 30 (38.5%), diabetes (9.7%), hypertension (26.7%), asthma (14.6%), and chronic obstructive pulmonary disease (2.4%). Overall, 1188 participants (83.1%) reported receiving at least 2 COVID-19 vaccine doses. Median (IQR) time from symptom onset to enrollment was 3 (2-5) days and to study drug receipt was 5 (3-7) days, with 60% receiving the study drug within 5 days of symptom onset (eFigure 2 in Supplement 3 ). eTable 1 in Supplement 3 presents baseline symptom prevalence and severity.
The median (IQR) time to recovery was 11 (11-12) days in the ivermectin group and 12 (11-12) days in the placebo group. The posterior probability for benefit was .65 for the primary outcome of time to recovery, with an HR of 1.02 (95% credible interval [CrI], 0.92-1.12), where HR greater than 1 indicates faster symptom resolution with ivermectin ( Table 2 and Figure 2 A). This posterior probability was below the prespecified threshold of .95 ( Supplement 2 ). The data do not provide evidence of a conclusive treatment benefit when using a bayesian noninformative prior, no prior, with various approaches to imputing missing symptom data, or when restricting the analysis to participants who received the drug within 2 or 3 days of symptom onset and across severity of symptoms reported on day 1 ( Table 2 , Figure 3 , and eFigures 3 and 4 in Supplement 3 ). The probability that ivermectin reduced symptom duration by 24 hours was less than 0.1%.
Hospitalizations and deaths were uncommon, with 7 events (including 1 death not attributable to COVID-19 or treatment) in the ivermectin group and 2 events (no deaths) in the placebo group (eFigure 5A in Supplement 3 ). Statistical comparisons were uninformative due to the few events. The composite secondary outcome of urgent care or ED visits, hospitalizations, or death was not shown to differ with ivermectin compared with placebo (5.5% [39/708] vs 5.8% [42/722]; HR, 0.97 [95% CrI, 0.60-1.45]; P value for efficacy = .55) ( Table 2 , Figure 2 B, and eFigure 5B in Supplement 3 ). The difference in the amount of time spent feeling unwell with COVID-19 was estimated as 1 hour faster with ivermectin (95% CrI, 9 hours better to 8 hours worse) than placebo ( Figure 2 C). The COVID Clinical Progression Scale scores at days 7, 14, and 28 did not meet prespecified thresholds for beneficial treatment effect ( Supplement 3 ). For example, by day 7, a total of 582 of 677 participants (86%) in the ivermectin group and 600 of 693 (87%) in the placebo group were not hospitalized and did not report limitation of activities (eFigure 6 in Supplement 3 ).
Interaction tests for heterogeneity of treatment effect showed no overall influence of the putative treatment effect modifiers, even when all subgroup analyses across symptom severity were not adjusted for multiple comparisons (eFigure 7 in Supplement 3 ). The overall effect of timing from symptom onset to receipt of the study drug was not significant ( P = .19 for heterogeneity). Similarly, no evidence existed for a different treatment effect of ivermectin compared with placebo for severity of symptoms, sex, age, BMI, calendar time, or vaccination status (eFigure 8 in Supplement 3 ).
Among participants who reported taking the study drug at least once, adverse events were similar in both groups (53/635 [8.3%] in the ivermectin group and 41/652 [6.3%] in the placebo group with adverse events) (eTable 2 in Supplement 3 ). Adverse events reported more than twice, only in the ivermectin group, included cognitive impairment (n = 6), blurred vision (n = 6), light sensitivity to eye (n = 5), photophobia (n = 4), and dizziness (n = 5). Serious adverse events were rare, with 6 in the ivermectin group and 4 in the placebo group. The death in the ivermectin group was reported to be an accident and not attributable to the study drug or COVID-19.
Among a largely vaccinated outpatient population with mild to moderate COVID-19, treatment with ivermectin, with a targeted maximum dose of 600 μg/kg daily for 6 days, compared with placebo was not shown to improve time to recovery in more than 1200 participants in the US during a period of Omicron variant/subvariant circulation. No evidence of benefit was observed for secondary clinical outcomes, including the composite of hospitalization, death, or acute care visits. Hospitalization and death were uncommon in this largely vaccinated population. These findings do not support the use of ivermectin in outpatients with COVID-19.
Multiple large double-blind randomized clinical trials have failed to identify a clinically meaningful benefit of ivermectin when used at a targeted dose of 400 μg/kg daily for 3 days. 6 , 17 This large clinical trial addresses a potential gap in knowledge by testing (1) a higher daily dose (targeted maximum dose of 600 μg/kg) and (2) a longer (6-day) duration of ivermectin. Due to the lack of early-phase studies or animal-model studies to determine optimal dosing for a therapeutic drug, the appropriate dosing of ivermectin for COVID-19 was never determined. Modeling studies and a proof-of-concept clinical study have suggested that doses up to 600 μg/kg daily may achieve levels sufficient for in vitro antiviral activity 18 , 19 ; however, a phase 2 trial testing ivermectin, 600 μg/kg daily for 7 days, and assessing a virologic end point of oropharyngeal SARS-CoV-2 polymerase chain reaction test result did not show measurable antiviral activity and was stopped for futility. 21 With weight-based dosing, there is additional variability in the range for dosing and, in this study, the dosing per weight strata was targeted to a maximum dose of 600 μg/kg; thus, the median dose across the study population of approximately 500 μg/kg is meaningfully higher than that achieved in studies that targeted a maximum dose of 400 μg/kg. For example, a previous study from the current platform trial that had a maximum targeted dose of ivermectin 400 μg/kg achieved a median dose of 343 μg/kg. The 600-μg/kg dose was safe and generally well tolerated, with a higher prevalence of the known self-resolving visual disturbances in the intervention group previously reported with similar doses of ivermectin for parasitic infections. 18 , 19
The notable difference in baseline characteristics between these 2 cohorts is the completed vaccination rate, which was 83% for this study and 47% for the prior ivermectin 400 μg/kg group. 16 Hospitalizations and COVID-19–related clinical events were less common in this largely vaccinated cohort. The incidence of acute care visits, hospitalizations, or death was similar with ivermectin (5.5%) and placebo (5.8%), which was a result also observed in the 2 previous randomized trials of ivermectin 400 μg/kg in the US. 6 , 16
This trial has several strengths. This was a double-blind, randomized, placebo-controlled nationwide trial with 93 enrolling sites and a call center that recruited participants from all 50 US states. The ivermectin 600 μg/kg group of the platform trial enrolled rapidly due to ongoing Omicron variant/subvariant surges and largely included vaccinated people, thus representing a highly relevant study population that also addresses a weakness of many other studies that excluded vaccinated people. Furthermore, standard-of-care therapies were allowable in this study, although utilization was low.
This study has limitations. Due to infrequent hospitalization, this study cannot assess the effect of the intervention on this clinical outcome. Also, due to the remote nature of the trial, 60% of participants received the study drug within 5 days of symptom onset. Most outpatient COVID-19 antiviral trials have limited enrollment to participants within 5 days of symptom onset. 1 , 2 In this trial, no evidence of a differential treatment effect was observed based on shorter time to study drug receipt. Lastly, the primary end point–adjusted model did not include underlying comorbidities. Treatment effect was putatively expected to differ based on age and BMI, and these were included as covariates and evaluated for heterogeneity of treatment effect.
Among outpatients with mild or moderate COVID-19, treatment with ivermectin, with a targeted maximum dose of 600 μg/kg daily for 6 days, was not shown to improve time to sustained recovery compared with placebo. These findings do not support the use of ivermectin in outpatients with COVID-19.
Corresponding Author: Susanna Naggie, MD, MHS, Duke Clinical Research Institute, Duke University School of Medicine, 300 W Morgan St, Ste 800, Durham, NC 27701 ( [email protected] ).
Accepted for Publication: February 1, 2023.
Published Online: February 20, 2023. doi:10.1001/jama.2023.1650
Correction: This article was corrected on May 16, 2024, because participants were erroneously excluded from the anaysis.
Author Contributions: Drs Lindsell and Stewart had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Naggie, Boulware, Lindsell, Stewart, Kavtaradze, Gentile, Felker, McCarthy, Rothman, Wilson, Remaly, Collins, Thicklin, Ginde, Castro, Hernandez.
Acquisition, analysis, or interpretation of data: Naggie, Boulware, Lindsell, Stewart, Slandzicki, Lim, Cohen, Kavtaradze, Amon, Gabriel, Felker, Jayaweera, Sulkowski, Rothman, DeLong, Wilder, Dunsmore, Adam, Thicklin, Hanna, Castro, McTigue, Shenkman, Hernandez.
Drafting of the manuscript: Naggie, Boulware, Lindsell, Stewart, Amon, McCarthy, Thicklin.
Critical revision of the manuscript for important intellectual content: Boulware, Lindsell, Stewart, Slandzicki, Lim, Cohen, Kavtaradze, Gabriel, Gentile, Felker, Jayaweera, Sulkowski, Rothman, Wilson, DeLong, Remaly, Wilder, Collins, Dunsmore, Adam, Hanna, Ginde, Castro, McTigue, Shenkman, Hernandez.
Statistical analysis: Lindsell, Stewart, McCarthy.
Obtained funding: Naggie, Lindsell, Gabriel, Jayaweera, Collins, Hernandez.
Administrative, technical, or material support: Naggie, Lindsell, Slandzicki, Lim, Cohen, Amon, Gabriel, Jayaweera, Wilson, DeLong, Remaly, Wilder, Dunsmore, Adam, Hanna, McTigue, Hernandez.
Supervision: Naggie, Lindsell, Kavtaradze, Amon, Gabriel, Gentile, Felker, Jayaweera, Rothman, Collins, Castro, Hernandez.
Conflict of Interest Disclosures: Dr Naggie reported receiving grants from the National Institutes of Health (NIH) during the conduct of the study and receiving grants from Gilead Sciences and AbbVie; receiving personal fees from Pardes Biosciences and Silverback Therapeutics for consulting; serving as a scientific advisor for and having stock options in Vir Biotechnology; receiving personal fees from and serving on a data and safety monitoring board for Personal Health Insights; and serving on an event adjudication committee for Bristol Myers Squibb/PRA Health Sciences outside the submitted work. Dr Boulware reported receiving grants from the NIH (#U24TR001608) as co-chair of the ACTIV-6 trial steering committee during the conduct of the study. Dr Lindsell reported receiving grants to the institution from the National Center for Advancing Translational Sciences (NCATS) to the institution during the conduct of the study and grants to the institution from NIH and Department of Defense and research funds to the institution from the CDC, bioMerieux, AstraZeneca, AbbVie, Entegrion Inc, and Endpoint Health outside the submitted work; having a patent for risk stratification in sepsis and septic shock issued to Cincinnati Children's Hospital Medical Center; and having stock options in Bioscape Digital unrelated to the current work. Dr Stewart reported receiving grants from Duke University as a subaward for ACTIV-6 from NIH during the conduct of the study and grants from NIH supported by grants from NCATS and NIDDK and research support from the Abdominal Core Health Quality Collaborative, a 501(c)(3) nonprofit organization, outside the submitted work. Dr Lim reported receiving a subaward from NCATS to the institution during the conduct of the study. Dr Gentile reported receiving personal fees from Duke University for ACTIV-6 protocol development committee membership during the conduct of the study. Dr Felker reported receiving grants from NIH during the conduct of the study. Dr Jayaweera reported receiving grants from NCATS during the conduct of the study and grants from Gilead, ViiV Healthcare, and Janssen and personal fees from Theratechnologies outside the submitted work. Dr Sulkowski reported receiving grants to the institution from Janssen, Vir, and GSK; personal fees for serving on a scientific advisory board from GSK, AbbVie, Antios, Assembly Bio, Atea, Gilead; personal fees for serving on a data and safety monitoring board from Precision Bio and Immunocore; personal fees as an editor for Journal of Viral Hepatitis ; and personal fees from NIH (K24DA034621) outside the submitted work. Dr Rothman reported receiving grants from the NIH during the conduct of the study and spouse owning a small amount of stock in Moderna. Dr Wilson reported receiving grants from NCATS (3U24TR001608) during the conduct of the study. Dr DeLong reported receiving grants from NCATS (3U24TR001608) during the conduct of the study. Dr Collins reported receiving grants from NCATS during the conduct of the study and personal fees from Vir Biotechnology and Enanta Pharmaceuticals and grants from NHLBI outside the submitted work. Dr Adam reported receiving US Government funding through Operation Warp Speed during the conduct of the study. Dr Hanna reported receiving grants from US Biomedical Advanced Research & Development Authority contract to Tunnell Government Services for consulting services during the conduct of the study and personal fees from Merck & Co and AbPro outside the submitted work. Dr Ginde reported receiving contracts from NIH during the conduct of the study and grants from NIH, Centers for Disease Control and Prevention, Department of Defense, Faron Pharmaceuticals, and AbbVie outside the submitted work. Dr Castro reported receiving grants from NIH during the conduct of the study and grants from ALA, PCORI, AstraZeneca, Gala Therapeutics, Genentech, GSK, Novartis, Pulmatrix, Sanofi-Aventis, Shionogi, and Theravance and personal fees from Genentech, Teva, Sanofi-Aventis, Merck, Novartis, Arrowhead, Allakos, Amgen, OM Pharma, Pfizer, Pioneering Medicines, GSK, and Regeneron and having stock options in Aer Therapeutics outside the submitted work. Dr McTigue reported receiving grants from University of Pittsburgh for ACTIV-6 research funding during the conduct of the study. Dr Hernandez reported receiving grants from AstraZeneca, Merck, and Pfizer outside the submitted work. No other disclosures were reported.
Funding/Support: ACTIV-6 is funded by the NCATS (3U24TR001608-06S1). Additional support for this study was provided by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (contract No.75A50122C00037). The Vanderbilt University Medical Center Clinical and Translational Science Award from NCATS (UL1TR002243) supported the REDCap infrastructure.
Role of the Funder/Sponsor: NCATS participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement : See Supplement 5 .
Additional Contributions: We thank Samuel Bozzette, MD, PhD, and Eugene Passamani, MD (National Center for Advancing Translational Sciences), for their roles in the trial design and protocol development. We also thank the ACTIV-6 data monitoring committee and clinical events committee members for their contributions: data monitoring committee : Clyde Yancy, MD, MSc (Northwestern University Feinberg School of Medicine); Adaora Adimora, MD (University of North Carolina, Chapel Hill); Susan Ellenberg, PhD (University of Pennsylvania); Kaleab Abebe, PhD (University of Pittsburgh); Arthur Kim, MD (Massachusetts General Hospital); John D. Lantos, MD (Children’s Mercy Hospital); Jennifer Silvey-Cason (participant representative); Frank Rockhold, PhD (Duke Clinical Research Institute); Sean O’Brien, PhD (Duke Clinical Research Institute); Frank Harrell, PhD (Vanderbilt University Medical Center); Zhen Huang, MS (Duke Clinical Research Institute); clinical events committee : Renato Lopes, MD, PhD, MHS; W. Schuyler Jones, MD; Antonio Gutierrez, MD; Robert Harrison, MD; David Kong, MD; Robert McGarrah, MD; Michelle Kelsey, MD; Konstantin Krychtiuk, MD; Vishal Rao, MD (Duke Clinical Research Institute, Duke University School of Medicine). Elizabeth E.S. Cook ( Duke Clinical Research Institute) provided editorial support.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- All Summits
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
The coronavirus lab leak hypothesis is damaging science
By John P. Moore Aug. 2, 2024

W here and when the Covid-19 pandemic began — in Wuhan, China in late 2019 — is well known. How it began is a matter of heated controversy. There are two competing hypotheses, one of which is hindering the process of scientific discovery and could hold back the development of vaccines and other antiviral agents in the U.S.
The zoonosis hypothesis proposes that SARS-CoV-2, the virus that causes Covid-19, was naturally transmitted from an animal to one or more humans in a so-called wet market in Wuhan selling fresh produce, meat, fish, and live animals. The lab leak hypothesis posits that the virus was modified (possibly through gain of function maneuvers), or even created, in the Wuhan Institute for Virology (WIV) and somehow escaped the laboratory.
advertisement
Many politicians, pundits, and the general public now favor the lab leak idea. Most scientists, particularly virologists, do not. This schism threatens their legitimate and ultimately socially important work, as outlined in a peer-reviewed publication published on August 1 in the Journal of Virology that was written by 41 virologists. I am one of them.
The zoonosis hypothesis is solidly evidence based. Viruses often spill over from animals to humans, although usually as dead-end events without the sustained human-to-human transmission that sparks a pandemic. Wildlife coronaviruses have long been poised to infect humans . An estimated 66,000 people are infected with SARS coronaviruses each year due to human-bat contact, almost all resulting in asymptomatic infections with little or no further transmission.
Related: Listen: Anthony Fauci on presidents, bird flu, and turning down a multimillion-dollar job
That said, zoonotic transfer of three different coronaviruses (MERS-CoV, SARS-CoV-1, and SARS-CoV-2) from other animals to humans have resulted in epidemics or pandemics in the past 25 years. The 2002-2003 SARS-CoV-1 outbreak started in a Chinese wet market .
The influenza pandemic of 1918, which began from an animal-human cross-over, most likely from a pig in the U.S. heartland , killed an estimated 50 million people worldwide .
The illegal wildlife trade and wet markets are a $20 billion global industry with clear zoonosis risks. The more that humans and “exotic” animals mingle in close proximity, the greater the risk of viral transmission. There is the potential for a devastating pandemic with the H5N1 avian influenza virus entering birds and cattle and, sporadically, humans, in the U.S.
The lab leak hypothesis, in contrast, is essentially evidence-free: It relies on a chain of events that are unproven and highly speculative. A recent New York Times guest essay by Alina Chan, a molecular biologist at the Broad Institute of M.I.T. and Harvard, reiterates arguments first made in 2020 through 2022, but presents no new evidence.
The online and scientific literature support the zoonotic transfer hypothesis and/or counter the notion that a lab leak occurred.
Related: Lawmakers, as part of ‘lab leak’ Covid inquiry, press to bar EcoHealth from federal research funds
Five of seven reports from the U.S. intelligence community favor the zoonotic origin of SARS-CoV-2, based on declassified scientific evidence and investigations. These five reports found no evidence that Wuhan Institute for Virology possessed SARS-CoV-2 or a closely related virus before the end of December 2019, and conclude that it is unlikely that SARS-CoV-2 was engineered .
Yet the lab leak hypothesis is now dominating discussions in the public square. It is being promoted by right-wing politicians and media celebrities, and even embraced by high-profile newspapers like The New York Times . The Heritage Foundation , a conservative think tank, has accepted the lab leak as established fact, dismissing the zoonosis hypothesis on dubious grounds. That matters , as the report outlines future government policies on relations with China.
Dr. Anthony Fauci, former director of the National Institutes of Allergy and Infectious Diseases (NIAID), testified in June 2024 before the House of Representatives subcommittee investigating the Covid-19 pandemic. He stated that people should keep an open mind on the competing hypotheses, pending definitive proof for one or the other. Despite taking a balanced position, Fauci was viciously abused , even told that he should be “prosecuted” and imprisoned for “ crimes against humanity ” because NIAID had sent grant funds for coronavirus research to the Wuhan Institute for Virology via an EcoHealth Alliance subcontract .
My concern, and that of many other virologists, is that the evidence-light lab leak hypothesis is damaging the virology research community at a time when it has an essential role to play in the face of pandemic threats. The attacks on Fauci are far from unique. Coronavirus virologists have been falsely accused of engineering SARS-CoV-2, allowing it to escape from a lab due to inadequate safety protocols, being participants in an international cover-up, and taking grants as bribes from NIAID for favoring the zoonosis hypothesis. There is mounting harassment, intimidation, threats and violence towards scientists that are particularly vile in the online space .
Sign up for First Opinion
The smartest thinkers in life sciences on what's happening — and what's to come
In a survey conducted by Science magazine , of 510 researchers publishing coronavirus research, 38% received insults, threats of violence, doxing (publicly providing personally identifiable information about an individual), and even face-to-face threats. A second survey of 1,281 scientists found that 51% had experienced at least one form of harassment, sometimes repeatedly for years.
As a result, scientists have withdrawn from social media platforms, rejected public speaking opportunities, and taken steps to protect themselves and their families. Some have even diverted their work to less controversial topics.
There are now long-term risks that fewer experts will help combat future pandemics; and that scientists will be less willing to communicate the findings of sophisticated, fast-moving research on global health topics. Pandemic preparation research has already been deferred, diverted or abandoned . Most worrisome is that the next generation of scientists has well-founded fears about becoming researchers on emerging viruses and pandemic science .
All virologists embrace the need for laboratory safety. None of them ignore the implications of the lab leak hypothesis — that there could be a future escape of a dangerous virus from a research laboratory. However, lab leak anxiety underpins proposals for policies that would unnecessarily restrict research on vaccines and antiviral agents in the U.S. The overarching concern here is that the lab leak narrative fuels mistrust in science and public health infrastructures. The increasingly virulent and widespread anti-science agenda damages individual scientists and their institutions, and hinders planning to counter future epidemics and pandemics.
Science is humanity’s best insurance policy against threats from nature, but it is a fragile enterprise that must be nourished and protected. Scientific organizations need to develop programs to counter anti-science and protect the research enterprise in the face of mounting hostility.
And the rhetoric being thrown at virologists must be toned down. Viruses are the real threats to humanity, not virologists.
John P. Moore, Ph.D., is a professor of microbiology and immunology at Weill Cornell Medicine in New York City. This essay is adapted from a longer article written with 40 colleagues that was published in the Journal of Virology .
LETTER TO THE EDITOR
Have an opinion on this essay submit a letter to the editor here ., about the author reprints, john p. moore.
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

Performance-enhancing drugs or placebos? The myth at the heart of anti-doping

Letters on Sonya Massey’s death, vaccine injuries, and much more

STAT Plus: Sarepta demanded Duchenne patient advocacy group censor video critical of the company

STAT Plus: Health Care's Colossus: How UnitedHealth harnesses its physician empire to squeeze profits out of patients

Mount Sinai mounted aggressive campaign to stifle debate over revelations about its controversial brain research

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Health Topics
- Brochures and Fact Sheets
- Help for Mental Illnesses
- Clinical Trials
COVID-19 and Mental Health
What is covid-19.
COVID-19 is a disease caused by a virus named SARS-CoV-2. COVID-19 most often affects the lungs and respiratory system, but it can also affect other parts of the body. Some people develop post-COVID conditions, also called Long COVID . These symptoms can include neurological symptoms such as difficulty thinking or concentrating, sleep problems, and depression or anxiety.
Why is NIMH studying COVID-19 and mental health?
Both SARS-CoV-2 and the COVID-19 pandemic have significantly affected the mental health of adults and children. Many people experienced symptoms of anxiety , depression , and substance use disorder during the pandemic. Data also suggest that people are more likely to develop mental illnesses or disorders in the months following COVID-19 infection. People with Long COVID may experience many symptoms related to brain function and mental health .
While the COVID-19 pandemic has had widespread mental health impacts, some people are more likely to be affected than others. This includes people from racial and ethnic minority groups, mothers and pregnant people, people with financial and housing insecurity, children, people with disabilities, people with preexisting mental illnesses or substance use problems, and health care workers.
How is NIMH research addressing this critical topic?
NIMH is supporting research to understand and address the impacts of the pandemic on mental health. This includes research to understand how COVID-19 affects people with existing mental illnesses across their entire lifespan. NIMH also supports research to help meet people’s mental health needs during the pandemic and beyond. This includes research focused on making mental health services more accessible through telehealth, digital tools, and community-based interventions.
NIMH is also working to understand the unique impacts of the pandemic on specific groups of people, including people in underserved communities and children. For example, NIMH supports research investigating how pandemic-related factors, such as school disruptions, may influence children’s brain, cognitive, social, and emotional development.
Where can I learn more about COVID-19 and mental health?
- NIMH video: Mental Illnesses and COVID-19 Risks
- NIMH Director’s Messages about COVID-19
- NIMH events about COVID-19
- NIMH news about COVID-19
Where can I learn more about Long COVID and COVID-19?
- NIH page on Long COVID
- NIH RECOVER Initiative
- CDC COVID-19 resources
How can I find help for mental health concerns?
If you have concerns about your mental health, talk to a primary care provider. They can refer you to a qualified mental health professional, such as a psychologist, psychiatrist, or clinical social worker, who can help you figure out the next steps. Find tips for talking with a health care provider about your mental health.
You can learn more about getting help on the NIMH website. You can also learn about finding support and locating mental health services in your area on the Substance Abuse and Mental Health Services Administration (SAMHSA) website.
Last Reviewed: May 2024
Unless otherwise specified, the information on our website and in our publications is in the public domain and may be reused or copied without permission. However, you may not reuse or copy images. Please cite the National Institute of Mental Health as the source. Read our copyright policy to learn more about our guidelines for reusing NIMH content.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Hum Vaccin Immunother
- v.20(1); 2024
- PMC11232646
Top 100 cited research on COVID-19 vaccines: A bibliometric analysis and evidence mapping
a Child Rehabilitation Department, Gansu Rehabilitation Center Hospital, Lanzhou, Gansu, China
b Evidence-Based Medicine Center, Lanzhou University, Lanzhou, Gansu, China
c School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu, China
Mingyue Zhang
Yongjia zhou.
d School of Nursing, Gansu University of Chinese Medicine, Lanzhou, Gansu, China
Jinhui Tian
e College of Traditional Chinese Medicine, Gansu Health Vocational College, Lanzhou, Gansu, China
Associated Data
The outbreak of the COVID-19 has seriously affected the whole society, and vaccines were the most effective means to contain the epidemic. This paper aims to determine the top 100 articles cited most frequently in COVID-19 vaccines and to analyze the research status and hot spots in this field through bibliometrics, to provide a reference for future research. We conducted a comprehensive search of the Web of Science Core Collection database on November 29, 2023, and identified the top 100 articles by ranking them from highest to lowest citation frequency. In addition, we analyzed the year of publication, citation, author, country, institution, journal, and keywords with Microsoft Excel 2019 and VOSviewer 1.6.18. Research focused on vaccine immunogenicity and safety, vaccine hesitancy, and vaccination intention.
Introduction
The Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), also known as coronavirus disease 2019 (COVID-19), which is a disease characterized by respiratory distress, fever, cough, fatigue, pneumonia, and muscle pain. 1 Patients with severe infection may also develop neurological manifestations, such as acute cerebrovascular disease, skeletal muscle damage, and disturbance of consciousness. 2 The virus has also been reported to cause loss of smell and taste. 3 In general, fatigue, muscle aches, and headaches have a higher incidence in all subsequent stages. 4 And it carries a high risk of death in older people with cardiovascular disease (coronary artery disease, heart failure, and arrhythmias) and lung disease (chronic obstructive pulmonary disease). 5 SARS-CoV-2 is transmitted primarily from person to person through short-range airborne aerosols, respiratory droplets, and directly or indirectly contacted with infectious respiratory droplets. 6 The persistent COVID-19 pandemic has devastating consequences on populations, social structures, and global economic growth. 7 Moreover, studies have shown that control measures such as the use of masks, physical distancing, testing of exposed or symptomatic individuals, contact tracing, and quarantine are not sufficient to stop the transmission of COVID-19 caused by SARS-CoV-2. 8 Mass vaccination of at-risk populations and the subsequent general population is the single most effective public health measure to mitigate the coronavirus disease (COVID-19) pandemic. 9
Although there have been articles on COVID-19 vaccines from a bibliometric perspective, the top 100 most-cited articles on COVID-19 vaccines to date have not been studied. 10–14 Citation analysis was one of the bibliometric analysis methods that has been used to quantify the relative importance of scientific articles by examining citations attributed to the paper. 15 The total number of citations for published articles indicate the importance of published articles in that area of practice. 16 This study analyzes the bibliometric characteristics of the top 100 cited articles on COVID-19 vaccines since the outbreak of COVID-19, and discusses the research hotspots and trends in this field, as well as the collaboration between authors and institutions, so as to provide reference for subsequent research.
Data sources and search strategies
The Web of Science (WOS) is a comprehensive multidisciplinary database that includes all high-impact scientific journals and world-class indexes 17 , 18 and has the title of “academic recognition as one of the most comprehensive bases for several areas of scientific knowledge.” 19 Compared with Scopus or MEDLINE/PubMed, the Web of Science (WOS) database can extract more complete information for bibliometric analysis. Therefore, we conduct a comprehensive search of the web of science (WOS) core collection database on November 29, 2022.The specific search strategy is explained in Appendix 1. Descriptors are defined from the Medical Subject Headings (MeSH) and Emtree directories. 19 The period is 2020-present (November 29, 2022) and there were no language restrictions. In addition, we include only articles and reviews. In addition, we also rank the articles according to their relevance and select the literature related to the COVID-19 vaccine online. A total of 6,957 publications are identified, and then we sort the 6,957 retrieved publications from highest to lowest in terms of total citation volume. The most recent article ranks higher if the total number of citations is the same.
Data extraction and bibliometric analysis
Two reviewers (LY and CX) extract all the data including title, country, institution, journal, author, etc, and discuss with the third reviewer (JT) in case of disagreement. Then we import the data into Microsoft Excel 2019 for statistical and descriptive analysis. Journal impact factors (IF) and journal category quartiles (Q) are from the 2022 Journal Citation Report (JCR). 20 In addition, it is used to construct evidence maps and this study is presented in the form of bubble charts, pie charts, and histograms. 21 We use VOSviewer1.6.18 (Van Eck and Waltman, Leiden University, Netherlands) for bibliometric analysis and mapping processes, and to construct and visualize bibliometric networks. 22–24 We use it to construct cooperative network maps of countries, authors, and institutions as well as co-emergence networks of author keywords, to better understand the framework and evolutionary track of scientific research and identify research frontiers and hot spots. 25 In the visual network diagram generated by VOSviewer1.6.18, each node represents a different element (author, country, organization, and keyword), the size of the node represents the frequency of occurrence of the element or the number of publications, the lines between the nodes represents cooperative or co-occurrence relationships, and the thickness of the lines between the nodes indicates the degree of connection. 16 , 20 , 26 The node colors indicate different clusters.
Basic characteristics of the data
Table S 1 lists the top 100 most-cited articles in the field of COVID-19 vaccines, in descending order according to the number of citations in the WOS Core database. In the top 100 cited publications, 86 articles and 14 reviews were published in English. The number of citations ranged from 345 to 8,381. The total number of citations reached 78,881, with an average of 788.81 citations per article.
Publication year and citations
The 100 articles identified were published between 2020 and 2022. The relationship between the number of publications (x-axis), the average number of citations (y-axis), the total number of citations (bubble size), and the year of publication (different colors) was shown in Figure S1. In the data label, the former represented the annual number of publications, the middle was the average number of citations, and the latter represented the total number of citations. The total number of citations (46,878) and the number of publications (65) was the highest in 2021, the average number of citations (1004) was the highest in 2020, and 2022 was the lowest in both the number of publications (7) and the average number of citations (557).
WoS categories
Figure 1 compares the average citations of 100 articles based on different WOS categories. The 100 publications were distributed across 29 WOS categories, with an average citation of 346.69 as the boundary. There were 12 species above the border. The top three by the number of articles were Medicine, General & Internal ( n = 43), Medicine, Research & Experimental ( n = 15), and Immunology( n = 13). In terms of the total number of citations and the average number of citations, the highest was Medicine, General & Internal ( n = 36197, n = 842). Interestingly, Medicine, General & Internal were far ahead of the runners-up in terms of the number of articles, total citations, and average citations. Overall, there were fewer green bubbles than red bubbles, suggesting that only a few categories contributed to the overall increase in average citations.

Comparison of each WoS category’s average number of citations with average citations of the top 100 cited articles. Note: The X-axis referred to different categories, the Y-axis represented the average number of citations, and the bubble size stood for the corresponding number of articles. In the data label, the former value was the Y value, and the latter was the value of the bubble size.
Journal of publication
The top 100 cited publications were published in 37 different journals (Table S2). We can see that these journals were administered by the USA, the UK, the Netherlands, Switzerland, and China Mainland. 17 journals were run by the USA and 15 in the UK, accounting for more than 86%. In terms of the number of publications, the New England Journal of Medicine published the most ( n = 24), followed by the Lancet ( n = 15), Nature Medicine ( n = 7), and Nature ( n = 7), accounting for 53% of the top 100 articles. In terms of journal types, 53.7% of the journals published only one article. The highest impact factor was the Lancet (168.9), and the lowest was the Journal of Multidisciplinary Healthcare (3.3). Although the New England Journal of Medicine ranked first in both the number of publications and the number of citations, it did not have the highest IF. In addition, in terms of the category quartile of journals, there were 31 journals belonging to Q1, accounting for more than 84%.
Authorship impact on COVID-19 vaccine research
Of the 618 authors who contributed to the top 100 most-cited articles, 556 published just one article, about 90% of all authors. Table 1 shows only 17 authors with ≥ 3 publications, of which 10 were from the USA, 3 from Israel, 2 from Germany, and 2 from the UK. In addition, we confirmed that Swanso Kena A, and Dormitzer Philip R from Pfizer in the USA, as well as Shi Pei-Yong from the University of Texas System were the most prolific authors in this field, with 4 publications. Meanwhile, Swanso Kena A and Dormitze Philip R have the highest citations (2708), suggesting that they may be the most influential authors in the top 100 most-cited COVID-19 vaccine studies.
The most prolific authors in COVID-19 vaccine research.
| SCR | Author | Institutions | Country | NP | TC |
|---|---|---|---|---|---|
| 1 | Swanson, Kena A. | Pfizer | USA | 4 | 2708 |
| 2 | Dormitzer, Philip R. | Pfizer | USA | 4 | 2708 |
| 3 | Shi, Pei-Yong | University of Texas System | USA | 4 | 2642 |
| 4 | Tuereci, Oezlem | BioNTech SE | Germany | 3 | 2344 |
| 5 | Sahin, Ugur | BioNTech SE | Germany | 3 | 2344 |
| 6 | Reis, Ben | Boston Children’s Hospital | USA | 3 | 1902 |
| 7 | Pollard, Andrew J. | University of Oxford | UK | 3 | 990 |
| 8 | Lipsitch, Marc | Harvard University | USA | 3 | 1902 |
| 9 | Larson, Heidi J. | University of London | UK | 3 | 1989 |
| 10 | Krammer, Florian | Icahn School of Medicine at Mount Sinai | USA | 3 | 1877 |
| 11 | Hernan, Miguel A. | Harvard University | USA | 3 | 1902 |
| 12 | Freedman, Laurence | Chaim Sheba Medical Center | Israel | 3 | 1460 |
| 13 | Dagan, Noa | Harvard Medical School | USA | 3 | 1902 |
| 14 | Cooper, David | Pfizer | USA | 3 | 2387 |
| 15 | Barda, Noam | Harvard Medical School | USA | 3 | 1902 |
| 16 | Balicer, Ran D. | Clalit Health Services | Israel | 3 | 1902 |
| 17 | Alroy-Preis, Sharon | Israel Minist Health | Israel | 3 | 1609 |
SCR: Standard Competition Ranking based on the number of Publication; NP: Number of Publications; TC: Total Citations; The number of publications ≥ 3.
As shown in Figure 2 , we constructed a cooperative cluster analysis for 618 authors who participated in the top 100 most-cited articles. Only 103 authors were associated with others and formed a total of 6 clusters.

Collaborative network and cluster distribution of authors in the top 100 articles on COVID-19 vaccines.
Top productive institutions in COVID-19 vaccine
Figure 3 and Table 2 show the institutions that were most active in the top 100 most cited COVID-19 vaccine studies. A total of 346 institutions and universities around the world have collaborated independently or in small groups in this area. Table 2 lists only 18 institutions with publication volume ≥ 4, of which 9 were from the USA, 4 were from Israel, 3 were from the UK, and the remaining 2 were from Germany and China respectively. Figure 3 is a collaborative network composed of 67 institutions associated with other institutions with more than 2 publications. The network was composed of 67 nodes, 305 links, and 6 clusters. We found that these studies were mainly the result of cooperation between researchers from universities in Europe, Asia, and the USA. The UK’s University of Oxford and the University of London were the most prolific in the field with 12 publications, followed by Pfizer and Harvard University in the USA with 10. They have played a bigger role than other institutions in the publication of these studies, and we can also say that universities in the USA and the UK have played a prominent role in the publication of research on COVID-19 vaccines. In addition, Pfizer from the USA had the highest cited times of 10,999, followed by BioNTech from Germany with the cited times of 10,249. In other words, Pfizer and BioNTech have published papers that have the greatest impact on citations in the scientific community.

Collaborative network and cluster distribution of institutions in the top 100 articles on COVID-19 vaccines (the number of publications ≥ 3).
The most prolific institutions in COVID-19 vaccine research.
| SCR | Institutions | Country | NP | TC |
|---|---|---|---|---|
| 1 | University of Oxford | UK | 12 | 7094 |
| 2 | University of London | UK | 12 | 6705 |
| 3 | Pfizer | USA | 10 | 10999 |
| 4 | Harvard University | USA | 10 | 9188 |
| 5 | BioNTech | Germany | 8 | 10249 |
| 6 | University of Texas System | USA | 7 | 3829 |
| 7 | Tel Aviv University | Israel | 7 | 3053 |
| 8 | Vanderbilt University | USA | 5 | 5664 |
| 9 | University of Washington | USA | 5 | 1730 |
| 10 | University of Maryland | USA | 5 | 7936 |
| 11 | NIH National Institute of Allergy & Infectious Diseases | USA | 5 | 6987 |
| 12 | National Institute of Food & Drug Control | China | 5 | 3115 |
| 13 | Imperial College London | UK | 5 | 3735 |
| 14 | Cincinnati Children’s Hospital Medical Center | USA | 5 | 8979 |
| 15 | Ben Gurion University | Israel | 5 | 2991 |
| 16 | Technion Israel Institute of Technology | Israel | 4 | 1400 |
| 17 | Hebrew University of Jerusalem | Israel | 4 | 1870 |
| 18 | Fred Hutchinson Cancer Center | USA | 4 | 5953 |
a Standard Competition Ranking based on the number of Publication; b Country of institutions; c Number of publications; d Total Citations; The number of publications ≥ 4.
National distribution and cooperation
Figure 4a shows the countries of the corresponding authors of the top 100 articles, who come from 25 different countries. The USA topped the top 100 most-cited COVID-19 vaccine studies with 33 articles, followed by the UK with 16 articles and Israel with 11 articles. In addition, authors from Russia, India, Turkey, Norway, Malaysia, and Canada did not collaborate with other countries, but only within their own countries. As shown in Figure 4a , although the USA was the country with the largest number of publications, its international cooperation rate is relatively low.

Country analysis. (a) Corresponding Author’s Country (Where MCP represents the number of coauthored papers with authors from other countries; SCP represents the number of papers coauthored by authors of the same nationality; MCP Ratio indicates the ratio of international cooperation.) (b) National collaborative networks and cluster distribution in the top 100 articles on COVID-19 vaccines.
Figure 4b includes 48 countries that were involved in COVID-19 vaccine research in partnership with other countries, forming four clusters. Cluster 1(red) was made of six European countries represented by Belgium and Russia, four Asian countries represented by India, six African countries represented by Burkina Faso, and two South American countries represented by Colombia. Cluster2 (green) was consisted mainly of 14 countries with a core of the USA, UK, and Israel. Cluster3 (blue) was consisted mainly of nine countries, including Germany, South Africa, Brazil, Canada, Turkey, Australia, Argentina, Austria, and Chile. Cluster 4 (yellow) was consisted of six Asian countries represented by China and Egypt from Africa. Not only that but Figure 4b also reveals the cooperation between countries, with the USA and the UK (link strength = 18) having the closest cooperation, followed by the USA and Germany (link strength = 12) and the UK and Germany (link strength = 9).
Research hotspot and co-occurrence keyword clustering network analysis
The top 100 most-cited articles involved 210 keywords. We showed the density of the main keywords in Figure 5a , and the frequency was greater than or equal to 2. The graph showed 55 nodes, with the brightest spot relating to COVID-19 and vaccine hesitancy, followed by vaccines, immunogenicity, vaccine confidence, and vaccine safety. In Figure 5b , 55 keywords that appear more than twice were included and classified into 5 clusters in the map. Cluster1 (red) mainly included 23 keywords such as coronavirus, immunogenicity, acute respiratory syndrome, spike, antibody-response, mRNA vaccine, immune response, neutralizing antibody, protective immunity, rituximab, etc, which were mainly related to immunological research of COVID-19 vaccine. Cluster 2 (green) mainly consisted of 9 keywords, such as infection, vaccine safety, risk, workers, children, and heparin-induced thrombocytopenia, which mainly studied the safety and risk after vaccination. Cluster 3 (blue) included vaccine hesitancy, influenza, vaccination, immunization, intention, communication, decision, pandemic, and public health willingness, which looked at people’s willingness to be immunized against vaccines during influenza pandemic. Cluster 4 (yellow) included vaccine confidence, influenza vaccine, adults, vaccination, vaccine acceptance, antibody, COVID-19 vaccine, and measles. It focused on adults’ attitudes toward COVID-19 vaccines. Cluster 5(purple) was composed of 7 keywords. They were COVID-19, SARS-Cov-2, vaccine, convalescent plasma therapy, healthcare workers, monoclonal antibodies, and vaccine rejection. The cluster focused on vaccine rejection and the use of novel therapeutic monoclonal antibodies. In conclusion, the research hotspots of the top 100 cited articles on the COVID-19 vaccine mainly concentrated on vaccine immunology, vaccine safety, vaccine hesitancy, people’s attitude toward vaccines, and vaccine rejection.

Keyword analysis. (a) Density map of main keywords; (b) Clustering distribution of keywords co-occurrence network in the top 100 articles on COVID-19 vaccines (keyword co-occurrence frequency ≥ 2).
The top 100 most-cited articles may be highly recognized articles in a certain field, and bibliometric analysis of these articles can quantitatively determine their main research concerns and dynamic change information. 15 Through the analysis of the years of these 100 articles, it is found that the average amount of citations in 2020 is the highest, the number of publications in 2021 is the highest, while the number of publications and the average number of citations in 2022 is both the lowest, which may be related to the publication time of the article.
A total of 37 journals publishes the top 100 articles cited on the COVID-19 vaccine, 95% of which are included by SCI. In addition, high-impact medical journals such as Nature, Science, JAMA, Nature Medicine, and Cell also participate in the publication of the top 100 articles on the COVID-19 vaccine, indicating that the vaccine has received global attention. In addition, we can also find that the Lancet, Natural Medicine, and Nature journals are not only the most productive journals but also the journals with the highest citation times and journal impact. Therefore, we can say how much attention the topic is getting. Focus not only on the number of articles but also on the quality of articles. Based on the research focus and the number of citations accumulated, the most cited articles provide more in terms of the general interest of the research. 27
A total of 346 institutions and 49 countries contribute to the publication of the top 100 cited articles on COVID-19 vaccines. After analyzing 18 institutions with more than 4 publications, we found that half of them is from the USA, and affiliate with universities. The cooperative network diagram of institutions show that different institutions are connected but not closely. From the perspective of national contribution, most of the countries participating in the top 100 published articles are developed countries, and the USA is the country with the largest number of published articles. And because the USA has been developing well in the medical field and even other fields, we can undoubtedly conclude that the USA still has the greatest influence in this field and is in a leading position in this field. In our analysis of cooperation networks in 48 countries, we found that these countries are not very well connected to the rest of the world. Of the 49 countries, 30 are from developing countries, and 20 published only one paper, which may be due to international differences in economic levels. Research has shown that funding is one of the potential mechanisms for encouraging and enhancing productivity, and developed countries have the most potential funding institutions for scientific research. 28 Consequently, we should be encouraged to study high productive country and less activity countries of international cooperation between the author, in order to promote the research in the future better.
Swanson Kena A and Dormitzer Philip R from Pfizer in the USA as well as Shi Pei-Yong from the University of Texas System in the USA are the authors of the top 100 cited articles on the COVID-19 vaccine with 4 articles published, and they are also the authors with the most cited articles. Pfizer is the world’s leading research-based biomedical and pharmaceutical company with its headquarters in New York, USA. The University of Texas system is the second largest state public university system in the USA after the University of California system. Some studies show that an excellent platform is the basis of the author’s research, and the author’s research results can represent and enhance the influence of the institution or even the country. 21
In bibliometrics, keyword co-occurrence can reflect different research hotspots and topics. 15 According to the analysis of 5 clusters consisting of 55 keywords with co-occurrence frequency greater than or equal to 2, we found that cluster 1 (red) consists of 23 keywords, which mainly study the immunology of the COVID-19 vaccine. Studies by Warsey M et al. demonstrated that the use of viral vectors to induce an immune response against spike proteins could protect humans from this disease. 29 It has also been shown that a single dose can cause humoral and cellular responses to SARS-CoV-2, and strengthening immunity can improve the neutralizing antibody titer and anti-VOC activity. 30 , 31 Moreover, allogenic prime-booster vaccination increases the immunogenicity of either vaccine alone or the homologous prime-booster combination. 32 Cluster 2(green) consists of 9 keywords and focuses on the safety of the COVID-19 vaccine. Many articles on vaccine safety can be retrieved from the WOS Core collection database, and it can be found that most vaccine adverse reactions are mild and transient local or systemic, but a small number of serious adverse events can occur, such as myocarditis, thrombocytopenia, disseminated intravascular coagulation and thrombosis. 33–35 Cluster 3 include 9 keywords related to the intention and vaccine hesitancy for COVID-19 or influenza vaccination. Vaccine hesitancy, a global phenomenon that is a barrier to full vaccination against highly infectious diseases, 36 , 37 dates back to the 1800s, 38 and many factors influence willingness to vaccinate. The most common reasons for vaccine hesitation or refusal include inadequate perception of disease risk, low confidence in vaccine safety and efficacy, previous experience, certain religious beliefs, education, and income levels, public health policies, social factors, and media communication. 36 , 39 In addition, other studies have shown that vaccine hesitancy is associated with gender, race, age, economics (higher hesitancy among women, young people, black people, and economically disadvantaged groups), and fear of injection, 40 , 41 and that hesitancy was higher among healthcare workers among nurses than among doctors. 37 Cluster 4 consists of 8 keywords, which mainly study vaccine confidence and acceptability. In Vaccines (Basel), 16 February 2021, Sallam M. entitled “COVID-19 Vaccine Hesitancy Worldwide: A concise systematic review of vaccine acceptance rates” 36 notes that there are significant differences in vaccine acceptance rates in different countries and regions of the world, ranging from 38% in the northeast to 49% in the west. Acceptance is higher in some Asian countries and lowers in the Middle East, Eastern Europe, and Russia. The rapid development, political interference, and misinformation that have dominated the vaccine discussion have undermined confidence in the rigor of the approval process and in the use of the vaccine itself. Emphasizing transparency and adherence to scientific standards throughout the process of vaccine development, approval and distribution can restore confidence. Cluster 5 (purple) consists of seven keywords that may be associated with vaccine rejection in healthcare workers. Hospital healthcare workers are at high risk during the outbreak. Studies have found that healthcare workers who believe they were immune to COVID-19 and those who are confident they will not get it have the highest rates of COVID-19 vaccine refusal, healthcare providers who do not care for COVID-19-positive patients have higher rates of vaccination refusal, and nurses are more likely to reject vaccines than doctors. 37 , 42
In addition, an analysis of the top 100 cited studies of COVID-19 vaccines revealed the following problems in the development of COVID-19 vaccines. ① At present, there are few or no vaccines developed specifically for infants, the elderly and pregnant women, which will increase the risk of infection in these groups to a certain extent. ② Most of these literatures focus on the efficacy and safety of vaccines, but do not pay attention to the production cost of vaccines, which may add a certain economic burden to the society. ③ Due to the normalization of the COVID-19 epidemic, the demand for SARS-CoV-2 vaccines has declined sharply, which has led to the lack of subjects for SARS-CoV-2 vaccines and it is difficult to develop new vaccines.
Although the epidemic has become normalized, considering that coronavirus has become an epidemic pathogen that seriously threatens human beings for several times, it is recommended to pay attention to the improvement and establishment of public health defense system and emergency response system, Improve the construction of a universal coronavirus emergency vaccine research and development platform, a universal respiratory virus emergency vaccine research and development platform, and even a universal infectious disease pathogen emergency vaccine research and development platform to avoid the situation of delayed vaccines in the face of epidemic again. In the future, some vaccines should be developed specifically for special populations. In addition, the pandemic is a battle that seriously threatens human lives and affects social economy. In the future, we should not only pay attention to the efficacy and safety of vaccines, but also consider the production cost, and strive to develop low-cost, effective and safe vaccines.
The study provides a quantitative and qualitative analysis of the top 100 most-cited articles on COVID-19 vaccines. According to the analysis results of bibliometrics, developed countries, especially the USA, are the biggest contributors, and universities are the main body of research in this field. The number of publications on COVID-19 vaccines in the New England Journal, the Lancet, Natural Medicine, and Nature shows how much attention the topic is getting. In addition, the collaboration network map showed that the collaboration among countries, institutions, and authors is fragmented. We should be encouraged to study high productive country and less activity countries of international cooperation between the author, in order to promote the research in the future better. Research hotspots mainly focus on vaccine safety (adverse reactions), immunogenicity, vaccine hesitancy, vaccine vaccination intention, and medical staff’s attitude toward vaccination. To have an in-depth understanding of research hotspots and trends in this field, this study identified and analyzed the countries, institutions, authors, and keywords involved in the 100 most influential articles on the COVID-19 vaccine, which may provide a reference value for later research. However, this paper also has certain limitations. First of all, the data in this paper only came from the Web of science, which may cause data bias. Secondly, although we have standardized and unified the authors, institutions, and keywords involved in this study, we still cannot rule out the possible bias caused by our subjective factors.
List of Abbreviations
Supplementary material, acknowledgments.
LY and CX conducted data integration and mapping and wrote the manuscript. JT and XL were responsible for the study concept and design and supervised the study. MZ, YZ, ZH, LL, and YL were responsible for data collection and collation. All authors reviewed and approved the final submitted version.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2370605

IMAGES
VIDEO
COMMENTS
Computer scientists, data scientists, physicists and mathematicians have joined public health professionals and virologists to confront the largest pandemic in the century by capitalizing on the large-scale 'big data' generated and harnessed for combating the COVID-19 pandemic. In this paper, we review the newly born data science approaches ...
Top Topics Discussed in COVID-19 Data Science Based Research Papers (a) Top topics in non-peer-reviewed data science based COVID-19 ... Qian K., Liu J., Zheng H., and Li X., " Covid-19 and computer audition: An overview on what speech & sound analysis could contribute in the SARS-CoV-2 Corona crisis," 2020, arXiv:2003.11117. [PMC free ...
Herein, we provide an expert overview of the key computational methods and their applications for the discovery of COVID-19 small-molecule therapeutics that have been reported in the research literature. We further outline that, after the first year the COVID-19 pandemic, it appears that drug repurposing has not produced rapid and global solutions.
Abstract. This paper focuses on addressing the urgent need for efficient and accurate automated screening tools for COVID-19 detection. Inspired by existing research efforts, we propose two ...
The main purpose of this work is to investigate and compare several deep learning enhanced techniques applied to X-ray and CT-scan medical images for the detection of COVID-19. In this paper, we ...
A special prize for outstanding research in high-performance computing (HPC) applied to COVID-19-related challenges sheds light on the computational science community's efforts to fight the ...
This research paper aims to (1) summarize the digital technologies used during the COVID-19 pandemic to mitigate the transmission of the COVID-19; (2) establish the extent to which digital technology applications have facilitated mitigation of the spread of COVID-19; and (3) explore the facilitators and barriers that impact the usability of ...
Yet, the majority of COVID-19 research papers (53%) have 4 or fewer authors. ... as Computer Science research in COVID-19 is most commonly characterised within the sub-disciplines Machine Learning ...
With high transmissibility and no effective vaccine or therapy, COVID-19 is now a global pandemic. Government-coordinated efforts across the globe have focused on containment and mitigation, with varying degrees of success. Countries that have maintained low COVID-19 per-capita mortality rates appear to share strategies that include early surveillance, testing, contact tracing, and strict ...
The year 2020 experienced an unprecedented pandemic called COVID-19, which impacted the whole world. The absence of treatment has motivated research in all fields to deal with it. In Computer Science, contributions mainly include the development of methods for the diagnosis, detection, and prediction of COVID-19 cases. Data science and Machine Learning (ML) are the most widely used techniques ...
Since the appearance of the COVID-19 disease, several researches have been proposed in different fields such as medicine, economy and biology. In computer science, several approaches and systems have been developed to cope with this disease. This paper aims at reviewing the latest work coping with the emerging infectious disease COVID-19.
Impact of COVID-19 on the Computer Science Research Community (opens in new tab) ... 3/20 - Added link to sample code to search MAG for COVID-19 papers using Fields of Study along with, publication title and abstract term matching; Opens in a new tab. Related Items. May 4, 2021
The reviewed research articles mainly emphasize the study of accuracies of different models (ML / DL / ML+DL) for the classification of COVID-19 v/s non-COVID-19 and other pulmonary diseases. In the field of medical science, machine learning models are found to be useful for the classification of medical images.
This section focuses on profiling COVID-19 research using defined measures and explores the following aspects: (1) Analyze the research field of COVID-19 publications and their citation behavior. (2) Detect topics variation over time and explore their inner connections. (3) Identify innovative papers and topics. 3.1.
2 Department of Computer Science and Engineering, Aditya Institute of Technology ... In this paper, a state-of-the-art analysis of the ongoing machine learning (ML) and deep learning (DL) methods in the diagnosis and prediction of COVID-19 has been done. ... Finally some important research directions for further research on COVID-19 are ...
This paper presents the prediction and analysis of COVID-19 using various machine learning algorithms. In the present study, ML-based enhanced model is implemented to predict the possible threat of COVID-19 all over the world and the algorithms used in these models classifies the COVID patients based on several subsets of features and predicts ...
Our analysis shows that: There is increasing author participation in both CS and AI conferences from the COVID-19 impacted regions in the past ten years, from about 6% to over 20% in 2019. The disease could impact one-fifth of conference attendees in both AI and CS (21.3%). May to early September is the period most CS conferences occur ...
Nowadays, the appearance of common symptoms, such as cough, fever, and loss of smell and taste, is the starting point of a battle against the coronavirus. The first standard method of COVID-19 infection assertion has become the RT-PCR test, which is however an uncomfortable solution for both patients and medical staff due to its high cost, timeliness, and false-negative result issue. This has ...
A fourth project, a collaboration led by UChicago Medicine's Maryellen Giger, was funded by the organization in spring. The awards were part of $5.4 million in funding distributed by DTI, after their inaugural call for proposals in March. The group also provides AI software tools and a "data lake" of COVID-19 datasets to aid researchers ...
During COVID-19, papers with more first-time collaboration were found to be more novel and international collaboration did not hamper novelty as it had done in the normal periods. The findings suggest the necessity of reaching out for distant resources and the importance of maintaining a collaborative scientific community beyond nationalism ...
Introduction. Academic conferences are one of the primary forms of dissemination of research output and scientific work. Particularly in the discipline of Computer Science, many conferences are highly prestigious with the reputation of some on par with peer reviewed journals (Vardi 2009; Vrettas and Sanderson 2015); some notable conferences having acceptance rates as low as 20% (Fathalla et al ...
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on ...
Factors Affecting Computer Science Student's Academic Performance During Covid-19 Abstract : In this research, the factors that affect the computer science student's academic performance are investigated in the scenario of online learning induced by the COVID-19 pandemic. It is found that while staying at home, and getting tutored online, the academic achievement of the students of Computer ...
Design, Setting, and Participants The ongoing Accelerating COVID-19 Therapeutic Interventions and Vaccines 6 (ACTIV-6) platform randomized clinical trial was designed to evaluate repurposed therapies among outpatients with mild to moderate COVID-19. A total of 1432 participants older than 30 years with confirmed COVID-19 experiencing at least 2 ...
Drones, robots, and AVs technology not only ensure minimum human interaction but also can be beneficial to access contagious COVID-19 patients. Wearables, making use of the Bluetooth and GPS technology, is another efficient way to monitor individual's health and their day-to-day stress levels in isolation.
The zoonosis hypothesis proposes that SARS-CoV-2, the virus that causes Covid-19, was naturally transmitted from an animal to one or more humans in a so-called wet market in Wuhan selling fresh ...
Learn about NIMH research on the mental health impacts of COVID-19 and find additional resources on COVID-19 and mental health. ... This includes research to understand how COVID-19 affects people with existing mental illnesses across their entire lifespan. NIMH also supports research to help meet people's mental health needs during the ...
COVID-19 is moderately infectious with a relatively high mortality rate, but the information available in public reports and published literature is rapidly increasing. ... Continued research into the virus is critical to trace the source of the outbreak and provide evidence for future outbreak ... Science. 2003; 300 (5627):1961-1966. [Google ...
The outbreak of the COVID-19 has seriously affected the whole society, and vaccines were the most effective means to contain the epidemic. This paper aims to determine the top 100 articles cited most frequently in COVID-19 vaccines and to analyze the research status and hot spots in this field through bibliometrics, to provide a reference for future research.