Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Water Filtration Using Plant Xylem
Contributed equally to this work with: Michael S. H. Boutilier, Jongho Lee
Affiliation Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts, United States of America
* E-mail: [email protected]
- Michael S. H. Boutilier,
- Jongho Lee,
- Valerie Chambers,
- Varsha Venkatesh,
- Rohit Karnik

- Published: February 26, 2014
- https://doi.org/10.1371/journal.pone.0089934
- Reader Comments
Effective point-of-use devices for providing safe drinking water are urgently needed to reduce the global burden of waterborne disease. Here we show that plant xylem from the sapwood of coniferous trees – a readily available, inexpensive, biodegradable, and disposable material – can remove bacteria from water by simple pressure-driven filtration. Approximately 3 cm 3 of sapwood can filter water at the rate of several liters per day, sufficient to meet the clean drinking water needs of one person. The results demonstrate the potential of plant xylem to address the need for pathogen-free drinking water in developing countries and resource-limited settings.
Citation: Boutilier MSH, Lee J, Chambers V, Venkatesh V, Karnik R (2014) Water Filtration Using Plant Xylem. PLoS ONE 9(2): e89934. https://doi.org/10.1371/journal.pone.0089934
Editor: Zhi Zhou, National University of Singapore, Singapore
Received: October 17, 2013; Accepted: January 23, 2014; Published: February 26, 2014
Copyright: © 2014 Boutilier et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by the James H. Ferry, Jr. Fund for Innovation in Research Education award to R.K. administered by the Massachusetts Institute of Technology. SEM imaging was performed at the Harvard Center for Nanoscale Systems, a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the National Science Foundation under NSF award no. ECS-0335765. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The scarcity of clean and safe drinking water is one of the major causes of human mortality in the developing world. Potable or drinking water is defined as having acceptable quality in terms of its physical, chemical, and bacteriological parameters so that it can be safely used for drinking and cooking [1] . Among the water pollutants, the most deadly ones are of biological origin: infectious diseases caused by pathogenic bacteria, viruses, protozoa, or parasites are the most common and widespread health risk associated with drinking water [1] , [2] . The most common water-borne pathogens are bacteria (e.g. Escherichia coli , Salmonella typhi , Vibrio cholerae ), viruses (e.g. adenoviruses, enteroviruses, hepatitis, rotavirus), and protozoa (e.g. giardia) [1] . These pathogens cause child mortality and also contribute to malnutrition and stunted growth of children. The World Health Organization reports [3] that 1.6 million people die every year from diarrheal diseases attributable to lack of access to safe drinking water and basic sanitation. 90% of these are children under the age of 5, mostly in developing countries. Multiple barriers including prevention of contamination, sanitation, and disinfection are necessary to effectively prevent the spread of waterborne diseases [1] . However, if only one barrier is possible, it has to be disinfection unless evidence exists that chemical contaminants are more harmful than the risk from ingestion of microbial pathogens [1] . Furthermore, controlling water quality at the point-of-use is often most effective due to the issues of microbial regrowth, byproducts of disinfectants, pipeline corrosion, and contamination in the distribution system [2] , [4] .
Common technologies for water disinfection include chlorination, filtration, UV-disinfection, pasteurization or boiling, and ozone treatment [1] , [2] , [5] . Chlorine treatment is effective on a large scale, but becomes expensive for smaller towns and villages. Boiling is an effective method to disinfect water; however, the amount of fuel required to disinfect water by boiling is several times more than what a typical family will use for cooking [1] . UV-disinfection is a promising point-of-use technology available [1] , yet it does require access to electricity and some maintenance of the UV lamp, or sufficient sunlight. While small and inexpensive filtration devices can potentially address the issue of point-of-use disinfection, an ideal technology does not currently exist. Inexpensive household carbon-based filters are not effective at removing pathogens and can be used only when the water is already biologically safe [1] . Sand filters that can remove pathogens require large area and knowledge of how to maintain them [1] , while membrane filters capable of removing pathogens [2] , [4] suffer from high costs, fouling, and often require pumping power due to low flow rates [6] that prevents their wide implementation in developing countries. In this context, new approaches that can improve upon current technologies are urgently needed. Specifically, membrane materials that are inexpensive, readily available, disposable, and effective at pathogen removal could greatly impact our ability to provide safe drinking water to the global population.
If we look to nature for inspiration, we find that a potential solution exists in the form of plant xylem – a porous material that conducts fluid in plants [7] . Plants have evolved specialized xylem tissues to conduct sap from their roots to their shoots. Xylem has evolved under the competing pressures of offering minimal resistance to the ascent of sap while maintaining small nanoscale pores to prevent cavitation. The size distribution of these pores – typically a few nanometers to a maximum of around 500 nm, depending on the plant species [8] – also happens to be ideal for filtering out pathogens, which raises the interesting question of whether plant xylem can be used to make inexpensive water filtration devices. Although scientists have extensively studied plant xylem and the ascent of sap, use of plant xylem in the context of water filtration remains to be explored. Measurements of sap flow in plants suggest that flow rates in the range of several liters per hour may be feasible with less than 10 cm-sized filters, using only gravitational pressure to drive the flow [7] .
We therefore investigated whether plant xylem could be used to create water filtration devices. First, we reason which type of plant xylem tissue is most suitable for filtration. We then construct a simple water filter from plant xylem and study the resulting flow rates and filtration characteristics. Finally, we show that the xylem filter can effectively remove bacteria from water and discuss directions for further development of these filters.
Materials and Methods
Branches were excised from white pine growing on private property in Massachusetts, USA, with permission of the owner and placed in water. The pine was identified as pinus strobus based on the 5-fold grouping of its needles, the average needle length of 4.5 inches, and the cone shape. Deionized water (Millipore) was used throughout the experiments unless specified otherwise. Red pigment (pigment-based carmine drawing ink, Higgins Inks) was dissolved in deionized water. Nile-red coated 20 nm fluorescent polystyrene nanospheres were obtained from Life Technologies. Inactivated, Alexa 488 fluorescent dye labeled Escherichia coli were obtained from Life Technologies. Wood sections were inserted into the end of 3/8 inch internal diameter PVC tubing, sealed with 5 Minute Epoxy, secured with hose clamps, and allowed to cure for ten minutes before conducting flow rate experiments.
Construction of the Xylem Filter
1 inch-long sections were cut from a branch with approximately 1 cm diameter. The bark and cambium were peeled off, and the piece was mounted at the end of a tube and sealed with epoxy. The filters were flushed with 10 mL of deionized water before experiments. Care was taken to avoid drying of the filter.
Filtration and Flow Rate Experiments
Approximately 5 mL of deionized water or solution was placed in the tube. Pressure was supplied using a nitrogen tank with a pressure regulator. For filtration experiments, 5 psi (34.5 kPa) pressure was used. The filtrate was collected in glass vials. For dye filtration, size distribution of the pigments was measured for the input solution and the filtrate. Higgins pigment-based carmine drawing ink, diluted ∼1000× in deionized water, was used as the input dye solution. For bacteria filtration, the feed solution was prepared by mixing 0.08 mg of inactivated Escherichia coli in 20 mL of deionized water (∼1.6×10 7 mL −1 ) after which the solution was sonicated for 1 min. The concentration of bacteria was measured in the feed solution and filtrate by enumeration with a hemacytometer (inCyto C-chip) mounted on a Nikon TE2000-U inverted epifluorescence microscope. Before measurement of concentration and filtration experiments, the feed solution was sonicated for 1 min and vigorously mixed.
Xylem structure was visualized in a scanning electron microscope (SEM, Zeiss Supra55VP). Samples were coated with gold of 5 nm thickness before imaging. To visualize bacteria filtration, 5 mL of solution at a bacteria concentration of ∼1.6×10 7 mL −1 was flowed into the filter. The filter was then cut longitudinally with a sharp blade. One side of the sample was imaged using a Nikon TE2000-U inverted epifluorescence microscope and the other was coated with gold and imaged with the SEM. Optical images were acquired of the cross section of a filter following filtration of 5 mL of the dye at a dilution of ∼100×.
Particle Sizing
Dynamic light scattering measurements of particle size distributions were performed using a Malvern Zetasizer Nano-ZS.
Xylem Structure and Rationale for use of Conifer Xylem
The flow of sap in plants is driven primarily by transpiration from the leaves to the atmosphere, which creates negative pressure in the xylem. Therefore, xylem evolution has occurred under competing pressures of providing minimal resistance to the flow of sap, while protecting against cavitation (i.e. nucleation) and growth of bubbles that could stop the flow of sap and kill the plant, and to do this while maintaining mechanical strength [7] . The xylem structure comprises many small conduits that work in parallel and operate in a manner that is robust to cavitation [7] , [8] ( Figure 1 ). In woody plants, the xylem tissue is called the sapwood, which often surrounds the heartwood (i.e. inactive, non-conducting lignified tissue found in older branches and trunks) and is in turn surrounded by the bark ( Figure 1b,c ). The xylem conduits in gymnosperms (conifers) are formed from single dead cells and are called tracheids ( Figure 1c ), with the largest tracheids reaching diameters up to 80 µm and lengths up to 10 mm [7] . Angiosperms (flowering plants) have xylem conduits called vessels that are derived from several cells arranged in a single file, having diameters up to 0.5 mm and lengths ranging from a few millimeters to several meters [7] . These parallel conduits have closed ends and are connected to adjacent conduits via “pits” [8] ( Figure 1d,e ). The pits have membranes with nanoscale pores that perform the critical function of preventing bubbles from crossing over from one conduit to another. Pits occur in a variety of configurations; Figure 1d,e shows torus-margo pit membranes that consist of a highly porous part shaped like a donut (margo) and an impermeable part in the center called torus, occurring in conifers [8] . The porosity of the pit membranes ranges in size from a few nanometers to a few hundred nanometers, with pore sizes in the case of angiosperms tending to be smaller than those in gymnosperms [8] , [9] . Pit membrane pore sizes have been estimated by examining whether gold colloids or particles of different sizes can flow through [8] , [10] . Remarkably, it was observed that 20 nm gold colloids could not pass through inter-vessel pit membranes of some deciduous tree species [10] , indicating an adequate size rejection to remove viruses from water. Furthermore, inter-tracheid pit membranes were found to exclude particles in the 200 nm range [8] , as required for removal of bacteria and protozoa.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
a) Structure of xylem vessels in flowering plants and tracheids in conifers. Longer length of the vessels can provide pathways that can bypass filtration through pit membranes that decorate their circumference. b) Photograph of ∼1 cm diameter pine ( pinus strobus ) branch used in the present study. c) Scanning electron microscope (SEM) image of cut section showing tracheid cross section and lengthwise profile. Scale bar is 40 µm. d) SEM image showing pits and pit membranes. Scale bar is 20 µm. e) Pit membrane with inset showing a cartoon of the pit cross-section. The pit cover has been sliced away to reveal the permeable margo surrounding the impermeable torus. Arrow indicates observed hole-like structures that may be defects. The margo comprises radial spoke-like structures that suspend the torus, which are only barely visible overlaying the cell wall in the background. Scale bar is 1 µm. f) Dependence of area amplification, defined as the pit membrane area divided by the nominal filter area, on the tracheid aspect ratio L / D and fractional area α occupied by pit membranes.
https://doi.org/10.1371/journal.pone.0089934.g001
Since angiosperms (flowering plants, including hardwood trees) have larger xylem vessels that are more effective at conducting sap, xylem tissue constitutes a smaller fraction of the cross-section area of their trunks or branches, which is not ideal in the context of filtration. The long length of their xylem vessels also implies that a large thickness (centimeters to meters) of xylem tissue will be required to achieve any filtration effect at all – filters that are thinner than the average vessel length will just allow water to flow through the vessels without filtering it through pit membranes ( Figure 1a ). In contrast, gymnosperms (conifers, including softwood trees) have short tracheids that would force water to flow through pit membranes even for small thicknesses (<1 cm) of xylem tissue ( Figure 1a ). Since tracheids have smaller diameters and are shorter, they offer higher resistance to flow, but typically a greater fraction of the stem cross-section area is devoted to conducting xylem tissue. For example, in the pine branch shown in Figure 1b used in this study, fluid-conducting xylem constitutes the majority of the cross-section. This reasoning leads us to the conclusion that in general the xylem tissue of coniferous trees – i.e. the sapwood – is likely to be the most suitable xylem tissue for construction of a water filtration device, at least for filtration of bacteria, protozoa, and other pathogens on the micron or larger scale.
The resistance to fluid flow is an important consideration for filtration. Pits can contribute a significant fraction (as much as 30–80%) [7] , [8] of the resistance to sap flow, but this is remarkably small considering that pit membrane pore sizes are several orders of magnitude smaller than the tracheid or vessel diameter. The pits and pit membranes form a hierarchical structure where the thin, highly-permeable pit membranes are supported across the microscale pits that are arranged around the circumference of the tracheids ( Figure 1a ). This arrangement permits the pit membranes to be thin, offering low resistance to fluid flow. Furthermore, the parallel arrangement of tracheids with pits around their circumference provides a high packing density for the pit membranes. For a given tracheid with diameter D and length L , where pit membranes occupy a fraction α of the tracheid wall area, each tracheid effectively contributes a pit membrane area of πDLα /2, where the factor of 2 arises as each membrane is shared by two tracheids. However, the nominal area of the tracheid is only πD 2 /4, and therefore, the structure effectively amplifies the nominal filter area by a factor of 2 α ( L / D ) ( Figure 1f ). The images in Figure 1c indicate that typical D ∼ 10–15 µm and α ∼ 0.2 yield an effective area amplification of ∼20 for tracheid lengths of 1–2 mm. Therefore, for a filter made by cutting a slice of thickness ∼ L of the xylem, the actual membrane area is greater by a large factor due to vertical packing of the pit membranes. Larger filter thicknesses further increase the total membrane area, but the additional area of the membrane is positioned in series rather than in parallel and therefore reduces the flow rate, but potentially improves the rejection performance of the filter due to multiple filtration steps as shown in Figure 1a .
Construction of the Xylem Filter and Measurement of Flow Rate
a) Construction of xylem filter. b) Effect of applied pressure on the water flux through the xylem filter. c) Hydrodynamic conductivity of the filter extracted at each measured pressure using the total filter cross-section area and thickness as defined by Equation 1 . Error bars indicate ±S.D. for measurements on three different xylem filters.
https://doi.org/10.1371/journal.pone.0089934.g002
Biologists have performed similar flow rate measurements by cutting a section of a plant stem under water, flushing to remove any bubbles, and applying a pressure difference to measure the flow rate [11] , [12] . Xylem conductivities of conifers [7] typically range from 1–4 kg s −1 m −1 MPa −1 , which compares very well with the conductivities measured in our experiments. Lower conductivities can easily result from introduction of bubbles [11] or the presence of some non-conducting heartwood. We can therefore conclude that the flow rate measurements in our devices are consistent with those expected from prior literature on conductivity of conifer xylem.
Filtration of Pigment Dye
After construction of the filter, we tested its ability to filter a pigment dye with a broad particle size distribution. The red color of the feed solution disappeared upon filtration ( Figure 3a ) indicating that the xylem filter could effectively filter out the dye.
a) Feed solution of a pigment dye before filtration (left), compared to the filtrate (right). b) Size distribution of the pigment particles in the feed and filtrate solutions measured by dynamic light scattering. c) Dependence of the rejection on the particle size estimated from the data in (b). d) Cross-section of the xylem filter after filtration. Scale is in centimeters and inches.
https://doi.org/10.1371/journal.pone.0089934.g003
Since the dye had a broad pigment size distribution, we investigated the size-dependence of filtration by quantifying the pigment size distribution before and after filtration using dynamic light scattering. We found that the feed solution comprised particles ranging in size from ∼70 nm to ∼500 nm, with some larger aggregates ( Figure 3b ). In contrast, the filtrate particle size distribution peaked at ∼80 nm, indicating that larger particles were filtered out. In a separate experiment, we observed that 20 nm fluorescent polystyrene nanoparticles could not be filtered by the xylem filter, confirming this size dependence of filtration. Assuming that pigment particles 70 nm or less in size were not rejected, the size distributions before and after filtration enable calculation of the rejection performance of the xylem filter as a function of particle size ( Figure 3c ). We find that the xylem filter exhibits excellent rejection for particles with diameters exceeding 100 nm, with the estimated rejection exceeding 99% for particles over 150 nm. Smaller particles are expected to pass through the larger porosity of the pit membrane: SEM images in Figure 1e indicate sub-micron spacing between the radial spoke-like margo membrane through which the pigment particles can pass, although the porosity is difficult to resolve in the SEM image.
After filtration, we cut the xylem filter parallel to the direction of flow to investigate the distribution of dye in the filter. We observed that the dye was confined to the top 2–3 millimeters of the xylem filter ( Figure 3d ), which compares well with the tracheid lengths on the millimeter scale expected for coniferous trees [7] . These results show that the majority of the filtration occurred within this length scale, and suggests that the thickness of the xylem filter may be reduced to below 1 cm while still rejecting the majority of the dye.
Filtration of Bacteria from Water
Finally, we investigated the ability of the xylem filter to remove bacteria from water. As a model bacterium, we used fluorescently labeled and inactivated Escherichia coli bacteria that are cylindrical in shape with a diameter of ∼1 µm. Use of fluorescently labeled bacteria enabled easy enumeration of their concentrations, and also allowed us to track the location in the xylem filter where they were trapped. Since filtration is dominated by size-exclusion at this length scale, we do not expect modification with the dye to significantly affect filtration characteristics. Filtration using three different xylem filters showed nearly complete rejection of the bacteria ( Figure 4a ). Using a hemacytometer to count the bacteria, we estimate that the rejection was at least 99.9%.
a) Concentrations of bacteria in the feed and filtrate solutions. Inset shows fluorescence images of the two solutions. Scale bar is 200 µm. Error bars indicate ±S.D. for experiments performed on three different xylem filters. b) Fluorescence image of xylem filter cross-section showing accumulation of bacteria over the margo pit membranes. Scale bar is 20 µm. c) Low-magnification fluorescence image shows that bacteria are trapped at the bottoms of tracheids within the first few millimeters of the top surface. Scale bar is 400 µm. Arrow indicates top surface of the xylem filter and also the direction of flow during filtration. Autofluorescence of the xylem tissue also contributes to the fluorescence signal in (b) and (c). d), e) SEM images showing bacteria accumulated on the margo pit membranes after filtration. Scale bars are 10 µm and 2 µm, respectively.
https://doi.org/10.1371/journal.pone.0089934.g004
To investigate the mechanism of filtration, the xylem filter was cut parallel to the direction of flow after filtration. When examined under a fluorescence microscope, we observed that the bacteria accumulated over the donut-shaped margo pit membranes ( Figure 4b ). This distribution is consistent with the expectation that the bacteria are filtered by the porous margo of the pit membranes. The distribution of trapped bacteria was not uniform across the cross section of the filter. Similar to the case of the dye, bacteria were observed only within the first few millimeters from the end through which the solution was infused (indicated by the white arrow in Figure 4c ). In addition, the low-magnification fluorescence image shows that the bacteria had accumulated primarily over pit membranes at the bottom of the tracheids, which is again not unexpected. Further investigation by SEM clearly showed individual bacterial cells accumulated on the pit membranes over the porous margo ( Figure 4d,e ). These results confirm the pit membranes as the functional units that provide the filtration effect in the xylem filter.
Wood has been investigated recently as a potential filtration material [13] , showing moderate improvement of turbidity. While we showed filtration using freshly cut xylem, we found that the flow rate dropped irreversibly by over a factor of 100 if the xylem was dried, even when the xylem was flushed with water before drying. We also examined flow through commercially available kiln-dried wood samples cut to similar dimensions. Wood samples that exhibited filtration showed two orders of magnitude smaller flow rates than in the fresh xylem filter, while those that had high flow rates did not exhibit much filtration effect and seemed to have ruptured tracheids and membranes when observed under SEM. Wetting with ethanol or vacuuming to remove air did not significantly increase the flow rate in the wood samples that exhibited the filtration effect, suggesting that the pit membranes may have a tendency to become clogged during drying. These results are consistent with literature showing that the pit membranes can become irreversibly aspirated against the cell wall, blocking the flow [14] . In fact, the pit membranes in the SEM images ( Figure 1d,e and Figure 4d,e ), which were acquired after drying the samples, appear to be stuck to the walls. Regardless, our results demonstrate that excellent rejection (>99.9%) of bacteria is possible using the pit membranes of fresh plant xylem, and also provide insight into the mechanism of filtration as well as guidelines for selection of the xylem material.
Peter-Varbanets et al. [2] have outlined the key requirements for point-of-use devices for water disinfection: a) performance (ability to effectively remove pathogens), b) ease of use (no time-consuming maintenance or operation steps), c) sustainability (produced locally with limited use of chemicals and non-renewable energy), and d) social acceptability. Meeting all of these requirements has proved to be challenging, but point-of-use methods that have been successfully used for low-cost water treatment in developing countries include free-chlorine/solar disinfections, combined coagulant-chlorine disinfection, and biosand/ceramic filtrations [5] . While chlorine is a very effective biocide, its reaction with organic matter can produce carcinogenic by-products [15] and some waterborne pathogens such as Cryptosporidium parvum and Mycobacterium avium are resistant to the chlorine [16] . Solar disinfection based on ultraviolet irradiation can effectively inactivate C. parvum , but this requires low turbidity of source water [17] and is not effective for control of viruses [16] . Filtration based on biosand and ceramic filters is also effective at removing pathogens, but the effectiveness against viruses is low or unknown [18] . Coagulation combined with chlorine disinfection removes or inactivates viruses and pathogens effectively. However, necessity of an additional filtration step and relatively high cost are potential barriers for practical use [18] . Among these methods, a review on field studies by Sobsey et al. [5] suggested that biosand and ceramic filtration are the most effective methods in practice, because once the apparatus is installed, the effort for use and dosage is significantly reduced and therefore promotes persistent use compared to disinfection approaches. Although membrane-based filtration is the most widely used for water treatment in industrialized nations and the cost of membranes has significantly decreased, membranes are still unaffordable to poor communities in the developing world [2] . Ultrafiltration systems run by hydrostatic pressure [19] and some recently invented point-of-use devices using ultrafiltration membranes may provide water to developing regions at reasonable cost [2] . However, membranes still require specialized chemicals and processes for manufacture, and need cleaning or replacement.
Xylem filter technology could be an attractive option for low-cost and highly efficient point-of-use water treatment by filtration, overcoming some of the challenges associated with conventional membranes. Xylem filters could provide the advantage of reduced human effort compared to existing point-of-use water treatment options, requiring only simple periodic filter replacement. In addition, the pressures of 1–5 psi used here are easily achievable using a gravitational pressure head of 0.7–3.5 m, implying that no pumps are necessary for filtration. The measured flow rates of about 0.05 mL/s using only ∼1 cm 2 filter area correspond to a flow rate of over 4 L/d, sufficient to meet the drinking water requirements of one person [20] . This is comparable to chlorination and biosand filtration, which have the highest production rates of prevalent point-of-use water treatment methods, and far exceeds typical production rates for solar disinfection. Xylem filters could potentially be produced locally and inexpensively, and disposed of easily owing to their biodegradability. The high flow rates and low cost would certainly help address the issues of maintenance and replacement. For example, 200 filters of 10 cm 2 area and 0.5 cm thickness could be packaged into a volume of about 1 L, which will be inexpensive and last a few years even with weekly replacement. Furthermore, as suggested by the dye filtration experiment, xylem filters should be able to significantly reduce water turbidity, enhancing the aesthetic qualities of the drinking water, which is hardly achieved by chlorination and solar disinfection.
Wood is an easily available material. While use of fresh xylem does not preclude its use as a filter material, dried membranes have definite practical advantages. Therefore, the process of wood drying and its influence on xylem conductivity needs further study. In particular, processes that yield intact yet permeable xylem tissues that can be stored long-term will be helpful for improving the supply chain if these filters are to be widely adopted. In addition, flow through xylem of different plants needs to be studied to identify locally available sources of xylem, which will truly enable construction of filters from locally available materials. In the present study, we report results using xylem derived from only one species. These xylem filters could not filter out 20 nm nanoparticles, which is a size comparable to that of the smallest viruses. It will be interesting to explore whether there exist any coniferous species that have pit membranes with smaller pore sizes that can filter out viruses, or whether conifer xylem can be impregnated with particles such as carbon black to improve rejection of viruses. In their absence, angiosperms with short vessels that yield practical filter lengths may be the best alternative due to their smaller pit membrane pore sizes [8] . Further exploration of xylem tissues from different plants with an engineering perspective is needed to construct xylem filters that can effectively reject viruses, exhibit improved flow rates, or that are amenable to easy storage. It is also conceivable that plants could be selected or developed for enhanced filtration characteristics, as has been the norm in agriculture for enhancement of many desirable characteristics including resistance to pests, flavor, or productivity.
Conclusions
Plant xylem is a porous material with membranes comprising nanoscale pores. We have reasoned that xylem from the sapwood of coniferous trees is suitable for disinfection by filtration of water. The hierarchical arrangement of the membranes in the xylem tissue effectively amplifies the available membrane area for filtration, providing high flow rates. Xylem filters were prepared by simply removing the bark of pine tree branches and inserting the xylem tissue into a tube. Pigment filtration experiments revealed a size cutoff of about 100 nm, with most of the filtration occurring within the first 2–3 mm of the xylem filter. The xylem filter could effectively filter out bacteria from water with rejection exceeding 99.9%. Pit membranes were identified as the functional unit where actual filtration of the bacteria occurred. Flow rates of about 4 L/d were obtained through ∼1 cm 2 filter areas at applied pressures of about 5 psi, which is sufficient to meet the drinking water needs of one person. The simple construction of xylem filters, combined with their fabrication from an inexpensive, biodegradable, and disposable material suggests that further research and development of xylem filters could potentially lead to their widespread use and greatly reduce the incidence of waterborne infectious disease in the world.
Acknowledgments
The authors thank Yukiko Oka for assistance with preparation of illustrations and Sunandini Chopra for help with dynamic light scattering measurements.
Author Contributions
Conceived and designed the experiments: MSHB JL VV VC RK. Performed the experiments: MSHB JL VV VC. Analyzed the data: MSHB JL RK. Contributed reagents/materials/analysis tools: VC. Wrote the paper: MSHB JL RK.
- View Article
- Google Scholar
- 3. World Health Organization. Available: http://www.who.int/water_sanitation_health/mdg1/en/ . Accessed 2014 Jan 14.
- 13. Sens ML, Emmendoerfer ML, Muller LC (2013) Water filtration through wood with helical cross-flow. Desalination and Water Treatment.
- 14. Petty JA (1972) Aspiration of bordered pits in conifer wood. Proceedings of the Royal Society Series B-Biological Sciences 181: 395-+.
- 18. Lantagne DS, Quick R, Mintz ED (2006) Household water treatment and safe storage options in developing countries: A review of current implementation practices. Woodrow Wilson International Center for Scholars. Environmental Change and Security Program.
- 20. Reed B, Reed B (2011) How much water is needed in emergencies. World Health Organization. Available: http://www.who.int/water_sanitation_health/publications/2011/tn9_how_much_water_en.pdf . Accessed 2014 Jan 14.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 March 2021
Engineering and characterization of gymnosperm sapwood toward enabling the design of water filtration devices
- Krithika Ramchander ORCID: orcid.org/0000-0001-7156-1356 1 ,
- Megha Hegde 2 ,
- Anish Paul Antony ORCID: orcid.org/0000-0002-5627-0291 2 ,
- Luda Wang 1 nAff3 nAff4 ,
- Kendra Leith 2 ,
- Amy Smith 2 &
- Rohit Karnik ORCID: orcid.org/0000-0003-0588-9286 1
Nature Communications volume 12 , Article number: 1871 ( 2021 ) Cite this article
12k Accesses
14 Citations
175 Altmetric
Metrics details
- Developing world
- Fluid dynamics
Naturally-occurring membranes in the xylem tissue of gymnosperm sapwood enable its use as an abundantly-available material to construct filters, with potential to facilitate access to safe drinking water in resource-constrained settings. However, the material’s behavior as a filter is poorly understood, and challenges such as short shelf life have not been addressed. Here, we characterize the operational attributes of xylem filters and show that the material exhibits a highly non-linear dependence of flow resistance on thickness upon drying, and a tendency for self-blocking. We develop guidelines for the design and fabrication of xylem filters, demonstrate gravity-operated filters with shelf life >2 years, and show that the filters can provide >3 log removal of E. coli , MS-2 phage, and rotavirus from synthetic test waters and coliform bacteria from contaminated spring, tap, and ground waters. Through interviews and workshops in India, we use a user-centric approach to design a prototype filtration device with daily- to weekly-replaceable xylem filters, and uncover indicators of social acceptance of xylem as a natural water filter. Our work enhances the understanding of xylem as a filtration material, and opens opportunities for engineering a diverse range of low-cost, biodegradable xylem-based filtration products on a global scale.
Similar content being viewed by others

Exclusion-zone water inside and outside of plant xylem vessels
Effective pathogen removal in sustainable natural fiber Moringa filters
A bio-based nanofibre hydrogel filter for sustainable water purification
Introduction.
Diarrheal diseases caused by microbial contamination of water and poor sanitation are a global problem. In 2019, diarrheal diseases accounted for 1.5 million deaths per year, primarily in resource-limited settings amongst children under the age of five 1 . Majority of the deaths (57.8%) are caused by bacterial pathogens, while water-borne viruses and protozoa account for 33.8% and 8.3% of the fatalities, respectively 2 . Household water treatment (HWT) methods like chlorination, solar disinfection, and filtration can significantly reduce the risk of diarrheal diseases 3 , 4 . However, the adoption of these methods in resource-constrained settings is often hindered by their limited availability in remote locations, incompatibility with local sociocultural practices, high cost, or lack of suitable financing schemes 3 , 4 , 5 . In addition, the common perception that water that appears clear is safe for drinking, and the difficulty in appreciating the link between diarrheal diseases and poor water quality, also impede uptake 3 , 4 , 5 . Novel water treatment technologies that are inexpensive, readily available, socially acceptable, and effective against water-borne pathogens have the potential to address these challenges and improve access to safe drinking water.
Gymnosperm (non-flowering plants like conifers) wood, a common material that is widely available and traded across the globe 6 , presents the intriguing possibility of creating inexpensive, sustainable, and socially acceptable filters to address this challenge 7 , 8 , 9 , 10 , 11 . The gymnosperm sapwood consists largely of xylem tissue that conducts sap, with longitudinally-oriented conduits called tracheids up to 10-mm long that are interconnected by ‘pit membranes’ with pore size ranging from 100 to 500 nm (Fig. 1a and b ) 12 . Fluid flowing through a transverse section of a branch that is thicker than a single tracheid must therefore pass through the pit membranes, which can act as physical sieves that trap particulate contaminants present in water 7 (Fig. 1c ). Compared to most angiosperms (flowering plants), the short length of tracheids and their high proportion in the cross-section makes gymnosperm sapwood better-suited to creating compact filters.
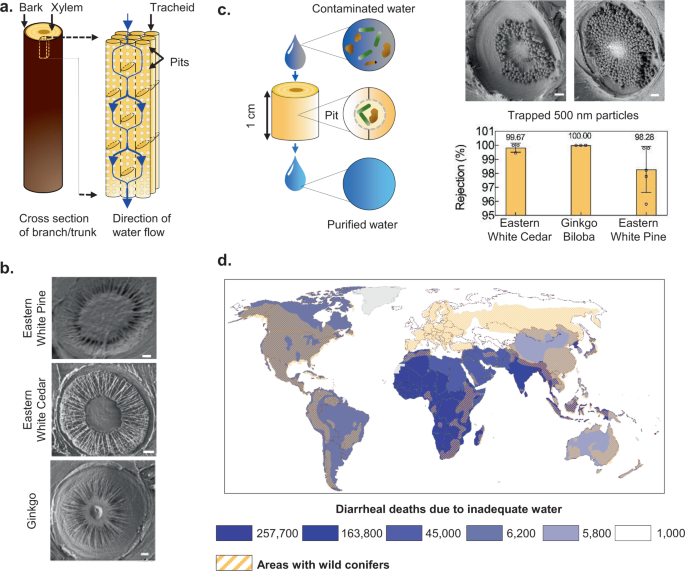
a Illustration of gymnosperm xylem structure. b Scanning Electron Microscopy (SEM) images of pit membranes in different gymnosperms. Scale bar, 1 μm. c Schematic depiction and SEM images of filtration of 500-nm particles by xylem pit membranes in a section of a branch (ginkgo, 1-cm diameter, 0.25-inch thickness). Scale bar, 1 μm. The bar graph shows rejection of 500-nm particles by fresh xylem filters made from different tree species (1-cm diameter, 0.25-inch thickness). Mean ± s.d.; n = 3 different filters; see “Methods” for details). d The global distribution of wild conifers overlaid on the number of diarrheal deaths caused by inadequate (contaminated) water in 2016 within each colored region illustrates the potential for scaling and impact of xylem-based filtration devices. The numbers in the legend represent the total diarrheal deaths for the regions shaded in the same color (world map by Diana Beltekien adapted from https://ourworldindata.org/world-region-map-definitions under Creative Commons BY license; see “Methods” for figure construction details and references).
The unique structure of gymnosperm xylem gives rise to two interesting questions: (a) is the xylem a suitable material for water filtration, and (b) if so, how can it be engineered to create practically useful water filters. Previous studies have reported that pit membranes in pine xylem can filter bacteria from deionized water and incorporation of silver nanoparticles in xylem can enhance removal of bacteria 7 , 10 . However, several other material characteristics of xylem that are critical for practical water filtration applications, such as its structural stability over the course of its shelf- and operational life, susceptibility to different foulants present in water, and mechanisms of fouling, remain to be explored. While the hydraulic properties of xylem have been well-characterized in the context of sap transport in plants 13 , 14 , 15 , xylem’s functional attributes as a water filter, such as flow rate, filtration capacity, and variation in flow rate over time, particularly with contaminated water as the fluid medium and in the absence of active transport mechanisms that regulate flow in plants, are currently not well understood. A known challenge with xylem filters is that their permeance (defined as flow rate per unit area per unit pressure difference) drops by a factor of ~100 upon drying, which limits their usability in dry state 7 . Wet filters have reasonable permeance, but have limited shelf life due to their propensity for degradation and are heavy to transport. Thus, identifying the underlying mechanism that leads to this behavior and developing methods for preserving xylem in dry state are critical for their supply and distribution, particularly to remote, low-resource settings where they are most needed. In addition, simple and inexpensive methods for filter design and manufacture that help tailor xylem’s functional attributes to suit practical needs are required to facilitate technology translation.
Here, we investigate the material attributes of xylem to reveal a unique nonlinear dependence of permeance on filter thickness, intrinsic tendency for ‘self-blocking’, and susceptibility to organic and dust foulants in water. By studying the filtration capacity, permeance, and its variation over the operational lifetime with different water qualities, we characterize the performance of these filters and evaluate their suitability for practical water filtration applications. Literature reports and our field trips to India revealed that, to be useful in households in resource-limited settings, xylem filters should (a) process at least 8 L of water to meet the daily drinking water requirement (see Supplementary Note 1 ), (b) have flow rates of at least 1 L/h, (c) effectively remove contaminants 16 , (d) function reliably with contaminated water, (e) operate under gravity with heads <1 m to minimize operation costs and space requirements, and (f) be easy to access and use 16 (Supplementary Note 1 ). By combining our insights on material behavior with an understanding of how the filter’s geometry (thickness, area) affects its performance, we develop a simple, inexpensive manufacturing method that can be performed in resource-limited settings to transform gymnosperm xylem into a dry-preservable, biodegradable, lightweight filter that meets the aforementioned metrics. Further, through field studies in India, we demonstrate the practical utility of this technology by manufacturing filters locally, validating their performance with natural water sources, and presenting evidence for positive user reception toward xylem filters. To illustrate potential for translation into a practically useful product, we develop a functional device prototype using a user-centered design approach.
Our work enhances the understanding of xylem as a water filtration material and presents the engineering tools necessary for creating a diverse range of xylem-based filtration products. The ability to create filters from different gymnosperms, widespread availability of gymnosperm xylem 17 (Fig. 1d ), low cost, natural appeal, ease of transport and distribution, and the traditional comfort associated with wood, could help xylem filters lower the barriers of access, affordability, and social acceptance, and thereby facilitate access to safe drinking water.
Nonlinear dependence of permeance on filter thickness
The blockage of xylem filters upon drying is related to the physiological function of pit membranes that have evolved to protect the plant against cavitation (i.e., nucleation of vapor bubbles) that could severely disrupt sap flow 12 . In gymnosperms, surface tension forces of a receding liquid meniscus (corresponding to an advancing vapor bubble) pull the pit membrane toward an aperture in the cell wall; water-mediated adhesive forces cause the pit membrane to seal against the cell wall, thereby isolating any cavitated conduits 18 , 19 . While the exact mechanism underlying this phenomenon, referred to as ‘pit aspiration’, remains to be elucidated, it relies on the presence of water to mediate adhesion 19 . Similar to cavitation, drying induces the formation of liquid–vapor interfaces in the xylem, which triggers pit aspiration and reduces the permeance (with prior work reporting 100× drop in flow rate for 1-inch-thick filters 7 ).
To retain some permeance in dried filters, we examined the effect of reducing filter thickness. Traditionally, Darcy’s law, which is commonly applied to porous media and predicts a linearly inverse relation between thickness and permeance (i.e., permeability, defined as permeance normalized by thickness, is constant), has been used to model the permeance of xylem 20 , 21 . Since filters have to be thicker than the conduit length to ensure contaminant removal and gymnosperm conduits are typically <0.22-inch long 14 , we expected that reducing filter thickness from 1 to 0.25 inch would lower flow resistance without compromising the rejection ability. Experiments with 1-μm microspheres (used as proxy for E. coli , justification provided in “Methods”) confirmed that the rejection ability of 0.25-inch-thick filters made from Eastern white pine ( Pinus strobus ) was comparable to 0.50- and 1-inch-thick filters (Fig. 2a ). However, in stark contrast to fresh filters where Darcy’s law was followed, the inverse dependence of permeance on thickness in dried filters was highly nonlinear; permeability dropped abruptly on increasing filter thickness beyond 0.25 inch (Fig. 2b ).
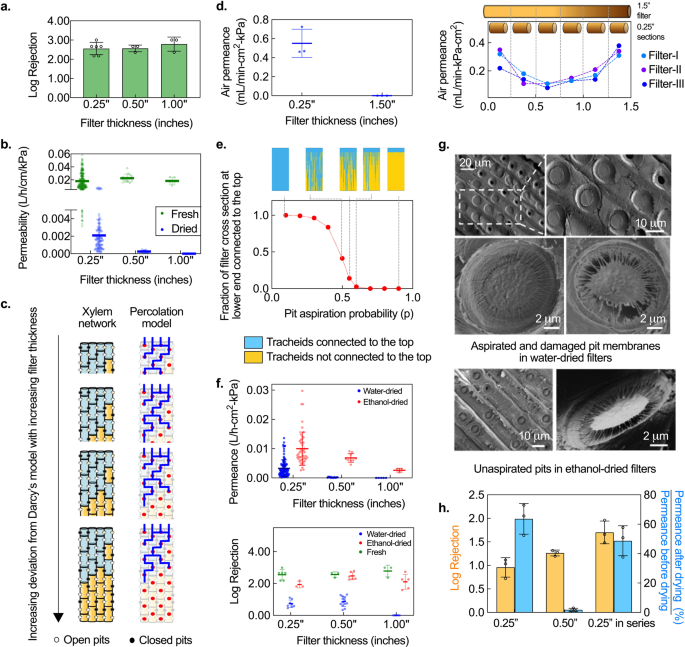
a Rejection of 0.25-inch-thick fresh filters is comparable to thicker ones. Mean ± s.d. and individual data points are shown; n = 5, 3, 3 different filters with 0.25-, 0.50-, and 1-inch thickness. b Permeability (permeance normalized by thickness) is constant with filter thickness in fresh filters, but drops abruptly with increasing thickness in the case of dried filters. Mean ± s.d. and individual data points are shown; n = (280,118), (15,15), and (5,5) different filters (numbers denote fresh and water-dried, respectively) with 0.25-, 0.50-, and 1-inch thickness, respectively. c Schematic of the physical structure of xylem and the proposed percolation-based model (tracheids depicted as red dots) for filters of different thickness, illustrating percolation-governed length dependence. Black dots represent blocked tracheid connections (pit aspiration probability, p = 0.35 for all filters). Blue shading and lines represent tracheids and flow pathways that are connected to the top surface; there is no flow pathway from the top to bottom surface for the two thickest filters. d Air permeance of water-dried filters. 0.25-inch-thick water-dried filters are permeable to air whereas 1.5-inch-thick filters are not but 0.25-inch sections cut from 1.5-inch-thick blocked filters are permeable, consistent with percolation-governed permeance. Left: Individual data points and mean ± s.d. are shown; n = 3 different filters. Right: Each data point corresponds to a single measurement on a 0.25-inch-thick section. e Simulation results show that interconnectivity of tracheids to the top face decreases with pit aspiration probability. Inset depicts interconnected (blue) and isolated (yellow) tracheids at different pit aspiration probabilities. f Ethanol-drying reduces pit aspiration and improves permeance and rejection over water-drying in a thickness-dependent manner. Individual data points and mean ± s.d. are shown. Top: n = (118, 41), (15, 9), and (5,3) different filters (numbers denote water-dried and ethanol-dried, respectively) with 0.25-, 0.50-, and 1.00-inch thickness, respectively. Bottom: n = (8, 3, 6), (13, 6, 3), and (5, 5, 3) different filters (numbers denote water-dried, ethanol-dried, and fresh filters, respectively) with 0.25-, 0.50-, and 1.00-inch thickness, respectively. g SEM images reveal aspirated or damaged pit membranes in water-dried filters, but not in ethanol-dried filters. h Two 0.25-inch-thick water-dried filters stacked in series perform significantly better than 0.25-inch and 0.50-inch-thick water-dried filters. Mean ± s.d; n = 3 different filters.
To explain this observation, we note that the permeability of dried xylem filters is a function of not only the flow resistance of tracheids and pit membranes 13 , 14 , but also the tracheid interconnectivity 22 . The length scale over which tracheids maintain connectivity depends on the degree to which the pit membranes get blocked during drying, and corresponds to cluster size in percolation theory 22 , 23 , 24 . Filters much thicker than this length scale of connectivity will be impermeable to flow, while those that are thinner, will have non-zero permeance (Fig. 2c ). When filter thickness is comparable to this length scale, a highly nonlinear dependence of permeance on thickness that deviates strongly from Darcy’s law, is expected. The experimental results suggest that the length scale of connectivity in dried filters was ~0.25 inch, which also implies that any 0.25-inch section of a longer, impermeable filter should have non-zero permeance. This hypothesis was confirmed through experiments where 1.5-inch dried filters made from Eastern white pine were completely blocked, but 0.25-inch sections cut from the same blocked filters were permeable to flow (Fig. 2d ).
The non-zero permeance of 0.25-inch sections in dried filters indicates that some pit membranes remain open (unaspirated) even after drying, i.e., the probability of pit aspiration blocking off a tracheid-tracheid interconnection upon drying is <1. We built a probabilistic model based on percolation theory 23 to capture the flow characteristics of a dried filter and better understand the dependence of permeance on filter thickness. We modeled the xylem as a node-edge network, where the tracheids and pits correspond to the nodes and edges, respectively (Fig. 2c ). The model associated a probability p for an edge being broken 23 , which in the case of a xylem filter represents the likelihood of connectivity between two tracheids being broken by pit aspiration. Simulations of this percolation model in a simplified, 2-D xylem network using MATLAB corroborated experimental observations; for a given pit aspiration probability, the connectivity (and thus the permeance) dropped to zero beyond a critical filter thickness (Supplementary Fig. 1a ). Further, the model suggested that the converse should also be true, i.e., for a given filter thickness, there exists a critical probability p = p c , at which there is transition from zero to non-zero permeance (Fig. 2e , see Supplementary Note 2 for model details).
Dry preservation of xylem filters
The knowledge of how xylem permeance is affected by thickness and pit aspiration probability ( p) suggests two methods that could be used for preserving permeance in dried filters. First, permeance in dried filters may be retained by restricting their thickness to below a certain threshold value but above the tracheid length (~0.25 inches for Eastern white pine). Second, methods could be implemented to mitigate pit aspiration and thereby retain permeance in thicker filters.
Previous studies have shown that pit aspiration can be reduced by replacing the sap in the xylem with non-aqueous solvents, like alcohols, as it precludes water-mediated adhesion between the pit membrane and the cell wall during drying 18 , 19 , 25 . To evaluate whether treatment with non-aqueous solvents can improve permeance, we compared the permeance of filters (made from Eastern white pine) that were dried after flushing with ethanol (‘ethanol-dried’) to those that were flushed with water before drying (‘water-dried’). Ethanol-dried filters exhibited higher permeance than their water-dried counterparts (Fig. 2f ); the effect was more pronounced for thicker filters (0.5- and 1.0-inch) where water-dried filters were almost completely blocked whereas ethanol-dried filters retained permeance. Similar effects on permeance were observed on treating filters with other alcohols like isopropanol (see Supplementary Fig. 1b–f and Supplementary Note 3 for more details on solvent-based preservation). When benchmarked against commercial microfiltration membranes with similar pore size, the permeance of 0.25-inch ethanol-dried filters was comparable. The permeance range for commercial membranes is 0.002–0.05 L/h.cm 2 .kPa 26 , 27 , 28 , 29 , 30 while 95% of the ethanol-dried filters from among 47 filters made from different Eastern white pine trees in Cambridge, MA, tested over a 2-year period consistently had permeance >0.005 L/h.cm 2 .kPa (Supplementary Fig. 1b ).
The rejection performance of ethanol-dried filters with 1-μm microspheres was significantly better than water-dried filters ( p < 0.001 for 0.25-, 0.50-, and 1-inch filters, respectively) and comparable to fresh filters ( p = 0.02, 0.59, and 0.08 for 0.25-, 0.50-, and 1-inch filters, respectively) (Fig. 2f ). Scanning electron microscopy (SEM) confirmed that the pit membranes in ethanol-dried filters were unaspirated and intact, whereas those in water-dried filters were aspirated or damaged, consistent with their low permeance and rejection (Fig. 2g ) 25 . Nevertheless, the rejection performance of water-dried filters could be improved by stacking multiple filters in series (Fig. 2h ). Two 0.25-inch water-dried filters in series had better rejection than a single 0.25-inch filter (1.70 ± 0.24 log versus 0.95 ± 0.16 log ( p = 0.015)), with the log rejection being additive, and higher rejection and permeance recovery than a 0.50-inch filter (1.70 ± 0.24 log versus 1.26 ± 0.06 log ( p = 0.04), and 48.8 ± 4.9% versus 1.8 ± 1.0% ( p = 0.0013), respectively). Stacking could be also used in conjunction with solvent treatment to further improve the rejection performance of filters, which offers opportunities for tailoring the rejection capability of xylem to suit different applications.
Both methods of dry preservation, thickness control and non-aqueous solvent treatment, offer different advantages and disadvantages with respect to rejection performance, ease of implementation, and reliability. Thickness control could be particularly useful where access to solvents is difficult or expensive, whereas solvent treatment is likely to be more robust but relatively expensive to perform, although the cost may be reduced by solvent recovery and reuse. The solvent used for dry-preservation must be certified as food-grade and the level of residual solvent in dried filters should be maintained within the permissible limits for human consumption as prescribed by food safety standards 31 .
Self-blocking and its control
In membrane-based filters, fouling due to contaminants in the feed water determines the filter’s volumetric capacity, i.e., the total amount of water that can be processed before the filter needs to be replaced 32 . Surprisingly, we observed a decrease in the flow rate of xylem filters that eventually led to blockage after a certain period of time even when filtering uncontaminated, deionized (DI) water (Fig. 3a ). After ruling out several potential mechanisms that could be responsible for this behavior (see Supplementary Note 4 and Supplementary Fig. 2a and b ), we observed that filters soaked in DI water (without flow) over similar time durations were not blocked, indicating that fluid flow played an important role in the underlying mechanism leading to blockage (Supplementary Fig. 2c and d ). Furthermore, SEM imaging revealed an apparent deposition of material on the pit membranes of the blocked filters (Fig. 3b ; compare this to pit membranes in unblocked ethanol-dried filters shown in Fig. 2g ). Deposition of material even with DI water indicated that the material must originate from the filter itself. Xylem is composed of cellulose and hemicellulose fibers and hydrophobic lignin polymers, of which hemicellulose fibers are highly amorphous and relatively easily soluble in water 33 . We therefore hypothesized that the dissolution of hemicellulose fibers in DI water and their convective re-deposition on the pit membranes gives rise to self-blocking of xylem filters. Analysis of the water filtered through the xylem filters under atomic force microscopy (AFM) revealed the presence of dissolved solids (Fig. 3b ) and further Fourier transform infrared spectroscopy (FTIR) measurements confirmed the presence of hemicellulose, validating our hypothesis (Fig. 3c ) 34 .
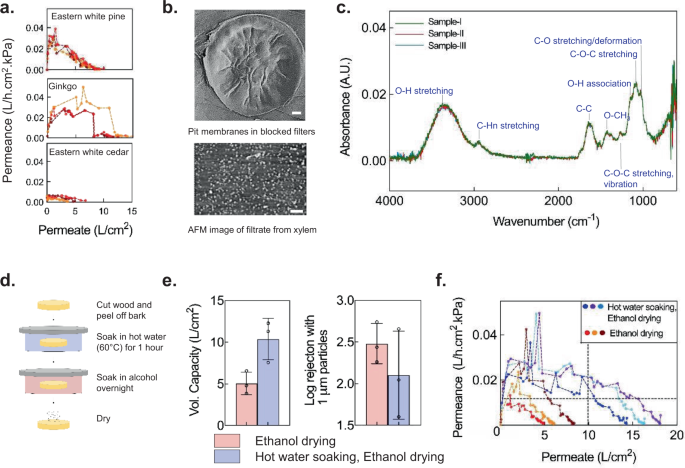
a Permeance of 0.25-inch-thick ethanol-dried filters made from different gymnosperm species decreases with permeate volume when filtering deionized water; n = 3 different filters, denoted by different colors. b Microfibrils are covered by deposited material in pit membranes of blocked filters (SEM image, top) and filtrate dried on a surface contains particulates (AFM image, bottom), suggesting dissolution and deposition of organic material within the filter. Scale bars, 2 μm. c FTIR spectra of different samples of filtered water indicate that hemicellulose leaches out of xylem filters. Modes corresponding to FTIR peaks are specified. A.U. stands for Absorbance Unit. d – f Hot-water soaking improves volumetric capacity (vol. capacity) and retains rejection (0.375-inch-thick filters). Mean ± s.d. are indicated in ( e ); n = 3 different filters. Different colors denote different filters in ( f )). Data were obtained with 1-cm diameter Eastern white pine filters operated under 1-m gravity head. The horizontal dashed line denotes the permeance (0.01 L/h.cm 2 .kPa) corresponding to the target flow rate of 1 L/h with a 10-cm 2 filter area and 1-m gravity head, whereas the vertical dashed line corresponds to a volumetric capacity of 100 L, which is achieved by the hot water soaked and ethanol-dried filters while maintaining the target permeance.
Self-blocking of xylem imposes an intrinsic limit on filter life and its volumetric capacity. However, it could also safeguard users against the risk of using a filter degraded by prolonged exposure to contaminated water or trapped microbes and signal the need for filter replacement. The ability to regulate self-blocking is therefore important, as it can help balance performance and safety. Broadly, self-blocking may be regulated by fixing the molecules within the xylem (which could also reduce degradation), or prior removal of the material responsible for the behavior. Effect on structural integrity of pit membrane (critical for rejection performance) and ease of implementation in low-resource settings are considerations that govern the choice of such methods.
We leveraged the solubility of hemicellulose in water to develop a simple process for mitigating self-blocking by soaking the filters in hot water to remove hemicellulose. We identified optimal temperature and duration of soaking to improve volumetric capacity without compromising structural integrity of the pit membranes; soaking the filters in hot water at 60–65 °C and atmospheric pressure for 1 h before ethanol-drying doubled the capacity while maintaining its ability for filtration (Fig. 3d, e , Supplementary Fig. 2e ). In practice, the volumetric capacity of filters will also be limited by the fouling due to external water contaminants. Consequently, the necessity for measures to minimize self-fouling will be low if external contaminant load is high, and hot water soaking may not be needed. It is to be noted that this soaking process is different from industrial hydrolysis of hemicellulose that is typically performed at high temperature and pressure for extraction of chemical derivatives such as sugars 35 .
Eastern white pine filters fabricated using hot water soaking and ethanol-drying could maintain permeance >0.01 L/h.cm 2 .kPa while filtering at least 11 L/cm 2 of DI water (Fig. 3f ). Thus, in the absence of fouling due to constituents in the feed water, filters with 10-cm 2 area (3.6-cm diameter) would achieve flow rates >1 L/h and volumetric capacity of ~100 L under gravity-driven operation with 1 m head (see Supplementary Fig. 2f for variation in permeance with permeate filtered for intermittent and continuous operation and Supplementary Fig. 2g for scaling of flow rate with filter area). However, we observed that the rejection performance of 0.25-inch-thick filters was sensitive to the variability in filter thickness, which is expected if the filter thickness approaches the length of the xylem conduits (tracheids) in Eastern white pine 36 . To circumvent this issue, the filter thickness was increased to 0.375 inches for all filters in subsequent studies.
Effect of water quality
Constituents in water such as humic acids or colloids typically cause fouling of membrane filters, reducing the flow rate with time. Understanding how such constituents affect the flow rate and volumetric filtration capacity of xylem filters is therefore essential to better inform how xylem filters would perform in practical settings. The World Health Organization (WHO) prescribes two kinds of synthetic test waters to evaluate the performance of household water treatment technologies 37 : a general test water (GTW) representing high-quality groundwater or rainwater, and a challenge test water (CTW) with aggressive water specifications to represent turbid surface water (see Supplementary Fig. 3a for composition of GTW and CTW). With GTW, the volumetric capacity and peak permeance (highest permeance over the course of operation) of xylem filters (fabricated by hot-water soaking and ethanol-drying) were sufficient to meet the target metrics (flow rate >1 L/h and volumetric capacity >8 L). However, filter performance varied significantly with water quality; both peak permeance and capacity with CTW (0.022 ± 0.020 L/h.cm 2 .kPa and 6.07 ± 4.40 L/cm 2 , respectively) were an order of magnitude lower than those with GTW (0.002 ± 0.001L/h.cm 2 .kPa and 0.58 ± 0.47 L/cm 2 , respectively; Fig. 4a ).
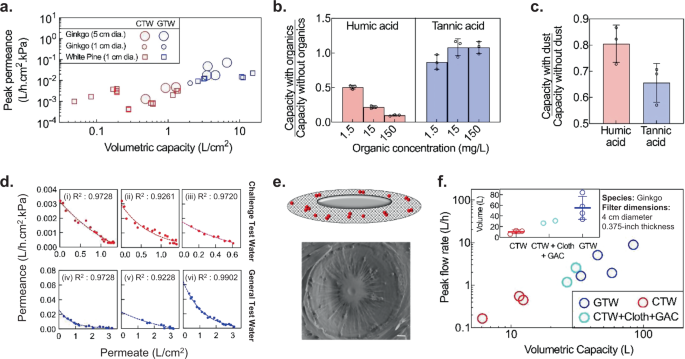
a Peak permeance and volumetric capacity normalized by area for general test water (GTW) and challenge test water (CTW). Each data point represents a different filter (one measurement per filter) with 0.375-inch thickness operated under 1-m gravity head. b , c Filter capacity is most susceptible to humic acid, followed by dust and tannic acid (1-cm diameter, 0.375-inch-thick Eastern white pine filters operated under 1-m gravity head; mean ± s.d.; n = 3 different filters) (see “Methods” for experiment details). In b , either humic acid or tannic acid is added to water. In c , water contains 70 mg/L dust or no dust in either 15 mg/L humic acid or tannic acid. d Decrease in filter permeance with CTW (red) and GTW (blue) is well-fitted by the intermediate fouling model (dashed lines). Each graph represents measurements on a single filter under 1-m gravitational head. (i)–(v): Eastern white pine, 1-cm diameter, 0.375-inch thickness. (vi): Ginkgo, 4-cm diameter, 0.375-inch thickness. e Schematic illustrating the deposition of foulant particles (red) on the pit membrane in the intermediate fouling model, which is consistent with foulant deposition observed by SEM in a partially fouled ginkgo filter. Scale bar, 1 μm. f Pre-treatment with cloth and granular activated carbon (GAC) improves the peak flow rates and volumetric capacity of ginkgo filters with GTW and CTW at 1-m gravity head. Inset shows mean ± s.d. of the volumetric capacity; n = 3, 2, and 4 different filters with CTW, CTW + Cloth + GAC, and GTW, respectively.
The deterioration in performance with CTW could be attributed to one or more of the water quality parameters that differ between CTW and GTW, which are (a) higher turbidity, (b) higher concentration of organics, and (c) the larger size of organic contaminants in CTW. To identify the key foulants that cause deterioration, we measured filtration capacity of xylem while selectively adding different constituents at varying concentrations. Xylem filters were most susceptible to fouling by humic acids (present in decomposed organic matter) followed by particulates (dust) (Fig. 4b and c ). By contrast, tannic acid did not impact filter capacity significantly, demonstrating that the filters have a low susceptibility for fouling with small, homogeneous organic molecules.
Fouling is a well-researched topic in membrane filtration and several fouling models have been developed to understand the nature of interaction between the foulants and membrane surface and aid membrane design, operation, and fouling control 38 . Based on the goodness of fit of different models to experimental data with GTW and CTW, we identified that the fouling behavior in xylem filters is best explained by the ‘intermediate blocking’ model. This model has commonly been used to represent the fouling of polymeric micro/ultrafiltration membranes by biological and organic contaminants 39 , 40 , 41 , 42 (Fig. 4d , see Supplementary Note 5 and Supplementary Fig. 3b,c for detailed comparison with other fouling models). In this model, foulant particles deposit randomly on the pit membranes and result in exponential decrease in permeance. SEM images of partially fouled filters were in agreement with this fouling mechanism (Fig. 4e ). The fouling model helps predict the change in filter permeance with time for a given contaminant load; consequently, it can be used for estimating volumetric capacity, filter lifetime, and replacement frequency for different water qualities.
Knowledge that humic acid and dust particles adversely impact filter performance offers the possibility of mitigating their impact through approaches ranging from pre-treatment of water to chemical modification of xylem. To keep filter manufacturing simple and inexpensive, and accommodate variations in contaminant type and load, we explored pre-treatment methods that can be easily integrated in-line with xylem filters when the water quality is poor. Specifically, we investigated cloth pre-filtration and granular activated carbon (GAC) adsorption to reduce the load of dust and humic acid, respectively 43 . Both these methods have been commonly used for household water treatment, but have limited efficacy in removing bacterial or viral pathogens from water 44 , 45 . After studying the adsorption kinetics of various types of GACs, we designed a GAC column to reduce humic acid content in CTW by 95% (see Supplementary Note 6 , Supplementary Fig. 3d for GAC column design). When used in conjunction with cloth pre-filtration, the GAC column improved the performance of xylem filter with CTW significantly (Fig. 4f ); on average, capacity and flow rates increased by a factor of ~3× and 5×, respectively.
In practice, pre-filtration is not essential for the operation of the filter; it is an option which, in conjunction with water quality, determines the flow characteristics. The decision whether to incorporate pre-treatment and the choice of pre-treatment would be governed by the tradeoff between the added convenience of longer filter lifetime or lower filter replacement frequency, cost, and the complication of an added replaceable component, plus the need to remove any chemical contaminants that may be present in the water (cost estimates for xylem filters and GAC provided in Supplementary Note 7 ). The replacement frequency of the cloth or the GAC module would vary depending on the type of cloth/GAC used, configuration of GAC module, and water quality. While the cloth pre-filter could be washed or replaced once it is dirty, the GAC might need replacement once every few months (1.5–6 months; see Supplementary Note 7 for estimates on GAC replacement frequency). The reduced lifetime or slower flow rates even with newly-replaced xylem filters could be used as an indicator for pre-filtration module replacement.
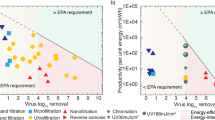
In summary, these studies demonstrate that xylem filters offer promise for practical translation. Filters made from Ginkgo biloba (ginkgo) with an area of 13 cm 2 (4-cm diameter) using the fabrication protocol shown in Fig. 3d , operated under a 1-m gravity head could (a) process ~55 ± 21 L of GTW without pre-filtration and 28 ± 3 L of CTW with GAC and cloth pre-filtration, which is more than sufficient to meet the daily drinking water requirement of a household, (b) yield peak flow rates of 1.5–9 L/h depending on water quality (see Supplementary Fig. 3e–g for variation of flow rates over filter lifetime), (c) reject 99.76 ± 0.25% of 1-µm particles. Further, these filters had a shelf life of at least 1 year (the permeance of filters stored for 1 year was 0.0074 ± 0.0003 L/h.cm 2 .kPa and the rejection of 1-μm microspheres was 99.92 ± 0.05%) and could be transported easily due to their lightweight (~7–8 g).
Microbiological performance
To assess the potential health impact of xylem filters and their effectiveness in reducing the risk of diarrheal diseases, we tested the filters’ ability to remove E. coli , MS-2 phage, and rotavirus (the single largest causal organism of diarrhea 2 ) from water. Xylem filters (4-cm diameter, 0.375-inch thickness, stored for 2 years, no pre-filtration) made from ginkgo were operated under a 1.2-m gravity head with GTW containing WHO-prescribed concentrations of E. coli (≥10 6 CFU/mL) and MS-2 phage (≥10 5 PFU/mL) 37 and NSF-prescribed concentrations of rotavirus (≥10 4 PFU/mL) 46 . E. coli and MS-2 phage were dosed simultaneously in the same test solution while rotavirus removal was tested separately. The bacteria and virus removal was tested at the start of filter operation and when permeance declined to 75, 50, and 25% of the initial value. After the first sampling point at the start of filter operation, dust was added to the test solution to accelerate clogging 47 , 48 (refer to “Methods” for further details on test procedure). The filters showed >4-log removal of rotavirus and >3-log removal of E. coli and MS-2 phage (Fig. 5a , data provided in Supplementary Table 1 ). With such rejection performance, xylem filters would fall under the ‘comprehensive protection (high pathogen removal)’ category ( ★ ★ ) as per the WHO scheme for classifying water treatment technologies (Fig. 5b ) 48 . Since the virus particles are smaller than the expected pore size of the filters (MS-2 phage and rotavirus are 24 48 and 70 nm 49 in diameter, respectively, while the pore size is 100–500 nm 12 ), the results suggest that the mechanism of virus removal is likely to be adsorption-driven. Virions can adsorb on cellulose-based materials 50 , with cellulose nitrate reported to remove virions that are much smaller than the filter pore size 51 . We hypothesize that the relatively slow flow rate and the large thickness of xylem filters could facilitate adsorption and removal of viruses.
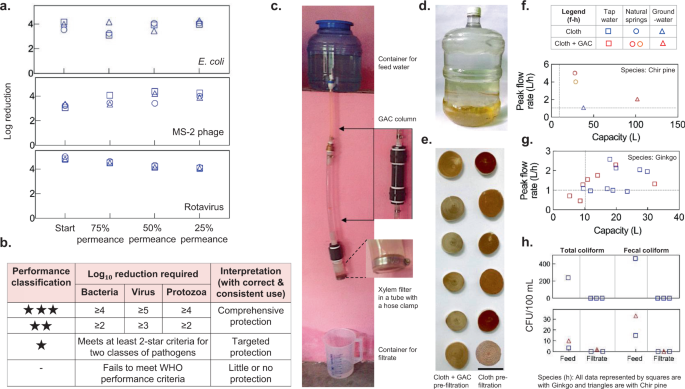
a Microbial removal performance of xylem filters (ginkgo, 4-cm diameter, 0.375-inch thickness, no pre-filtration) when operated under 1.2-m gravity head with general test water containing E. coli (≥10 6 CFU/mL) and MS-2 phage (≥10 5 PFU/mL) dosed simultaneously, or rotavirus (≥10 5 PFU/mL). Rejection was measured at the start of filter operation and when permeance dropped to 75, 50, and 25% of initial permeance (see “Methods” for further details). Different symbols indicate different filters. b World Health Organization (WHO) scheme for classification of household water treatment technologies. c Field setup for testing filter performance. d Tap water sample (New Delhi, India) used for testing. e Xylem filters used with granular activated carbon (GAC) show reduced deposits after filtration with tap water. Scale bar, 4 cm. f – h Chir pine and ginkgo filters show peak flow rates and capacity exceeding 1 L/h and 10 L, respectively, indicated by dashed lines in ( f ) and ( g ), and absence of coliform in the filtered water. Legend is shown at the top. In h , chir pine and ginkgo filters were used for tap water and groundwater studies, respectively. All data are obtained with 4-cm diameter, 0.375-inch-thick filters operated under 1-m head (see “Methods” for details on field testing of filters). Each data point represents a different filter (one measurement per filter).
Technology translation
Motivated by encouraging results from the lab-based studies, we conducted field studies to assess the ability of xylem filters to function with natural water and facilitate access to safe drinking water in resource-constrained settings. We focused on India, which has the highest water-borne illness mortality rate in the world with more than 160 million people lacking access to safe and reliable water 2 , 52 . In particular, we targeted low-income communities in urban slums (Delhi and Bengaluru) and rural villages (Uttarakhand). We assessed filter performance in the field, developed a functional prototype device through user-centric design, and examined aspects of social acceptance and user preferences to gauge the potential of xylem filters to lower existing barriers for HWT adoption.
Xylem filters made from gingko trees in US and those manufactured in India with indigenous Pinus roxburghii (chir pine) using local resources for all fabrication steps such as cutting, hot water soaking, and dry preservation, were tested with water from natural springs, municipal taps, and tubewells (groundwater) (which were the primary sources of drinking water in Uttarakhand, Delhi, and Bengaluru, respectively; see Supplementary Table 2 for water quality information). Xylem filters with 4-cm diameter mounted by simply clamping the xylem filters in a tube (Fig. 5c ) and operated under 1-m gravity head yielded peak flow rates exceeding 1 L/h and filtration capacities exceeding 10 L in most cases, with either cloth pre-filtration or cloth and GAC pre-filtration (Fig. 5d–h , see Supplementary Fig. 4a-d for variation of flow rates over filter lifetime). With cloth pre-filtration, xylem filter capacity ranged from ~40 L with groundwater to 12–30 L with turbid tap water. The benefits of adding a GAC pre-filtration module varied with water quality; GAC improved filter capacity from 38 to 102 L with groundwater and yielded a capacity of ~30 L with spring water, but did not improve xylem filter performance with tap water. No total or fecal coliform bacteria were detected in the filtrate for 5 out of the 6 xylem filters tested (3 filters operated with GAC and 3 filters operated without GAC) (Fig. 5h , Supplementary Fig. 4e–g ). These results confirmed that xylem filters could remove coliform bacteria and function in realistic settings with replacement on a daily to weekly basis depending on the operating conditions.
Analogous to other membrane filters, xylem filters have to be housed in a device for HWT. A wide range of device configurations could be designed to suit different use cases, water quality, resources available, and user preferences. As an illustrative example, we built a functional, first-generation device prototype based on the feedback gathered through 600 semi-structured interviews and surveys, 53 focus group discussions, and 2 hands-on co-design workshops with over 1000 target users (see “Methods” for user study protocol). Product attributes desired by users revealed through these efforts included: (a) ease of operation (filling and extracting water from the device, replacement of filter cartridge, etc.), (b) low cost, and (c) aesthetic appeal. Combining these inputs with existing guidelines for fabricating household water filters 16 , we developed a device consisting of a top container for feed water, a screw-on holder that houses a xylem filter and allows for easy replacement, and a bottom receptacle with a dispenser to collect filtered water and a cover to minimize the risk of recontamination. The device height was optimized such that users could fill the water in the top receptacle conveniently and the water head was sufficient to yield adequate flow rates (Fig. 6a–c , Supplementary Fig. 5a-f , see Supplementary Note 1 for other device configurations). Some challenges encountered during device design included obtaining a leak-proof seal between the holder and xylem filter due to irregularities on the wood surface, and preventing air entrapment in the tubing and filter holder that could obstruct flow. These challenges were overcome by cutting the wood at high speeds using a cold saw to obtain a smooth surface finish, using O-rings with appropriate compliance to conform to the wood surface, proper sizing of tubing and connectors to avoid bubble entrapment, and providing a vent in the holder for releasing any trapped air. This device showed 99.76 ± 0.41% rejection of 1-µm particles in lab studies and filtered 10 L of tap water in Delhi at flow rates >1 L/h (Fig. 6c ). Flow rates can be improved further by clamping the filter on the side instead of the face, which prevents complete utilization of the peripheral filter cross-sectional area containing the xylem (the effective filtration area in the device was 7 cm 2 ; see Supplementary Fig. 5d-f for alternate holder configurations; see Supplementary Note 8 for details on resources required for manufacturing filters and filtration devices).
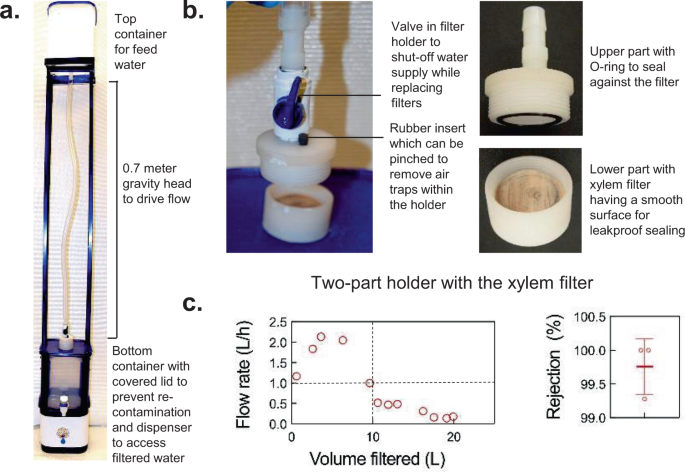
a , b Device prototype and its components. c Device flow rate with tap water in New Delhi and rejection of 1-μm particles measured in the laboratory are consistent with prior characterization of xylem filters. Filters were made from ginkgo of 4-cm diameter and 0.375-inch thickness. For the rejection performance, mean±s.d and individual data points are shown; n = 3 different filters.
Although performance is important to the success of any technology, its adoption by the target population is critical to achieve impact. In addition to lack of awareness, the adoption of water treatment technologies in resource-constrained settings faces three main barriers: access, affordability, and acceptance. Xylem filters could lower these barriers in the following ways. First, the abundant availability of gymnosperms, local access to other raw materials required for filter fabrication (primarily alcohol for dry preservation), simplicity of the manufacturing process, and open access to this technology (filter fabrication has not been patented to facilitate adoption) could provide an opportunity for local manufacture of these filters, making them more accessible to local communities (see Supplementary Note 8 for a list of equipment that can be used in low-resource settings). Due to their low weight (<10 g) and volume (~13 cm 3 ), xylem filters could be shipped easily (even by post to remote locations) and stocked in local shops to facilitate access. Second, our preliminary cost estimation studies suggest that, in comparison to conventional filter cartridges that cost USD 5–10 and require replacement every 3–6 months, xylem filters could cost USD 0.06–0.10 and require replacement every 1–5 days with a comparable cost per liter water filtered (see Supplementary Note 7 for cost estimation details and Supplementary Fig. 6 for cost comparison with currently available commercial filters in India). Such amortization of filter replacement costs could significantly lower the barrier to affordability for low-income households, where ‘pay-as-you-go’ or ‘buying less, but more often’ model is preferred over longer-term filter replacements 53 . In Indian urban slums where reverse-osmosis (RO) filtered water cans costing USD 0.28–0.56 per 20 L or government-operated water booths which provide RO water at USD 0.06–0.10 per 20 L are the only options, xylem filters could provide a viable HWT alternative for those who cannot afford or access these options easily. They could also facilitate sustained usage of HWT amongst those who are unable to purchase cans or fetch water from the booths on a regular basis to realize effective health outcomes. Furthermore, it could offer the opportunity to involve local suppliers, e.g., in small stores, in the daily distribution of filters. Finally, the traditional comfort associated with using wood for fuels and utensils could facilitate the social acceptance of xylem filters. During our field studies, 40% of the 300 survey respondents cited natural appeal and simplicity as the primary attributes they like about xylem filters, suggesting positive prospects for user reception (Supplementary Note 1 ).
The need for frequent filter replacement creates the risk of lack of user compliance and disruption of sustained usage, which could be exacerbated if pre-filtration units that require replacement at a different frequency than the xylem filters are used. Technological and product design improvements (such as engineering filters with longer lifetime, designing holders for easy filter replacement) or supply-related solutions (such as filters being available at stores that provide groceries or other regularly-purchased items) could help mitigate the risk of disruption of sustained usage, whereas the decrease in flow rate of filters with time mitigates the risk of lack of user compliance in replacing the filters. These efforts would nevertheless have to be complemented with behavior change interventions. The motivators for behavior change and thus, the nature of these interventions, would depend on the local social, cultural and environmental characteristics, such as water quality (whether water has visible coloration, odor, or poor taste), perceived health risk associated with water quality, education level of the population, etc. 3 , 54 , 55 , 56 , 57 , 58 , 59 . The influence of these characteristics on HWT adoption has been extensively studied in literature and this knowledge, in addition to field studies with user groups, could be leveraged to facilitate sustained adoption of xylem filters (see Supplementary Note 9 for factors that affect behavior change and sustained adoption of HWT and examples of behavior change interventions for xylem filters) 54 , 55 , 56 , 57 , 58 , 59 , 60 .
This work provides new insights into gymnosperm xylem from the perspective of its use as a material for water filtration—namely, the interplay between permeance and rejection governed by percolation, the intriguing ‘self-blocking’ behavior arising from dissolution and re-deposition of hemicellulose, and elevated propensity for fouling in the presence of large organic molecules and dust. We leveraged these insights to develop engineering methods for preserving xylem filters in dry state, mitigating self-blocking, and obtaining practically useful performance with different water qualities (see Table 1 for a summary of the effect of the key design, manufacturing, and operating parameters on filter performance). To demonstrate potential for practical utility and translation, filters were fabricated using locally available gymnosperms in India and filter performance was validated with natural water sources used for drinking. As an example of how xylem filters could be incorporated in filtration devices, a gravity-operated, functional device prototype for household drinking water treatment was developed using user-centered design approaches. Finally, evidence gathered from user research and preliminary cost estimation studies was used to show how xylem filters could potentially reduce the barriers of access, affordability, and social acceptance to serve as an attractive HWT option for low-income communities that are at the highest risk of water-borne diseases.
With >3-log removal for bacteria and virus (>4-log removal for rotavirus), xylem filters can provide ‘comprehensive protection’ against water-borne pathogens as per WHO’s performance criteria for household water treatment technologies 48 and have potential for reducing the health burden of water-borne diseases (Fig. 7a ). The contaminant removal ability of xylem filters could be improved further due to fouling (Supplementary Note 10 ), by stacking filters or using other approaches. Use of silver nanoparticles in xylem to enhance the removal of bacteria and methylene blue (a commonly used industrial dye that causes water pollution) using xylem 10 and chemical modification of xylem surface for copper adsorption has been reported 9 . Incorporating suitable sorbents such as zeolites or ferrous oxide into pre-filtration modules could enhance removal of viruses, arsenic, or other pollutants 61 , 62 . More generally, xylem filters could be used synergistically with other water treatment methods, e.g., in conjunction with chlorine to remove protozoan cysts that are relatively chlorine-resistant. Beyond gymnosperms, plants like primitive angiosperms that have short xylem conduits and nanoscale pit membrane pores and are not prone to aspiration 63 , or ubiquitous bamboo nodes 64 , could be explored for filtration applications.
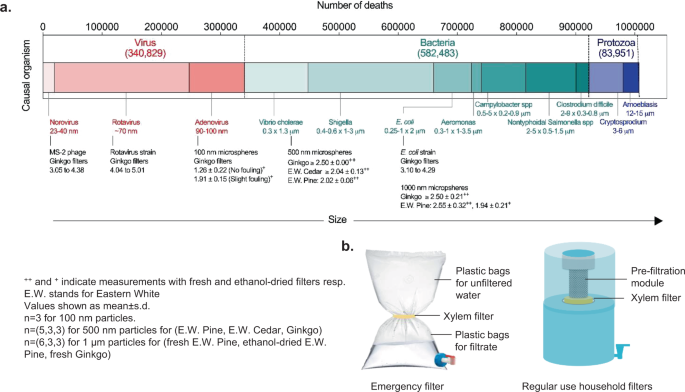
a Comparison of diarrheal mortality burden due to different water-borne pathogens 2 arranged by size to the measured log rejection of particles of different size by single 0.375-inch-thick xylem filters (except for rejection experiments with 500-nm microspheres, where filter thickness was 0.25 inch) made from different tree species. Each n corresponds to a different filter. b Illustrations of possible configurations of xylem-based filtration devices.
Some limitations of xylem filters for household water treatment currently are (a) the higher replacement frequency of xylem in comparison to current filtration technologies could deter sustained usage, (b) heterogeneity in xylem structure within and across gymnosperm species could create variability in filter performance, and (c) a possibility that degradation of filters under certain conditions could cause their rejection performance to deteriorate and prevent them from getting blocked posing a health risk to users (timescales for degradation would depend on the feed water quality, type of wood used for manufacturing the filter, and local environmental factors and could be of the order of 1–2 weeks 65 , 66 or less since bacteria are directly seeded on pit membranes during filtration). Mitigation of these risks will require: (a) pilot studies of filter performance under different operational conditions (and measures such as increasing filter thickness or providing guidance as to when xylem filters are suitable for use if filter degradation is found to be an issue), (b) standardization of sources of wood and development of methods to test and assure quality control 67 , (c) safety assessment of xylem filters for human use (e.g., assessing the filtrate for presence of organic compounds leached from xylem and evaluating their safety for human consumption, noting that cellulose, hemicellulose, lignin, and pectin occur naturally in many foods, and that plant material contributes to organic content in drinking water), (d) designing filter holders for maximal ease of replacement along with ready local availability of filters, and (e) behavior change interventions to facilitate sustained usage (see Supplementary Note 9 and 11 for more information on these topics). To facilitate uptake and further advances in xylem filter technology, we have not patented this technology and have created design guide for fabricating xylem filters (Supplementary Note 11 ) and compendium enlisting the geographic availability, structural characteristics, and degradation properties of different gymnosperms (Supplementary Data 1 ). It is important to use non-toxic sapwood for making xylem filters (see Supplementary Note 11 for toxicity-related information).
Xylem filters present a HWT solution with unique characteristics (see Table 2 for comparison with other low-cost water treatment technologies). The availability of wood is not a bottleneck for scaling and large-scale dissemination—the sapwood used for making these filters is a low-grade by-product of the timber industry; we estimate that ~0.01% of the timber used in softwood global trade could be used to make a billion filters annually 68 . The compactness, lightweight, and long shelf life of xylem filters could enable easy shipping to remote locations and bulk storage for prolonged use. Due to the worldwide availability of gymnosperms and simplicity of the filter fabrication process, xylem filters offer potential for local manufacture of a wide variety of water treatment and other filtration products, ranging from compact filter pouches for use in emergencies to household filtration devices (Fig. 7b , see Supplementary Note 12 for further details on HWT for emergency use). This opens up the possibility for involvement of micro-entrepreneurs at a global scale 69 , implementation of innovative business models, and engagement of local communities in filter distribution 70 , 71 and manufacture to raise awareness about importance of safe drinking water and encourage adoption (see Supplementary Note 13 for further details on potential avenues for engaging local communities and micro-enterprises). In addition, the characterization, modeling, and engineering of xylem filters is not limited to household water filters and has the potential to be applied to different filtration needs, such as for disaster and emergency use (Fig. 7b ), microfiltration for assessment of water quality, and as an alternative to synthetic microfiltration membranes for some applications.
Cutting of xylem filters from branches
Straight sections of branches of appropriate diameters were excised from trees (Eastern white pine, Eastern white cedar, ginkgo or chir pine) using a hand pruner and immediately immersed in water to prevent drying (in winter, foliate branches were excised and stored indoors at room temperature for 2–3 days with one end immersed in water to restore xylem activity and improve permeance). Branches without leaves or sections near branch attachments were avoided due to their low hydraulic conductivity 74 , 75 . The natural direction of water flow in the branch was marked with a sharpie and the same flow direction was maintained in experiments. The branches were subsequently cut into smaller sections with the desired filter thickness using a band saw. The outer bark and the cambium were peeled off by hand to obtain a fresh xylem filter. The filters were stored in deionized (DI) water for up to 8 h before experiments or subsequent processing steps.
Water flow rate and volumetric capacity measurements
Xylem filters were mounted in PVC (polyvinyl chloride) tubes with 0.375-, 1.5-, and 1.875-inch inner diameters (McMaster-Carr, part numbers 5233K63, 5233K77, and 53945K22) for 1-, 4-, and 5-cm-diameter filters, respectively. The tubing was connected to a nitrogen tank and regulator set at 10 psi (69 kPa) pressure, or to a water-filled tank providing a 1-m head (specified in figure captions). For nitrogen gas-driven measurement of permeance of ~1-cm-diameter filters, the tube was filled with 5 mL of DI water and the time required for the filter to process the water was used to obtain the flow rate. In gravity-driven experiments to measure permeance and capacity of 4–5-cm-diameter filters, the volume of fluid processed by the filters was monitored with time by collecting it in a cylinder with 20 mL graduations to obtain flow rates. For experiments with DI water containing Ca 2+ and K + ions, calcium chloride dehydrate (ACS reagent grade, CAS number 10035-04-8, Sigma-Aldrich) and potassium chloride (ACS reagent grade, CAS number 7447-40-71, Sigma-Aldrich) were dissolved in DI water at the desired concentrations. For experiments involving intermittent filter operation, filters made from ginkgo (4-cm diameter, 0.375-inch thickness) were operated for a duration of 7 days while processing 5 L of DI water per day. The filters were mounted in the device (shown in Supplementary Fig. 5a ) and operated under a gravitational pressure head of 1 m with 5 L of DI water added into the device every day. The filters stopped producing water after the 5 L water was filtered, till 5 L of water was again added on the following day. The time required to filter the 5 L of water was measured and used to calculate the average permeance to filter 5 L of water on a given day. Permeance was calculated by normalizing the flow rate by the filter cross-section area and the driving pressure. The permeate processed by the filter per unit area was calculated by normalizing the volume of the fluid processed by the filter by the filter cross-section area.
SEM imaging
Xylem structure was visualized using scanning electron microscopy (SEM, Zeiss Merlin High Resolution). Samples were prepared by cutting thin slices of dried filters in the direction of the flow (longitudinally) using a razor blade. No coating was applied on the samples. The slices were imaged under an extra high tension (EHT) voltage of 0.7–1.2 kV and a probe current of 75–90 pA. The depth-of-resolution mode was used for acquiring images. The images in Fig. 1c were obtained from ethanol-dried xylem filters made from ginkgo (1-cm diameter and 0.25-inch thickness) after filtration of ~5 mL DI water spiked with 500-nm carboxylate-modified latex microspheres with yellow-green fluorescence (Thermo Fischer Scientific) at a concentration of 10 9 particles/mL.
Rejection of fluorescent microspheres
Carboxylate-modified latex microspheres with yellow-green fluorescence (Thermo Fischer Scientific) with diameters of 100 nm, 500 nm, and 1 μm were used. The microspheres were sonicated for 1 min using a VWR Ultrasonic Mixer at room temperature (25 °C), suspended in DI water at 10 6 mL −1 concentration, and vortexed for 1–2 min to obtain a homogenous feed solution. The feed solution was filtered through xylem filters mounted in PVC tubing at 10 psi pressure as described in Methods sub-section “Water flow rate and volumetric capacity measurements”. The filtrate was collected in clean glass vials; a feed volume 3–4× of the filter volume was used.
10 µL of the feed or filtrate solutions were introduced into a hemacytometer (Neubauer-modified C-Chip, InCyto) and imaged with a Nikon TE2000U epifluorescence microscope using a ×20 objective for the 1-μm microspheres or ×40 objective for the 500- and 100-nm microspheres. Due to the limited depth of focus of the ×40 objective, images were acquired (by Andor iXon camera) with the focus at the mid-plane of the C-chip, and the particle count in this plane was used to estimate the concentration of the 100-nm microspheres. Particle counts over three draws of the feed/filtrate solution were averaged to calculate rejection as follows, where c feed and c filtrate are microsphere counts in the feed and filtrate solutions, respectively.
Rejection tests with the xylem filter device were conducted by mounting the xylem filter in holder (Fig. 6b ), connecting the holder to PVC tubing, filling the tubing and the holder with a fluospheres solution (concentration of ~10 6 mL −1 ) of volume 3–4× that of the filter and pressurizing the fluid through the holder at 10 psi gas pressure. The filtrate was collected in a glass beaker. The particle count in the feed and the filtrate were evaluated as described in Methods sub-section “Water flow rate and volumetric capacity measurements”.
As the mechanism of bacterial removal in xylem filters is physical sieving 7 , we expected the contaminant size to be the key determinant of the filtration characteristics and used 1-μm latex microspheres as surrogates for E. coli . Latex microspheres have been used as proxies to study the transport of E. coli in porous media due to the similarity in size and zeta potential 76 , 77 .
Treatment of xylem filters
For hot-water treatment, filters were soaked in covered glass containers filled with deionized water maintained at 60 °C using a temperature-controlled water bath for 1 h (tap water and a simple stove were used for filters fabricated in India). A wood to water volume ratio of 1:20 was used. Subsequently, filters were immersed in room-temperature DI water 4–5 min before subsequent processing. 200-proof ethanol (CAS number 64-17-5, Koptec) or isopropyl alcohol (ACS reagent grade, CAS number 67-63-0, Macron Chemicals) were used for alcohol treatment; ethanol with 99.9% purity (CAS number 64-17-5, Merck) was used for field studies in India. Ethanol–water mixtures of different concentrations were prepared by adding DI water to 200-proof ethanol (CAS number 64-17-5, Koptec). Xylem filters (freshly cut or hot-water soaked) of 1-cm and 4–5-cm diameter were treated by flushing and soaking, respectively (filter to ethanol/isopropyl alcohol volume ratio of 1:6–8). For flushing, 10 psi gas pressure was used to drive the desired volume of ethanol/isopropyl alcohol loaded in plastic tubing in which xylem filters were mounted, similar to permeance measurements described in Methods sub-section “Water flow rate and volumetric capacity measurements”. For soaking, filters were placed edge-on in appropriately sized glass vessels and completely immersed in ethanol for 24–48 h. Most of the filters (~90%) were soaked for more than 24 h, in which case the ethanol in the vessel was typically replaced with fresh ethanol after 24 h. Filters manufactured in the field were treated with ethanol only by soaking.
Drying of xylem filters
In laboratory experiments, all filters (soaked in water or in ethanol/isopropyl alcohol) were dried by placing edge-on on aluminum foil in an oven (VWR 1410 vacuum oven) at atmospheric pressure and 45 °C. To ensure complete drying, the filter weights were monitored (using Mettler Toledo AL104 scale) till they stabilized. Water-dried filters with 1-cm diameter and 0.25-, 0.5-, and 1.5-inch thickness required around 10, 25, and 50 h for complete drying. Ethanol treatment resulted in faster drying (~6–8 h for 1-cm diameter, 0.25-inch-thick filters). In field studies, filters were dried at 45 °C and atmospheric pressure for equivalent durations (at least 48 h) using an oven (Bajaj Majesty 1603 TSS Oven Toaster Grill) but weights were not monitored due to lack of access to appropriate instruments.
Air flow measurements
Air flow rates were measured using the setup illustrated in Supplementary Fig. 1d (inset). Xylem filters with 1-cm diameter were mounted in a PVC tube (0.375-inch inner diameter, McMaster-Carr part number 5233K63) secured with a hose clamp and supplied with nitrogen at 1 psi using a nitrogen cylinder and pressure regulator. The gas flowing through the filter was collected in an inverted 250-mL beaker fully immersed in water, and the time required for the gas to displace water from the inverted beaker was measured to obtain the flow rate (and permeance).
Rewetting of water-dried filters with ethanol
To obtain data shown in Supplementary Fig. 1e , freshly cut xylem filters with 1-cm diameter, 0.25-inch thickness were mounted in a PVC tube (0.375-inch inner diameter, part number 5233K63, McMaster-Carr), secured using a hose clamp and flushed with 5 mL of DI water at 10 psi nitrogen gas pressure. Flow rates were recorded and filters were dried in an oven at 45 °C and atmospheric pressure. Dried filters were flushed with 5 mL of 200-proof ethanol (CAS number 64-17-5, Koptec) and re-dried in the oven at 45 °C and atmospheric pressure. The flow rates of the dried filters were measured with DI water as described in Methods sub-section “Water flow rate and volumetric capacity measurements”. The ratio of flow rate after drying to that before was used to calculate permeance recovery.
Atomic force microscopy (AFM)
DI water was filtered through Eastern white pine filter (1-cm diameter, 0.375-inch thickness) at a gas pressure of 10 psi using the method described in Methods sub-section “Water flow rate and volumetric capacity measurements”. The filtrate was collected in a clean glass vial, dispersed and dried on a substrate, and analyzed under an Asylum-2 MFP-3D Coax AFM.
Eastern white pine xylem filter (1-cm diameter, 0.375-inch thickness) was placed in a glass vial filled with 20 mL deionized water maintained at 60 °C for 1 h. The filter was removed and the residual water was evaporated until the volume reduced to 5 mL to concentrate any extracts from the filter. The concentrate was analyzed using a Perkin Elmer FTIR spectrometer.
Preparation of synthetic test waters
GTW and CTW were prepared by adding the WHO-prescribed dosage of sea salts (Sigma-Aldrich, product number S9883), sodium bicarbonate (BioXtra, 99.5–100.5%, CAS number 144-55-8 procured from Sigma-Aldrich), and tannic acid (ACS reagent grade, CAS number 1401-55-4 obtained from Sigma-Aldrich) or humic acid (50–60%, CAS number 68131-04-4 procured from Alfa Aesar) in DI water (see Supplementary Fig. 3a for dosages) 37 . To achieve turbidity of 40 NTU in CTW, Arizona Test Dust (ISO 12103-1, A2 fine test dust obtained from Powder Technologies Inc.) was added to DI water at a concentration of 70 mg/L based on calibration reported in literature 78 .
Susceptibility of xylem filter to different contaminants
To determine the susceptibility of volumetric capacity to different organics, DI water having same alkalinity, salinity, and turbidity as CTW (1.5 g/L sea salts, 100–120 mg/L sodium bicarbonate, 70 mg/L Arizona test dust) and varying dosages (0, 1.5, 15, or 150 mg/L) of tannic or humic acid was used. To evaluate the effect of dust on capacity, DI water with the same alkalinity and salinity as CTW (1.5 g/L sea salts and 100–120 mg/L sodium bicarbonate), 15 mg/L of humic or tannic acid, and varying dosages of dust were used (0 and 70 mg/L). All measurements were performed with Eastern white pine filters (1-cm diameter and 0.375-inch thickness) operated under a 1-m gravity head. Volumetric capacity was measured as per the protocol specified in Methods sub-section “Water flow rate and volumetric capacity measurements”.
Cloth pre-filtration
A sediment pre-filter manufactured by Hindustan Unilever Limited (PureIt microfiber mesh) was used for removing visible dust particles. Feed water was first passed through the pre-filter, collected in a separated beaker, and subsequently poured into the container connected to the xylem filter for further filtration.
Activated carbon pre-filtration
Granular Activated Carbon (GAC, Activated Carbon Corporation) of 12 × 40 grain was sieved in a fume hood to obtain 30 × 40 grain size. Prior to usage, the GAC was soaked in tap water overnight and rinsed thoroughly under running tap water to remove dust that otherwise clogged the filters. The GAC column was fabricated by assembling off-the shelf components. The GAC was packed in a 5-cm diameter, 15-cm-long pipe threaded at both ends (MPT (male pipe thread) × MPT, product number 4677T45, procured from McMaster-Carr). A circular mesh with 5-cm diameter was cut from the PureIt microfiber mesh manufactured by Hindustan Unilever Limited and fixed to the lower end of the pipe using epoxy (3 M Scotch-Weld Epoxy Adhesive DP100 Plus) to keep the GAC granules in place. The top end of the pipe was attached to 0.375-inch diameter PVC tubing (McMaster-Carr, part numbers 5233K63) that was connected to the feed water container through a set of connectors, which consisted of a 2-inch coupling (FPT (female pipe thread) × FPT, part number 9499, Metropolitan Pipe and Supply), a 2-inch × 0.75-inch reducer (MPT × FPT, part number 9530, Metropolitan Pipe and Supply), and a 0.75-inch × 0.375-inch reducer (McMaster-Carr, product number 5372K154). The lower end of the pipe was threaded onto a cap (McMaster-Carr, part number 4880K807) and connected to a standard port valve (McMaster-Carr, part number 45975K32) to regulate the flow rate (and thus the GAC contact time). The valve was connected to the xylem filter. During experiments, care was taken to avoid formation of air bubbles. If formed, air bubbles were removed by squeezing the tubing. The humic acid concentration in the feed and the filtrate was measured by UV–Vis spectrometry (Agilent Cary 60). The absorbance was averaged over 300–500 nm wavelengths and absorbance at 700 nm was subtracted. Calibration curves were generated using different concentrations of humic acid in DI water (curves were linear). Dust was not used in experiments involving measurement of humic acid concentration due to interference with the UV–Vis measurement. DI water with 1.5 g/L of sea salts, 120 mg/L of sodium bicarbonate, and ~10 mg/L of humic acid was used as the test solution for comparing the performance of different GACs (Supplementary Fig. 3d ).
Testing xylem filters in field studies
Experiments in India were conducted with locally fabricated filters made from chir pine ( Pinus roxburghii ), as well as ginkgo ( Ginkgo biloba ) and Eastern white pine ( Pinus strobus ) filters fabricated in Massachusetts. Filters made in Massachusetts were transported to India by air in zip-locked pouches containing desiccant (Silica gel manufactured by Dry Packs, serial number 1203-61) to prevent condensation of moisture and blocking. Water was collected from different water sources in clean 20 L plastic cans. Before starting the experiment, the tube, holder, and device container surfaces were wiped with cotton soaked in isopropyl alcohol to prevent contamination of the feed water. The feed was introduced after 5–10 min to allow sufficient time for isopropyl alcohol evaporation. For microbiological testing, the feed and filtrate were collected in glass or plastic bottles that were previously disinfected in boiling hot water for 15–20 min. The filtrate for microbiological testing was collected during the filtration process as follows. Prior to collecting the filtrate for microbiological analysis, the bottom surface of the xylem filter, the hose clamp, and lower end of the tubing were wiped with cotton soaked in isopropyl alcohol (while filtration continued). Collection of the filtered sample was started after 10 min to allow sufficient time for any residual isopropyl alcohol to be flushed or evaporated. The microbiological tests were conducted by certified third-party labs (Uttarakhand Jal Sansthan for all the tests conducted in Uttarakhand (Supplementary Fig. 4e-g ) and Delhi Analytical Research Laboratory for tests conducted in Delhi (Fig. 5h )). The methods used for sampling and microbiological examination conformed to Indian Standards, IS:1622 (1981) for data in Fig. 5h and American Public Health Association (APHA) 22nd edition for data in Supplementary Fig. 4e–g .
Construction of xylem filters from trunks
Eastern white pine ( Pinus strobus ) trunks for constructing xylem filters were procured from a local sawmill in Essex, MA. Due to logistical issues, logs were used ~2 weeks after the tree was felled (partial drying of the wood within this duration could have compromised rejection). The bark was removed using a band saw. 10 × 4-cm 2 sections were cut from the sapwood (lighter in color than the heartwood) to obtain xylem filters. Hot-water soaking and ethanol treatment were performed as described in Methods sub-section “Treatment of xylem filters”. Rejection performance of these filters was tested as described in Methods sub-section “Rejection of fluorescent microspheres” on cylindrical 1-cm diameter filters cored out from the dried filters using a hammer-driven small hole punch with 0.375-inch hole diameter (McMaster-Carr, part number 3424A25). To avoid leaks, the xylem filter was sealed in the tubing using epoxy.
Construction of Fig. 1d
The number of diarrheal deaths for the year 2016 was taken as is from the Global Health Observatory data repository managed by the World Health Organization (WHO) 79 . The total deaths corresponding to each of the six WHO world regions (African region, region of the Americas, South-East Asia region, European region, Eastern Mediterranean region, and Western Pacific region) 80 were calculated by adding the deaths associated with the countries in that region. The number of diarrheal deaths was rounded to the nearest hundreds for ease of reading. The numbers were color-coded on the world map depicting the WHO world regions using Adobe Photoshop (the world map was obtained from an open access website, ‘Our World in Data’ (URL: https://ourworldindata.org/world-region-map-definitions , Product name: ‘World Map Region Definition’ > ‘World Health Organization’, Author: Diana Beltekien) and re-colored using Adobe Photoshop). The global distribution of wild conifers reported in literature 17 was re-sketched over this map in Microsoft Power Point (Version 16.37).
Statistical information and reproducibility
All graphs were plotted using GraphPad Prism (Version 8.4.3). The error bars in all figures represent positive and negative standard deviations from the mean and calculated using in-built functions in GraphPad Prism v8. The n values for the data are included in the figure captions. The p -values for Fig. 2f and 2h were determined by performing two-sample, two-tailed t -tests on the data using Microsoft Excel (Version 16.37). The sample sets were considered unpaired with equal variance. Homogeneity in variance was determined using Levene’s test. For micrographs shown in Figs. 1 b, 2 g, 3b, and 4e , experiments were repeated independently 3, 5, 3, and 3 times, respectively, with similar results.
Field studies
Information on user needs and preferences for water filtration devices and feedback on xylem filtration device prototypes was gathered through 600 individual semi-structured interviews, 53 focus group discussions, and 2 hands-on co-design workshops with over 1000 potential users. The research sample for the field studies included low-income rural households in the mountainous state of Uttarakhand, India, and the urban slum households from Bengaluru and Delhi and other stakeholders in the water filter supply chain, including, filter vendors, manufacturers, NGO staff, and local health officials. The ‘National Ethical Guidelines for Biomedical and Health Research Involving Human Participants’ guidelines published by the Indian Council of Medical Research (ICMR) were followed while conducting user research in India. These guidelines suggest that social and behavioral research for health applications should be approved by the ethics committee for the researchers’ institution, which in our case would be the Massachusetts Institute of Technology Committee on the Use of Human Subjects (MIT COUHES). All human-subjects research procedures were approved by MIT COUHES under protocol 1612798762. Informed consent of the participants was obtained prior to data collection.
Different sampling strategies were followed for different data collection methods. Potential segments with potentially different needs were stratified based on a variety of factors including the geography, types of water sources, proximity to town, and heterogeneity of the population in terms of water-related practices. Villages were selected based on these criteria with the assistance of local partner NGOs. For semi-structured interviews, simple random sampling was used within the selected villages. In general, 20–30 subjects were interviewed for each segment 81 . For focus group discussions and design workshops, a combination of non-probability sampling techniques such as convenience sampling and snowball sampling techniques were used. For key informant interviews, a purposeful sampling strategy was used to select participants. Data saturation was also considered in the studies. The homogeneity of the population with regards to water usage practices was assessed with the help of local experts and data was considered saturated when no new information or themes were observed. Pen and paper were used to record the data. Photos were taken and voice recorders were also used with participant consent. A translator was present during data collection. Researchers were not blind to experimental condition and study hypothesis. No data points were omitted while performing data analysis.
Microbiological performance as per WHO protocol
The microbiological performance of xylem filters was tested by a third-party laboratory (Quality Filter Testing (QFT) Laboratory LLC, 1041 Glassboro Road Suite E-4, Williamstown, New Jersey 08094, USA; ISO 17025 laboratory certified by the International Association of Plumbing and Mechanical Officials (IAPMO) to test water filters as per National Sanitation Foundation (NSF)/American National Standards Institute (ANSI) standards). Xylem filters (4-cm diameter, 0.375-inch thickness) made from ginkgo ( Ginkgo biloba ) wood using hot water treatment and ethanol-based dry preservation at Massachusetts Institute of Technology (MIT), USA, and stored for 2 years were mounted in 1.5-inch diameter PVC (polyvinyl chloride) tubes (McMaster-Carr, part number 5233K77) and secured in place using hose clamps, and were shipped to the third-party laboratory. In the third-party laboratory, the tubing was connected to a water-filled tank providing a 1.2-m head. E. coli (ATCC 11229) and MS-2 phage (ATCC-15597-B1, with host organism E. coli ATCC-15597) were dosed in GTW at concentrations ≥10 6 CFU/mL and ≥10 5 PFU/mL, respectively, as per the ‘WHO International Scheme to Evaluate Household Water Treatment Technologies’ 48 . For rotavirus removal, rotavirus strain SA-11 (ATCC-VR-899) was spiked in GTW at concentration ≥10 4 PFU/mL (as specified by NSF Protocol P231 for Microbiological Water Purifiers) 46 . The removal of bacteria and virus was tested at the start of filter operation and when permeance declined to 75, 50, and 25% of the initial value. Flow rates were measured by monitoring the volume of fluid processed by the filters over time. After the first sample at the start of filter operation, dust was added to achieve accelerated clogging of the filter (dust concentration raised turbidity to 120 NTU and 130 NTU for E. coli /MS-2 phage and rotavirus, respectively, which is above what is required for CTW). Prior to collecting the filtrate for microbiological analysis, the bottom surface of the xylem filter, the hose clamp, and lower end of the tubing were wiped with cotton soaked in ethanol (while filtration continued). Collection of the filtered sample was started after 10 min to allow sufficient time for any residual ethanol to be flushed or evaporated. E. coli and MS-2 phage were assayed using Standard Method 9222 and 9224 published by American Public Health Association (APHA) for the Examination of Water and Wastewater. The log-reduction values were determined by measuring the bacteria/virus count in the feed solution ( C feed ), and filtrate ( C filtrate ) using the following equation:
This method was used for experiments with results shown in Fig. 5a .
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data that support the findings of this study are available in the main text, figures, and Supplementary information. Field study results are presented in aggregate to protect privacy of survey respondents. Data for number of diarrheal deaths shown in Fig. 1d was obtained from the Global Health Observatory data repository managed by the World Health Organization (URL (accessed on 14 June 2019): https://apps.who.int/gho/data/view.main.INADEQUATEWATERv?lang=en , Dataset title: ‘Number of diarrhea deaths from inadequate water (2016)’ within the section ‘Burden of disease from inadequate water in low- and middle-income countries’.
Code availability
Codes used in the percolation model in this study are available from the corresponding author on reasonable request.
World Health Organization (WHO). The top 10 causes of death. http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
Troeger, C. et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18 , 1211–1228 (2018).
Article Google Scholar
World Health Organization (WHO). Scaling Up Household Water Treatment Among Low-Income Populations . WHO/HSE/WSH/09.02 (2009).
Sobsey, M. D., Stauber, C. E., Casanova, L. M., Brown, J. M. & Elliott, M. A. Point-of-use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 42 , 4261–4267 (2008).
Article ADS CAS PubMed Google Scholar
Clasen, T., Schmidt, W.-P., Rabie, T., Roberts, I. & Cairncross, S. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ https://doi.org/10.1136/bmj.39118.489931.BE (2007).
Devadoss, S. & Aguiar, A. H. Effects of global trade liberalization on softwood lumber markets. Appl. Econ. 38 , 2351–2360 (2006).
Boutilier, M. S. H., Lee, J., Chambers, V., Venkatesh, V. & Karnik, R. Water filtration using plant xylem. PLoS ONE 9 , e89934 (2014).
Article ADS PubMed PubMed Central CAS Google Scholar
Vitas, S. et al. Rejection of micron-sized particles using beech wood xylem. Environ. Sci. Water Res. Technol. 5 , 944–955 (2019).
Article CAS Google Scholar
Vitas, S., Keplinger, T., Reichholf, N., Figi, R. & Cabane, E. Functional lignocellulosic material for the remediation of copper(II) ions from water: Towards the design of a wood filter. J. Hazard. Mater. 355 , 119–127 (2018).
Article CAS PubMed Google Scholar
Che, W. et al. Wood-based mesoporous filter decorated with silver nanoparticles for water purification. ACS sustain. Chem. Eng. 7 , 5134–5141 (2019).
CAS Google Scholar
Potash, B. R. Characterization and preservation techniques of plant xylem as low cost membrane filtration devices. 235–260, https://doi.org/10.5334/pb-47-4-235 (2014).
Sperry, J. S. Evolution of water transport and xylem structure. Int. J. Plant Sci. 164 , S115–S127 (2003).
Pittermann, J., Sperry, J. S., Hacke, U. G., Wheeler, J. K. & Sikkema, E. H. Inter-tracheid pitting and the hydraulic efficiency of conifer wood: the role of tracheid allometry and cavitation protection. Am. J. Bot. 93 , 1265–1273 (2006).
Article PubMed Google Scholar
Hacke, U. G., Sperry, J. S. & Pittermann, J. Analysis of circular bordered pit function II. Gymnosperm tracheids with torus-margo pit membranes. Am. J. Bot. 91 , 386–400 (2004).
Sperry, J. S., Hacke, U. G. & Pittermann, J. Size and function in conifer tracheids and angiosperm vessels. Am. J. Bot. 93 , 1490–1500 (2006).
Peter-Varbanets, M., Zurbrügg, C., Swartz, C. & Pronk, W. Decentralized systems for potable water and the potential of membrane technology. Water Res. 43 , 245–265 (2009).
Farjon, A. A Handbook of the World’s Conifers Vol. I (Brill Academic Publishers, 2010).
Liese, W. & Bauch, J. On the closure of bordered pits in conifers. Wood Sci. Technol. 1 , 1–13 (1967).
Comstock, G. L. & Côté, W. a. Factors affecting permeability and pit aspiration in coniferous sapwood. Wood Sci. Technol. 2 , 279–291 (1968).
Sperry, J. S., Donnelly, J. R. & Tyree, M. T. A method for measuring hydraulic conductivity and embolism in xylem. Plant. Cell Environ. 11 , 35–40 (1988).
Tyree, M. T. A dynamic model for water flow in a single tree: evidence that models must account for hydraulic architecture. Tree Physiol. 4 , 195–217 (1988).
Loepfe, L., Martinez-Vilalta, J., Piñol, J. & Mencuccini, M. The relevance of xylem network structure for plant hydraulic efficiency and safety. J. Theor. Biol. 247 , 788–803 (2007).
Article PubMed MATH Google Scholar
Sahimi, M. & Sahini, M. Applications of Percolation Theory (CRC Press, 2014).
Mrad, A., Domec, J. C., Huang, C. W., Lens, F. & Katul, G. A network model links wood anatomy to xylem tissue hydraulic behaviour and vulnerability to cavitation. Plant Cell Environ. 41 , 2718–2730 (2018).
Ramchander, K. Development of Xylem-based Water Filters (Massachusetts Institute of Technology, 2016).
Sterlitech. PTFE membrane filters, laminated, 0.45 micron, 300 × 300 mm, 5/pk. https://www.sterlitech.com/ptfe-laminated-membrane-filter-ptfe0453005.html . Accessed on 24th July 2019.
Sterlitech. Cellulose acetate membrane filters, 0.45 mciron, 25 mm, 100/pk. https://www.sterlitech.com/cellulose-acetate-membrane-filter-ca04525100.html . Accessed on 24th July 2019.
Sterlitech. Nylon membrane filters, 0.45 micron, 90 mm, 25/pk. https://www.sterlitech.com/nylon-membrane-filter-ny459025.html . Accessed on 24th July 2019.
Sterlitech. Nylon membrane filters, 0.1 mciron, 90 mm, 25/pk. https://www.sterlitech.com/nylon-membrane-filter-ny019025.html . Accessed on 24th July 2019.
Sterlitech. Polycarbonate (PCTE) membrane filters, gray, 0.1 mciron, 47 mm, 100/pk. https://www.sterlitech.com/hydrophilic-polycarbonate-membrane-filter-pct0147100.html . Accessed on 24th July 2019.
International Council for Harmonisation. Guidance for Industry Q3C. U.S. Dep. Heal. Hum. Serv. Food Drug Adm. 9765 , 1–8 (2017).
Gao, W. et al. Membrane fouling control in ultrafiltration technology for drinking water production: a review. Desalination 272 , 1–8 (2011).
Chen, H. & Chen, H. in Biotechnology of Lignocellulose 25–71 (Springer, 2014).
Yang, H., Yan, R., Chen, H., Lee, D. H. & Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86 , 1781–1788 (2007).
Yu, Y., Lou, X. & Wu, H. Some recent advances in hydrolysis of biomass in hot-compressed water and its comparisons with other hydrolysis methods. Energy Fuels 22 , 46–60 (2008).
Bannan, M. W. Length tangential diameter and length/ width ratio of conifer tracheids. Can. J. Bot. 43 , 967–984 (1965).
World Health Organization (WHO). WHO Intetnational Scheme to Evaluate Household Water Treatment Technologies Harmonized Testing Protocol: Technology Non-specific (2014).
Iritani, E. A review on modeling of pore-blocking behaviors of membranes during pressurized membrane filtration. Dry. Technol. 31 , 146–162 (2013).
Hlavacek, M. & Bouchet, F. Constant flowrate blocking laws and an example of their application to dead-end microfiltration of protein solutions. J. Memb. Sci. 82 , 285–295 (1993).
Mondal, S. & De, S. A fouling model for steady state crossflow membrane filtration considering sequential intermediate pore blocking and cake formation. Sep. Purif. Technol. 75 , 222–228 (2010).
Yuan, W., Kocic, A. & Zydney, A. L. Analysis of humic acid fouling during microfiltration using a pore blockage-cake filtration model. J. Memb. Sci. 198 , 51–62 (2002).
Ho, C. C. & Zydney, A. L. A combined pore blockage and cake filtration model for protein fouling during microfiltration. J. Colloid Interface Sci. 232 , 389–399 (2000).
Çeçen, F. & Aktaş, Ö. Activated Carbon for Water and Wastewater Treatment . https://doi.org/10.1002/9783527639441 (2011).
Hijnen, W. A. M., Suylen, G. M. H., Bahlman, J. A., Brouwer-Hanzens, A. & Medema, G. J. GAC adsorption filters as barriers for viruses, bacteria and protozoan (oo)cysts in water treatment. Water Res. 44 , 1224–1234 (2010).
Comprehensive Initiative on Technology Evaluation at MIT. Household Water Filter Evaluation Sustainability Report 1–24 (2015).
NSF International. NSF Protocol P231 - Microbiological Water Purifiers (2014).
NSF International. NSF/ANSI 53: Drinking Water Treatment Units - Health Effects Vol. 29, 11–12 (2019).
World Health Organization (WHO). WHO International Scheme to Evaluate Household Water Treatment Technologies Harmonized Testing Protocol: Technology Non-Specific . 1–5 (2014).
Kapikian, A. Z. & Shope, R. E. Medical Microbiology (University of Texas Medical Branch at Galveston, 1996).
Junter, G. A. & Lebrun, L. Cellulose-based virus-retentive filters: a review. Rev. Environ. Sci. Biotechnol. 16 , 455–489 (2017).
Article CAS PubMed PubMed Central Google Scholar
Cliver, D. O. Virus interactions with membrane filters. Biotechnol. Bioeng . 10 , 877–889 (1968).
WaterAid. The water gap. 1–24, https://doi.org/10.1029/2010GL044571 (2018).
Banerjee, A. V. & Duflo, E. The economic lives of the poor. J. Econ. Perspect. https://doi.org/10.1257/jep.21.1.141 (2007).
Daniel, D., Marks, S. J., Pande, S. & Rietveld, L. Socio-environmental drivers of sustainable adoption of household water treatment in developing countries. npj Clean Water 1 , 1–6 (2018).
Hunter, P. R. Household water treatment in developing countries: comparing different intervention types using meta-regression. Environ. Sci. Technol. 43 , 8991–8997 (2009).
Inauen, J., Hossain, M. M., Johnston, R. B. & Mosler, H. J. Acceptance and use of eight arsenic-safe drinking water options in Bangladesh. PLoS ONE 8 , e53640 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Lantagne, D. S., Quick, R. & Mintz, E. D. Household water treatment and safe storage options in developing countries: a review of current implementation practices. Navig 99 , 17–38 (2006).
Google Scholar
Loharikar, A. et al. Long-term impact of integration of household water treatment and hygiene promotion with antenatal services on maternal water treatment and hygiene practices in Malawi. Am. J. Trop. Med. Hyg. 88 , 267–274 (2013).
Article PubMed PubMed Central Google Scholar
Ram, P. K. et al. Bringing safe water to remote populations: An evaluation of a portable point-of-use intervention in rural Madagascar. Am. J. Public Health 97 , 398–400 (2007).
World Health Organization (WHO). Scaling Up Household Water Treatment Among Low-Income Populations . http://www.who.int/household_water/research/household_water_treatment/en/index.html (2009).
Ryan, J. N. et al. Field and laboratory investigations of inactivation of viruses (PRD1 and MS2) attached to iron oxide-coated quartz sand. Environ. Sci. Technol. 36 , 2403–2413 (2002).
Xu, Y. H., Nakajima, T. & Ohki, A. Adsorption and removal of arsenic(V) from drinking water by aluminum-loaded Shirasu-zeolite. J. Hazard. Mater. 92 , 275–287 (2002).
Barker, D. Flowering Plant Origin, Evolution, & Phylogeny (JSTOR, 1996).
André, J. P. A study of the vascular organization of bamboos (Poaceae-Bambuseae) using a microcasting method. IAWA J. 19 , 265–278 (1998).
Eriksson, K.-E. L., Blanchette, R. A. & Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components . https://doi.org/10.1016/0300-9629(91)90156-7 (Springer Science & Business Media, 2012).
Burnes, T. A., Blanchette, R. A. & Farrell, R. L. Bacterial biodegradation of extractives and patterns of bordered pit membrane attack in pine wood. Appl. Environ. Microbiol. 66 , 5201–5205 (2000).
Arkhurst, B. Identification and Evaluation of Techniques for Quality Control of Low-Cost Xylem Filters (Massachusetts Institute of Technology, 2018).
AJOT Maritime News. Global trade of softwood lumber has gone up 66 percent in seven years. https://www.ajot.com/news/global-trade-of-softwood-lumber-has-gone-up-66-percent-in-seven-years (2017).
Potters for Peace. Ceramic Water Filter Project . https://www.pottersforpeace.org/ceramic-water-filter-project (2019).
Dolan, C., Johnstone-Louis, M. & Scott, L. Shampoo, saris and SIM cards: seeking entrepreneurial futures at the bottom of the pyramid. Gend. Dev. 20 , 33–47 (2012).
Neuwirth, B. Marketing Channel Strategies in Rural Emerging Markets: Unlocking Business Potential (2012).
World Health Organization (WHO). Results of Round II of the WHO International Scheme to Evaluate Household Water Treatment Technologies . http://www.who.int/household_water/scheme/household-water-treatment-report-round-1/en/#.VtmmIDJjI5c.mendeley (2019).
World Health Organization (WHO). Results of Round I of the WHO International Scheme to Evaluate Household Water Treatment Technologies . http://www.who.int/household_water/scheme/household-water-treatment-report-round-1/en/# (2016).
Rioux, D. in Histology, Ultrastructure and Molecular Cytology of Plant-microorganism Interactions 211–225. https://doi.org/10.1007/978-94-009-0189-6_12 (1996).
Schulte, P. J. Branch junctions and the flow of water through xylem in Douglas-fir and ponderosa pine stems. J. Exp. Bot. 54 , 1597–1605 (2003).
Passmore, J. M., Rudolph, D. L., Mesquita, M. M. F., Cey, E. E. & Emelko, M. B. The utility of microspheres as surrogates for the transport of E. coli RS2g in partially saturated agricultural soil. Water Res. 44 , 1235–1245 (2010).
Radu, A. I., van Steen, M. S. H., Vrouwenvelder, J. S., van Loosdrecht, M. C. M. & Picioreanu, C. Spacer geometry and particle deposition in spiral wound membrane feed channels. Water Res. 64 , 160–176 (2014).
Comprehensive Initiative on Technology Evaluation (Massachusetts Institute of Technology). Household Water Filter Evaluation, Suitability Report - Field Research in Ahmedabad, India. (2015).
World Health Organization (WHO). Burden of Disease from Inadequate Water in Low- and Middle-income Countries . http://apps.who.int/gho/data/view.main.INADEQUATEWATERv?lang=en (2018).
Wikipedia. WHO Regions . https://en.wikipedia.org/wiki/WHO_regions (2021).
Griffin, A. & Hauser, J. R. The voice of the customer. Mark. Sci. 12 , 1–27 (1993).
Download references
Acknowledgements
The authors are grateful for support from the Abdul Latif Jameel Water and Food Systems Lab through the J-WAFS Solutions Program sponsored by Community Jameel. We also thank the MIT Tata Center for Technology and Design, Rasikabhai L. Meswani Fellowship, and J-WAFS Grant for Water and Food Projects in India for funding this research. We thank Himmotthan Society, People’s Science Institute, Shramyog, and Pan Himalayan Grassroots in Uttarakhand, Essmart in Bengaluru, Surinder Nagar, R. Akhilesh Kumar, and translators for facilitating the field studies in India. We thank MIT Facilities, particularly, Daniel Caterino, Sogna Scott, and Todd Gillan for providing access to wood samples. This work made use of the MRSEC Shared Experimental Facilities at MIT, supported by the National Science Foundation under award number DMR-1419807 and was in particular, aided by Research Specialist, Patrick Boisvert. We would also like to thank Prof. Daniel Frey and Dr. Michael Bono from Department of Mechanical Engineering at MIT, Dr. Eric Verploegen from D-Lab, Dr. Chintan Vaishnav from MIT Sloan School of Management, Dr. James K Wheeler from UCSC, and Prof. Jonathan Schilling from the University of Minnesota for their insightful suggestions. We also thank the many other people who contributed to various aspects of the work on xylem filters.
Author information
Present address: Institute of Microelectronics, School of Electronics Engineering and Computer Science, Peking University, Beijing, China
Present address: Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China
Authors and Affiliations
Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA
Krithika Ramchander, Luda Wang & Rohit Karnik
D-Lab, Massachusetts Institute of Technology, Cambridge, MA, USA
Megha Hegde, Anish Paul Antony, Kendra Leith & Amy Smith
You can also search for this author in PubMed Google Scholar
Contributions
K.R. performed lab and field experiments, data analysis, simulations for percolation theory, and cost estimation. L.W. performed FTIR and AFM measurements. K.R. and R.K. conceived and designed the experiments. R.K. supervised the studies. M.H. and K.L. designed user studies, with inputs from all authors. M.H., K.R., and A.P.A. performed user studies and analyzed data from user studies. K.L. and A.S. supervised user studies. K.R. and A.P.A. designed and fabricated filter devices. K.R. and R.K. wrote the paper with inputs from all authors.
Corresponding authors
Correspondence to Krithika Ramchander or Rohit Karnik .
Ethics declarations
Competing interests.
The authors declare no competing (financial/non-financial) interests. The authors have not filed patent applications on xylem filters and the technology and filter designs are open-source. R.K. is involved with a start-up company related to water. R.K. currently does not currently have financial interest in the company; the company does not have any rights to xylem filters and is not working on xylem filters, although it is possible that this may change in the future.
Additional information
Peer review information Nature Communications thanks Amy Bilton, Sushanta Mitra, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ramchander, K., Hegde, M., Antony, A.P. et al. Engineering and characterization of gymnosperm sapwood toward enabling the design of water filtration devices. Nat Commun 12 , 1871 (2021). https://doi.org/10.1038/s41467-021-22055-w
Download citation
Received : 22 May 2020
Accepted : 22 February 2021
Published : 25 March 2021
DOI : https://doi.org/10.1038/s41467-021-22055-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Solar distillation meets the real world: a review of solar stills purifying real wastewater and seawater.
- Thirugnanasambantham Arunkumar
- Ravishankar Sathyamurthy
- Sang Joon Lee
Environmental Science and Pollution Research (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- DOI: 10.1038/NPG.ELS.0001302
- Corpus ID: 28142555
Xylem Structure and Function
- A. Myburg , R. Sederoff
- Published 19 April 2001
- Biology, Environmental Science
63 Citations
What makes the wood exploring the molecular mechanisms of xylem acclimation in hardwoods to an ever-changing environment, photosynthesis-related functions of vasculature-associated chlorenchymatous cells, evolution of plant conducting cells: perspectives from key regulators of vascular cell differentiation, plant glucose transporter structure and function, axial xylem architecture of larix decidua exposed to co 2 enrichment and soil warming at the 1 treeline 2, xylan in the middle: understanding xylan biosynthesis and its metabolic dependencies toward improving wood fiber for industrial processing, growth, nodulation, and anatomical characterization of calopogonium mucunoides desv., a tropical legume, in copper-contaminated soil, two complementary mechanisms underpin cell wall patterning during xylem vessel development[open], plant and pathogen nutrient acquisition strategies, changes in root xylem anatomy of peanut genotypes with different drought resistance levels under early‐season drought, 3 references, biochemistry and molecular biology of lignification., xylogenesis: initiation, progression, and cell death., recent advances in understanding lignin biosynthesis., related papers.
Showing 1 through 3 of 0 Related Papers
- Search Menu
- Sign in through your institution
- Special Issues
- Advance articles
- High Impact Research
- Why Publish with AoB?
- Author Guidelines
- Submission Site
- Open Access Policies
- Self-Archiving Policy
- Benefits of Publishing Open Access
- Quarterly Newsletter
- About Annals of Botany
- About the Annals of Botany Company
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, biomechanics of the vessel elements, engineering model, literature cited.
- < Previous
Biomechanical Model of the Xylem Vessels in Vascular Plants
- Article contents
- Figures & tables
- Supplementary Data
GEBRAN N. KARAM, Biomechanical Model of the Xylem Vessels in Vascular Plants, Annals of Botany , Volume 95, Issue 7, June 2005, Pages 1179–1186, https://doi.org/10.1093/aob/mci130
- Permissions Icon Permissions
• Background and Aims The xylem, or water transport system, in vascular plants adopts different morphologies that appear sequentially during growth phases. This paper proposes an explanation of these morphologies based on engineering design principles.
• Methods Using microscopic observations of the different growth stages, an engineering analysis of the xylem vessels as a closed cylinder under internal pressure is carried out adopting pressure vessel design concepts.
• Key Results The analysis suggests that the xylem vessel structural morphology follows the ‘constant strength’ design principle, i.e. all of the material within the wall of the xylem is loaded equally to its maximum allowable stress capacity, and the amount of material used is therefore systematically minimized. The analysis shows that the different structural designs of the xylem vessel walls (annular, helical, reticulate and pitted) all quantitatively follow the constant strength design principle.
• Conclusions The results are discussed with respect to growth and differentiation. It is concluded that the morphology of the xylem vessel through the different phases of growth seems to follow optimal engineering design principles.
The xylem is the principal water conducting tissue in a vascular plant. It is divided between primary and secondary xylem. Primary xylem consists of an early part, the protoxylem, which differentiates and matures among actively elongating plant organs, and a later part, the metaxylem, which initiates during the growth of the primary body of the plant but matures after elongation has ceased. Secondary xylem forms during secondary growth stages and initiates after all elongation has been completed. Cell walls in the tracheary elements of the xylem have a variety of secondary wall thickenings. Different types appear in an ontogenetic sequence with annular thickening occurring first, followed in order by helical, scalariform, reticulate and pitted thickening. Occurrence of the secondary wall types depends on the growth and maturity of the tracheary element and cannot be assigned distinctly to any one type of xylem. Two types of tracheary elements can be distinguished: tracheids and vessel elements. Tracheids appear mainly in woody plants and are connected laterally through multiple pits. Vessels appear in both woody and non-woody plants and are built of numerous vessel members joined at their ends. They are typically found in vascular bundles inside different plant organs ( Fig. 1 ).

Micrograph of vascular bundle in a grass stem showing large open vessels (metaxylem), early formed collapsed vessels (protoxylem) and phloem.
In this study, the biomechanics of vessel elements are analysed from an engineering point of view. An engineering model is derived by assuming that the design of the wall thickening follows a constant strength design principle, which, by loading all parts to their maximum allowable stress capacity, minimizes the amount of material used. The quantitative and qualitative predictions of this engineering model are compared with the structural designs of plant xylem vessel cell walls as reported in the published literature and as observed on some herbaceous and non-woody vascular plants to provide a new functional explanation for the different observed types of secondary wall thickening: annular, helical, reticulate and pitted.
From an engineering point of view, the xylem is the water distribution network that transmits water from the root collection system to the main consumers, the leaves, in the upper parts of the plant. Transpiration of the leaf mesophyll cells causes a water potential difference between the leaf and the xylem, resulting in water transport. During the growing season the water is lifted up to the leaves by negative pressures, less than atmospheric, created by transpiration. Vessels, made of elongated hollow cells connected end to end ( Zimmerman, 1983 ), are subjected to high internal negative pressures ( Choat et al ., 2003 ) that must be resisted by their walls to prevent cell collapse. Microscopic investigation shows that primary vessel walls are reinforced by the secondary wall, a lignified cell-wall thickening, deposited on the inside of the primary wall of the cell ( Wooding and Northcote, 1964 ; Bierhorst and Zamora, 1965 ; Ray, 1972 ; Neushul, 1974 ) ( Fig. 2 ). Secondary wall first appears as an annular thickening. Subsequently, the thickening becomes helical, followed by interconnected helices, coils or scalariform thickening. The final structure, encountered in the last ontogenetic stage, has reticulate or net-like thickening, or uniform thickening with staggered pits in pitted vessels ( Fig. 2 ) ( Bierhorst and Zamora, 1965 ; Jensen and Salisbury, 1972 ; Ray, 1972 ; Neushul, 1974 ; Esau, 1977 ; Fahn, 1990 ). The type of secondary wall thickening is determined by the dimension (diameter) of the vessel and the maturity and growth stage of the plant (ontogeny). The literature contains many descriptions and discussions of the secondary wall patterns without an exact analysis or explanatory model. The mechanical engineering design problem of the xylem vessel element is treated in the next section to provide a possible explanation for these observations.

Types of vessel elements with different secondary wall thicknening patterns. (A) Annular; (B) annular/helical; (C) double helical opposite curl; (D) double helical same curl; and (E) pitted (after Ray, 1972 ; permission requested from Holt, Reinhart and Winston Inc.).
In addition to mechanically resisting the water pressure, the xylem also has to satisfy other design constraints such as plant growth strains, hydraulic conductivity and connectivity between adjacent cells. These will not be considered in the derivation of the engineering model.
The vessel element is analysed as a closed-end cylinder, of uniform radius, R , and wall thickness, t , subjected to an internal pressure, p , which can be either negative or positive; engineers call such a structure a ‘pressure vessel’ ( Fig. 3 ).

Xylem vessel modelled as a pressurized tube (see text for detail).
The primary cell wall is a layer made of randomly orientated cellulose microfibrils in a relatively visco-elastic matrix that allows extension and elongation ( Bodig and Jane, 1982 ; Niklas, 1992 ). Some re-orientation of the microfibrils is thought to take place under elongation stresses during growth. There is evidence that microfibrils get deposited mostly along the transverse or hoop direction before some get realigned along the fibre axis ( Niklas, 1992 ) due to elongation stresses. For the purposes of the analysis the primary wall is considered to be homogeneous and isotropic with a maximum allowable stress of σ * both axially and transversely.
The secondary wall layer of xylem tracheids is known to consist of highly orientated cellulose microfibrils with a structure stabilized by lignin ( Mark, 1967 ; Bodig and Jane, 1982 ; Niklas, 1992 ; Reiterer et al ., 1999 ). The secondary wall of tracheids contributes over 80 % of the cell wall thickness and provides mechanical support to the plant. The same structure of highly orientated cellulose microfibrils is usually assumed for the secondary wall layer of xylem vessels in woody and non-woody plants, albeit the structural support function to the plant is not required. The cellulose microfibrils in the secondary wall of vessels are expected to be more-or-less aligned along the direction of deposition (annular, helical, etc.) with varying amounts of lignin deposited along the microfibrils. These factors result in differing strengths between the primary and secondary layers. In order to allow for this possible difference, a strength ratio, r , is introduced where the maximum allowable stress of the secondary wall layer along the direction of its deposition would be r σ * . The secondary wall layer, which is deposited along circular, spiral or net-like patterns, acts as a unidirectional reinforcement and hence only its axial properties are relevant to the analysis. The anisotropy of the secondary wall does not affect the model. It is worthwhile noting that the strength ratio, r , could be derived from basic composite theory as r = E sw / E pw ; where E sw is the modulus of elasticity of the secondary wall, and E pw is the modulus of elasticity of the primary wall, when both walls are subjected to the same strains. The use of this strength ratio would account in the most general way for the difference in properties between the primary and secondary walls. When r = 1, the secondary and primary walls are assumed to have the same maximum allowable stress.
Note that a positive pressure (cell pressure greater than atmospheric) leads to tensile stresses in the wall while a negative pressure (cell pressure less than atmospheric) leads to compressive stresses. If the maximum allowable stress in the primary wall material is σ * , then the available resisting hoop force per unit length will be σ * t . Depending on the relative magnitudes of the pressure, the allowable stress in the wall material and the geometry of the tube, three cases will be encountered, each of which is analysed below.
Case I. σ * t ≥ pR , which implies that the tube can readily resist the hoop and longitudinal stresses caused by the internal pressure.
Equation 2 can be readily solved as an equality for the minimum A / s and an appropriate reinforcement can be designed.

Secondary wall thickening modelled as helical winding (case III; see text for detail).
Note that eqns (3) – (6) are identical for α and −α, which means that the reinforcement is equally effective whether the winding is from left to right or right to left; for a helix this translates into positive or negative curl (by the right hand rule).
When reinforcing helices of opposite curls are provided then the added area of the reinforcement also resists shears and the available shear resistance increases with the reinforcement ratio. Inequality (8) can be rewritten to give the limiting wall thickness before a double helix with opposite curls is required.
The above analysis is based on the ‘constant strength’ design principle ( Flugge, 1966 ): the optimum design is that which stresses all material to the maximum allowable stress. Xylem vessels formed during growth and elongation of the plant, the protoxylem, are subjected to high longitudinal tensile stresses ( Paolillo and Rubin, 1991 ; Niklas, 1992 ) in addition to the negative internal pressure: they fail by rupture of the primary wall and collapse under excessive elongation and internal pressure ( Figs 1 and 2 ). The primary xylem vessels that mature after elongation ceases, the metaxylem, are subject only to internal pressure: negative in the normal situation of leaf transpiration and upward ‘pulling’ of water, and positive during the rare occasions of suppressed transpiration or very high humidity ( Zimmerman, 1983 ). Failure can occur either by cell wall yielding or buckling: cell wall yield refers to material failure while buckling refers to a geometric instability failure (for instance, a light plastic ruler bowing out of shape when compressed at its ends or a bent straw kinking on the compressive face). Yielding of the vessel cell wall can occur under the combination of tensile (positive pressure) or compressive (negative pressure) hoop and longitudinal stresses. Buckling can occur under the combined effect of the hoop and longitudinal compressive stresses due to positive pressure; this type of failure is similar to that of a closed-end cylindrical shell under external hydrostatic pressure.
Vessel element dimensions and cell wall properties
| Plant type | Wall thickness, (μm) | Radius, (μm) | Length, (mm) | Modulus of elasticity, (GPa) | Yield strength, σ (MPa) | Source of data |
|---|---|---|---|---|---|---|
| Lily stem (Lilium) | 0·5–1·0 | 15–25 | 0·5–1·0 | – | – | SEM investigation |
| Monocotyledons | – | – | 0·76–3·96 | – | – | |
| Plant stems and roots | – | – | – | 2·8–7·0 | 50–120 (tensile) 6–11 (shear) | |
| Iris leaf | – | – | – | 2·7–7·1 | – | . (1988) |
| Hardwoods | – | 20–350 | 0·2–1·3 | – | – | |
| Wood | – | – | – | 35 | 120 | |
| Oak latewood ( ) | 1–6 | 30–100 | 0·2–0·4 | – | – | , |
| Plant type | Wall thickness, (μm) | Radius, (μm) | Length, (mm) | Modulus of elasticity, (GPa) | Yield strength, σ (MPa) | Source of data |
|---|---|---|---|---|---|---|
| Lily stem (Lilium) | 0·5–1·0 | 15–25 | 0·5–1·0 | – | – | SEM investigation |
| Monocotyledons | – | – | 0·76–3·96 | – | – | |
| Plant stems and roots | – | – | – | 2·8–7·0 | 50–120 (tensile) 6–11 (shear) | |
| Iris leaf | – | – | – | 2·7–7·1 | – | . (1988) |
| Hardwoods | – | 20–350 | 0·2–1·3 | – | – | |
| Wood | – | – | – | 35 | 120 | |
| Oak latewood ( ) | 1–6 | 30–100 | 0·2–0·4 | – | – | , |
–, information not available;
scanning electron microsope.
Dimensions and geometry of annular and helically reinforced xylem vessels in non-woody plants
| Source of data | Wall thickness, (μm) | Thickening spacing, (μm) | Thickening area, (μm ) | Estimated angle, α (degrees) | Radius of vessel, (μm) |
|---|---|---|---|---|---|
| , fig. 8.10a | 0·38 | 1·82 | 0·87 | 10–20 | 3·60 |
| , fig. 8.12a | 0·38 | 3·50 | 1·56 | 10–17 | 4·25 |
| , p.409 | 0·5 | 6·0 | 4·22 | 12–17 | 5·0 |
| , fig. 27 | 0·5 | 1·81 | 0·91 | 0–3 | 6·88 |
| SEM investigation ( ) | 0·54 | 5·36 | 3·06 | 9 | 15·71 |
| SEM investigation ( ) | 0·91 | 6·97 | 3·24 | 0 | 7·88 |
| Source of data | Wall thickness, (μm) | Thickening spacing, (μm) | Thickening area, (μm ) | Estimated angle, α (degrees) | Radius of vessel, (μm) |
|---|---|---|---|---|---|
| , fig. 8.10a | 0·38 | 1·82 | 0·87 | 10–20 | 3·60 |
| , fig. 8.12a | 0·38 | 3·50 | 1·56 | 10–17 | 4·25 |
| , p.409 | 0·5 | 6·0 | 4·22 | 12–17 | 5·0 |
| , fig. 27 | 0·5 | 1·81 | 0·91 | 0–3 | 6·88 |
| SEM investigation ( ) | 0·54 | 5·36 | 3·06 | 9 | 15·71 |
| SEM investigation ( ) | 0·91 | 6·97 | 3·24 | 0 | 7·88 |
Scanning electron microscope.
Using these typical numbers in eqn (13) the ration p y / p cr varies from 0·6 to 4·0; we expect yielding to dominate in some cases while buckling would dominate in others similarly to the behaviour of tubular structures encountered in nature ( Karam and Gibson, 1994 ). The clarification of this issue requires detailed experimental measurements on specific samples, and falls beyond the scope of this work. In the rest of this paper it will be assumed that the main purpose of the secondary wall reinforcement thickening is to prevent yielding of the walls.
Geometry of secondary wall thickening
The botanical literature gives a wide range of descriptions of secondary wall thickening patterns in xylem vessels ( Goodwin, 1942 ; Wooding and Northcote, 1964 ; Jensen and Salisbury, 1972 ; Ray, 1972 ; Neushul, 1974 ; Esau, 1977 ; Fahn, 1990 ). Here those descriptions are compared with the geometries suggested by the engineering model developed in the previous section. It is noted that the optimum design from the structural perspective must also ensure the cell opening is large enough to maintain hydraulic efficiency, allowing the flow of fluid between cells. For case II where only hoop reinforcement is needed, the optimal geometry of the secondary wall is given by equally spaced rings or annuli as determined from eqn (2) . For case III when only a little reinforcement is needed, i.e. A / s as determined from eqn (5) is small, inclined rings or a helical thickening will do. If more reinforcement is required, a second helix with the same curl can be added with the condition that s , the spacing, has to be larger than the thickness of the helix, so that helices do not merge. If the shear resistance of the xylem wall is exceeded, as well as its hoop and longitudinal resistance, then the addition of a second helix of opposite curl will be required. As the pressure or the radius increase, eqn (5) calls for larger reinforcement ratios, A / s . Noting that the addition of similar-curl helices to other closely spaced helices will result in a total material bridging and a less-than-optimal uniform secondary wall thickening, or no added shear resistance for example, the only possible solution is to add helices with an opposite curl, resulting in a net-like or reticulate pattern. This can be attained by simply bridging the spires of the already present helices. The different reinforcement design patterns are show in Fig. 5 . All the secondary wall patterns reported in the literature ( Fig. 2 ) correspond to a design solution from Fig. 5 . or to a combination of two solutions.

Different thickening patterns obtained from helical winding combinations. (A) Annular thickening; (B) single helix; (C) double helices; and (D) reticulated pattern (opposite curl helices).
Figure 6 shows scanning electron micrographs of the xylem of a grass stem ( Elytrigia repens ) where different secondary wall thickening patterns can be seen to agree qualitatively with the predictions of the model ( Fig. 5 ). A complete set of optical photomicrographs was presented by Goodwin (1942) in his seminal work, showing all the different types of secondary wall thickening. Bierhorst and Zamora (1965) described secondary wall pattern formation and compiled observations on more than 1350 species; Meylan and Butterfield (1972) also presented scanning electron micrographs of wood xylem vessel elements, showing with exceptional detail the net-like or reticulate pattern of the secondary wall similar to Fig. 5D . Classical botany texts and publications contain a wealth of such micrographs that can be seen to follow the optimal design solutions. Note that the pitted vessel type reported in many works as the last form of reinforced xylem ( Jensen and Salisbury, 1972 ; Ray, 1972 ; Neushul, 1974 ; Esau, 1977 ) ( Figs 2E and 6D ) corresponds to a heavily thickened reticulate pattern ( Fig. 5D ), where the secondary wall approaches a uniform thickening layer. The regularly staggered pits correspond to unthickened parts of the xylem with the primary wall autolysed to allow side-to-side transmission of water from one vessel to the other or to the surrounding parenchyma. Figure 7 shows schematically the evolution from the reticulate to the pitted pattern.

Micrographs of thickening patterns in xylem of Elytrigia repens . (A) Annular thickening; (B) single helix; (C) double helices; and (D) pitted pattern.

Sketch showing transition from reticulate to pitted pattern.
Theoretical predictions and experimental observations
In a given plant species it is reasonable to assume that the primary cell walls of vessels would have more or less the same average thickness, t , as well as the same average wall stress, σ * , across the existing range of diameters and lengths. Using this assumption eqn (5) suggests the need for larger reinforcement ratios, A / s , as the radius, R , or the pressure, p , increase in the xylem vessel. This prediction is verified by the observations of Goodwin (1942) and Esau (1977) who show a progression of thickening patterns, from annular to reticulate, as the radius of the vessel increases and the primary wall thickness remains more or less constant. Equation (6) provides a direct way of verifying the mechanical model against geometric measurements. t , s , A and α were estimated from scanning electron microscope observations performed in this study and from published photomicrographs of vessel sections in mostly herbaceous plants ( Table 2 ). The dimensionless factor st / rA was then calculated and compared with theoretical predictions of eqn (6) in Fig. 8 for r = 1; the data points show the same trend as predicted by eqn (6) and agreement is relatively good. Note that the last entry in Table 2 does not show in Fig. 8 because it falls under case II where only hoop reinforcement is needed in the form of circumferential rings: the observed angle is zero as predicted by the model, st / A > 1 and eqn (6) is not applicable.

Helical thickening winding angle plotted against dimensionless reinforcement ratio ( eqn 6 ).
Growth, differentiation and secondary wall thickening
The secondary wall thickening pattern has been used as an index of growth and differentiation in xylem vessels ( Goodwin, 1942 ; Wooding and Northcote, 1964 ; Bierhorst and Zamora, 1965 ; Jensen and Salisbury, 1972 ; Ray, 1972 ; Neushul, 1974 ; Esau, 1977 ; Zimmerman, 1983 ; Fahn, 1990 ; Paolillo and Rubin, 1991 ). The protoxylem develops and matures with the growing plant and has a smaller diameter than the metaxylem, which matures after the plant has finished its primary growth phase ( Fig. 1 ). Having a smaller diameter, the protoxylem only needs annular and helical reinforcement while the metaxylem, functioning at the same pressure but with a larger diameter, will require more reinforcement resulting in a reticulate pattern. Putting aside hydraulic considerations, one reason why the plant only develops small diameter xylem (protoxylem) in its early growth phase is the need to allow for growing strains, which can reach as high as 13 % in a functional xylem, before the primary cell wall is ruptured and the vessel in question is rendered useless ( Paolillo and Rubin, 1991 ; Niklas, 1992 ) ( Figs 1 and 2 ). The annular and helical reinforcement patterns do not offer as much resistance to elongation as a reticulate pattern. The helical and annular reinforcement, having the lowest longitudinal stiffness among secondary wall patterns, will resist growth the least and stay functional for the longest time possible.
Concluding remarks
The constant strength design principle has been used by mechanical engineers to optimize the design of engineering pressure vessels ( Harvey, 1980 ). Application of this principle has led to metallic pressure vessels reinforced with coil-layer, ribbon or wire-wrapping and helical corrugations, and to fibre-reinforced plastic pressure vessels manufactured with optimized helical winding patterns ( Harvey, 1980 ; Rolston, 1990 ; Shevchenko et al ., 1993 ; Contech, 1994 ). Here, a simple mechanical model of the xylem vessel as a pressure vessel has been utilized to show that the constant strength principle can be used to explain secondary wall thickening patterns in cell walls. Experimental observations of the geometry and relative dimensions of the reinforcement thickening agree well with the predictions of the model. Mechanical design considerations underlie many observed characteristics of the xylem vessels and tracheids, which are best described as composite hierarchical systems. Recent experimental results by Reiterer et al . (1999) and Choat et al . (2003) point to the existence of optimal orientation angles of microfibrils in the secondary wall. A modelling approach based on optimization principles similar to the one presented above for secondary cell wall deposition patterns could be used to analyse and understand these microstructural systems as well ( Gibson et al ., 1995 ).
The author acknowledges the financial support of US NSF (grants number MSS-9202202 and EID 9023692) for part of this work and the invaluable contribution of Professor Lorna Gibson of the Materials Science and Engineering Department at MIT.
Bierhorst DW, Zamora PM. 1965 . Primary xylem elements and element associations of angiosperms. American Journal of Botany 52 : 657 –710
Blahovec J. 1988 . Mechanical properties of some plant materials. Journal of Materials Science 23 : 3588 –3593.
Bodig J, Jayne BA. 1982 . Mechanics of wood and wood composites . New York: Van Nostrand Reinhole Co.
Cheadle VI. 1943 . The origin and certain trends of specialization of the vessel in the monocotyledon. American Journal of Botany 30 : 11 –17.
Choat B, Ball M, Luly J, Holtum J. 2003 . Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species. Plant Physiology 131 : 41 –48.
Contech Construction Products Inc. 1994 . Ultra Flo storm Sewer pipe, UF-101, ed.5 . Middletown, Ohio.
Esau K. 1977 . Anatomy of seed plants , 2nd ed. New York: John Wiley and Sons.
Fahn A. 1990 . Plant anatomy , 4th ed. Oxford: Pergamon Press.
Flugge W. 1966 . Stresses in shells . New York: Springer-Verlag.
Gibson LJ, Ashby MF. 1988 . Cellular solids . Oxford: Pergamon Press.
Gibson LJ, Ashby MF, Easterling KE. 1988 . Structure and mechanics of the iris leaf. Journal of Materials Science 23 : 3041 –3048.
Gibson LJ, Ashby MF, Karam GN, Wegst U, Shercliff HR. 1995 . The mechanical properties of natural materials. II. Microstructures for mechanical efficiency Proceedings of the Royal Society of London Series A 450 : 141 –162.
Goodwin RH. 1942 . On the development of xylary elements in the first internode of Avena in dark and light. American Journal of Botany 29 : 818 –828.
Harvey JF 1980 . Pressure component construction . New York: Van Nostrand Reinhold Co.
Illston JM, Dinwoodie JM, Smith AA. 1979 . Concrete, timber and metals . New York: Van Nostrand Reinhold International.
Jensen WA, Salisbury FB. 1972 . Botany: an ecological approach . Belmont, CA: Wadsworth Publishing Co. Inc.
Karam GN, Gibson LJ. 1994 . Biomimicking of animal quills and plant stems: natural cylindrical shells with foam cores. Materials Science and Engineering C Biomimetic Materials, Sensors and Systems C2 : 113 –132.
Kollàr L, Dulàcska E. 1984 . Buckling of shells for engineers . New York: John Wiley and Sons.
Mark RE. 1967 . Cell wall mechanics of tracheids , Yale University Press.
Meylan BA, Butterfield BG. 1972 . Perforation plate development in Knightia excelsa R. Br: a scanning electron microscope study. Australian Journal of Botany 20 : 79 –86.
Neushul M. 1974 . Botany . Santa Barbara, CA: Hamilton Pub. Co.
Niklas KJ. 1992 . Plant biomechanics . Chicago IL: The University of Chicago Press.
Paolillo Jr DJ, Rubin G. 1991 . Relative elemental rates of elongation and the protoxylem-metaxylem transition in hypocotyls of soybean seedlings. American Journal of Botany 78 : 845 –854.
Ray PM. 1972 . The living plant . New York: Holt, Rinehart and Winston Inc.
Reiterer A, Lichtenegger H, Tschegg S, Fratzl P. 1999 . Experimental evidence for a mechanical function of the cellulose microfibril angle in wood cell walls. Philosophical Magazine A 79 : 2173 –2184.
Roberts LW. 1976 . Cytodifferentiation in plants . Cambridge: Cambridge University Press.
Rolston JA. 1990 . Filament winding . In: Bader M, Smith W, Isham A, Rolston JA, Metzner A, eds. Processing and fabrication technology. Delaware composites design encyclopedia 3 . Lancaster, PA: Technomic Pub. Co. Inc., 193–204.
Shevchenko YuN, Merzlyakov VA, Galishin AZ, Novikov SV, Los AO, Yukhimets PS. 1993 . Determination of the limiting stress-strain state of helically corrugated pipes. Problemy Prochnosti 7 : 48 –53 (English translation 507–511).
Siau JF. 1984 . Transport processes in wood . Berlin: Springer-Verlag.
Wooding FBP, Northcote DH. 1964 . The development of the secondary wall of the xylem in Acer pseudoplatanus . Journal of Cell Biology 23 : 327 –337.
Zimmerman MH. 1983 . Xylem structure and the ascent of sap . Berlin, Heidelberg: Springer-Verlag.
| Month: | Total Views: |
|---|---|
| December 2016 | 1 |
| January 2017 | 1 |
| February 2017 | 19 |
| March 2017 | 10 |
| April 2017 | 11 |
| May 2017 | 6 |
| June 2017 | 3 |
| July 2017 | 1 |
| August 2017 | 9 |
| September 2017 | 6 |
| October 2017 | 9 |
| November 2017 | 13 |
| December 2017 | 51 |
| January 2018 | 63 |
| February 2018 | 54 |
| March 2018 | 137 |
| April 2018 | 87 |
| May 2018 | 76 |
| June 2018 | 69 |
| July 2018 | 73 |
| August 2018 | 41 |
| September 2018 | 59 |
| October 2018 | 55 |
| November 2018 | 39 |
| December 2018 | 52 |
| January 2019 | 34 |
| February 2019 | 42 |
| March 2019 | 40 |
| April 2019 | 43 |
| May 2019 | 35 |
| June 2019 | 29 |
| July 2019 | 33 |
| August 2019 | 33 |
| September 2019 | 30 |
| October 2019 | 34 |
| November 2019 | 58 |
| December 2019 | 22 |
| January 2020 | 27 |
| February 2020 | 39 |
| March 2020 | 17 |
| April 2020 | 24 |
| May 2020 | 24 |
| June 2020 | 49 |
| July 2020 | 30 |
| August 2020 | 49 |
| September 2020 | 52 |
| October 2020 | 32 |
| November 2020 | 80 |
| December 2020 | 46 |
| January 2021 | 43 |
| February 2021 | 92 |
| March 2021 | 116 |
| April 2021 | 63 |
| May 2021 | 38 |
| June 2021 | 44 |
| July 2021 | 34 |
| August 2021 | 44 |
| September 2021 | 61 |
| October 2021 | 70 |
| November 2021 | 74 |
| December 2021 | 39 |
| January 2022 | 42 |
| February 2022 | 39 |
| March 2022 | 74 |
| April 2022 | 61 |
| May 2022 | 59 |
| June 2022 | 46 |
| July 2022 | 47 |
| August 2022 | 37 |
| September 2022 | 45 |
| October 2022 | 48 |
| November 2022 | 31 |
| December 2022 | 17 |
| January 2023 | 47 |
| February 2023 | 50 |
| March 2023 | 56 |
| April 2023 | 52 |
| May 2023 | 55 |
| June 2023 | 26 |
| July 2023 | 19 |
| August 2023 | 20 |
| September 2023 | 24 |
| October 2023 | 34 |
| November 2023 | 29 |
| December 2023 | 45 |
| January 2024 | 21 |
| February 2024 | 20 |
| March 2024 | 29 |
| April 2024 | 26 |
| May 2024 | 29 |
| June 2024 | 30 |
| July 2024 | 27 |
| August 2024 | 11 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1095-8290
- Print ISSN 0305-7364
- Copyright © 2024 Annals of Botany Company
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Xylem: Structure and Function
- Conference paper
- Cite this conference paper

- Jörg J. Sauter 6
Part of the book series: Progress in Botany/Fortschritte der Botanik ((BOTANY,volume 48))
131 Accesses
2 Citations
This article endeavours to compile structural and functional results on xylem. Of the manifold aspects of xylem, the present review is primarily devoted to tracheary elements (vessels, tracheids), other cells being excluded. Results are still coming form a wide and fairly diversified field, which has necessitated extreme abbreviation to include as many of them as possible. This review covers mainly papers which appeared from 1983 to 1985. Especial consideration was given to the differentiation of tracheary elements, vascularization of organs, functional xylem anatomy, water conduction, and translocation in xylem sap.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Similar content being viewed by others

The recurrent evolution of extremely resistant xylem

In vivo pressure gradient heterogeneity increases flow contribution of small diameter vessels in grapevine

Xylem Development in Trees: From Cambial Divisions to Mature Wood Cells
Albinger, G., Beiderbeck, R.: Z. Pflanzenphysiol. 112 , 443–448 (1983).
Google Scholar
Alfen, N.K. Van, Mcmillan, B.D., Turner, V.: Plant Physiol. 73 , 1020–1023 (1983).
Aloni, R.: Planta 150 , 255–263 (1980).
CAS Google Scholar
Aloni, R., Zimmermann, M.H.: Differentiation 24 , 203–208 (1983); Bot. Gaz. 145, 50–54 (1984).
Ashworth, E.N., Abeles, F.B.: Plant Physiol. 76 , 201–204 (1984).
PubMed CAS Google Scholar
Atkins, C.A., Pate, J.S., Peoples, M.B., Joy, K.W.: Plant Physiol. 71 , 841–848 (1983).
Baas, P.: New Perspectives in Wood Anatomy. The Hague: Nijhoff/Junk 1982a; Systematic, phylogenetic, and ecological wood anatomy — History and perspectives, 29-53. In: New Perspectives in Wood Anatomy, ed. P. BAAS. The Hague: Nijhoff/Junk 1982b.
Baas, P., Carlquist, S., IAWA Bull. N.S. 6 , 349–353 (1985).
Baas, P., Werker, E., Fahn, A.: IAWA Bull. N.S. 4 , 141–159 (1983).
Barabé, D., Labreque, M.: Can. J. Bot. 61 , 1718–1726 (1983); ibid. 62, 1971–1983 (1984).
Barajas-Morales, J.: IAWA Bull. N.S. 6 , 355–364 (1985).
Batenburg, L.H., M0ELI0N0, B.M.: Acta Bot. Neerl. 31 , 215–220 (1982).
Benayoun, J.: Ann. Bot. 52 , 189–200 (1983).
Benayoun, J., Catesson, A.M., Czaninsky, Y.: Ann. Bot. 47 , 687–698 (1981).
Beusichem, M.L. Van: Z. Pflanzenphysiol. 109 , 449–458 (1983).
Bel, A.J.E. Van: Plant Sci. Lett. 35 , 81–85 (1984).
Bel, A.J.E. Van, Van der SCHOOT, C.: Plant Sci. Lett. 19 , 101–107 (1980).
Bel, A.J.E. Van, Van Leeuwenkamp, P., Van der Schoot, C.: Z. Pflanzenphysiol. 104 , 117–128 (1981).
Bonsen, K.J., Terwelle, B.J.H.: Bot. Jahrb. Syst. 105 , 49–71 (1984).
Bosshard, H.H., Kucera, L.J., Stocker, U.: Vierteljahresschr. Naturforsch. Ges. Zürich 127 /1, 29–48 (1982).
Boyer, J.S.: Annu. Rev. Plant Physiol. 36 , 473–516 (1985).
Braun, H.J.: Ber. Dtsch. Bot. Ges. 96 , 29–47 (1983); IAWA Bull. N.S. 5 , 275–294 (1984); Ber. Dtsch. Bot. Ges. 98 , 239–244 (1985).
Burgess, J., Linstead, P.: Planta 160 , 481–489 (1984).
Calkin, H.W., Gibson, A.C., Nobel, P.S.: Can. J. Bot. 63 , 632–637 (1985).
Carlquist, S.: Aliso 10 , 383–395 (1983a); ibid. 10, 413–425 (1983b); ibid. 10, 427–441 (1983c); Am. J. Bot. 70, 578–590 (1983d); Bot. J. Linnean Soc. 88, 257–277 (1984a); Plant. Syst. Evol. 144, 103–118 (1984b); Ann. Missouri Bot. Gard. 71, 232–242 (1984c); Aliso 10, 505–525 (1984d); ibid. 573–582 (1984e); ibid. 10, 583–602 (1984f); System Bot. 10, 174–183 (1985a); Bull. Torrey Bot. Club 112, 59–69 (1985b); Brittonia 37, 58–75 (1985c); Aliso 11, 37–68 (1985d).
Carlquist, S., Eckart, V.M.: Aliso 10 , 527–546 (1984).
Carlquist, S., Hoekman, D.A.: IAWA Bull. N.S. 6 , 319–347 (1985).
Carlquist, S., Eckart, V.M., MICHENER, D.C.: Aliso 10 , 397–412 (1983); ibid. 10, 547 - 572 (1984).
Castro, M.A.: Iawa Bull. N.S. 6 , 35–38 (1985).
Catesson, A.-M.: Iawa Bull. N.S. 4 , 89–101 (1983).
Catesson, A.-M., Czaninski, Y., Roland, J.C.: Iawa Bull. N.S. 4 , 70–71 (1983).
Cheadle, V.I., Kosakai, H.: Phyta, Studies on Living & Fossil Plants, Pant Comm. Vol., 45–57 (1982).
Clarkson, D. T., Williams, L., Hanson, J.B.: Planta 162 , 361–369 (1984).
Cortes, P.M., Sinclair, T.R.: J. Exp. Bot. 36 , 12–24 (1985).
Crombie, D.S., Milburn, J.A., Hipkins, M.F.: Planta 163 , 27–33 (1985).
Czaninski, Y., Monties, B.: C.R. Acad. Sci. Paris 295 , 551–556 (1982).
Czanin-Ski, Y., Imberty, A., Catesson, A.-M.: Biol. Cell 51 , 34a (1984).
Demason, D.A., Wilson, M.A.: Can. J. Bot. 63 , 1907–1913 (1985).
Dickison, W.C., Phend, K.D.: IAWA Bull. N.S. 6 , 3 - 22 (1985).
Dickson, R.E., Vogelmann, T.C., Larson, P.R.: Plant Physiol. 77 , 412–417 (1985).
Dodd, R.S.: Iawa Bull. N.S. 5 , 253–257 (1984).
Edwards, W.R.N., Booker, R.E.: J. Exp. Bot. 35 , 551–561 (1984).
Elmore, G.S., Ewers, F.W.: IAWA Bull. N.S. 6 , 303–307 (1985a); Am. J. Bot. 72, 814 (1985b).
Essiamah, S.K., Eschrich, W.: Forstarchiv 53 , 133–135 (1982); IAWA Bull. N.S. 6 , 97–106 (1985).
Ewers, F.: Iawa Bull. N.S. 6 , 309–317 (1985).
Ewers, F.W., Zimmermann, M.H.: Physiol. Plant. 60 , 453–458 (1984a); Can. J. Bot. 62 , 940–946 (1984b)
Falconer, M.M., Seagull, R.W.: Protoplasma 125 , 190–198 (1985a); ibid 128, 157–166 (1985b).
Ferguson, I.B., Eiseman, J.A., Leonard, J.A.: Ann. Bot. 51 , 823–833 (1983).
French, J.C., Tomlinson, P.B.: Am. J. Bot. 10 , 756–771 (1983).
French, J.C., Clancy, K., Tomlinson, P.B.: Am. J. Bot. 70 , 1386–1400 (1983).
Fukazawa, K.: Iawa Bull. N.S. 5 , 65–73 (1984).
Fukazawa, K., Imagawa, H.: Ultraviolet and fluorescence microscopic studies of lignin, 20–23. In: 1983 International Symposium on Wood and Pulping Chemistry, Vol. 1. Japanese Technical Assoc. of the Pulp and Paper Industry 1983.
Gasson, P.: Iawa Bull. N.S. 6 , 219–237 (1985).
Gibson, A.C., Calkin, H.W., Nobel, P.S.: Am. J. Bot. 71 , 564–574 (1984); Iawa Bull. N.S. 6 , 293–302 (1985a).
Gibson, A.C., Calkin, H.W., Raphael, D.O., NOBEL, P.S.: Proc. Royal Soc. Edinburgh 86B , 81–92 (1985b).
Goldberg, R., Catesson, A.-M., Czaninski, Y.: Z. Pflanzenphysiol. 110 , 267–279 (1983)
Goldberg, R., LE, T., Catesson, A.-M.: J. Exp. Bot. 36 , 503–510 (1985).
Gomez-Vazquez, B.G., Engleman, E.M.: IAWA Bull. N.S. 4 , 207–212 (1983).
Gottwald, H.: Iawa Bull. N.S. 4 , 161–178 (1983).
Grace, J., Tyree, M.T.: Plant Cell Environ. 7 , 615–618 (1984).
Gray, H.R., Erickson, P.I., Stone, J.F.: J. Exp. Bot., 36 , 1320–1324 (1985).
Grison, R., Pilet, P.-E., J. Plant Physiol. 118 , 189–199 (1985a); ibid. 218, 201–208 (1985b).
Grosser, D.: Holzforsch. 37 , 327–330 (1983); ibid. 38, 55–59 (1984); ibid. 39, 63–65 (1985a); ibid. 39, 189–194 (1985b).
Hardiman, R.T., Jacoby, B.: Physiol. Plant. 61 , 670–674 (1984).
Hawkins, S.W., Philips, R., Plant Sci. Lett. 32 , 221–224 (1983).
Herth, W.: Planta 164 , 12–21 (1985).
Hill, J.F.: Am. J. Bot. 70 , 934–939 (1983).
Imagawa, H.: Res. Bull. Coll. Exp. For. Hokkaido Univ. 42 , 149–178 (1985a); ibid. 42, 585–594 (1985b).
Imberty, A., Goldberg, R., Catesson, A.-M.: Planta 164 , 221–226 (1985).
Jeschke, W.D.: J. Plant Physiol. 117 , 267–285 (1984).
Jeschke, W.D., Atkins, C.A., PATE, J.S.: J. Plant Physiol. 117 , 319–330 (1985).
Kirchoff, B.K., Fahn, A.: Can. J. Bot. 62 , 2432–2440 (1984a); ibid. 62, 2580–2586 (1984b).
Koek-Noorman, J., Topper, S.M.C., Terwelle, B. J.H.: Iawa Bull. N.S. 5 , 317–329 (1984a); ibid. 5, 330–334 (1984b).
Kramer, P.J.: Water Relations of Plants. 489 pp. New York: Academic Press 1983.
Kuöera, L.J., Bariska, M.: Forstarchiv 53 , 136–141 (1982)
Lachaud, S.: Can. J. Bot. 12 , 2692–2697 (1981); ibid. 6, 1768–1774 (1983).
Lachaud, S., Bonnemain, J.-L.: Can. J. Bot. 159 , 1222–1230 (1981); ibid. 6, 869–876 (1982).
Larson, P.R.: Am. J. Bot. 71 , 1201–1210 (1984a); ibid. 1201–1220 (1984b).
Larson, P.R., Fisher, D.G.: Can. J. Bot. 61 , 1040–1051 (1983).
Lynch, J., Läuchli, A.: Planta 161 , 295–301 (1984).
Maksymowych, A.B., Orkwiszewski, A.J., Maksymowych, R.: Am. J. Bot. 70 , 1289–1296 (1983).
Masuda, H., Fukuda, H., Komamine, A.: Z. Pflanzenphysiol. 112 , 417–426 (1983).
Mcneil, D.L., Larue, T.A.: Plant Physiol. 74 , 227–232 (1984).
Meichenheimer, R.D., Larson, P.R.: Ann. Bot. 52 , 491–502 (1983); J. Exp. Bot. 36, 320–329 (1985).
Metcalfe, C. R., Chalk, L.: Anatomy of the Dicotyledons, 2nd ed., Vol. II, Wood Structure and Conclusion of the General Introduction. Oxford: University Press 1983.
Milburn, J.A., Johnson, R.P.C.: Planta 69 , 43–52 (1966).
Milburn, J.A., O’malley, P.E.R.: Can. J. Bot. 62 , 2101–2106 (1984).
Miller, A.R., Roberts, L.W.: J. Exp. Bot. 35 , 691–698 (1984).
Miller, A.R., Pengelly, W.L., Roberts, L.W.: Plant Physiol. 75 , 1165–1166 (1984).
Miller, A.R., Crawford, D.L., Roberts, L.W.: J. Exp. Bot. 36 , 110–118 (1985).
Miller, D.M.: Plant Physiol. 77 , 162–167 (1985a); Plant Physiol. 77, 168–174 (1985b).
Miller, R.B.: J. Arnold Arbor. 56 , 20–102 (1975).
Minocha, S.C.: J. Exp. Bot. 35 , 1003–1015 (1984).
Moreno, J., Garcia-Martinez, J.L.: Physiol. Plant. 59 , 669–675 (1983).
Muhammad, A.F.: IAWA Bull. N.S. 5 , 217–223 (1984).
Munns, R.: J. Exp. Bot. 36 , 1032–1042 (1985).
Newbanks, D., Bosch, A., Zimmermann, M.H.: Phytopathology 73 , 1060–1063 (1983).
Nobel, P.S.: Biophysical Plant Physiology and Ecology. San Francisco: Freeman 1983.
Nobel, P.S., Jordan, P.W.: J. Exp. Bot. 34 , 1379–1391 (1983).
Oever, L. Van den, Baas, P., Zandee, M.: IAWA Bull. N.S. 2 , 3–24 (1981)
Ohtani, J.: Res. Bull. Coll. Exp. For. Hokkaido Univ. 40 , 323–386 (1983); IAWA Bull. N.S. 6, 43–51 (1985).
Ohtani, J., Meylan, B.A., Butterfield, B.G.: N. Z. J. Bot. 21, (1983); IAWA Bull. N.S. 5, (1984a); ibid. 5, 9–12 (1984b).
O’malley, P.E.R., Milburn, J.A.: Can. J. Bot. 61 , 3100–3106 (1983).
Outer, R.W. Den: Acta Bot. Neerl. 34 , 11–113 (1985).
Outer, R.W. Den, Van Veenendaal, W.L.H.: Acta Bot. Neerl. 31 , 265–274 (1982).
Parameswaran, N., Liese, W.: Holz Roh. Werkst. 40 , 145–155 (1982).
Parker, W.C., Pallardy, S.G.: Can. J. Bot. 63 , 1266–1270 (1985).
Pate, J.S., Atkins, C.A.: Plant Physiol. 71 , 835 - 840 (1983).
Pate, J.S., Peoples, M.B., Atkins, C.A.: Plant Physiol. 74 , 499–505 (1984).
Peoples, M.B., Pate, J.S., Atkins, C.A.: J. Exp. Bot. 36 , 567–582 (1985).
Pettersson, S.: Physiol. Plant. 61 , 663–669 (1984).
Phillips, R., Hawkins, S.W.: J. Exp. Bot. 36 , 119–128 (1985).
Pizzolato, T.D.: Am. J. Bot. 70 , 17–29 (1983a); ibid. 70, 1173–1187 (1983b).
Porandowski, J., Rakowski, K., Wodzicki, T.: Acta Soc. Bot. Pol. 51 , 203–214 (1982).
Pulawska, Z.: Acta Soc. Bot. Pol. 51 , 361–376 (1982).
Quirk, J.T., Miller, R.B.: IAWA Bull. N.S. 6 , 200–212 (1985).
Radin, J.W., Eidenbock, M.P.: Plant Physiol. 75 , 372–377 (1984).
Rana, M.A., Gahan, P.B.: Planta 157 , 307–316 (1983).
Reiss, H.-D., Schnepf, E., HERTH, W.: Planta 160 , 428–435 (1984).
Richter, H.G.: Iawa Bull. N.S. 6 , 187–199 (1985).
Roland, J.C., Mosiniak, M.: IAWA Bull. N.S. 4 , 15–26 (1983).
Rosendahl, L.; Physiol. Plant. 60 , 215–220 (1984).
Rury, P.M.: Iawa Bull. N.S. 6 , 365–397 (1985).
Russell, S.H., Evert, R.F.: Planta 164 , 448–458 (1985).
Russin, W.A., Evert, R.F.: Am. J. Bot. 71 , 1398–1415 (1984); ibid. 72, 487–500 (1985a); ibid. 72, 1232–1247 (1985b).
Saks, Y., Aloni, R.: Ann. Bot. 56 , 771–778 (1985).
Salim, M., Pitman, M.G.: J. Exp. Bot. 35 , 869–881 (1984a); Physiol. Plant. 61 , 263–270 (1984b).
Salleo, S., Logullo, M.A., Siracusano, L.: Ann. Bot. 54 , 543–552 (1984).
Salleo, S., Logullo, M.A., Oliveri, F.: J. Exp. Bot. 36 , 1–11 (1985).
Sandford, A.P., Grace, J.: J. Exp. Bot. 36 , 298–311 (1985).
Sauter, J.J.: Z. Pflanzenphysiol. 101 , 399–411 (1981); J. Plant Physiol. 116 , 331–342 (1984).
Savidge, R.A., Mutumba, G.M. C., Heald, J.K., Wareing, P.F.: Plant Physiol. 71 , 434–436 (1983).
Schmid, R., Baas, P.: Iawa Bull. N.S. 5 , 197–215 (1984).
Sheriff, D. W., Whitehead, D.: Plant Cell Environ. 7 , 53–62 (1984).
Siau, J.F.: Transport Processes in Wood. 345 pp. Berlin, Heidelberg, New York: Springer 1984
Smart, C.C., Amrhein, N.: Protoplasma 124 , 87–95 (1985). Sperry, J.S.: Am. Fern J. 73 , 65–72 (1983); Iawa Bull. N. S. 6 , 283–292 (1985); Plant Physiol. 80 , 110–116 (1986).
Takano, T., Fukazawa, K., Ishida, S.: Res. Bull. Coll. Exp. For. Hokkaido Univers. 40 , 709–722 (1983).
Thompson, R.G., Tyree, M.T., Logullo, M.A., Salleo, S.: Ann. Bot. 52 , 399–406 (1983).
Tomlinson, P.B.: Development of stem conducting tissues in monocotyledons, 1–51. In: Contemporary Problems in Plant Anatomy, eds. R.A. White, W.L. Dickison. Orlando: Academic Press 1984.
Tomlinson, P.B., Vincent, J. R.: J. Arnold Arbor. 65 , 191–214 (1984).
Tromp, J.: Plant Soil 71 , 401–413 (1983).
Tromp, J., Ovaa, J.C.: Z. Pflanzenphysiol. 102 , 249–255 (1981); Physiol. Plant. 62 , 209–214 (1984); J. Plant Physiol. 119 , 301–309 (1985).
Tyree, M.T.: Plant Physiol. 73 , 277–285 (1983).
Tyree, M.T., Dixon, M.A.: Plant Physiol. 72 , 1094–1099 (1983); Physiol. Plant. 65 , 397–405 (1986).
Tyree, M.T., Graham, M.E.D.Cooper, K.E., BAZOS, L.J.: Can. J. Bot. 61 , 2105–2111 (1983).
Tyree, M.T., Dixon, M.A., Thompson, R.G.: Plant Physiol. 74 , 1046–1049 (1984a).
Tyree, M.T., Dixon, M.A., Tyree, E.L., Johnson, R.: Plant Physiol. 25 , 988–992 (1984b).
Urquhart, A.A., Joy, K.W.: Plant Physiol. 69 , 1226–1232 (1982).
Villalba, R.: Iawa Bull. N.S. 6 , 119–130 (1985).
Vogelmann, T.C., Dickson, R.E., LARSON, P.R.: Plant Physiol. 77 , 418–428 (1985).
Werker, E., Baas, P.: Iawa Bull. N.S. 2 , 69–76 (1981).
Wheeler, E. A.: Iawa Bull. N.S. 4 , 2–3 (1983).
White, R.A., Dickison, W.C.: Contemporary Problems in Plant Anatomy. Orlando: Academic Press 1984.
Wilkes, J., Abbott, D.: Appita 37 , 231–232 (1983).
Wodzicki, T.J., Zajaczkowski, S.: Folia Forest. Pol. A 25 , 5–23 (1983).
Wodzicki, T.J., Rakowski, K., Starck, Z., PORANDOWSKI, J., ZAJACZKOWSKI, S.: Acta Soc. Bot. Pol. 51 , 187–201 (1982).
Wolterbeek, H.TH., Van Luipen, J., De Bruin, M.: Physiol. Plant. 61 , 599–606 (1984).
Yeo, A.R., Yeo, M.E., Caporn, S.J.M., Lachno, D.R., Flowers, T.J.: J. Exp. Bot. 36 , 1099–1109 (1985).
Yoshizawa, N., Koike, S., Idei, T.: Bull. Utsunomiya Univ. Forests 20 , 59–76 (1984).
Yoshizawa, N., Itoh, T., Shimaji, K.: Iawa Bull. N.S. 6 , 131–138 (1985a).
Yoshizawa, N., Matsumoto, S., Idei, T.: IAWA Bull. N.S. 6 , 245–253 (1985b).
Yoshizawa, N., Koike, S., Idei, T.: Mokuzai Gakkaishi 325–333 (1985c).
Yumoto, M., Ishida, S., Fukazawa, K.: Res. Bull. Coll. Exp For. Hokkaido Univ. 40 , 409–454 (1983).
Zee, G.A. Van, Schurer, K.: J. Exp. Bot. 34 , 1636–1651 (1983).
Zim-Mermann, M.H.: Xylem Structure and the Ascent of Sap. 143 pp. Berlin Heidelberg New York: Springer 1983.
Zimmermann, M.H., Jeje, A.A.: Can. J. Bot. 59 , 1882–1892 (1981).
Zimmermann, M.H., Milburn, J.A.: Transport and storage of water, 135–151. In: Encyclopedia of Plant Physiology, N.S. Vol. 12 B, eds. O.L. Lange, P.S. Nobel, C.B. Osmond, H. Ziegler. Berlin, Heidelberg, New York: Springer 1982.
Zimmermann M.H., Potter, D.: Iawa Bull. N.S. 3 , 103–109 (1982).
Zimmermann, M. H., Sperry, J.S.: J. Arnold Arbor. 64 , 599 - 609 (1983).
Zimmermann, M.H., Mccue, K.F., Sperry, J.S.: J. Arnold Arbor. 63 , 83–95 (1982).
Download references
Author information
Authors and affiliations.
Botanisches Institut der Universität Kiel, Olshausenstr. 40, D-2300, Kiel, Germany
Prof. Dr. Jörg J. Sauter
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Zellenlehre, Universität Heidelberg, Im Neuenheimer Feld 230, D 6900, Heidelberg, Germany
H.-Dietmar Behnke
Lehrstuhl für Allgemeine Botanik, Ruhr-Universität, Postfach 10 21 48, D 4630, Bochum 1, Germany
Institut für Allgemeine Botanik und Botanischer Garten, Universität Hamburg, Ohnhorststr. 18, D 2000, Hamburg 52, Germany
Klaus Kubitzki
Lehrstuhl für Geobotanik, Systematisch-Geobotanisches Institut der Universität, Untere Karspüle 2, D 3400, Göttingen, Germany
Michael Runge
Institut für Botanik und Mikrobiologie, Technische Universität München, Arcisstr. 21, D 8000, München 2, Germany
Hubert Ziegler
Rights and permissions
Reprints and permissions
Copyright information
© 1986 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper.
Sauter, J.J. (1986). Xylem: Structure and Function. In: Behnke, HD., Esser, K., Kubitzki, K., Runge, M., Ziegler, H. (eds) Progress in Botany. Progress in Botany/Fortschritte der Botanik, vol 48. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-71668-3_25
Download citation
DOI : https://doi.org/10.1007/978-3-642-71668-3_25
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-642-71670-6
Online ISBN : 978-3-642-71668-3
eBook Packages : Springer Book Archive
Share this paper
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Plant Sci
On the Efficacy of Water Transport in Leaves. A Coupled Xylem-Phloem Model of Water and Solute Transport
Gen sakurai.
1 Institute for Agro-Environmental Sciences, National Agriculture and Food Research Organization, Tsukuba, Japan
Stanley J. Miklavcic
2 Phenomics and Bioinformatics Research Centre, University of South Australia, Mawson Lakes, SA, Australia
Associated Data
The data underpinning the results presented in figures herein are available from the authors upon request.
In this paper, we present and use a coupled xylem/phloem mathematical model of passive water and solute transport through a reticulated vascular system of an angiosperm leaf. We evaluate the effect of leaf width-to-length proportion and orientation of second-order veins on the indexes of water transport into the leaves and sucrose transport from the leaves. We found that the most important factor affecting the steady-state pattern of hydraulic pressure distribution in the xylem and solute concentration in the phloem was leaf shape: narrower/longer leaves are less efficient in convecting xylem water and phloem solutes than wider/shorter leaves under all conditions studied. The degree of efficiency of transport is greatly influenced by the orientation of second-order veins relative to the main vein for all leaf proportions considered; the dependence is non-monotonic with efficiency maximized when the angle is approximately 45° to the main vein, although the angle of peak efficiency depends on other conditions. The sensitivity of transport efficiency to vein orientation increases with increasing vein conductivity. The vein angle at which efficiency is maximum tended to be smaller (relative to the main vein direction) in narrower leaves. The results may help to explain, or at least contribute to our understanding of, the evolution of parallel vein systems in monocot leaves.
1. Introduction
Vascular plants, particularly the dicot group of angiosperms, have evolved complicated hierarchical venation systems. The first-order so-called main vein of the system enters the leaf at the petiole and extends to the apex. Second-order veins diverge from this first-order vein. In turn, higher order veins branch off from their lower order neighbors to form a complex reticulated vein network extending across the entire leaf (Esau, 1953 ; Sack and Scoffoni, 2013 ). The detailed structure of the network differs notably from species to species, and potentially even from variety to variety within the same species. It is generally accepted that the whole-of-plant hydraulic system is significantly influenced by the hydraulic conductance of the plant's leaves (Sack and Holbrook, 2006 ). It follows then that leaf vasculature plays an important role in the response of a plant to water stress. In its evolution, however, a plant has also come to utilize this physical infrastructure to translocate to the rest of the plant, via a parallel phloem system connected to the petiole, photosynthetic products produced across the leaf (Roth-Nebelsick et al., 2001 ).
An underlying feature of note is that the network of xylem vessels runs parallel to the network of phloem vessels. Therefore, to properly appreciate the functionality of the vein architecture as a whole it is important to represent both the network's ability to conduct water from the petiole to the lamina and its capability to transport sucrose from the lamina to the petiole. This can only be achieved by allowing for the existence of both the phloem and the xylem sub-systems. In other words, it is important to represent both transport mechanisms as their respective roles perform in parallel. This is one of the objectives of our ongoing efforts. We note, however, that as the xylem and phloem networks do perform different functions it is equally interesting to identify conditions under which the two act independently, as it is to identify conditions under which the two influence each other, and naturally to understand the reasons for these opposing effects.
A large number of studies have reported on the relationship between leaf conductivity and vein architecture in conjunction with leaf shape. At a very basic level, leaf conductivity depends principally on vein density (Sack and Frole, 2006 ; Sommerville et al., 2012 ) and vein diameter (Cochard et al., 2004 ). But it has also been suggested that vein hierarchical architecture and vein tapering contribute to leaf conductivity (McKown et al., 2010 ). To what extent this is the case is still to be confirmed, but the number of connections among veins may be important (Brodersen et al., 2012 ). As for leaf shape, it is possible that the shape of lobed leaves confers the benefit of a high degree of conductivity by shortening the pathway toward sites of evaporation (Nicotra et al., 2011 ). Despite a high level of interest there has not been a systematic study of the effect of leaf shape or other important geometric characteristics, such as vein angle, on leaf water conductivity.
The majority of theoretical contributions to the study of plant vascular systems has focused on transport in stems or shoots. Early models considered xylem and phloem as isolated systems (Goeschl et al., 1976 ; Lhomme et al., 2001 ; Thompson and Holbrook, 2003 ; Steppe et al., 2006 ; Seki et al., 2015 ). More recent studies, however, explore the effects of the mutual influence of phloem and xylem in stems and roots of plants (Boersma et al., 1991 ; Daudet et al., 2002 ; Hölttä et al., 2006 , 2009 ; Lacointe and Minchin, 2008 ; Foster and Miklavcic, 2013 , 2014 , 2016 , 2017 , 2019 ; Nikinmaa et al., 2013 ). These models confirmed the considerable influence of the phloem-xylem interaction on water transport (and in some cases solute transport). While some theoretical studies included a leaf component in a whole-of-plant transport system, no detailed description of leaf venation was featured; leaf transport properties were usually represented by a single parameter corresponding to a whole-of-leaf phloem and/or xylem conductance or resistance contribution.
Only a few studies focus attention on detailed modeling of water transport in leaves. But even these models considered the xylem as an isolated pathway, in a similar fashion to early stem models. Meinzer et al. (Meinzer and Grantz, 1990 ; Meinzer et al., 1992 ) used the well-known Ohm's law analogy to calculate sugarcane leaf conductance based on measurements of transpiration flow and water potentials. This analogy was utilized by subsequent authors (Zwieniecki et al., 2004 , 2006 ). Xylem conductivity in another monocot plant, tall fescue Festuca arundinacea Schreb., was measured and compared to a theoretical estimate (Martre et al., 2001 ). The much more detailed model study of leaf vascular systems by Cochard et al. ( 2004 ) was then utilized by McKown et al. ( 2010 ) to study the impact of altering venation architecture traits. Measured pressure differences were used to model hydraulic architecture in dicot leaves of Laurus nobilis (Zwieniecki et al., 2002 ), while the pressure distribution in the xylem of a pine needle vein has also been modeled (Zwieniecki et al., 2006 ). North et al. ( 2013 ) developed a model for a monocot plant, tank bromeliad Guzmania lingulata , using leaky cable theory. What is common to all these studies is that the models employed only considered the xylem network.
In this paper we present and explore a detailed leaf model that features both the xylem and phloem networks. The xylem network model is the same as earlier models [particularly the model of Cochard, McKown and colleagues (Cochard et al., 2004 ; McKown et al., 2010 )]. Our additional phloem network parallels the xylem network and is intimately coupled to the latter via additional conduits, as in an actual leaf; the phloem sap is modeled to flow according to well-defined hydraulic and osmotic pressure gradients in the phloem network. The coupled xylem-phloem network system we have adopted aims to imitate the hierarchical architecture of angiosperms.
The focus of this paper is on evaluating the effect of leaf shape and vein geometry on the functionality of both the xylem and the phloem networks. In our study, we consider, specifically, the effect of changing leaf shape (the length-to-width ratio), vein angle (and consequently vein density), and individual vein conductivities. We address the question of what is the resulting distribution of sucrose (a 2D leaf area concentration map) and the resultant hydraulic pressure pattern across the leaf, where the latter is used here as an index that characterizes the transport of both water and sucrose out of the leaf.
2.1. Physical and Mathematical Model
The coupled xylem-phloem system we adopt is a significant extension of, but analogous to, the model developed by Cochard et al. ( 2004 ) and further utilized by McKown et al. ( 2010 ). In the original model the complex leaf vein architecture is presented as a two-dimensional network comprising xylem veins of different sizes (i.e., vein orders). The transport of fluid in the xylem and phloem systems is here modeled as a system of equations, founded on Darcy's law of plug flow (Batchelor, 1967 ): μ u = − k ∇ p , expressing the fact that in the conduit between two consecutive nodes the fluid velocity, u , is proportional to the pressure gradient across the conduit joining those nodes, with the vein conductance ( k /μ) being the coefficient of proportionality; here k is a fluid permeability (m 2 ) and μ is the fluid viscosity (Pa s), and the negative sign is consistent with flow from a point of high to a point of low hydraulic pressure. One of the main developments featured in our extended model is the addition of a parallel network of phloem veins, connected to the two-dimensional xylem network at corresponding nodes. In our model, every node (vein confluence) of the two-dimensional xylem system (blue points in the upper grid in Figure 1 ) is connected to a corresponding node in the phloem network (green points in the lower grid in Figure 1 ). In this phloem network we factored in the transport of sucrose solutes as well as water. The sucrose transport is influenced both by diffusive effects due to concentration differences between nodes, and by convective influences determined by hydraulic pressure differences between those nodes (see below).

The xylem (upper blue) and phloem (lower green) networks, and their connections. The black arrows indicate the possible flow directions (d) to or from a single node to nearest and next nearest neighbor nodes within each network (these are numbered d = 1 to d = 8). The orange conduit connects a node in the xylem network to its corresponding partner node in the phloem network. The light blue arrow indicates transpiration from a xylem node, while the light green arrow indicates sucrose loading to a phloem node. In the xylem network, only water is transported, while in the phloem network both water and sucrose are transported. In our computations, the nodes are numbered in a grid-like fashion: ( i, j ) = (1, 1)…( N, M ), as indicated by the red numbering. The identities of nearest and next-nearest neighbor nodes to node ( i, j ) are identified at the leaf creation stage and recorded for later recall.
At the ( i, j )th xylem node (hereafter abbreviated to ij , for i = 1, …, N, j = 1, …, M ), we apply the mass conservation constraint of zero net water flux (mmol s −1 ) out of the xylem node (i.e., total influx is balanced by total efflux). The conservation constraint asserts that the sum of the fluxes in the eight xylem conduit directions (see Table 1 and Figure 1 ), plus the flux from the xylem node to its corresponding phloem node, F i j - c x y l , and the transpiration flux, F i j - T x y l , is equal to zero.
Xylem flux directions (d).
| (1) | North | |
| (2) | North-east | |
| (3) | East | |
| (4) | South-east | |
| (5) | South | |
| (6) | South-west | |
| (7) | West | |
| (8) | North-west |
In the above equation, the transpiration flux ( F i j - T x y l ) is given as the product of a transpiration rate, E ij (m s −1 ), and the 2D area per grid point (i.e., area per transpiring node), a ij (m 2 ): F i j - T x y l = E i j a i j ( Figure 1 ). In the original model of Cochard et al. ( 2004 ), a xylem node only possessed links with its four nearest neighbor nodes. In our extension, we also consider connections with next nearest neighbor xylem nodes, as indicated in the figure.
Arguing in a completely analogous fashion and therefore at a consistent level of approximation, we have, at the ij th phloem node, the zero sum of water fluxes in the eight lateral directions, { F i j - 1 p h , … , F i j - 8 p h } , as well as the cross flow from the phloem node to its corresponding xylem node, F i j - c p h ( = - F i j - c x y l ):
Invoking a discrete, cross-sectional area-integral version of Darcy's law (expressed in current nomenclature) the flux between two consecutive xylem nodes is determined from the relation
In Equation (4), F i j - d x y l is the (signed) water flux from xylem node ij to the xylem node connected to it in the direction d . Lastly,
is the conductance of the conduit between those nodes in terms of its cross-sectional area, A , length, l , permeability k , and fluid viscosity, μ. L i j - d x y l is the local water permeability.
In the phloem, fluid motion is driven by hydraulic and osmotic pressure influences. Darcy's law must then be modified to include an osmotic pressure contribution resulting from a concentration difference:
where R is the universal gas constant (8.314 J mol −1 K −1 ), T is temperature (in degrees K), and C is the local concentration (mol m −3 ) of solute (sucrose) inside the sieve tube. The parameter γ is a proportionality constant.
In discrete form, the cross-sectional area-integral of the flux in the phloem becomes
where, in direct analogy with the xylem case, F i j - d p h is the (signed) fluid volume flux from phloem node ij to the phloem node connected to it in the direction d (one of eight neighbors). The position dependent parameter σ ij − d is called the reflection coefficient (Katchalsky and Curran, 1965 ; Kramer and Boyer, 1995 ; Foster and Miklavcic, 2014 , 2016 ). In Equation (6), σ ij − d = 0 since the solute movement is assumed not to be impeded (Kramer and Boyer, 1995 ). K i j - d p h = L i j - d p h A i j - d p h is the fluid conductance of the phloem conduit between the ij th node and its neighbor in the direction d , L i j - d p h is its water permeability.
The (signed) volume flux of water from the xylem to the phloem, F i j - c x y l , (or vice versa) is determined by the difference between the hydraulic pressure at the xylem node and the sum of hydraulic pressure and osmotic pressure at the corresponding phloem node, multiplied by the conductance of the conduit linking those nodes. This is expressed by the relation:
K ij − c is the conductance of the conduit between the two nodes, defined as in Equation (4) and σ ij − c is a reflection coefficient for this pathway, which is here set to unity (i.e., σ ij − c = 1). From this we see that even if the hydraulic pressures in the xylem and phloem networks are equal, the presence of a solution in the phloem will drive fluid from the xylem to the phloem network.
We note in passing that at the scale of a typical leaf it is legitimate to ignore the influence of gravity when calculating water fluxes.
In direct analogy with the water fluxes, we assume a conservation of sucrose fluxes. Namely, we specify that the sum of all sucrose fluxes into and out from a given node in the eight lateral directions ( S ij −1 , ..., S ij −8 ), plus a contribution from sucrose loading into the sieve tube ( S ij − L ) should be equal to zero:
In the above equation, the sucrose loading into the sieve tube ( S ij − L ) is calculated as S ij − L = Λ ij a ij , where Λ ij is the local sucrose loading rate per unit area and a ij is the 2D grid area assigned to that node ij . We do not assume there to be any local depletion of sucrose due to consumption by metabolic processes, i.e., we assume no unloading of sucrose into leaf mesophyll tissue (although this may easily be added). On the other hand we do assume photosynthetic activity (sucrose production) at all nodal points and in all veins. Sucrose flow is driven by a combination of convection, which is proportional to the total pressure difference between neighboring phloem nodes, and diffusion, which depends on the sucrose concentration difference between those same two phloem vein nodes. This sum is expressed by the equation,
S ij − d is defined as the mass flux (mol s −1 ) from node ij to the neighbor node in the d direction, F i j - d p h is the corresponding volume flow of water, σ ij − d = 0, and D su is the free diffusion sucrose diffusivity (m 2 s −1 ), and v μ is the molal volume of water. All the symbols used in the model are summarized in Table 2 .
Summary of parameters and function variables (at node ij , subscripts not shown).
| Xylem hydraulic pressure | MPa | |
| Phloem total pressure | MPa | |
| Π | Phloem osmotic pressure | MPa |
| Xylem water permeability | m | |
| μ | Fluid (water) viscosity | Pa s |
| Phloem sucrose concentration | mol m | |
| Xylem water flux | mmol s | |
| Phloem water flux | mmol s | |
| Phloem sucrose flux | mol s | |
| Xylem conductance | mmol s MPa | |
| Phloem conductance | mmol s MPa | |
| Phloem/xylem connection conductance | mmol s MPa | |
| Transpiration flux | mmol s | |
| Sucrose loading flux | mol s | |
| Transpiration rate | mmol s m | |
| Λ | Sucrose loading rate | mol s m |
| Area per grid point | m | |
| Phloem vein cross-sectional area | m | |
| Distance between nodes | m | |
| Sucrose diffusivity | m s | |
| σ | Reflection coefficient | - |
| Molal volume of water | m mmol | |
| Temperature of the leaf | K | |
| Universal Gas constant | MPa m mol K |
2.2. Boundary Conditions
At every node, except the petiole, we adopt Dirichlet-type boundary conditions in which we assume fixed values of transpiration flow at all xylem nodes, and fixed sucrose loading at phloem nodes. By adopting Dirichlet boundary conditions, we essentially circumvent the need to calculate the water potential in the mesophyll wherein conductances in the narrow transpiration pathway between xylem conduits and mesophyll are uncertain. For the transpiration rate, E ij , we set (for comparative reasons) a baseline or reference value of −2.00 mmol s −1 m −2 , although in our exploration we also consider lower values. For the sucrose loading rate, Λ ij , we assigned a reference value of 2.78 × 10 −7 mol s −1 m −2 , although in this case too we consider lower values. With this choice of parameters we arbitrarily assume that approximately one-third of all photosynthate production is loaded into phloem sieves given an ordinal leaf photosynthesis production of 1.00 × 10 −6 mol (CO 2 ) s −1 m −2 .
At the petiole, the hydraulic pressure in the xylem we arbitrarily set to 0.00 MPa, while the pressure in the phloem was set to 0.20 MPa. With regard to sucrose transport, we adopt a zero Neumann boundary condition at the petiole, which is equivalent to stating that the sucrose concentration at the petiole is equal to the sucrose concentration at the exterior phloem “node” connected immediately to it.
The choices of these types of boundary conditions, as well as the actual values set, are purely for pragmatic reasons as we wish to explore the effects of other variables on water and solute transport. In subsequent studies we shall adopt more physically relevant conditions as we move from the present steady-state model to a time-dependent model.
2.3. Leaf and Vein Structure
In the present paper we shall often refer to so-called baseline or reference models of leaf shape and vein architecture. These correspond to the Laurel leaf shape and vein structure adopted and studied in the work of Cochard et al. ( 2004 ). The first-order vein is marked by the red line in Figure 2 , and depicts the main vein connected directly to the petiole at the base of the leaf. The second-order veins shown in purple in Figure 2 , branch directly off from the first-order vein at regular intervals and at a finite, acute angle, as measured from the main vein. Higher order veins (third, fourth, and fifth order veins) are arranged on and aligned with a rectangular lattice of nodal points (blue dots in Figure 2 ). They are distinguished by their frequency of occurrence as well as by their cross-sectional areas and permeabilities. The third-order veins (marked by orange lines in Figure 2 ) are distributed, both vertically and horizontally, at a frequency of every six nodal points. The two narrowest veins, the fourth and fifth order veins, marked by yellow and black lines, respectively in Figure 2 , occur at the same frequency, but with their respective networks displaced by one rectangular grid unit so as to appear alternately in a similar rectangular pattern. On the main vein, which is the leaf's symmetry axis, the internal node which is connected directly to the petiole is set a distance of 5 mm from the petiole.

Vein architecture of the model leaf. In our simulations, the angle of the second-order vein to the main vein is varied. Accordingly, the vein distances L v 1 and L v 2 are also varied (alternately) to ensure a constant total vein length.
In our study we considered the effect on the xylem hydraulic pressure, the phloem pressure, and the phloem distribution of sucrose, of varying the angle of the second-order veins relative to the main vein direction. In order to make a reasonable comparison of quantities under different simulation conditions the total lengths of the veins of all orders were kept fixed. Two factors need considering in implementing this vein length constraint.
First, increasing the angle θ of the second-order veins reduces their lengths, since the distance from the main vein to the edge of the leaf along a second-order vein becomes shorter. Therefore, to maintain the total length of second-order veins at all angles we reduced the interval between the second-order veins and increased their number as the angle of the veins increased. As a reference state we assign the case of θ = 0° where the second-order veins are perpendicular to the first-order vein; the vein spacing was then set at 10.00 mm, while the distance between the very first second-order vein and the petiole was set at 5.00 mm ( Figure 2 ). The intervals, L v 1 and L v 2 , between connection points of alternate second-order veins (shown in Figure 2 ) were then adjusted (to different extents) in order to keep constant the total length of second-order veins. The total length of second-order veins was set at 0.74 ± 0.01 m S.D.).
2.4. Parameter Settings
The main aim of the study was to evaluate the effects of changing leaf shape and changing vein angle on the indices that reflect water flow into and through leaves, as well as on the sucrose flow from leaves. We designed three (virtual) leaf shapes that differ in their length-to-width ratio. These are illustrated in Figures 3 – 5 . As mentioned earlier, the reference leaf shape with which we compare other shapes and vein architectures is that of the Laurel leaf described in Cochard et al. ( 2004 ). For this leaf we have assumed a leaf length of 16.00 cm and maximum leaf width of 6.47 cm. The other cases we consider are either wider (8.09 cm) or narrower (5.39 cm) leaves, with leaf lengths being, respectively, 80 and 120% of the length of the reference leaf shape. In all three cases the leaf areas are maintained at 73.35 cm 2 . For each leaf shape, our simulations were conducted assuming the following 2 nd order vein angles (differing from each other by approximately 6°): 0.00°, 7.13°, 14.03°, 21.80°, 30.96°, 36.87°, 40.60°, 45.00°, 49.40°, 53.13°, 59.06°, 68.20°, and 75.96°. The irregular increments are due to the need to adapt angles to our discrete grid. The vein angle of θ = 0° corresponds to our reference state of perpendicular 2 nd order veins.

Leaf area distributions of xylem hydraulic pressure. In this and the next three figures, (A) depicts the wide leaf, (B) shows the reference leaf, while (C) shows the narrow leaf distribution. In addition, second-order veins are 45° to the main vein (i.e., 45° to the reference state of perpendicular veins). Parameter conditions under which the simulations were performed are summarized in Table 3 and the core values in Table 4 .

Leaf area distribution of sucrose concentration in the phloem network. (A) Wide leaf, (B) reference leaf, and (C) narrow leaf. Details as in Figure 3 .
As for the choice of values of individual vein conductances, we defer to the values adopted in (Cochard et al., 2004 ). Consequently, the 5 th order veins have the lowest conductance values, with the conductances increasing with decreasing vein order in correspondence with the envisaged increases in vein diameter. The specific reference values are tabulated in Table 3 .
Xylem vein conductivities; phloem conductivities are a factor of 3/100 lower.
| MPa ) | ||
|---|---|---|
| 1 | 1.00 × 10 | 15.50 |
| 2 | 5.00 × 10 | 11.30 |
| 3 | 6.00 × 10 | 7.50 |
| 4 | 4.00 × 10 | 7.20 |
| 5 | 4.00 × 10 | 4.34 |
Apart from studying the effect of leaf shape and vein angle we also consider the influence of 2 nd order vein conductance. To this end we performed simulations with 2 nd order vein conductances that were 5 times larger and 5 times smaller than the reference value given in Table 3 .
It is important to point out here that, as suggested by Daudet et al. ( 2002 ), we have assigned values for the conductances in the phloem network that were smaller than the conductances of corresponding veins in the xylem by a uniform factor of 3/100 (Not being the focus of this paper, with this single choice of relation between the two networks it is not possible to draw any significant conclusions on the interaction between the two networks).
A uniform value of conductance for the conduits between the phloem and xylem networks was set at 0.50 mmol s −1 MPa −1 (Daudet et al., 2002 ). Other fixed parameters are the gas constant as R = 8.31 × 10 −6 MPa m 3 mol −1 K −1 , sucrose diffusion coefficient as D s u = 5 . 22 × 1 0 - 10 m 2 s −1 , water volume per mmol as v μ = 18 . 00 × 1 0 - 9 m 3 mmol −1 , and leaf temperature as T = 293.00 K.
Finally, under the reference simulation condition, the sucrose loading rate in the phloem network was set initially to be uniform across the grid. However, given that leaf conductance, hydraulic pressure, and sucrose concentration may vary if the loading rate is not uniform, we considered also a case of non-uniform sucrose loading profile: a linear gradient starting with a 50% higher loading rate at the leaf base than the average, linearly decreasing to a 50% lower loading rate at the leaf tip. The average loading rate in both cases was kept the same. The summary of the parameter values of the base experimental setting and optional settings are shown in Table 4 .
Summary of system parameters with core and additional values.
| Inter-vein interval ( 1; 2) | (mm) | 10.00 | 15.00 |
| Sucrose loading gradient (∂ − /∂ ) | (%) | 0.00 (uniform) | 1.0 (linear gradient) |
| Uniform transpiration rate ( ) | (mmol s m ) | -2.00 | -1.00; 0.00 |
| Phloem/xylem conduit conductance ( − ) | (mmol s MPa ) | 0.5 | 0.0005 |
| Second-order vein conductance ( = ) | (m mmol s MPa ) | 5.00 × 10 | 1.00 × 10 ; 2.50 × 10 |
In this work, we present the majority of our results in terms of the average leaf xylem hydraulic pressure, defined as
An analogous expression applies to the average phloem pressure, 〈 p l e a f p h 〉 .
By solving Equations (2), (3), and (7), we determined values for p i j x y l , p i j p h , and C ij for each node. Equations (2), (3), and (7) were solved numerically using the Matlab (Mathworks, 2020 ) non-linear system solver fsolve , with the default setting employing the Trust-Region Dogleg Method.
3.1. Pressure and Solute Distribution Patterns
Figure 3 shows the 2D distribution of the xylem hydraulic pressure for our three leaf shapes: the wide leaf ( Figure 3A ), the reference leaf ( Figure 3B ), and the narrow leaf ( Figure 3C ), assuming that 2 nd order veins are 45° to the main vein (i.e., 45° to the reference state of perpendicular veins). Although the boundary conditions and total vein lengths were identical in these three cases, the pressure distributions were different. While all three cases showed similar qualitative behavior: the xylem hydraulic pressure was lower at the leaf tip and higher closer to the petiole, the rate of decrease of hydraulic pressure (from the petiole to the leaf tip) was greater for narrower leaves. In contrast, Figure 4 shows the distribution of hydraulic pressure in the phloem network. An inverse correspondence to that of the xylem hydraulic pressure distribution is apparent, the phloem pressure increased from the leaf petiole to the leaf tip. Once again we found that the narrower was the leaf, the steeper was the gradient. On the other hand, the area-average phloem pressure in the three leaves varied only marginally from 0.33 MPa for the wide leaf, to 0.34, and 0.36 MPa, respectively, for the reference leaf and the narrow leaf.

Leaf area distribution of hydraulic pressure in the phloem network. (A) Wide leaf, (B) reference leaf, and (C) narrow leaf. Details as in Figure 3 .
The data in Figures 3 , ,4 4 shows that while water in the xylem flowed from the petiole out toward the extremities, water in the phloem flowed in the opposite direction, from the extremities to the petiole. This suggests there was a degree of circulation between the two networks, on a length scale of the whole leaf.
Figure 5 shows the sucrose concentration profiles in the three leaf shapes. The predicted sucrose concentration pattern mimicked that of the pressure pattern in the phloem: higher at the extremities and lower at the petiole. However, the solute concentration gradient appears somewhat different in shape compared with the pressure gradient suggested in Figure 4 , with the concentration being more uniform over a larger part of the leaf before decreasing very rapidly over the one-third leaf section closest to the petiole. This pattern is reflected also in the larger differences between the area-average sucrose concentrations found for the three leaf shapes: 159.30, 169.37, and 181.82 mol m −3 for the widest, the reference and the narrowest leaf, respectively.
3.2. Effect of Vein Angle and Leaf Shape
In this section, we compare the effect of varying the angle of second-order veins (with respect to the main vein) on the xylem hydraulic pressure, phloem hydraulic pressure and sucrose concentration. As mentioned earlier the reference state (θ = 0) has 2 nd order veins being perpendicular to the main vein. Figure 6 shows the effect on the distribution of hydraulic pressure in the xylem for a leaf of a given shape (the reference shape) as a result of varying the angle of orientation of second-order veins. Note that increasing θ (from θ = 0 corresponding to perpendicular 2 nd order veins) means veins become more aligned with the main vein. Although 13 different θ angles were sampled, only four case distributions are shown here. All cases are summarized in Figures 7 , ,8, 8 , where we have plotted area-average quantities against vein angle.

Leaf area distribution of xylem hydraulic pressure and its response to varying angle of second-order veins. The resulting patterns for four θ angles are shown in (A–D) . The definition of the angle θ is shown adjacent to (D) . The pressure scale is given on the right. Other parameter details are as in Figure 3 .

The functional relationship between leaf area-average, xylem hydraulic pressure and second-order vein angle. The different curves refer to the narrower/longer leaf (blue dashed line), the reference leaf (green dotted line), and the wider/shorter leaf (red solid line). The parameter values for these results are as in Figure 3 .

The functional relationship between leaf area-average, hydraulic pressure in the phloem, and second-order vein angle. Curve descriptions and simulation conditions are as in Figure 3 .
While the quantitative differences in the area-average xylem hydraulic pressure, over the angle range studied, were not great, it is nevertheless significant that the effect of a monotonic decrease in 2 nd order vein angle was a non-monotonic response. This non-monotonic response itself appeared to be non-uniform across the leaf as can be observed in Figure 6 , where close to the petiole and close to the main vein, the response appeared to be directly proportional. Elsewhere, e.g., toward the leaf edge, the effect was clearly non-monotonic. Given that the area-averages in Figures 7 , ,8 8 were themselves non-monotonic, it would appear that the observed non-uniform effect away from the symmetry line dominated.
The summary relationships between second-order vein angle and leaf area-average xylem hydraulic pressure is shown in Figure 7 . In concert with the specific case of 45° aligned 2 nd order veins (shown in Figures 3 – 5 ), the area-averaged hydraulic pressure was generally lower the narrower was the leaf, at all angles. We also noted that the peak in the average pressure was around 45°, although the angle at which the peak was reached was not constant (varying from 40.60° for the widest leaf to 53.13° for the narrowest leaf). Moreover, it is distinctive that the shapes of the curves were not symmetric about the peak position, with a steeper decay in average hydraulic pressure for larger angles (i.e., for veins more acutely aligned with the main vein).
As may be expected, the response of the leaf area-averaged phloem hydraulic pressure ( Figure 8 ) was approximately the inverse of that of the xylem hydraulic pressure. The average phloem pressure was higher in the narrowest leaf, at all angles of 2 nd order veins. This is consistent with the earlier finding that the solute concentration was higher generally in the narrowest leaf. Interpreting the pressure as a potential for transporting photosynthate solutes out of the leaf via the phloem (by diffusion), the results suggest that narrow leaves have (and probably need) a greater potential to convey these solutes than do wider leaves. Moreover, leaves attain a still higher pressure by increasing or decreasing the angle of their 2 nd order veins relative to the roughly 45° orientation.
3.3. Effect of Vein Angle and Leaf Shape Under Other Conditions
Steady state simulations investigating the effect of 2 nd order vein angle on the leaf hydraulics were also performed under other external condition settings (see the final column in Table 4 ). These included different pressure boundary condition at the petiole, evaporation rates, E , sucrose loading rate, S ij − L , and vein conductances. As these results appear qualitatively similar to the results shown above, we simply comment here on the simulation outcomes and relegate the figures to the Supplementary Material .
With regard to the influence of transpiration rate, we considered rates of transpiration reduced (in magnitude) from our reference value of −2.00 mmol s −1 m −2 to E = −1.00 and to E = 0.00 mmol s −1 m −2 (i.e., no transpiration). Even under the extreme condition of no transpiration, the results of leaf area-average xylem hydraulic pressure and area-average phloem total pressure were qualitatively the same as found with a transpiration setting of E = −2.00 mmol s −1 m −2 ) (see Supplementary Figures 1 – 3 ). This suggests that the nature of the response is intrinsic to the leaf itself and not a consequence of transpiration.
Adjusting the pressure boundary condition from P = 0.0 MPa to P = −2.0 MPa, while maintaining the same difference in pressure between xylem and phloem at the petiole (so the phloem pressure was then −1.8 MPa) resulted only in a magnitude change in the average xylem pressure but no relative difference in either the trend with 2 nd order vein angle or the relation between the different leaf shapes ( Supplementary Figure 4 ).
Thirdly, as another external condition that might influence the behavior of the pressure distributions, we considered a non-uniform (linear gradient) sucrose loading rate, ranging from 50% higher than the average at the leaf base to 50% lower at the leaf tip. Although the distribution pattern of sucrose concentration with this uneven sucrose loading was different from that found with a uniform distribution (see Supplementary Figure 5 ), the relationships between the leaf area-average xylem hydraulic and phloem hydraulic pressure, the vein angle (or leaf shape) were similar to those found with a uniform sucrose loading ( Supplementary Figures 6 , 7 ).
Since previous whole-leaf models treated only the xylem network (Meinzer and Grantz, 1990 ; Meinzer et al., 1992 ; Cochard et al., 2004 ; Zwieniecki et al., 2004 , 2006 ; McKown et al., 2010 ) we thought, in our fourth exercise, to see whether the hydraulic response in the leaf xylem to a variation of 2 nd order vein angle was influenced by the connection to the phloem network. We considered one case where the conductance of the phloem/xylem conduit connection was significantly reduced (by three orders of magnitude) to virtually isolate the xylem and phloem networks. Interestingly, the leaf area-average xylem hydraulic pressures were largely unaltered ( Supplementary Figure 8 ). As for the leaf area-average phloem pressure, although the magnitudes were lower than under a higher xylem/phloem conductance, the resulting dependencies on 2 nd order vein angle (or leaf shape) were qualitatively similar ( Figure 9 ). It is a little premature to draw any general conclusion from this single result as the outcome may be a consequence of the particular set of all parameters.

The functional relationship between leaf area-average, hydraulic pressure in the phloem and second-order vein angle. Curve descriptions and simulation conditions are as in this figure except with a xylem/phloem conduit conductance of K i j - c = 5 × 1 0 - 4 mmol s −1 m −2 (i.e., reduced by a factor of 10 3 ).
To address the question of whether the higher order veins play a critical role in the xylem hydraulic pressure distribution, in our fifth effort we repeated our simulations for the two cases of either both 4th and 5th order veins having the higher of the two original conductivities ( Supplementary Figures 9 , 10 ), or both having the lower of the two original conductivities ( Supplementary Figures 11 , 12 ). Although the magnitudes were (again) different in either case, the qualitative behavior of the leaf area-average xylem hydraulic pressure to 2 nd order vein angle (and leaf shape) were similar. The leaf area-average phloem hydraulic pressure followed suit. Somewhat unsurprisingly, increasing the conductances of the higher order veins, such as fifth order veins ( Supplementary Figure 9 ), demonstrates a weakening of the influence of the 2 nd order veins. As higher order veins become more conductive the dependence of the 2 nd order vein angle becomes less pronounced since the distribution of water (and solutes) is more equally shared between 2 nd order and higher order veins. As could be expected, by lowering the conductivities of higher order veins (relative to 2 nd order veins) the effect of 2 nd order vein angle ( Supplementary Figure 11 ) was enhanced.
Somewhat on the same theme, we evaluated the leaf response for different conductances of the 2 nd order veins themselves. Figure 10 shows the dependence on 2 nd order vein angle of the leaf area-average xylem hydraulic pressure for a reduced 2 nd order vein conductance of one-fifth that of the core model setting. While the overall magnitudes were very low, it is nevertheless distinguishable in that, within a class of leaf shape, the response to a change of angle was weakened: the degree of variation was 2, 1.3, and 0.9% for the wide leaf, the reference leaf and the narrow leaf, respectively. This result was also consistent with the diminished role of 2 nd order veins when the conductances of the higher order veins were increased relatively speaking ( Supplementary Figures 9 , 10 ). As previously, the phloem hydraulic pressure shows an inverted response (see Supplementary Figure 13 ).

The functional relationship between leaf area-average, xylem hydraulic pressure, and second-order vein angle. Curve descriptions and simulation conditions are as in Figure 7 except with a uniform second-order vein conductance of K xyl = 100 K ph /3 = 1 × 10 −4 mmol s −1 m −2 (i.e., a factor of one-fifth that of the core value).
Not surprisingly, in the contrasting case of an increase in 2 nd order vein conductance, the dependence on vein orientation was enhanced, as shown in Figure 11 for which the conductance of second-order veins was increased by a factor of 5. The estimated degrees to which the average xylem pressure changed with angle were 11, 12, and 13% for the wide leaf, the reference leaf, and the narrow leaf, respectively. The angle at which the xylem pressure peaked changed significantly: 49°, 68°, and 76°. Again the angular dependence of leaf area-average phloem pressure showed an inverted relationship to that of the leaf area-average xylem hydraulic pressure (see Supplementary Figure 14 ).

The functional relationship between leaf area-average, xylem hydraulic pressure, and second-order vein angle. Curve descriptions and simulation conditions are as in Figure 7 except with a uniform second-order vein conductance of K xyl = 100 K ph /3 = 2.5 × 10 −3 mmol s −1 m −2 (i.e., an increase by a factor of 5 of the core value).
4. Discussion
This study of a coupled xylem/phloem model of an angiosperm leaf with a reticulated vein network focused on the effect of specific geometrical features (leaf shape and second-order vein angle) on the hydraulic capability of the interconnected xylem and phloem system. The indices we have used to quantify these hydraulic capacities, namely leaf area-average xylem and phloem pressures, have some experimental relevance. For example, since most of the calculations were performed under the condition of constant transpiration, our results could be re-formulated as the experimentally acceptable definition of leaf conductance (Cochard et al., 2004 ; Zwieniecki et al., 2004 ; Sack and Holbrook, 2006 ; Prado and Maurel, 2013 ) by simply dividing the (constant) transpiration flux by the difference between the leaf-area average hydraulic pressure and the petiole pressure. Since the hydraulic pressure in the xylem has been determined for the coupled xylem-phloem system, the contributions of flows in both the xylem and the phloem have been included.
Based on our chosen indices, many interesting facts about the leaf response to factors such as leaf shape and vein angle come to light. These are discussed below. In this discussion it is necessary to keep in mind that the angiosperm model we have adopted, irrespective of the angle of the 2 nd order veins, remains that of a reticulate vein structure (vasculature) and is therefore exclusively pertinent to that of a dicot (eudicot, Rudall, 2007 ) leaf. Consequently, although we may in the discussion below speculate on implications for monocot leaves, the connection remains tenuous since leaves of true monocots have parallel vein structures connected to the petiole in a significantly different way. Here, we have not simulated monocot leaves.
We remark further that although convenient and of arguable experimental relevance, the boundary conditions of a stipulated constant transpiration rate in the xylem and a constant sucrose loading rate in the phloem network were somewhat artificial. Transpiration is firstly a time dependent phenomenon and secondly its magnitude is very much linked to the surrounding atmospheric condition. Setting a condition of given atmospheric pressure external to the leaf and monitoring the leaf's response in terms of the rate at which the leaf—as a whole—transpires would seem more in line with in vivo circumstances. But, as the focus of interest of the paper is on the internal movement of water we have chosen, for simplicity, to set the rate of transpiration as a boundary condition. Similarly, the production of photosynthetic compounds is also time dependent and a function of external variables (light intensity). However, here too we have simply set a fixed rate of production in order to explore the ramifications on the internal re-distribution of sucrose.
We should also point out that our study of the effect of 2 nd order vein angle was conducted under the constraint of constant total vein length. In this convolved system, we cannot alter vein angle at constant length without changing other structural variables (such as the spacing of connection points along the midrib vein and the total number of 2 nd order veins. On the other hand, by keeping the vein conductance and total vein length constant while altering the vein angle, we maintain the flow resistance through those veins; the hydrodynamic condition within the 2 nd veins remains unaffected (this is true as we have not included the feature of vein tapering).
To exhibit the contrast, in Supplementary Figure 15 , we show the case where we have altered vein angle in the absence of the total length constraint, instead keeping fixed the total vein number and the spacing of branching or connecting points. The different conditions resulted, for the long leaf, in a monotonic increase in average xylem pressure. For shorter and wider leaves there is an optimal vein angle. Presumably, this arises from the fact that at large θ angles a significant area of the bottom part of the leaf is not serviced by 2 nd order veins, so water distribution to this region must be performed by the more highly resistive, higher order veins.
As a final general comment, in earlier papers by Foster and Miklavcic (Foster and Miklavcic, 2013 , 2014 ) transport of water and solutes through the symplast and apoplast of living root tissues was treated as single pathway. Later efforts differentiated the two pathways Foster and Miklavcic ( 2013 ). In the present study of passive transport through parallel 2D networks, the leaf xylem forms part of the apoplast while the leaf phloem has been assumed to be solely the symplast. That is, we have modeled flow through the phloem as a single pathway when in real leaves the phloem (as well as surrounding living tissues such as the bundle sheath, sheath extension, the mesophyll, etc.) comprises both a symplastic pathway via cells that are interconnected by plasmadesmata and an apoplastic pathway through the porous cell wall region external to the contiguous plasmalemma network. The parameter values we have used to represent the conductivities of the phloem veins are therefore in some sense a weighted average of the conductances typical of the two phloem pathways. This idea has been discussed in detail in Foster and Miklavcic ( 2013 ). In a future model in which we include the mesophyll and other tissue elements we shall differentiate the two transport pathways.
For the same leaf shape (and vein conductivities) as that modeled by Cochard and co-workers (Cochard et al., 2004 ), we point out that our predicted hydraulic pressure distribution was qualitatively similar to their water potential distribution. Of course, since the detailed modeling of the vein network used in this study was somewhat different from that adopted in Cochard et al. ( 2004 ), the finer details of the distribution pattern were understandably slightly different.
We found the most important influencer of the xylem hydraulic pressure distribution to be the leaf shape. The leaf area-average xylem water pressure was lowest in the narrowest leaf, at all angles ( Figure 7 ). Since the leaf areas and total vein lengths were fixed properties in all leaf shapes adopted in this study, this outcome cannot be attributed to differences in either total transpiration volume per unit time or total length of travel of water. This finding was true under all conditions studied, as evidenced also by Figures 10 , ,11 11 (e.g., see also Supplementary Figures 1 , 4 , 6 , 9 , 11 ).
With regard to the angle of 2 nd order veins, we have consistently found that at a vein angle within a small angular range roughly centered at 45° ( Figure 7 ) the leaf area-average xylem hydraulic pressure is maximized. This finding was irrespective of external conditions of, say, transpiration and sucrose loading rates ( Supplementary Figures 1 , 4 , 6 , 8 ). However, we found that the response to vein angle exhibited a strong dependence on vein conductivity. For low 2 nd order vein conductances [lower than that adopted by Cochard et al. for a Laurel leaf (Cochard et al., 2004 )], the response overall became less dependent on angle, indeed largely independent of angle in the range 0−45° from the perpendicular. Even beyond the upper limit of the latter range (at which the xylem pressure was a maximum) the dependence of the decay with angle was weaker ( Figure 10 ). For high 2 nd order vein conductances, on the other hand, there was a strong linear dependence on angle up to a maximum pressure, which appeared at significantly larger angles [for the narrow leaf the angle at which the maximum occurred was 76° ( Figure 11 )]. Given other findings, these traits are relative the conductivities of higher order veins.
In the phloem, we have consistently found that the two-dimensional phloem pressure distribution showed an inverted behavior to that of the xylem hydraulic pressure distribution ( Figure 4 ). A similar inverted response is also exhibited by the sucrose distribution ( Figure 5 ). So, in a direct mirror reflection of the xylem hydraulic pressure, the narrower the leaf, the higher the phloem pressure, for all 2 nd order vein angles and under all the other conditions we've investigated, Figures 8 , ,9 9 (see also Supplementary Figures 2 , 3 , 7 , 10 , 12 – 14 ).
We infer from these results the important consequence that the angle of second-order veins, relative to the main vein, is a significant factor governing the efficient flow of water across a leaf, depending only on intrinsic vein properties such as the vein conductance and vein number and its connection with higher-order veins, but not on external factors such as transpiration rate or sucrose loading rate.
With our simulations were conveniently conducted at fixed rates of transpiration and sucrose loading (mostly uniformly across the leaf), it is possible to draw some further inferences.
To achieve the same constant rate of evaporation over the leaf, the xylem pressure must reach a much lower (more negative) value in the narrow leaf than in the case of the wider leaf case, This points to a less efficient xylem water transport system in narrow leaves. The (poor) efficiency is maximized when the veins are aligned roughly 45° to the main vein, although this depends on vein conductivity. The architecture is least efficient in transporting water when the 2 nd order veins are perpendicular to the main vein. Again, one imagines that narrow leaves would be more suited to having a parallel vein structure. Arguing similarly, the constant production of sucrose leads to a higher concentration distributed across the narrow leaf at steady state compared with the two wider leaves. This too suggests that narrow leaves are less effective in passively translocating photosynthates out through the petiole. Here, again it would be interesting to compare this narrow, reticulated leaf result with that of a monocot's parallel vein system to see whether there may be an evolutionary advantage of having the latter system in a leaf that is narrow.
As a final comment we might mention that one utility of a coupled xylem/phloem model is its potential to evaluate the degree to which water circulates between the xylem and the phloem networks. The degree to which plant nutrients are transported into the leaf in the xylem stream and delivered to sites in the phloem and mesophyll tissues will largely depend on this circulation flow. So, while membrane transporters of elemental nutrients are important to effect the transfer of nutrients into the symplast and hence living cells, the stream carrying the nutrients to membrane sites is critically important (Yamaji and Ma, 2014 ). Although we have not fully investigated this aspect here, our model represents an essential step to understanding this complex circulatory dynamic. In Supplementary Figure 16 , we show an area map of the difference between xylem water hydraulic pressure and phloem total pressure. The difference is non-uniform with the xylem pressure generally higher than the total phloem pressure particularly near the leaf petiole. The leaf area-average pressure difference is shown in Supplementary Figure 17 ). Any further comments will have to wait until for a more representative model of the complicated dynamics of water and solutes in the xylem and phloem (Foster and Miklavcic, 2016 , 2017 ; Sakurai et al., 2017 ).
5. Concluding Remarks
Despite the model resources developed in this work, it is not possible to draw authoritative conclusions on the evolutionary implications of leaf shape. As such, we cannot speculate on some questions such as why a leaf is broad or narrow. It is well-recognized that a very broad spectrum of leaf shapes in the angiosperm class of plants exist. The shape of leaves is a result of a complex combination, cooperation and competition between various external and internal influences. Which factor is most influential, if any, in determining the evolution of a given shape cannot be addressed here. For the moment, our model does not incorporate important external factors such as degree and duration of light intensity, temperature, air moisture, etc. On the other hand, what we can do is speculate, for a given shape, on what vein arrangement is optimal. In this regard our investigation has complemented the many early studies leaf shape evolution that have focused more on the edge shape of leaves, but also on the vasculature system (Cochard et al., 2004 ; Sack and Frole, 2006 ; McKown et al., 2010 ; Nicotra et al., 2011 ; Sommerville et al., 2012 ; Tsukaya, 2018 ). Those studies did not consider the contribution of vein angle, nor did they indicate that it could be one of the more important geometric characteristic. We believe that our model study contributes to the body of knowledge and improved understanding of how vein angles feature in an evolutionary perspective.
On the whole, our results suggest that it may be of some evolutionary hydraulic advantage for plants to have broader leaves. As for narrow leaves it appears that the disadvantage can be minimized if the 2 nd order veins (at least) are both highly conductive and arranged at small acute angles to the main vein. If the alignment is too great, however, (approaching a parallel vein system) the efficiency again decreases. Although we did not model the parallel vein system itself, one can conjecture that from this point a narrow leaf might well be better suited to have more a parallel 2 nd order vein arrangement.
In a future exercise it should be possible to compare our theoretical results with those measured on real leaves, although a comparison would require a good deal of quantitative anatomical and physiological information such as vein network structure and hierarchy and vein conductances. One means of bringing a comparison about would involve a series of measurements of water potential as a function of transpiration (and other external conditions) across many different species, and for us to compare our representative calculations based on the quantified vein architectures of those species. Alternatively, based on the higher level predictions of our model, we imagine the possibility of a collecting information on leaf size and shape, and leaf vein architecture from leaves from different locations around the world, and then correlate this information with the climate at their origin to see if our general conclusions (such as long thin veins having more acute 2 nd order vein angles) are verified.
The model we have utilized in this work presents a relatively simple description of actual vein architecture. No account has been taken of details such as the curving and tapering of veins, which are features of eucamptodromous venation. Moreover, branched veins as in craspedodromous and actinodromous vascular systems (Hickey, 1973 ) were not considered in this study. Secondly, we only simulated systems using (mostly) the vein conductivities referred to in the works of Cochard et al. ( 2004 ), although other 2 nd order vein conductivities were also considered. But to properly understand the functionality and evolution of angiosperm vein systems, we aim in our future investigations to entertain more accurate vascular architectures with measured vein conductivities for different species. Nevertheless, even with the simple geometric vein arrangement we have employed in the model that we have presented we are able to show some interesting consequences of leaf venation. The present study is our initial attempt toward understanding the evolution of the complicated vein systems and leaf shapes of plants. In future work, the model will be improved to also feature more tissue-specific details such as the bundle sheath surrounding veins and the mesophyll. That level of elaboration is needed in order to include a more detailed representation of both symplastic and apoplastic pathways for water and solutes.
Data Availability Statement
Author contributions.
The authors were equal contributors to the design of simulation experiments, analysis of results and drafting of the paper. SM designed the model. GS performed the simulations.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Dr. Herve Cochard, INRA, UMR-PIAF, Université Blaise-Pascal, for providing his K_leaf program code and accompanying experimental data (neither of which were actually used in this paper). We would also like to thank Galena Safonova and Kylie Foster for initial discussions and efforts during an early stage of this project.
Funding. This project was supported by the Australian Research Council (Discovery project grant DP200103168) and the Grains Research and Development Corporation and by JSPS KAKENHI grants (16H06296 and 19H03085).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.615457/full#supplementary-material
- Batchelor G. K. (1967). An Introduction to Fluid Dynamics . Cambridge: Cambridge University Press. [ Google Scholar ]
- Boersma L., Lindstrom F., Childs S. (1991). Model for steady state coupled transport in xylem and phloem . Agronomy J . 83 , 401–408. 10.2134/agronj1991.00021962008300020028x [ CrossRef ] [ Google Scholar ]
- Brodersen C. R., Roark L. C., Pittermann J. (2012). The physiological implications of primary xylem organization in two ferns . Plant Cell Environ . 35 , 1898–1911. 10.1111/j.1365-3040.2012.02524.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cochard H., Nardini A., Coll L. (2004). Hydraulic architecture of leaf blades: where is the main resistance? Plant Cell Environ . 27 , 1257–1267. 10.1111/j.1365-3040.2004.01233.x [ CrossRef ] [ Google Scholar ]
- Daudet F. A., Lacointe A., Gaudillere J., Cruiziat P. (2002). Generalized Münch coupling between sugar and water fluxes for modelling carbon allocation as affected by water status . J. Theoret. Biol . 214 , 481–498. 10.1006/jtbi.2001.2473 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Esau K. (1953). Plant Anatomy . New York, NY: John Wiley and Sons; 10.1097/00010694-195305000-00014 [ CrossRef ] [ Google Scholar ]
- Foster K. J., Miklavcic S. J. (2013). Mathematical modelling of the uptake and transport of salt in plant roots . J. Theoret. Biol . 336 , 132–143. 10.1016/j.jtbi.2013.07.025 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Foster K. J., Miklavcic S. J. (2014). On the competitive uptake and transport of ions through differentiated root tissues . J. Theoret. Biol . 340 , 1–10. 10.1016/j.jtbi.2013.09.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Foster K. J., Miklavcic S. J. (2016). Modeling root zone effects on preferred pathways for the passive transport of ions and water in plant roots . Front. Plant Sci . 7 :914. 10.3389/fpls.2016.00914 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Foster K. J., Miklavcic S. J. (2017). A comprehensive biophysical model of ion and water transport in plant roots. I. Clarifying the roles of endodermal barriers in the salt stress response . Front. Plant Sci . 8 :1326. 10.3389/fpls.2017.01326 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Foster K. J., Miklavcic S. J. (2019). A comprehensive biophysical model of ion and water transport in plant roots. II. Clarifying the roles of sos1 in the salt stress response in arabidopsis . Front. Plant Sci . 10 :1121. 10.3389/fpls.2019.01121 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goeschl J. D., Magnuson C., Demichele D. W., Sharpe P. J. (1976). Concentration-dependent unloading as a necessary assumption for a closed form mathematical model of osmotically driven pressure flow in phloem . Plant Physiol . 58 , 556–562. 10.1104/pp.58.4.556 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hickey L. J. (1973). Classification of the architecture of dicotyledonous leaves . Am. J. Bot . 60 , 17–33. 10.1002/j.1537-2197.1973.tb10192.x [ CrossRef ] [ Google Scholar ]
- Hölttä T., Mencuccini M., Nikinmaa E. (2009). Linking phloem function to structure: analysis with a coupled xylem-phloem transport model . J. Theoret. Biol . 259 , 325–337. 10.1016/j.jtbi.2009.03.039 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hölttä T., Vesala T., Sevanto S., Perämäki M., Nikinmaa E. (2006). Modeling xylem and phloem water flows in trees according to cohesion theory and Münch hypothesis . Trees 20 , 67–78. 10.1007/s00468-005-0014-6 [ CrossRef ] [ Google Scholar ]
- Katchalsky A., Curran P. F. (1965). Nonequilibrium Thermodynamics in Biophysics . Cambridge, MA: Harvard University Press. [ Google Scholar ]
- Kramer P. J., Boyer J. S. (1995). Water Relations of Plants and Soils . San Diego, CA: Academic Press; 10.1016/B978-012425060-4/50003-6 [ CrossRef ] [ Google Scholar ]
- Lacointe A., Minchin P. E. (2008). Modelling phloem and xylem transport within a complex architecture . Funct. Plant Biol . 35 , 772–780. 10.1071/FP08085 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lhomme J.-P., Rocheteau A., Ourcival J., Rambal S. (2001). Non-steady-state modelling of water transfer in a mediterranean evergreen canopy . Agric. Forest Meteorol . 108 , 67–83. 10.1016/S0168-1923(01)00218-0 [ CrossRef ] [ Google Scholar ]
- Martre P., Cochard H., Durand J.-L. (2001). Hydraulic architecture and water flow in growing grass tillers ( Festuca arundinacea Schreb.) . Plant Cell Environ . 24 , 65–76. 10.1046/j.1365-3040.2001.00657.x [ CrossRef ] [ Google Scholar ]
- Mathworks (2020). MATLAB version 9.8.0.1323502 (R2020a) . Natick, MA: The Mathworks, Inc. [ Google Scholar ]
- McKown A. D., Cochard H., Sack L. (2010). Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution . Am. Natural . 175 , 447–460. 10.1086/650721 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Meinzer F., Goldstein G., Neufeld H., Grantz D., Crisosto G. (1992). Hydraulic architecture of sugarcane in relation to patterns of water use during plant development . Plant Cell Environ . 15 , 471–477. 10.1111/j.1365-3040.1992.tb00998.x [ CrossRef ] [ Google Scholar ]
- Meinzer F., Grantz D. (1990). Stomatal and hydraulic conductance in growing sugarcane: stomatal adjustment to water transport capacity . Plant Cell Environ . 13 , 383–388. 10.1111/j.1365-3040.1990.tb02142.x [ CrossRef ] [ Google Scholar ]
- Nicotra A. B., Leigh A., Boyce C. K., Jones C. S., Niklas K. J., Royer D. L., et al.. (2011). The evolution and functional significance of leaf shape in the angiosperms . Funct. Plant Biol . 38 , 535–552. 10.1071/FP11057 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nikinmaa E., Hölttä T., Hari P., Kolari P., Mäkelä A., Sevanto S., et al.. (2013). Assimilate transport in phloem sets conditions for leaf gas exchange . Plant Cell Environ . 36 , 655–669. 10.1111/pce.12004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- North G. B., Lynch F. H., Maharaj F. D., Phillips C. A., Woodside W. T. (2013). Leaf hydraulic conductance for a tank bromeliad: axial and radial pathways for moving and conserving water . Front. Plant Sci . 4 :78. 10.3389/fpls.2013.00078 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Prado K., Maurel C. (2013). Regulation of leaf hydraulics: from molecular to whole plant levels . Front. Plant Sci . 4 :255. 10.3389/fpls.2013.00255 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roth-Nebelsick A., Uhl D., Mosbrugger V., Kerp H. (2001). Evolution and function of leaf venation architecture: a review . Ann. Bot . 87 , 553–566. 10.1006/anbo.2001.1391 [ CrossRef ] [ Google Scholar ]
- Rudall P. (2007). Anatomy of Flowering Plants . Cambridge: Cambridge University Press; 10.1017/CBO9780511801709 [ CrossRef ] [ Google Scholar ]
- Sack L., Frole K. (2006). Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees . Ecology 87 , 483–491. 10.1890/05-0710 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sack L., Holbrook N. M. (2006). Leaf hydraulics . Annu. Rev. Plant Biol . 57 , 361–381. 10.1146/annurev.arplant.56.032604.144141 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sack L., Scoffoni C. (2013). Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future . N. Phytol . 198 , 983–1000. 10.1111/nph.12253 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sakurai G., Yamaji N., Mitani-Ueno N., Yokozawa M., Ono K., Ma J. F. (2017). A model of silicon dynamics in rice: an analysis of the investment efficiency of si transporters . Front. Plant Sci . 8 :1187. 10.3389/fpls.2017.01187 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seki M., Feugier F. G., Song X.-J., Ashikari M., Nakamura H., Ishiyama K., et al.. (2015). A mathematical model of phloem sucrose transport as a new tool for designing rice panicle structure for high grain yield . Plant Cell Physiol . 56 , 605–619. 10.1093/pcp/pcu191 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sommerville K. E., Sack L., Ball M. C. (2012). Hydraulic conductance of acacia phyllodes (foliage) is driven by primary nerve (vein) conductance and density . Plant Cell Environ . 35 , 158–168. 10.1111/j.1365-3040.2011.02425.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Steppe K., De Pauw D. J., Lemeur R., Vanrolleghem P. A. (2006). A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth . Tree Physiol . 26 , 257–273. 10.1093/treephys/26.3.257 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thompson M. V., Holbrook N. M. (2003). Application of a single-solute non-steady-state phloem model to the study of long-distance assimilate transport . J. Theoret. Biol . 220 , 419–455. 10.1006/jtbi.2003.3115 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tsukaya H. (2018). “A consideration of leaf shape evolution in the context of the primary function of the leaf as a photosynthetic organ,” in The Leaf: A Platform for Performing Photosynthesis , eds W. W. Adams III and I. Terashima (Berlin: Springer; ), 1–26. 10.1007/978-3-319-93594-2_1 [ CrossRef ] [ Google Scholar ]
- Yamaji N., Ma J. F. (2014). The node, a hub for mineral nutrient distribution in graminaceous plants . Trends Plant Sci . 19 , 556–563. 10.1016/j.tplants.2014.05.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zwieniecki M. A., Boyce C. K., Holbrook N. M. (2004). Functional design space of single-veined leaves: role of tissue hydraulic properties in constraining leaf size and shape . Ann. Bot . 94 , 507–513. 10.1093/aob/mch173 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zwieniecki M. A., Melcher P. J., Boyce C. K., Sack L., Holbrook N. M. (2002). Hydraulic architecture of leaf venation in Laurus nobilis L . Plant Cell Environ . 25 , 1445–1450. 10.1046/j.1365-3040.2002.00922.x [ CrossRef ] [ Google Scholar ]
- Zwieniecki M. A., Stone H. A., Leigh A., Boyce C. K., Holbrook N. M. (2006). Hydraulic design of pine needles: one-dimensional optimization for single-vein leaves . Plant Cell Environ . 29 , 803–809. 10.1111/j.1365-3040.2005.01448.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A PXY-Mediated Transcriptional Network Integrates Signaling Mechanisms to Control Vascular Development in Arabidopsis
Affiliations.
- 1 Department of Plant Biology and Genome Center, University of California, Davis, California 95616.
- 2 Laboratory of Biochemistry, Wageningen University, 6708 WE, Wageningen, The Netherlands.
- 3 Department of Biosciences, Durham University, Durham DH1 3LE, United Kingdom.
- 4 National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University, Wuhan, Hubei 430070, China.
- 5 Department of Biology, University of North Carolina, Chapel Hill, North Carolina 27599.
- 6 School of Biological Science, University of Manchester, Manchester, M13 9PT, United Kingdom.
- 7 Department of Plant Biology and Genome Center, University of California, Davis, California 95616 [email protected] [email protected].
- PMID: 31806676
- PMCID: PMC7008486
- DOI: 10.1105/tpc.19.00562
The cambium and procambium generate the majority of biomass in vascular plants. These meristems constitute a bifacial stem cell population from which xylem and phloem are specified on opposing sides by positional signals. The PHLOEM INTERCALATED WITH XYLEM (PXY) receptor kinase promotes vascular cell division and organization. However, how these functions are specified and integrated is unknown. Here, we mapped a putative PXY-mediated transcriptional regulatory network comprising 690 transcription factor-promoter interactions in Arabidopsis ( Arabidopsis thaliana ). Among these interactions was a feedforward loop containing transcription factors WUSCHEL HOMEOBOX RELATED14 (WOX14) and TARGET OF MONOPTEROS6 (TMO6), each of which regulates the expression of the gene encoding a third transcription factor, LATERAL ORGAN BOUNDARIES DOMAIN4 (LBD4). PXY signaling in turn regulates the WOX14, TMO6, and LBD4 feedforward loop to control vascular proliferation. Genetic interaction between LBD4 and PXY suggests that LBD4 marks the phloem-procambium boundary, thus defining the shape of the vascular bundle. These data collectively support a mechanism that influences the recruitment of cells into the phloem lineage, and they define the role of PXY signaling in this context in determining the arrangement of vascular tissue.
© 2020 American Society of Plant Biologists. All rights reserved.
PubMed Disclaimer
Diagrammatic Representation of the Vascular…
Diagrammatic Representation of the Vascular Development TRN. (A) Representation of all the interactions…
Consequences of Removing the Feed-Forward…
Consequences of Removing the Feed-Forward Loop. (A) to (E) Morphology of vascular bundles…
Gene Expression Studies Supporting a…
Gene Expression Studies Supporting a Regulatory Relationship between WOX14 , TMO6 , and…
PXY and Auxin Signaling Regulate…
PXY and Auxin Signaling Regulate the Feedforward Loop. (A) and (B) qRT-PCR showing…
Genetic Interactions between LBD4 and…
Genetic Interactions between LBD4 and TDIF-PXY . (A) to (E) Analysis of pxy…
- The Shape of Rings to Come: Systems Approach to Xylem and Phloem Formation in Arabidopsis. Salomé PA. Salomé PA. Plant Cell. 2020 Feb;32(2):287-288. doi: 10.1105/tpc.19.00980. Epub 2019 Dec 18. Plant Cell. 2020. PMID: 31857441 Free PMC article. No abstract available.
Similar articles
- Plant vascular cell division is maintained by an interaction between PXY and ethylene signalling. Etchells JP, Provost CM, Turner SR. Etchells JP, et al. PLoS Genet. 2012;8(11):e1002997. doi: 10.1371/journal.pgen.1002997. Epub 2012 Nov 15. PLoS Genet. 2012. PMID: 23166504 Free PMC article.
- WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Etchells JP, Provost CM, Mishra L, Turner SR. Etchells JP, et al. Development. 2013 May;140(10):2224-34. doi: 10.1242/dev.091314. Epub 2013 Apr 11. Development. 2013. PMID: 23578929 Free PMC article.
- The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Etchells JP, Turner SR. Etchells JP, et al. Development. 2010 Mar;137(5):767-74. doi: 10.1242/dev.044941. Development. 2010. PMID: 20147378
- A brief history of the TDIF-PXY signalling module: balancing meristem identity and differentiation during vascular development. Etchells JP, Smit ME, Gaudinier A, Williams CJ, Brady SM. Etchells JP, et al. New Phytol. 2016 Jan;209(2):474-84. doi: 10.1111/nph.13642. Epub 2015 Sep 28. New Phytol. 2016. PMID: 26414535 Review.
- CLE peptides in vascular development. Qiang Y, Wu J, Han H, Wang G. Qiang Y, et al. J Integr Plant Biol. 2013 Apr;55(4):389-94. doi: 10.1111/jipb.12044. J Integr Plant Biol. 2013. PMID: 23473393 Review.
- Advances in understanding the graft healing mechanism: a review of factors and regulatory pathways. Wang L, Liao Y, Liu J, Zhao T, Jia L, Chen Z. Wang L, et al. Hortic Res. 2024 Jun 20;11(8):uhae175. doi: 10.1093/hr/uhae175. eCollection 2024 Aug. Hortic Res. 2024. PMID: 39108577 Free PMC article.
- Small Peptides: Orchestrators of Plant Growth and Developmental Processes. Lu S, Xiao F. Lu S, et al. Int J Mol Sci. 2024 Jul 11;25(14):7627. doi: 10.3390/ijms25147627. Int J Mol Sci. 2024. PMID: 39062870 Free PMC article. Review.
- Rhytidome- and cork-type barks of holm oak, cork oak and their hybrids highlight processes leading to cork formation. Armendariz I, López de Heredia U, Soler M, Puigdemont A, Ruiz MM, Jové P, Soto Á, Serra O, Figueras M. Armendariz I, et al. BMC Plant Biol. 2024 Jun 3;24(1):488. doi: 10.1186/s12870-024-05192-4. BMC Plant Biol. 2024. PMID: 38825683 Free PMC article.
- CcNAC6 Acts as a Positive Regulator of Secondary Cell Wall Synthesis in Sudan Grass ( Sorghum sudanense S.). Huang Y, Wu J, Lin J, Liu Z, Mao Z, Qian C, Zhong X. Huang Y, et al. Plants (Basel). 2024 May 14;13(10):1352. doi: 10.3390/plants13101352. Plants (Basel). 2024. PMID: 38794423 Free PMC article.
- Endogenous and environmental signals in regulating vascular development and secondary growth. Wang H. Wang H. Front Plant Sci. 2024 Apr 2;15:1369241. doi: 10.3389/fpls.2024.1369241. eCollection 2024. Front Plant Sci. 2024. PMID: 38628366 Free PMC article. No abstract available.
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
- Atanassov I.I., Atanassov I.I., Etchells J.P., Turner S.R. (2009). A simple, flexible and efficient PCR-fusion/Gateway cloning procedure for gene fusion, site-directed mutagenesis, short sequence insertion and domain deletions and swaps. Plant Methods 5: 14. - PMC - PubMed
- Bae S., Park J., Kim J.-S. (2014). Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30: 1473–1475. - PMC - PubMed
- Baima S., Forte V., Possenti M., Peñalosa A., Leoni G., Salvi S., Felici B., Ruberti I., Morelli G. (2014). Negative feedback regulation of auxin signaling by ATHB8/ACL5-BUD2 transcription module. Mol. Plant 7: 1006–1025. - PubMed
- Baima S., Nobili F., Sessa G., Lucchetti S., Ruberti I., Morelli G. (1995). The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 121: 4171–4182. - PubMed
Publication types
- Search in MeSH
Related information
- GEO DataSets
- Gene (GeneRIF)
- Nucleotide (RefSeq)
- Protein (RefSeq)
- Related Project
Grants and funding
- BB/E00380X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H019928/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full text sources.
- PubMed Central
- Silverchair Information Systems
Molecular Biology Databases
- Gene Ontology
- The Arabidopsis Information Resource
Research Materials
- NCI CPTC Antibody Characterization Program

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

designing for a more equitable world

Twitter Facebook Youtube LinkedIn
Xylem Water Filter

Developing low-cost water filters that exploit the natural filtration capabilities of xylem tissue in wood.
This project aims to address the largely unmet need to provide safe and affordable drinking water to low-income groups by developing low-cost water filters that exploit the natural filtration capabilities of xylem tissue in wood.
Why a xylem filter?
In 2013, Rohit Karnik, Associate Professor in the Mechanical Engineering department at MIT, came up with the idea of using the natural filtration capabilities of xylem tissue in wood to develop low-cost water filters. Further experiments showed that the sapwood xylem tissue from coniferous trees can be used to effectively remove E. coli bacteria and other microbes from water.
The key advantages of xylem as a water filter are that the water filter replacement costs are 10-100 times lower compared to existing gravity-driven filters, the xylem is lightweight and easy to transport, has good rejection of bacteria and protozoa, and can be manufactured locally with minimal infrastructure. Filtration devices developed from this material have the potential to act as disposable, low-cost household water filters and could also be distributed in humanitarian crises.
Field studies
In 2017, with support from J-WAFS (Abdul Latif Jameel World Water and Food Security Lab) at MIT, Dr. Karnik partnered with MIT D-Lab to conduct field studies and gain a better understanding of user needs and preferences regarding water filters, and also to find the potential market segment for this kind of filter.
Four field studies were conducted in different parts of India over 2 years, with over 400 potential users/community members and other stakeholders including staff of local NGO partners, filter vendors, and doctors. Through interviews, surveys, focus group discussions, and hands-on design workshops, rural and urban communities helped the team understand not only their needs and current practices related to water treatment, but also their design preferences for water filters, willingness to pay, and behavior change required for the sustained adoption of these filters.
What we've learned so far
Notable learnings from the field studies included the high acceptance of xylem filters as a natural, chemical-free filter and the preference for pay-as-you-go filters among the low-income population with weekly costs up to INR 25, which aligns well with the expected price of INR 5-20 for the filter elements. Local communities were also engaged in the field trials of filter prototypes. Necessary design changes were made based on user feedback.
A thorough literature review was conducted to understand the motivators for and barriers to behavior change in the adoption of household water treatment methods. Based on the literature and information gathered in the field from users and experts, a few behavior change interventions have been selected for implementation.
Amy Smith , Senior Lecturer, Department of Mechanical Engineering & MIT D-Lab Founding Director
Rohit Karnik , Associate Professor of Mechanical Engineering
Kendra Leith , Associate Director for Research
Megha Hegde , Research Associate
Anish Paul Antony , Postdoctoral Researcher
Krithika Ramchander , Research Assistant
- Publications
MIT engineers make filters from tree branches to purify drinking water
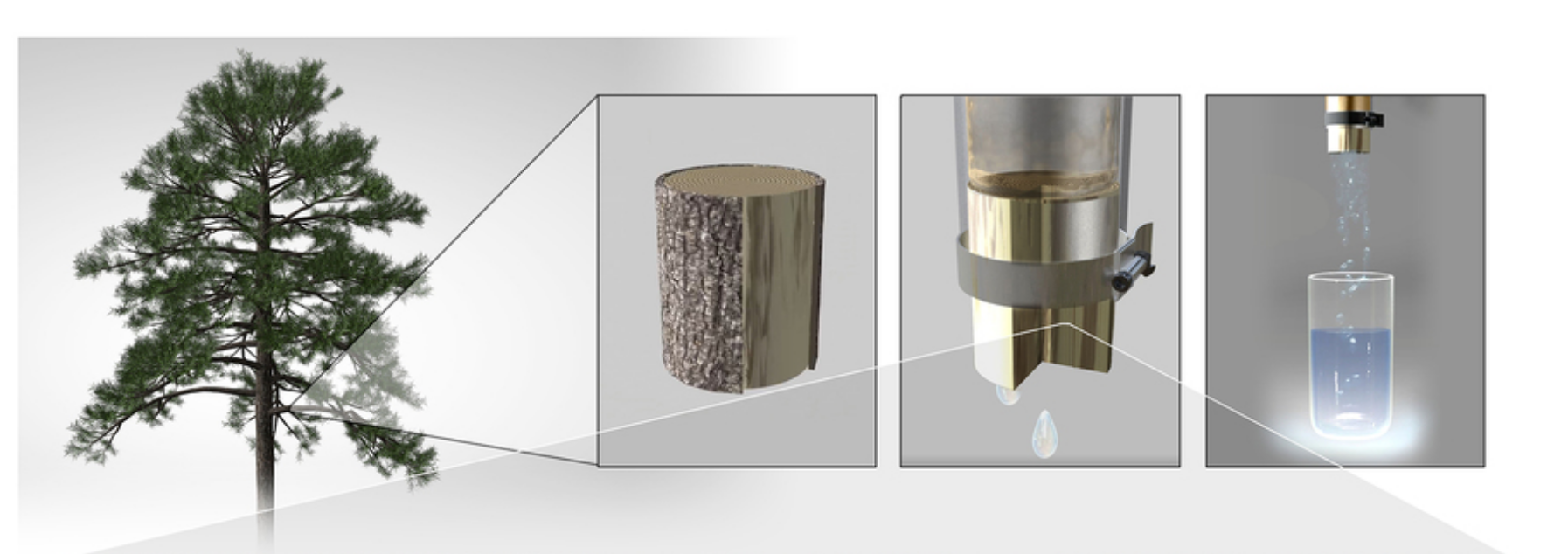
News | Mar 25, 2021 | Jennifer Chu
J-WAFS Grants $15,000 to MIT Who Are Developing Solutions for Water and Food Challenges in India

News | Jun 10, 2019 | Abdul Latif Jameel Water and Food Systems Lab
Student Spotlight: Engineering Low-cost Filters for Clean Water Access

News | Mar 11, 2019 | Andi Sutton
Reflections on Field Work Assessment for Xylem Water Filter Project – Uttarakhand, India

Blog | Sep 26, 2017 | Caroline Morris, MIT D-Lab UROP, Wellesley College '18
Assessment of user needs and preferences related to drinking water and water filters in Uttarakhand, India

Blog | Mar 27, 2017 | Megha Hegde, D-Lab Research Associate
Investigating user needs preferences for a low-cost water filter while gaining life perspective and cultural meaning in India

Blog | Mar 02, 2017 | Nupur Dokras, candidate MBA and SM Mechanical Engineering, MIT
Engineering and characterization of gymnosperm sapwood toward enabling the design of water filtration devices

Publication | Mar 25, 2021 | Krithika Ramchander, Megha Hegde, Anish Paul Antony, Luda Wang, Kendra Leith, Amy Smith, Rohit Karnik
REVIEW article
Secreted in xylem genes: drivers of host adaptation in fusarium oxysporum.

- Department of Botany, University of Delhi, New Delhi, India
Fusarium oxysporum ( Fo ) is a notorious pathogen that significantly contributes to yield losses in crops of high economic status. It is responsible for vascular wilt characterized by the browning of conductive tissue, wilting, and plant death. Individual strains of Fo are host specific ( formae speciales ), and approximately, 150 forms have been documented so far. The pathogen secretes small effector proteins in the xylem, termed as Secreted in Xylem (Six), that contribute to its virulence. Most of these proteins contain cysteine residues in even numbers. These proteins are encoded by SIX genes that reside on mobile pathogenicity chromosomes. So far, 14 proteins have been reported. However, formae speciales vary in SIX protein profile and their respective gene sequence. Thus, SIX genes have been employed as ideal markers for pathogen identification. Acquisition of SIX -encoding mobile pathogenicity chromosomes by non-pathogenic lines, through horizontal transfer, results in the evolution of new virulent lines. Recently, some SIX genes present on these pathogenicity chromosomes have been shown to be involved in defining variation in host specificity among formae speciales . Along these lines, the review entails the variability ( formae speciales , races, and vegetative compatibility groups) and evolutionary relationships among members of F. oxysporum species complex (FOSC). It provides updated information on the diversity, structure, regulation, and (a)virulence functions of SIX genes. The improved understanding of roles of SIX in variability and virulence of Fo has significant implication in establishment of molecular framework and techniques for disease management. Finally, the review identifies the gaps in current knowledge and provides insights into potential research landscapes that can be explored to strengthen the understanding of functions of SIX genes.
Introduction
Fusarium is a complex and an adaptive genus in Ascomycota that includes both pathogenic as well as non-pathogenic species ( Mandeel and Baker, 1991 ; Gordon and Martyn, 1997 ). Under this genus, species Fusarium oxysporum Schlechtendal (1824) emend. Snyder and Hansen (1940) ( Fo ) represents the most pervasive, anamorphic, and polytypic soil-borne pathogen ( O’Donnell and Cigelnik, 1997 ; O’Donnell et al., 1998 ) that is capable of infecting more than 150 plant species. The host range of Fo varies from vegetables (bottle gourd and tomato), flowers (tulips and carnations), field crops (cotton and chickpea) to plantation crops (banana, dates, and palms) ( Pietro et al., 2003 ; Rana et al., 2017 ; Edel-Hermann and Lecomte, 2019 ). Despite showing a broad host range, strains of Fo are highly host specific and are genetically and morphologically distinct ( Mandeel et al., 2005 ; Leslie and Summerell, 2008 ; Palmero et al., 2009 ; Edel-Hermann and Lecomte, 2019 ). Together, these host-specific forms constitute a consortium referred to as F. oxysporum species complex (FOSC) ( Edel-Hermann and Lecomte, 2019 ). FOSC consists of causative agents of vascular wilt, stem-, root-, and crown-rot diseases of economically imperative crops worldwide ( Weimer, 1944 ; Olivain and Alabouvette, 1999 ; Michielse and Rep, 2009 ; Gordon, 2017 ; Rana et al., 2017 ; Edel-Hermann and Lecomte, 2019 ). Based on its devastating impact on crop yield, Fo has been positioned fifth among the top 10 economically significant phytopathogenic fungi ( Dean et al., 2012 ).
Genome-wide analysis conducted on Fo has revealed a two-speed genome organization; separating genomic regions required for normal development of the pathogen from relatively fast-evolving regions required for pathogenesis ( Croll and McDonald, 2012 ; Raffaele and Kamoun, 2012 ; Dong et al., 2015 ; Fokkens et al., 2018 ). The host range and specificity of Fo are dictated by genes located on pathogenicity-associated genomic regions ( Ma et al., 2010 , 2015 ; Rep and Kistler, 2010 ; Williams et al., 2016 ). These pathogenicity-associated genes encode effector proteins, transcription factors (TFs), secreted enzymes, and proteins involved in secondary metabolism and signal transduction ( Rep et al., 2002 , 2004 ; Houterman et al., 2007 ; Van Der Does et al., 2008 ; Ma et al., 2010 ; Schmidt et al., 2013 ). Effector proteins either effectuate a compatible (virulence) response or, on interaction with their corresponding resistance ( R ) genes, result in incompatible (avirulence) reaction ( Flor, 1971 ; Jones and Dangl, 2006 ). The horizontal transfer of host-specificity genes to otherwise genetically distinct lineages result in the rapid emergence of new pathogenic lines with a wider host range ( Ma et al., 2010 ). Considering that non-pathogenic strains of Fo can colonize asymptomatic plants as endophytes ( Kuldau and Yates, 2000 ), the potential of these strains to evolve into new virulent lines is a matter of major concern ( Gordon and Martyn, 1997 ; Recorbet et al., 2003 ; Michielse and Rep, 2009 ). It is due to the evolution of new pathogens that management strategies for Fusarium wilt have not seen much success. On that account, a thorough understanding of the molecular basis of virulence in Fo is of primary importance as it will provide impetus to the development of efficient and effective disease control strategies. After providing an overview on the biology and variability of Fo pathogens, this review will focus, in particular, on Secreted in Xylem ( SIX ) genes, their diversity across formae speciales , their role in virulence and host specificity, and evolutionary relationships among Fo pathogens to better understand host–pathogen interactions and rapid emergence of new pathogenic strains.
Host–Pathogen Interaction
Fusarium wilt is a soil-borne disease that is characterized by wilted plants with yellow leaves and a marked reduction in crop yield. The pathogen thrives in warm climate and dry soil; hence, symptoms are severe at 25–30°C ( Zitter, 1998 ; Joshi, 2018 ). Fusarium , being an anamorphic fungus, produces asexual spores, namely, microconidia, macroconidia, and chlamydospores (dormant propagules) ( Gordon, 2017 ). Germination of these spores is triggered by secretion of exudates from host plant roots and sites of lateral root emergence or injury. Upon germination, the development of infection hypha is initiated that penetrates the root epidermis at the tip ( Bishop and Cooper, 1983 ). Thereafter, the hypha progresses intercellularly via root cortical cells until it enters the xylem tissue. Upon reaching the vascular tissue, the fungus branches profusely and produces microconidia and macroconidia that are transported acropetally by the transpirational pull of plant system ( Bishop and Cooper, 1983 ). Microconidia germinate, and the hyphae spread systemically throughout the host. However, contrasting results were obtained in a study by Michielse and Rep (2009) , wherein neither conidiophores nor microconidia were observed in xylem vessels of infected tomato and Arabidopsis plants. These results did not align with the conventional idea that microconidia play an important role in colonization ( Beckman, 1987 ). Generally, to prevent the spread of fungus, resistant plants produce antifungal compounds and occlude the lumen of the xylem vessel by tyloses ( VanderMolen et al., 1987 ; Zhang et al., 1993 ). This response in susceptible hosts is generally delayed till later stages of infection. Blockage of xylem vessels eventually results in browning of the vascular tissue, a prominent symptom of Fusarium wilt. Disease progression over time leads to leaf bending, chlorosis, wilting, and eventual death of the host ( Figure 1 ). At this stage, the fungus sporulates extensively on the surface of dead plant tissues. The disease spread to other hosts via infected plant parts, transplants or seeds, and contaminated soil ( Bishop and Cooper, 1983 ; Michielse and Rep, 2009 ; Gordon, 2017 ; Joshi, 2018 ).
Figure 1. Disease cycle of Fusarium oxysporum . (A) Secretion of root exudates by host plant triggers spore germination and the development of infection hypha prompting penetration of the root epidermis at the tip. (B) The hypha progresses intercellularly via the root cortical cells until it enters the xylem tissue, parenchymal cells, and vessels, through xylem pits. (C) The pathogen colonizes vascular vessels causing blockage and browning as a result of excessive mycelial growth. (D) The initial stage of infection shows symptoms at the stem base and slowly advancing upward, triggering withering of young leaves. (E) Marginal yellowing or complete chlorosis in mature leaves is observed. (F) Disease progression results in wilting and death of the host plant. Fungal spores (microconidia, macroconidia, and chlamydospores) are formed on dead plant tissue and remain dispersed in soil.
Concept of Formae speciales , Races, and Vegetative Compatibility Groups
Pathogenic Fusarium isolates are differentiated at subspecies level into assemblages termed as formae speciales (ff. spp.) ( Gordon, 1965 ; Armstrong and Armstrong, 1981 ; Baayen, 2000 ). A forma specialis (f. sp.) is composed of isolates capable of infecting a unique host. Individuals from a forma specialis are further subdivided into pathogenic races depending upon their varied virulence toward cultivars of the same host ( Correll, 1991 ). New races within a forma specialis emerge as a result of mutations in pathogenicity-associated genes. For instance, in race 1 of F. oxysporum f. sp. lycopersici (Fol) , whose isolates express three effector genes ( AVR1 , AVR2 , and AVR3 ), deletion of AVR1 resulted in emergence of race 2 and point mutation(s) in AVR2 eventuated in the evolution of race 3 ( Houterman et al., 2008 , 2009 ; Takken and Rep, 2010 ; Biju et al., 2017 ). Based on the capability of the isolates to undergo heterokaryosis, they can be grouped as vegetative compatibility groups (VCGs) ( Puhalla, 1985 ; Ploetz and Correll, 1988 ; Moore et al., 1993 ). The members of a particular VCG are clonal lineages and share similar pathological, physiological, and biological attributes ( Caten and Jinks, 1966 ). The relationship between races and VCGs of a forma specialis varies from simple to relatively complex ( Correll, 1991 ). In a rather simple relationship, isolates from one race (even from diverse geographical backgrounds) may correspond to a single VCG. For example, in F. oxysporum f. sp. niveum ( Fon ), all race 2 isolates belong to a single VCG ( Correll, 1991 ; Epstein et al., 2017 ). On the other hand, occurrence of isolates of different races within a single VCG or isolates of a single race belonging to different VCGs may add to the complexity of the relationship thereof. For instance, three VCGs of F. oxysporum f. sp. cubense ( Focub ) (0124, 0125, and 0128) contain isolates of races 1 and 2, while isolates of race 1 of Focub belong to eight different VCGs (0123, 0124, 0125, 0128, 01210, 01217, 01218, and 01220) ( Czislowski et al., 2018 ).
Polyphyletic Origin of Fusarium oxysporum Species Complex Members
Recent evolutionary studies have annulled the classical concept that most of the pathogenic isolates of Fo are monophyletic in origin. It is now well established that most formae speciales have evolved independently multiple times throughout the course of evolution pointing towards their para- or polyphyletic origin ( O’Donnell et al., 1998 , 1999 ). Remarkably, isolates from one forma specialis , race, or VCG may show close relatedness to the isolates of other formae speciales , races, or VCGs than their own members ( Kistler, 1997 ; Lievens et al., 2009a ). Conserved gene sequences and their combinations, mitochondrial or nuclear barcoding, and pathogenesis-related genes have been used to study the evolutionary relationships between different formae speciales , races, and VCGs. Studies based on IGS (intergenic spacer) region, vegetative compatibility, restriction fragment length polymorphism (RFLP), mitochondrial DNA (mtDNA) and isozyme polymorphism have demonstrated that Fol isolates may reflect genetically different evolutionary lines. For instance, phylogenetic analysis of Fol isolates by utilizing IGS rDNA sequences showed three well-supported clusters (A1, A2, and A3) ( Kawabe et al., 2005 ). The major cluster A2 consisted of Fol isolates along with representatives of other formae speciales ( melonis , batatas , and radicis-lycopersici ). Lievens et al. (2009b) studied the evolutionary relationships between Fol and F. oxysporum f. sp. radicis-lycopersici ( Forl ) by constructing phylogenetic tree employing pgx4 (exo-polygalacturonase) and Translation Elongation Factor 1α ( TEF-1 α) gene data and concluded that Fol comprises of three independent clonal lineages. Phylogenetic tree developed by exploiting data from the IGS region of rDNA resolved the isolates of Fol and Forl into five distinct lineages ( Cai et al., 2003 ). Interestingly, Fol VCG 0035 (lineage 5) isolates had more similarities to Forl isolates (lineage 4) compared with the isolates in other Fol lineages or VCGs ( Cai et al., 2003 ). About a decade later, Nirmaladevi et al. (2016) , through the ITS (internal transcribed spacer) region analysis, identified evolutionary relationships among Fol isolates and other formae speciales and concluded that Fol represents a polyphyletic forma specialis due to divergent evolution. A similar inference was drawn by employing mitochondrial small subunit ( mtSSU ) rDNA and TEF-1 α-based studies in forma specialis cubense . These phylogenetic studies showed that Focub consists of four clades containing members from various polytypic species ( O’Donnell et al., 1998 ). The results demonstrated a close relatedness of Focub isolates to other representative members of FOSC. Phylogenetic relationships between Focub and FOSC members, as well as between VCGs and Focub races, have revealed that the capacity of pathogens to trigger banana disease has evolved independently multiple times ( Fourie et al., 2009 ). The ability of Focub to inflict disease on a particular cultivar of banana is a polyphyletic trait ( Fourie et al., 2009 ). Multiple gene-genealogical studies established F. oxysporum f. sp. vasinfectum ( Fov ) as a polyphyletic forma specialis ( Skovgaard et al., 2001 ). The phylogenetic tree obtained from integrated TEF-1 α, NIR (nitrate reductase), PHO (acid phosphatase), and mtSSU rDNA sequences reported four different lineages of Fov that correlated with variations in their origin and virulence. The phylogenetic relationship deduced from TEF-1 α data of Phoenix -specific F. oxysporum f. sp. canariensis ( Focan ) isolates reported the presence of three lineages, confirming that Focan in Australia evolved independently ( Laurence et al., 2015 ). Depending on TEF-1 α phylogenies, isolates belonging to F. oxysporum f. sp. cucumerinum ( Foc ) and F. oxysporum f. sp. radicis-cucumerinum ( Forc ) were reported as genetically diverse and resolved in clades separate from other non-cucurbit-infecting formae speciales ( Lievens et al., 2007 ). Based on the phylogenetic tree obtained from 10 conserved gene dataset, Epstein et al. (2017) described F. oxysporum f. sp. apii as a polyphyletic forma specialis . Evaluation of genetic diversity among forma specialis betae isolates based on ITS , β-tubulin, and TEF-1 α phylogenies reported the polyphyletic origin of this forma specialis ( Hill et al., 2011 ). Isolates from F. oxysporum f. sp. melonis ( Fom ) were also reported to be polyphyletic based on the phylogenetic tree constructed using nuclear repetitive DNA sequences. The isolates were separated into different groups in the phylogenetic tree ( Namiki et al., 1994 ; Gordon and Martyn, 1997 ). The mtSSU rRNA and TEF-1 α phylogenies clustered F. oxysporum f. sp. vanillae isolates into different clades pointing toward a polyphyletic pattern of origin ( Pinaria et al., 2015 ). Similarly, F. oxysporum f. sp. lactucae was described as polyphyletic based on IGS phylogeny and VCGs ( Ogiso et al., 2002 ; Fujinaga et al., 2005 ; Pasquali et al., 2005 ).
Not all formae speciales are polyphyletic; a few monophyletic ones have also been reported. In a study by Baayen et al. (2000) , TEF-1 α, mtSSU rDNA , and amplified fragment length polymorphism (AFLP)-based phylogenies were assessed to identify the nature of origin in 89 isolates belonging to eight different formae speciales . The study revealed two formae speciales , tulipae and lilii , to be monophyletic and the remaining ones, asparagi , dianthi , gladioli , lini , opuntiarum , and spinaciae , to be polyphyletic in origin. Apart from lilii and tulipae ( Baayen et al., 2000 ), ciceris is also considered as a monophyletic forma specialis ( Jiménez-Gasco et al., 2002 ). Isolates of different F. oxysporum f. sp. ciceris ( Focic ) races shared similar sequences in the intronic region of TEF-1 α, β-tubulin, calmodulin, actin, and histone 3 genes by virtue of which they clustered together, separated from other non-pathogenic isolates and formae speciales suggesting a monophyletic origin of Focic ( Jiménez-Gasco et al., 2002 ). Identifying species boundary in FOSC is undeniably a challenge considering the lack of distinct morphological characters, ecological diversity, diverse genetic background, and dynamic host range of strains. FOSC members are devoid of sexual stages in their life cycle; however, horizontal gene transfer (HGT) within the complex may contribute to the observed genetic diversity.
Features of Fusarium oxysporum Genome
The genome sequences of 16 species (11 Fo and five Fusarium species) can be retrieved from the Joint Genome Institute (JGI) MycoCosm site, FungiDb (Fungi database), and GenBank National Centre for Biotechnology Information (NCBI) database ( Ma et al., 2010 , 2014 ; Ma L. J. et al., 2013 ; Williams et al., 2016 ; DeIulio et al., 2018 ). The complete genetic and physical maps of the pathogens provide an outstanding opportunity to investigate the variation in genome size and content within the genus ( Ma et al., 2010 ; Ma L. J. et al., 2013 ). Comparative genomic studies among the members of the genus have provided insight on the variation in genome size within the genus; Fol strain 4287 ( Fol-4287 ) has an average genome size of 61 Mb, whereas Fusarium verticillioides ( Fv ), Fusarium graminearum ( Fg ), and Fusarium solani ( Fs ) (syn. Nectria haematococca ) have 42-, 51-, and 36-Mb genome sizes, respectively ( Ma et al., 2010 ). Fv and Fg have comparable genome sizes, even though in the course of evolutionary diversification, Fol and Fv lineages share a common ancestor and have diverged earlier from the clade containing the Fg lineage ( Ma L. J. et al., 2013 ; O’Donnell et al., 2013 ). Furthermore, genome sequencing and gene mapping of Fusarium species have revealed a variable chromosome count, fluctuating between four in Fg and 17 in Fs ( Cuomo et al., 2007 ; Coleman et al., 2009 ; Ma et al., 2010 ).
The F. oxysporum genome is compartmentalized structurally and functionally into two components: a core genome that encodes housekeeping genes vital for survival and growth of the pathogen and an accessory genome encoding pathogenicity or virulence-associated genes ( Ma et al., 2010 ; Croll and McDonald, 2012 ; Schmidt et al., 2013 ). Till date, the genome of Fol-4287 remains the most exhaustively studied genome that has been mapped into complete chromosome sequences. Therefore, the Fol-4287 strain is used as a key point of reference for subsequent studies. Out of 15 chromosomes mapped in the genome assembly of Fol-4287 , 11 are designated as core chromosomes and four as accessory chromosomes. The core genome of Fol-4287 shows 80 and 90% similarity to Fg and Fv genomes, respectively, suggesting that they are highly syntenic across isolates and related species ( Ma et al., 2010 ).
The accessory genome, also termed as conditionally dispensable (CD) chromosomes, supernumerary (SP) chromosomes, or lineage-specific (LS) region, encompasses 19 Mb of the total genome size and includes chromosomes 3, 6, 14, 15, scaffold 27 of core chromosome 1, and scaffold 31 of core chromosome 2 ( Ma et al., 2010 ). Lineage-specific regions are rich in retro-elements including SINEs (short interspersed repeat elements), LINEs (long interspersed repeat elements), gypsy- and copia-like long terminal repeat retrotransposons, and DNA transposons [miniature inverted transposable elements (MITEs), hAT-like, Tc1-mariner, and Mutator-like] ( Ma et al., 2010 ). The LS region contains 95% of DNA transposons and 74% of all transposable elements (TEs) present in the Fol-4287 genome ( Ma et al., 2010 ) and may be specifically associated with pathogenic adaptation ( Ma et al., 2010 , 2015 ). The shared genomic region of Fol and Arabidopsis -infecting strain ( Fo-5176 ) (55 Mb) amounts to less than 2% of sequence divergence. Intriguingly, counterparts of most of the Fol LS region are missing in Fo-5176 ( Ma et al., 2010 ). Similarly, Fov also shows high sequence identity only to the core genomic region of Fol and not to the corresponding LS region ( Ma et al., 2010 ). On this account, comparison among the genomes of Fo pathogens link LS regions to host adaptation ( Ma et al., 2010 , 2015 ).
The observed variation in genome size in the genus is attributed to the activity of TEs, horizontal chromosome transfer (HCT), and deletion or fusion of genomic regions ( Kistler et al., 1995 ; Ma et al., 2010 ; Ma L. J. et al., 2013 ; Schmidt et al., 2013 ) (schematically represented in Figure 2 ). The activity of TEs can cause translocation, deletion, and complex arrangements of genetic material ( Davière et al., 2001 ; Schmidt et al., 2013 ). The increase in genome size of Fol has been attributed to the acquisition of LS chromosomes from other Fusarium species through horizontal transfer ( Ma et al., 2010 ).
Figure 2. Schematic representation of Fusarium oxysporum genome features. (A) Horizontal transfer, deletion, and duplication of the lineage-specific (LS) chromosome 14 (LS Chr14) alter pathogenicity and genome size. (B) The LS Chr14 structure showing the presence of class II transposable elements (TE class II) and Secreted in Xylem ( SIX ) genes. TE class II elements present in the promoter region of SIX are miniature impala (mimp) and mfot5 elements. SIX genes might be trapped between the internal resolution (IR) sites of TEs and subsequently transposed together. The mobilization of SIX genes due to the activity of TEs and HGT could result in variation in SIX profile in FOSC members. Fo , Fusarium oxysporum ; FOSC, F. oxysporum species complex; HGT, Horizontal gene transfer.
Horizontal Transfer of Mobile Pathogenicity Chromosome
Horizontal transfer provides a mechanism to transfer pathogenicity-associated genes/chromosomes from a pathogenic isolate to a non-pathogenic one, resulting in the generation of a new virulent lineage. Along these lines, the ability of Fol to cause disease on tomato has been presumed to be acquired through horizontal transfer of pathogenicity chromosome from other Fusarium species ( Ma et al., 2010 ). This was experimentally demonstrated via co-incubation studies. Chromosome 14 of Fol pathogenic strain ( Fol-007 ) marked with zeocin gene was co-incubated with a non-pathogenic Fo strain ( Fo-47 ) labeled with the hygromycin gene. Transfer of chromosome 14 during this co-cultivation experiment rendered Fo-47 pathogenic to tomato ( Ma et al., 2010 ). Similar outcomes in co-incubation experiments were obtained where LS chromosome were transferred from a pathogenic line ( Fol-4287 ) to a non-pathogenic line that rendered it pathogenic ( Vlaardingerbroek et al., 2016a ). Studies have also reported HCT in formae speciales other than Fol . van Dam et al. (2017) through co-cultivation experiments assessed HCT between cucurbit-infecting strains, in which Forc-016 was chosen as the donor strain and Fo-47 as the recipient. All strains obtained from the experiment exhibited the karyotype of Fo-47 strain along with an additional chromosome presumed to have been transferred from Forc-016 as a result of HCT. Besides the members of FOSC, HGT between other Fusarium species and Fo was also reported ( van Dam et al., 2017 ). Flower bulb-infecting strains, Fusarium proliferatum , Fusarium hostae , and Fusarium agapanthi , showed the presence of Fo -specific genes providing evidence for interspecific horizontal transfer due to shared habitat between their ancestors and F. oxysporum f. sp. hyacinthi or F. oxysporum f. sp. lilii strains. On the other hand, complete loss of chromosome 14 in pathogenic Fol-4287 strains compromised virulence on host plants ( Ma et al., 2010 ), whereas no effect on pathogenicity was observed on the deletion of core chromosome ( Vlaardingerbroek et al., 2016b ). Contrarily, strains with partial deletion of Fol-4287 chromosome 14 regions including effector genes were still pathogenic, implicating that loss of individual or a few genes results in only fractional loss of virulence ( Ma et al., 2010 ). Vlaardingerbroek et al. (2016b) further elaborated on these results. They showed that a part of the short arm (p arm) of the pathogenicity chromosome is adequate for inflicting disease on plants. Transfer of this portion of pathogenicity chromosome is sufficient to convert a non-pathogenic line to a pathogenic line. Interestingly, recipient strains of this portion of chromosome (short arm) were reported to be more virulent than strains that received complete pathogenicity chromosome (short and long arm). This suggested that the sequences present on the long arm (q arm) of the chromosome were possibly involved in suppressing virulence in non-pathogenic strains that received the complete chromosome ( Vlaardingerbroek et al., 2016b ). It was evident from these studies that LS chromosomes are significant for the development of new pathogenic lines. Owing to the limited availability of whole genome sequences of many formae speciales , it is difficult to trace the path of HCT between strains. More studies are needed to generate a curated database of genome assembly of pathogenic as well as non-pathogenic isolates. Analyses of the genome of isolates from different geographical backgrounds will shed light on how new pathogens evolve on the acquisition of mobile pathogenicity chromosomes from other lineages.
Pathogenicity Factors
Xylem-colonizing Fusarium pathogens employ both general and specific pathogenicity mechanisms to invade the host. While components of cell signaling pathways, such as cyclic adenosine monophosphate (cAMP), mitogen-activated protein kinase (MAPK), Ras (retrovirus-associated DNA sequences) proteins, G (guanine nucleotide-binding) protein, and cell wall-degrading enzymes, encompass the general factors regulating pathogenicity ( Di Pietro et al., 2001 ; Jain et al., 2002 , 2003 , 2005 ; Ma L. J. et al., 2013 ; Guo et al., 2016 ; Liu et al., 2016 ), effectors and host-specific toxins attribute specificity to pathogens. Effectors secreted by the pathogen facilitate its colonization by modulating immune response in the host plant ( Hogenhout et al., 2009 ). Secreted in Xylem (Six) proteins is one such example of effectors ( Rep et al., 2002 , 2004 ) whose detailed overview, structure, regulation, and diverse roles are dealt in further sections.
Secreted in Xylem Proteins
Rep et al. (2002) identified a small 12-kDa cysteine-rich fungal protein in the xylem sap proteome of tomato plants infected with Fol . Further structural analysis revealed that the observed 12-kDa protein corresponded to the central part (six of the eight cysteine residues) of the actual 30-kDa protein that they termed as Six1 ( Rep et al., 2004 ). Later, Houterman et al. (2007) identified a 22-kDa propeptide of Six 1 protein along with three new Six proteins, namely, Six2, Six3, and Six4, that were approximately 24, 16, and 24 kDa in size, respectively, with eight, two, and six cysteine residues, respectively ( Houterman et al., 2007 ). Van Der Does et al. (2008) and Schmidt et al. (2013) , through genomic analysis, identified genes that encode Six5, Six6, and Six7; and Six8, Six9, Six10, Six11, Six12, Six13, and Six14 proteins, respectively. Hitherto, 14 Six proteins have been recognized in Fol . These are small secreted proteins, and most of them contain cysteine residues in even numbers ( Rep et al., 2004 ; Rep, 2005 ; Houterman et al., 2007 ; Ma et al., 2010 ). Initially, SIX genes were considered to be limited to Fol , but later, homologs were identified in other formae speciales as well ( Supplementary Table 1 ). It is noteworthy to mention that non-pathogenic strains of Fo share a set of conserved putative effector genes with the pathogenic strains but carry fewer SIX genes ( van Dam et al., 2016 ; de Lamo and Takken, 2020 ).
Structure and Regulation of Secreted in Xylem Gene
The accessory chromosome 14 of Fol-4287 strain is dominated by TEs and has been predicted to predominantly harbor all 14 SIX genes ( Ma et al., 2010 ). The presence of TEs in the genome was also associated with clustering of SIX genes observed in TE-rich regions. Occasionally, SIX genes present in the vicinity of IR (inverted repeats) sites of class II TEs might get trapped and translocated together to a new location within class II TE-rich chromosomal subregions ( Schmidt et al., 2013 ). In accordance to that, the highly dynamic genomic location of AVR-Pita in rice blast fungus Magnaporthe oryzae was also attributed to the activity of TEs. Transposon insertion in AVR-Pita gene prevented the host from recognizing this avirulence protein ( Zhou et al., 2007 ). Multiple translocation events of AVR-Pita resulted in a cycle of loss and gain of recognition by resistant rice cultivars ( Chuma et al., 2011 ). Similarly, in Fol , deletion events caused by recombination between TEs led to the loss of an Fol avirulence gene ( AVR1 ) that eventuated in overcoming of resistance mediated by the cognate resistance gene ( Biju et al., 2017 ). Owing to the high density of TEs in LS regions of Fo pathogens, FOSC can prove useful as a model system to decipher relationships between virulence and TEs.
Structurally, SIX genes harbor two MITEs, namely, mimp (miniature impala; sized ≈220 nucleotides) and mFot5 (miniature Fot5 transposon) that vary in their distribution. While mfot5 has been reported to be present downstream of SIX9 or some mini-effector clusters, a portion of mimp is found consistently present in the promoter region of all SIX genes ( Schmidt et al., 2013 ). Hence, mimp can be exploited as a diagnostic feature in the detection of putative SIX genes. Overall, 103 mimp elements have been reported in Fol -4287 genome. Among these, only four are present on core chromosomes, while 54 are located on accessory chromosome 14, and the remaining 45 are on other accessory chromosomes ( Schmidt et al., 2013 ). Homologs of five SIX genes ( SIX1 , SIX2 , SIX6 , SIX7 , and SIX11 ) and an avirulence gene ( FomAVR2 ) were identified using mimp elements in melon- Fom pathosystem ( van Dam and Rep, 2017 ). Similarly, mimp elements were utilized to predict effector candidates in Fol ( Schmidt et al., 2013 ), legume-infecting strains such as Focic and F. oxysporum f. sp. pisi ( Williams et al., 2016 ), F. oxysporum f. sp. cepae ( Armitage et al., 2018 ), and race 1 and 4 of Focub ( Chang et al., 2020 ). Interestingly, deletion of mimp element from the promoter region of SIX genes ( SIX1 , SIX3 , and SIX5 ) neither altered gene expression nor affected pathogenicity of Fol ruling out the direct involvement of mimp in SIX gene expression ( Schmidt et al., 2013 ).
Virulence in Fo is considered a polygenic trait and requires TFs for the regulation of pathogenicity-related genes ( Husaini et al., 2018 ). The role of a TF Six gene expression 1 ( SGE1 ) (situated on the core genome), in modulating the expression of SIX genes ( SIX1 , SIX2 , SIX3 , and SIX5 ) has been confirmed in a study by Michielse et al. (2009b) suggesting the dependency of SIX expression on the core chromosome. In compliance with its transcriptional role, deletion of SGE1 in tomato-infecting Fol resulted in reduced pathogenicity, which is attributable to the lost expression of effector genes. SGE1 deletion mutants of Fol also exhibited a quantitative reduction in conidiation, confirming the major role of SGE1 during parasitic growth of the pathogen ( Michielse et al., 2009b ). Various orthologs of SGE1 have been reported in fungi such as Fv and Candida albicans ( Michielse et al., 2009a ; Brown et al., 2014 ). The retention of this gene in Fol indicates that it is a conserved TF that has developed as a SIX gene regulator ( Michielse et al., 2009b ).
Two TFs, Fusarium transcription factors ( FTF ) 1 and 2 , belonging to a Zn(II)2Cys6-type family factors, modulate the expression of SGE1 and SIX genes ( Niño-Sánchez et al., 2016 ; Van Der Does et al., 2016 ). While multiple copies of FTF1 are present on chromosome 14 of Fol-4287 and virulent strains of F. oxysporum f. sp. phaseoli ( Foph ), a single copy of FTF2 is present in all filamentous Ascomycetes ( de Vega-Bartol et al., 2011 ; Niño-Sánchez et al., 2015 , 2016 ; Van Der Does et al., 2016 ). Studies on SGE1 reported that the expression of SIX genes is dependent on the core chromosome, but the presence of FTF on the pathogenicity chromosome suggested that the SIX gene expression may also be controlled by the chromosome itself ( Michielse et al., 2009b ; Schmidt et al., 2013 ). FTF1 resembles the SIX genes in terms of having mimp in its promoter region ( Schmidt et al., 2013 ; Niño-Sánchez et al., 2016 ). Deletion mutants of FTF1 and FTF2 have implicated their role in the virulence of Foph ; however, their functions as direct regulators of SGE1 and SIX genes need further validation ( Niño-Sánchez et al., 2016 ).
Another important transcriptional regulator in Fom is FOW2 ( F. oxysporum f. sp. melonis gene for wilt syndrome 2) ( Imazaki et al., 2007 ). It is essentially required for the invasion and colonization of melon roots. Disruption of FOW2 induced loss of virulence in Fom ; however, it had no recognizable effect on vegetative development, conidiation, and carbon source utilization ( Imazaki et al., 2007 ).
Expression of SIX genes is very low in the absence of a living plant host ( Michielse et al., 2009b ). Under such conditions, the activity of SIX genes might be suppressed by the modification of chromatin to a closed/repressive state ( Schmidt et al., 2013 ). The repressive state is achieved by TE silencing that is guided by small RNAs transcribed from TEs. In Solanaceae members, MITEs in the vicinity of resistance genes have been shown to encode small RNAs that recruit methylation machinery to silence TEs ( Kuang et al., 2009 ). This strategy might serve as the first layer for SIX gene regulation where silencing of the MITEs in the vicinity of SIX genes creates a closed chromatin structure ( Schmidt et al., 2013 ). Clustering of SIX genes in class II TE-rich subregions of the accessory chromosome might have facilitated a coordinated expression of SIX genes during infection. The captured genes share the same genomic environment, i.e., closed or open chromatin structure allowing simultaneous transcriptional regulation of these genes ( Schmidt et al., 2013 ).
Roles of Secreted in Xylem Proteins/Genes
Secreted in xylem gene profile distinguishes formae speciales and races of fusarium oxysporum.
Fungicide treatment and soil solarization generally fail to control wilt infection in fields leaving use of resistant cultivar as the most reliable strategy of disease control ( Nirmaladevi et al., 2016 ). Breeding of resistant cultivars requires a thorough understanding of different formae speciales and races of pathogen emerging in the field, which will provide timely information of genes relevant for breeding programs. Members of FOSC are devoid of discernable morphological characters and exhibit genetic heterogeneity attributed to the polyphyletic origin ( Kistler, 1997 ) and horizontal transfer of pathogenicity-associated chromosomes ( Ma et al., 2010 ). Discrimination between pathogenic and non-pathogenic isolates relies on pathogenicity assays that are both time consuming and strenuous owing to abundance of formae speciales and races in FOSC ( Recorbet et al., 2003 ). On the other hand, standard molecular loci-based techniques used in fungal phylogenetics are also constrained by a weak correlation between pathogenicity and phylogenetic relations ( Fraser-Smith et al., 2014 ).
The above challenges can be addressed by techniques that employ specific sequences of DNA closely associated with pathogenicity ( Recorbet et al., 2003 ; van der Does and Rep, 2007 ; Lievens et al., 2008 ), such as SIX genes. In this regard, SIX genes can act as a sensitive and specific diagnostic marker as their array varies among members of different formae speciales and races ( Lievens et al., 2008 , 2009a ) (diagramatically represented in Figure 3 ). For instance, SIX6 gene was used as a molecular marker to differentiate cotton-specific pathogenic Fov isolates from non-pathogenic ones collected from the same geographical regions in Australia ( Chakrabarti et al., 2011 ). Likewise, three races (1, 2, and 3) of pathogenic Fol have been distinguished on the basis of specific array and number of SIX genes. Isolates of race 1 show the presence of three SIX genes ( SIX4 , SIX3 , and SIX1 ), while race 2 and 3 show two ( SIX3 and SIX1 ) and one ( SIX1 ) genes, respectively ( Rep et al., 2004 ; Houterman et al., 2008 , 2009 ; Van Der Does et al., 2008 ; Lievens et al., 2009a ; Takken and Rep, 2010 ; Kang et al., 2014 ). Furthermore, in Focub , race 1 (that infects Gros Michel cultivars of banana) and race 4 (pathogenic to Cavendish banana) were distinguished on the basis of presence, copy number, and sequence variability of SIX1 . Three copies and four sequence variants were observed in race 4 compared with one copy and two variants in race 1 ( Guo et al., 2014 ). Similarly, sequence variants of SIX8 have been used to further differentiate race 4 into tropical (TR4) and subtropical (STR4) races. TR4 race harbors four variants, unlike two in STR4 ( Fraser-Smith et al., 2014 ).
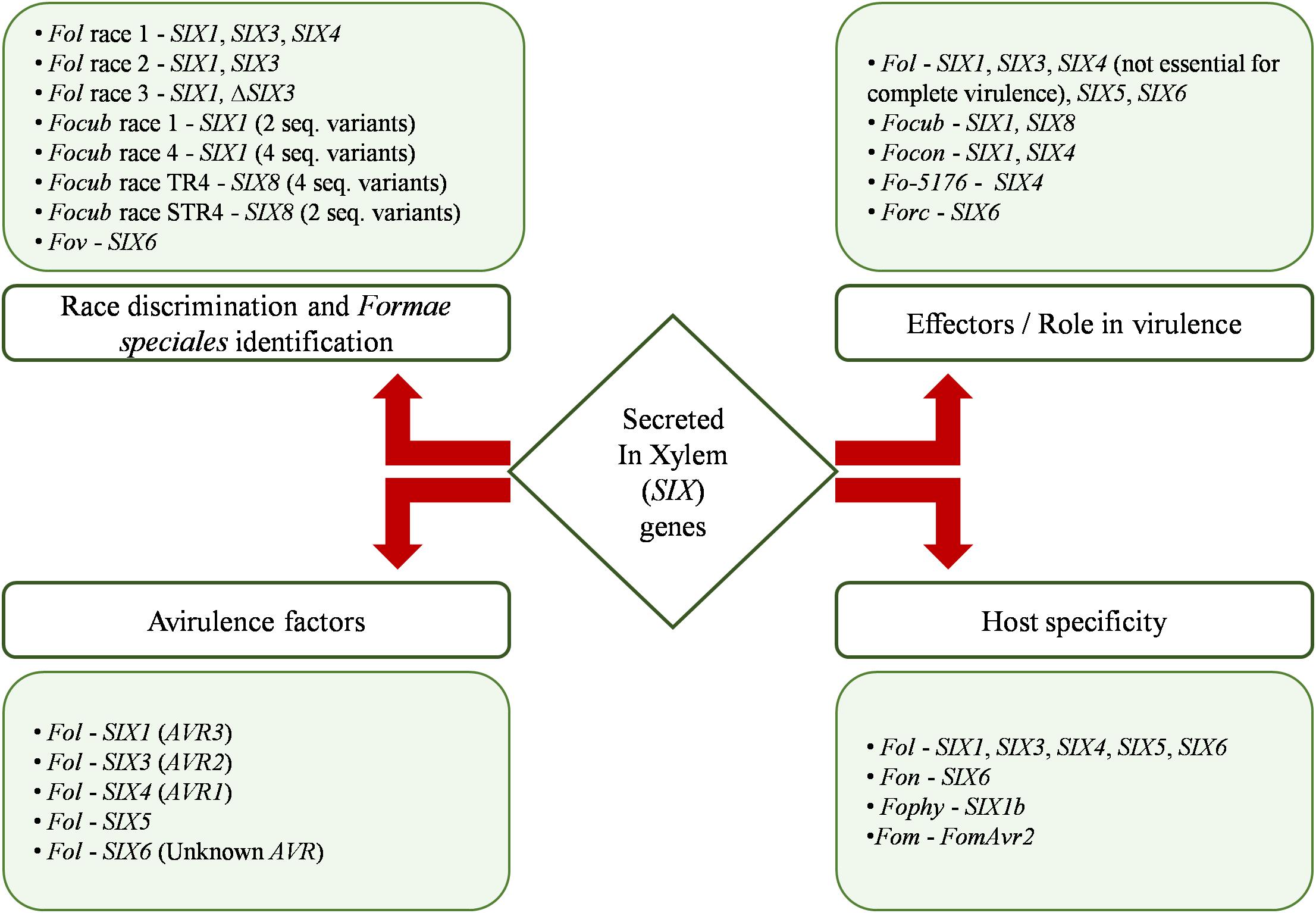
Figure 3. Diagrammatic summary of the roles of various Secreted in Xylem ( SIX ) genes in different formae speciales of Fusarium oxysporum species complex. Fol , Fusarium oxysporum f. sp. lycopersici ; Focub , F. oxysporum f. sp. cubense ; Fov , F. oxysporum f. sp. vasinfectum ; Focon , F. oxysporum f. sp. conglutinans ; Fo-5176 , Arabidopsis -infecting strain; Forc , F. oxysporum f. sp. radicis-cucumerinum ; Fon , F. oxysporum f. sp. niveum ; Fophy , F. oxysporum f. sp. physalis ; Fom, F. oxysporum f. sp. melonis ; TR4, tropical race 4; STR4, subtropical race 4; AVR, avirulence gene; SIX , Secreted in Xylem gene.
An account of the variation in the arsenal of SIX genes in various formae speciales reported so far is given in Supplementary Table 1 . The observed variation in SIX gene profile among formae speciales can be attributed to horizontal transfer of SIX genes among them. Since, LS chromosome 14 carries all SIX genes that reside within subregions of the chromosome rich in class II TEs ( Ma et al., 2010 ; Schmidt et al., 2013 ), it has been observed that a few genes or a cluster of physically linked SIX genes can be transferred to other strains ( Simbaqueba et al., 2018 ). The similarity in suite of effectors and low SIX sequence diversity in isolates of a forma specialis suggest that SIX genes have been transferred horizontally within and among formae speciales of Fo ( Lievens et al., 2009a ; Fraser-Smith et al., 2014 ; Laurence et al., 2015 ; Taylor et al., 2016 ; van Dam et al., 2016 ; Czislowski et al., 2018 ). In a recent study, the SIX genes profile of strains inhabiting asymptomatic banana plants differed from the known Focub SIX genes ( Lyons et al., 2019 ). Thus, a significant prospect will be to explore the differences in effector gene profile of pathogenic isolates and endophytic strains colonizing asymptomatic plants. It would aid in the accurate detection of different formae speciales , races, as well as endophytic strains that will be contributory in the management of diseases caused by Fo . Moreover, if horizontal transfer can be traced, elucidation of whether the functions of the acquired genes remain conserved in both donor and recipient formae speciales is necessary.
Virulence Function of SIX Genes
The presence of effector genes in formae speciales has been widely documented, but their function in pathogenesis has been experimentally validated only in a few (diagrammatically represented in Figure 3 ). The presence of SIX1 has been reported to be a prerequisite for complete virulence of the pathogens F. oxysporum f. sp. conglutinans ( Focon ) and Fol on cabbage and tomato, respectively ( Rep et al., 2004 ; Li et al., 2016 ). Widinugraheni et al. (2018) reported that SIX1 homolog contributes to virulence of Focub tropical race 4 toward the Cavendish banana. Like SIX1 , the role of Fol-SIX3 in complete virulence on host plants has also been demonstrated ( Van Der Does et al., 2008 ). The expression of SIX1 and SIX3 is spatiotemporally separated in Fol . While the expression of Fol-SIX1 is induced in the initial phases of root colonization, Fol-SIX3 is primarily expressed in the xylem during the later stages of hyphae growth ( Van Der Does et al., 2008 ). Likewise, the importance of SIX4 in virulence has been demonstrated by deletion studies in different strains. In Focon , deletion of SIX4 led to a reduction in disease severity on both resistant and susceptible cabbage plants comparative to the SIX4 -complemented and wild-type strains ( Kashiwa et al., 2013 ). Similarly, in Fo-5176 , SIX4 deletion mutants exhibited reduced fungal biomass that eventually resulted in reduced disease symptoms ( Thatcher et al., 2012 ). Deletion studies carried out in Fol have also highlighted the role of SIX5 as an effector ( Ma et al., 2015 ). Fol-ΔSIX5 displayed an apparent reduction in disease symptoms, and reintroduction of the gene restored pathogenicity in 75% of the mutants. Furthermore, knockout mutants of SIX6 in Fol and Forc exhibited compromised virulence confirming the role of SIX6 in pathogenicity ( Gawehns et al., 2014 ; van Dam et al., 2017 ). SIX6 plays a role in virulence by inhibiting a hypersensitive response (HR) ( Gawehns et al., 2014 ; De Wit, 2016 ). In Nicotiana benthamiana leaf cells (heterologous expression system), the transient expression of Fol-SIX6 , without its signal peptide, suppressed ion leakage and cell death induced by Avr2-I-2 interaction ( Gawehns et al., 2014 ). Like SIX1 , SIX8 is also required for virulence of Focub TR4 on Cavendish banana ( An et al., 2019 ). Similarly, SIX8 is involved in conferring virulence to Fo-5176 on Arabidopsis and cabbage plants ( Ayukawa et al., 2020 ).
Functional annotation is absent for most SIX genes. Information on how they contribute to virulence is still obscure. Some evidence suggest that they facilitate virulence by modulating hormonal pathways or defense response cascades ( Thatcher et al., 2012 ; Ma L. et al., 2013 ; Gawehns et al., 2014 ; Ma et al., 2015 ). The role of the SIX genes in virulence, their targets, and specific biological functions of their protein products warrant more research. The protein–protein interaction assay, such as co-immunoprecipitation, pull-down assays, and yeast two-hybrid, are used to identify putative targets of effector proteins ( Alfano, 2009 ; Rao et al., 2014 ). In case of transient interaction between effector and their targets, in planta subcellular effector localization can provide hints on target identity. Next-generation sequencing (NGS) technologies can be used to obtain sequences of putative effectors that can be screened for polymorphisms ( Alfano, 2009 ). Identification of effector targets and information on effector polymorphisms will improve our understanding on how the pathogen triggers disease or evade recognition by the host.
Secreted in Xylem Genes Act as Avirulence Determinants
Recognition of effector by cognate resistance ( R ) gene product of the plant results in induction of ETI (effector-triggered immunity) in the host ( Jones and Dangl, 2006 ). Effectors secreted by the pathogen serve as a two-edged sword ( Pradhan et al., 2020 ). While absence of the R gene in susceptible plants benefits the pathogen, their presence in tolerant plants triggers innate immunity characterized by HR-mediated cell death ( Flor, 1971 ; Jones and Dangl, 2006 ). However, ETI-mediated resistance to vascular wilt pathogen ( Fo ) does not include HR response; rather, it involves accumulation of tyloses, gums, phenolic compounds, and callose plugs in the xylem vessels, to preclude systemic spread of the pathogen ( Mes et al., 2000 ; Yadeta and Thomma, 2013 ).
Some SIX gene products such as Six1, Six3, and Six4 have been found to function as avirulence determinants in tomato- Fol pathosystem and, correspondingly, has been termed as Avr3, Avr2, and Avr1 proteins, respectively ( Rep et al., 2004 ; Houterman et al., 2008 , 2009 ; Takken and Rep, 2010 ; Ma et al., 2015 ). Four resistance genes have been identified in wild tomato cultivars ( Solanum pimpinellifolium and Solanum pennellii ) that confer resistance against Fol races. These resistance genes are I (Immunity), I-2 , I-3 , and I-7 ( Bohn and Tucker, 1939 ; Alexander and Tucker, 1945 ; Stall and Walter, 1965 ; McGrath et al., 1987 ; Scott and Jones, 1989 ; Lim et al., 2006 ). Individual expression of these genes in commercial cultivars of tomato resulted in the development of resistance against race 1, race 2, and race 3. The I gene that encodes an LRR-RLP protein (a class of receptor-like protein) was found to provide resistance against Fol race 1 upon recognizing AVR1 ( Houterman et al., 2008 ; Catanzariti et al., 2017 ). Both I-3 [a cell-surface S-receptor like kinase (SRLK)] and I-7 (LRR-RLP) proteins conferred resistance against race 3 by recognizing Avr3 and Avr7, respectively ( Rep et al., 2004 ; Lim et al., 2006 , 2008 ; Catanzariti et al., 2015 ; Gonzalez-Cendales et al., 2016 ). Likewise, I-2 (a cytoplasmic coiled-coil nucleotide-binding leucine-rich repeat protein) provided resistance against race 2 and race 1 by recognizing Avr2 ( Simons et al., 1998 ; Houterman et al., 2009 ). Later, Di et al. (2017) demonstrated that I-2 confers resistance by recognizing a specific epitope of Avr2. Ma et al. (2015) observed a new variant of the gene-for-gene model, where they observed that interaction between SIX5 and AVR2(SIX3) is required for I-2 -mediated resistance in tomato. While mutations in SIX5 led to evasion of recognition and also compromised the virulence of Fol , heterologous expression of AVR2 and I-2 in N. benthamiana leaves triggered I-2-mediated cell death ( Gawehns et al., 2014 ; Ma et al., 2015 ). Cao et al. (2018) , using the biomolecular fluorescence complementation assay, showed that Avr2 and Six5 interact at plasmodesmata, and Six5 facilitates cell-to-cell movement of Avr2, which in I-2-containing plants results in resistance. Interestingly, Avr1 also mediates the suppression of I-triggered responses. AVR1 gene in race 1 isolates enables the pathogen to overcome resistance response mediated by I-2 and I-3 despite the expression of AVR2 and AVR3 ( Rep et al., 2005 ; Houterman et al., 2008 ). This strategy has enabled the pathogen to circumvent the emergence of new races carrying AVR1 and AVR3 that would retain the virulence function of AVR3 while avoiding I-3 -initiated resistance ( Catanzariti et al., 2017 ). However, this suppression effect is strain-specific suggesting the involvement of an unknown fungal factor ( Houterman et al., 2008 ; Chellappan, 2014 ). Suppression of resistance response by AVR1 also established I as a gene of practical importance proposing that I-3 -mediated resistance was safeguarded by deployment of I ( Houterman et al., 2008 ). Nevertheless, AVR1 was not able to overcome resistance mediated by I-7 against race 3 ( Gonzalez-Cendales et al., 2016 ). I-7 is EDS1 (enhanced disease susceptibility1)-dependent and I-2 and I-3 are EDS1 independent ( Hu et al., 2005 ; Gonzalez-Cendales et al., 2016 ). The EDS1 signaling pathway is required for basal defense and systemic-acquired resistance ( Catanzariti et al., 2017 ). The fact that AVR1 suppresses I-2 and I-3 and not I-7 suggests that AVR1 is not a general suppressor of basal resistance ( Catanzariti et al., 2017 ). Recently, a new R-AVR interaction was recognized in melon- Fom pathosystem reported by Schmidt et al. (2016) wherein a novel AVR gene, FomAVR2 , is recognized by FOM2 in resistant melon plants. Similar to SIX genes, FomAVR2 encodes a small secreted protein with two cysteine residues and is found associated with a mimp element in the promoter region ( Schmidt et al., 2016 ).
Single nucleotide polymorphism serves as the source of genetic variation in SIX gene sequences. For instance, SIX genes in Focub were found to be present in multiple copies and showed the presence of sequence variants ( Fraser-Smith et al., 2014 ). The gain of forms and variation in sequence of pathogenicity-associated genes are presumed as adaptations by the pathogen to respond to rapidly changing environment and host ( Cuomo et al., 2007 ; Fraser-Smith et al., 2014 ; Maldonado et al., 2018 ). Houterman et al. (2009) demonstrated that Fol strains that are able to overcome I-2 -mediated resistance carry specific point mutations in AVR2 . These mutations in AVR2 resulted in amino acid change in the protein that led to the loss of its avirulence function. Till now, three AVR2 alleles have been described, each with one amino acid change at V41→M, R45→H, and R46→P in the protein ( Houterman et al., 2009 ; Biju et al., 2017 ). Additionally, a race 3 isolate showed the presence of an AVR2 gene with deletion of threonine residue at position 50 of the protein. This deletion also resulted in loss of avirulence function of Avr2 ( Biju et al., 2017 ). Di et al. (2017) analyzed the crystal structure of one of the AVR2 variants ( AVR2 R45H ) that is able to evade recognition by I-2 while retaining its virulence function. They identified two threonine residues in Avr2 protein (T53 and T145) that are required for virulence of Avr2 but not for recognition by I-2. The study revealed that the site of recognition by I-2 differs from the site required for maintenance of virulence function of Avr2. Avr2(Six3) facilitates virulence by suppression of pattern-triggered immunity (PTI) response, mainly, MAPK cascade, ROS burst, callose deposition, and growth inhibition ( Di et al., 2017 ). Similar to AVR2 , AVR1 , and AVR3 are presumed to be equally likely to undergo mutations that increase the probability of breakdown of resistance in tomato cultivars ( Takken and Rep, 2010 ). Such studies should be extrapolated to the remaining SIX genes to investigate if any variation in their sequence implies structural changes in their corresponding proteins, which may potentially increase the likelihood of evasion of SIX gene recognition by cognate R gene. Thus, it becomes important to uncover the mode of recognition of effector proteins. Additionally, as no direct interaction of avirulence gene with the host R gene is documented in Fol , efforts toward mining the targets of effector and cognate R-gene proteins need more impetus to understand disease resistance in host- Fo pathosystems. It will also be interesting to understand how the plant’s response to other vascular wilt fungi varies from Fo .
Furthermore, physical linkage of certain SIX genes observed in various formae speciales is deemed as important for the functions of the interacting pair of genes. As mentioned above, the AVR2(SIX3)–SIX5 linkage is one such example. These genes reside as a minicluster on chromosome 14 and their expression is under the control of the same bidirectional promoter present on the shared upstream region ( Schmidt et al., 2013 ). Another pair of effector genes, SIX8 – PSE1 , was identified in isolates capable of infecting Arabidopsis , and this pair was found to be associated with suppression of resistance in Arabidopsis ( Ayukawa et al., 2020 ). The mode of action of SIX8–PSE1 potentially involves suppression of a phytoalexin called camalexin. The SIX8 – PSE1 pair was found to be present in head-to-head orientation similar to the SIX3 – SIX5 gene pair. However, unlike SIX3 – SIX5 , no direct interaction between SIX8 – PSE1 was detected in yeast two-hybrid assays. Mutation in PSE1 , and not the SIX8 gene, resulted in evasion of recognition by the corresponding resistance protein suggesting that PSE1 is required to avoid detection ( Ayukawa et al., 2020 ). A conserved gene cluster SIX7/SIX10/SIX12 was also observed in formae speciales narcissi and gladioli ( Simbaqueba et al., 2018 ). However, the presence of SIX7 alone in formae speciales cubense and lilii suggests that SIX7 may be functionally and physically separable from SIX10 and SIX12 ( Simbaqueba et al., 2018 ). Clustering of SIX genes reflects cooperative interactions important to initiate (a)virulence functions. On this account, deletion studies either of any individual gene or a partial or a complete cluster can be done to elucidate the physical interactions among genes in a cluster and their individual roles in (a)virulence.
Secreted in Xylem Genes Confer Host Specificity
The capacity of fungal species to provoke disease on a specific host is referred to as host specificity. The basis of host specificity has been explained by molecular models like the guard and decoy hypothesis ( Van der Biezen and Jones, 1998 ; Dangl and Jones, 2001 ; van der Hoorn and Kamoun, 2008 ; Zipfel and Rathjen, 2008 ). These models have tremendously contributed in deciphering the role of effectors on the virulence of the pathogen as well as understanding the underpinnings of host–pathogen interactions ( van der Hoorn and Kamoun, 2008 ; Borah et al., 2018 ).
It is widely accepted that the factors that contribute to (a)virulence of a pathogen also determine its host specificity ( Li et al., 2021 ). In this regard, there are studies where SIX genes have been implicated in imparting host specificity to the pathogen. Avirulence genes of Fol and Fom that confer resistance to races of tomato and melon, respectively, function as host-specific factors ( Rep et al., 2004 ; Houterman et al., 2008 , 2009 ; Catanzariti et al., 2015 ; Schmidt et al., 2016 ). SIX6 gene from Fon has been known to operate as an avirulence gene in watermelon- Fon pathosystem providing host specificity to the pathogen ( Niu et al., 2016 ). The role of F. oxysporum f. sp. physalis ( Fophy ) SIX1 gene in specificity was demonstrated through complementation experiment where complementation strains of two homologs of Fophy-SIX1 (a and b) failed to overcome virulence loss in Fol-ΔSIX1 transformants ( Simbaqueba et al., 2018 ). Interestingly, SIX1b complementation restored avirulence of Fol on IL7-3 transgenic tomato lines carrying I-3 , demonstrating that Fophy-SIX1b is recognized by the resistance gene and functions as an avirulence factor. Similarly, complementation of Focon –Δ SIX1 mutant using Fol – SIX1 failed to rescue the virulence of Focon on cabbage suggesting a host-specific role of Fol – SIX1 ( Li et al., 2016 ).
Formae speciales of Fo are generally host specific but Forc shows an exceptional host range. Forc infects cucumber, melon, watermelon, squash, and gourd ( Vakalounakis, 1996 ; Punja and Parker, 2000 ; Cohen et al., 2015 ). Previous studies have demonstrated that forma specialis cucumerinum showed mild cross-pathogenicity toward melons ( Cafri et al., 2005 ) that was later corroborated in the findings of a study by van Dam et al. (2016) . They assessed cross-pathogenicity of cucurbit-infecting strains ( cucumerinum , radicis-cucumerinum , melonis , and niveum ) on resistant and susceptible cultivars of their corresponding hosts. The study revealed that Fom and Fon were highly host specific, whereas isolates of Forc displayed some degree of cross-pathogenicity toward musk melon ( van Dam et al., 2016 ). The genetic mechanism underlying the difference in host range was examined, and results showed that effector genes present on the mobile pathogenicity chromosome of Forc and Fom limit host range ( van Dam et al., 2017 ). A close comparison of the mobile pathogenicity chromosomes of Forc and Fom revealed that a single gene on mobile pathogenicity chromosome of Fom determined this difference in host range. This gene, upon introduction in Forc , rendered it non-pathogenic on cucumber suggesting that the gene functions as an avirulence factor ( Li et al., 2021 ). Overall, studies devoted to discerning the role of SIX genes in determining host-specificity in members of FOSC are sparse ( Table 1 ), obstructing our understanding of host specialization. Hence, more studies are required to identify gene(s) involved in limiting the host range of FOSC members.
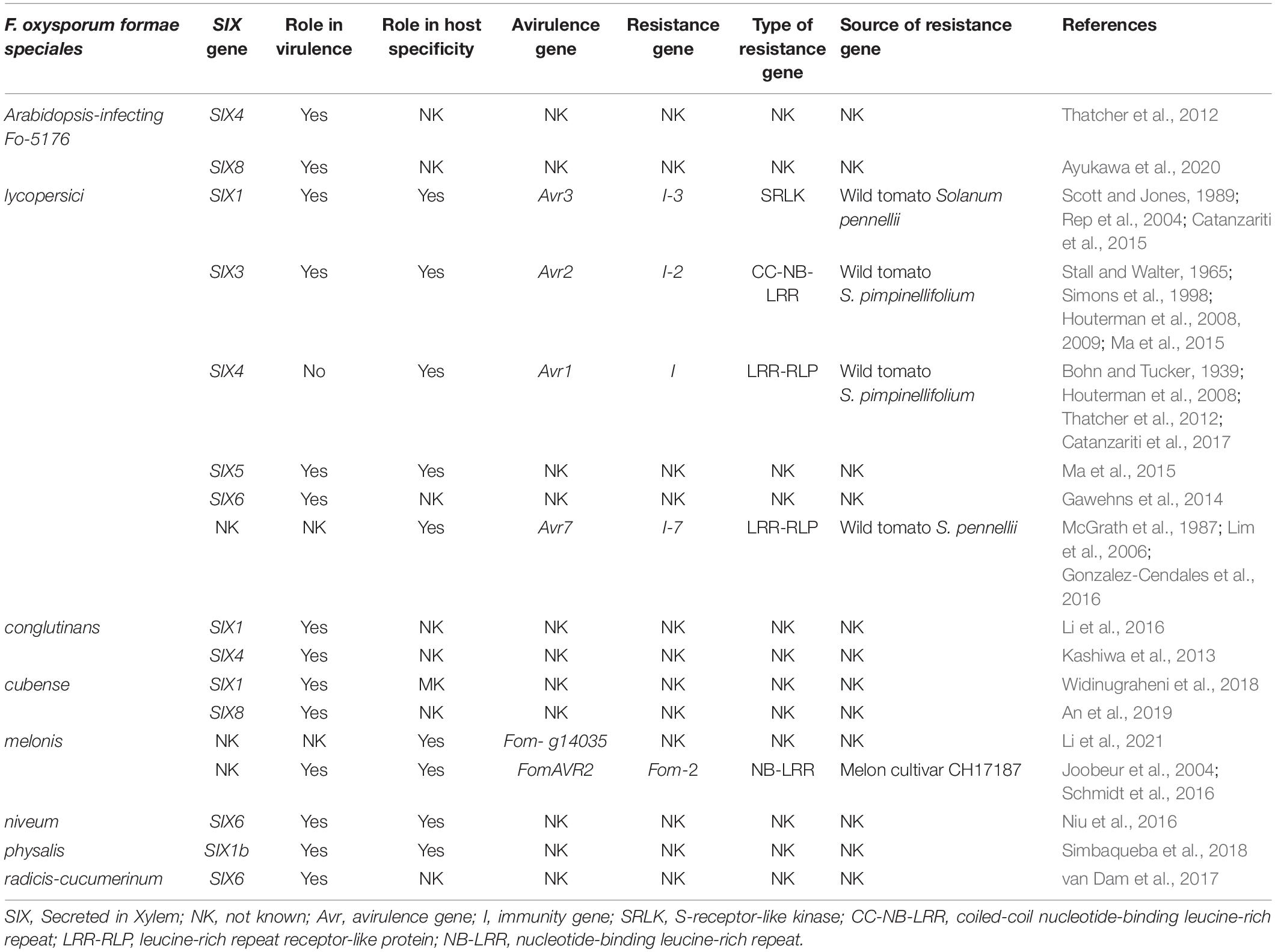
Table 1. Secreted in Xylem genes in formae speciale s of Fusarium oxysporum , their role in (a) virulence and host specificity.
Even though F. oxysporum infects a variety of plant species, the investigations on the molecular basis of pathogenicity are restricted to a limited number of hosts, mainly tomato, banana, melons, cucurbits, and cabbage. Genome sequencing of tomato and other cucurbit-infecting pathogens has provided insights on host–pathogen interactions, but a large number of formae speciales are yet left unexplored. Genome-based approaches are needed to elucidate mechanisms and understand the evolution of the pathogenicity. Transcriptomic and proteomic studies in infected plant tissues, the role of transposons and HGT in genome structure modulation, and emergence of host-specific pathogenicity are particular areas of interest. A significant prospect is to explore the differences in effector gene profile between different formae speciales , races, and non-pathogenic isolates. It would aid in the molecular detection of different formae speciales and pathogenic races that will assist significantly in the management of diseases caused by Fo . In addition, the biological functions of effector genes are still under investigation and require exhaustive research.
Author Contributions
PJ and RK jointly conceptualized and wrote the manuscript, contributing major parts of the literature survey. All the authors have collectively reviewed the manuscript and approved it.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors jointly acknowledge the Department of Biotechnology, Government of India, and Institution of Eminence, University of Delhi, India (Grant no. IoE/FRP/LS/2020/27). RK acknowledges the Research Council of the University of Delhi for the publication support. PJ and NS acknowledge the University Grants Commission, India, and NM, MR, and KS acknowledge the Council of Scientific and Industrial Research for Fellowships.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.628611/full#supplementary-material
Adusei-Fosu, K., and Dickinson, M. (2019). Development of pathogenicity assay and characterization of Fusarium oxysporum f. sp. elaeidis (FOE) based on Secreted In Xylem genes and EF-1α. J. Plant Pathol. 101, 1013–1024. doi: 10.1007/s42161-019-00332-4
CrossRef Full Text | Google Scholar
Alexander, L. J., and Tucker, C. M. (1945). Physiological specialization in the Tomato wilt fungus Fusarium oxysporum f. sp. lycopersici . J. Agric. Res. 70, 303–313.
Google Scholar
Alfano, J. R. (2009). Roadmap for future research on plant pathogen effectors. Mol. Plant Pathol. 10, 805–813. doi: 10.1111/j.1364-3703.2009.00588.x
PubMed Abstract | CrossRef Full Text | Google Scholar
An, B., Hou, X., Guo, Y., Zhao, S., Luo, H., He, C., et al. (2019). The effector six8 is required for virulence of Fusarium oxysporum f. sp. cubense tropical race 4 to cavendish banana. Fungal Biol. 123, 423–430. doi: 10.1016/j.funbio.2019.03.001
Armitage, A. D., Taylor, A., Sobczyk, M. K., Baxter, L., Greenfield, B. P., Bates, H. J., et al. (2018). Characterisation of pathogen-specific regions and novel effector candidates in Fusarium oxysporum f. sp. cepae . Sci. Rep. 8, 1–15. doi: 10.1038/s41598-018-30335-7
Armstrong, G. M., and Armstrong, J. K. (1981). “ Formae speciales and races of Fusarium oxysporum causing wilt disease,” in Fusarium: Disease, Biology, and Taxonomy , eds P. E. Nelson, T. A. Toussoun, and R. J. Cook (University Park, PA: Pennsylvania State University Press), 391–399.
Ayukawa, Y., Asai, S., Gan, P., Tsushima, A., Ichihashi, Y., Shibata, A., et al. (2020). A pair of effectors encoded on a conditionally dispensable chromosome of Fusarium oxysporum suppress host-specific immunity. BioRxiv 329052. [preprint]. doi: 10.1101/2020.10.06.329052
Baayen, R. P. (2000). Diagnosis and detection of host-specific forms of Fusarium oxysporum . Bull OEPP 30, 489–491. doi: 10.1111/j.1365-2338.2000.tb00935.x
Baayen, R. P., O’Donnell, K., Bonants, P. J. M., Cigelnik, E., Kroon, L. P. N., Roebroeck, E. J. A., et al. (2000). Gene genealogies and AFLP analysis in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 90, 891–900. doi: 10.1094/PHYTO.2000.90.8.891
Batson, A. M., Fokkens, L., Rep, M., and du Toit, L. J. (2020). Putative effector genes distinguish two pathogenicity groups of Fusarium oxysporum f. sp. spinaciae . Mol. Plant Microbe Interact. 34, 141–156. doi: 10.1094/MPMI-06-20-0145-R
Beckman, C. H. (1987). The Nature of Wilt Diseases of Plants. St. Paul, MN: American Phytopathological Society Press.
Biju, V. C., Fokkens, L., Houterman, P. M., Rep, M., and Cornelissen, B. J. (2017). Multiple evolutionary trajectories have led to the emergence of races in Fusarium oxysporum f. sp. lycopersici . Appl. Environ. Microbiol. 83, 2548–2516e. doi: 10.1128/AEM.02548-16
Bishop, C. D., and Cooper, R. M. (1983). An ultrastructural study of root invasion in three vascular wilt diseases. Physiol. Plant Pathol. 22, 15–13. doi: 10.1016/0048-4059(83)90018-8
Bohn, G. W., and Tucker, C. M. (1939). Immunity to Fusarium wilt in the tomato. Science 89, 603–604. doi: 10.1126/science.89.2322.603
Borah, N., Albarouki, E., and Schirawski, J. (2018). Comparative methods for molecular determination of host-specificity factors in plant-pathogenic fungi. Int. J. Mol. Sci. 19:863. doi: 10.3390/ijms19030863
Brown, D. W., Busman, M., and Proctor, R. H. (2014). Fusarium verticillioides SGE1 is required for full virulence and regulates expression of protein effector and secondary metabolite biosynthetic genes. Mol. Plant Microbe Interact. 27, 809–823. doi: 10.1094/MPMI-09-13-0281-R
Cafri, D., Katan, J., and Katan, T. (2005). Cross−pathogenicity between formae speciales of Fusarium oxysporum , the pathogens of cucumber and melon. J. Phytopathol. 153, 615–622. doi: 10.1111/j.1439-0434.2005.01029.x
Cai, G., Gale, L. R., Schneider, R. W., Kistler, H. C., Davis, R. M., Elias, K. S., et al. (2003). Origin of race 3 of Fusarium oxysporum f. sp. lycopersici at a single site in California. Phytopathology 93, 1014–1022. doi: 10.1094/PHYTO.2003.93.8.1014
Cao, L., Blekemolen, M. C., Tintor, N., Cornelissen, B. J., and Takken, F. L. (2018). The Fusarium oxysporum Avr2-Six5 effector pair alters plasmodesmatal exclusion selectivity to facilitate cell-to-cell movement of Avr2. Mol. Plant 11, 691–705. doi: 10.1016/j.molp.2018.02.011
Carmona, S. L., Burbano-David, D., Gómez, M. R., Lopez, W., Ceballos, N., Castaño-Zapata, J., et al. (2020). Characterization of Pathogenic and Nonpathogenic Fusarium oxysporum Isolates Associated with Commercial Tomato Crops in the Andean Region of Colombia. Pathogens 9:70. doi: 10.3390/pathogens9010070
Catanzariti, A. M., Do, H. T., Bru, P., de Sain, M., Thatcher, L. F., Rep, M., et al. (2017). The tomato I gene for Fusarium wilt resistance encodes an atypical leucine−rich repeat receptor−like protein whose function is nevertheless dependent on SOBIR 1 and SERK 3/BAK 1. Plant J. 89, 1195–1209. doi: 10.1111/tpj.13458
Catanzariti, A. M., Lim, G. T., and Jones, D. A. (2015). The tomato I−3 gene: a novel gene for resistance to Fusarium wilt disease. N. Phytol. 207, 106–118. doi: 10.1111/nph.13348
Caten, C. E., and Jinks, J. L. (1966). Heterokaryosis: its significance in wild homothallic Ascomycetes and Fungi imperfecti. Trans. Br. Mycol. Soc. 49, 81–93. doi: 10.1016/S0007-1536(66)80038-4
Chakrabarti, A., Rep, M., Wang, B., Ashton, A., Dodds, P., and Ellis, J. (2011). Variation in potential effector genes distinguishing Australian and non−Australian isolates of the cotton wilt pathogen Fusarium oxysporum f. sp. vasinfectum . Plant Pathol. 60, 232–243. doi: 10.1111/j.1365-3059.2010.02363.x
Chang, W., Li, H., Chen, H., Qiao, F., and Zeng, H. (2020). Identification of mimp-associated effector genes in Fusarium oxysporum f. sp. cubense race 1 and race 4 and virulence confirmation of a candidate effector gene. Microbiol. Res. 232:126375. doi: 10.1016/j.micres.2019.126375
Chellappan, B. V. (2014). Evolution of Races within Fusarium oxysporum f. sp. lycopersici. Ph.D. thesis. Amsterdam: University of Amsterdam.
Chuma, I., Isobe, C., Hotta, Y., Ibaragi, K., Futamata, N., Kusaba, M., et al. (2011). Multiple translocation of the AVR-pita effector gene among chromosomes of the rice blast fungus Magnaporthe oryzae and related species. PLoS Pathog. 7:e1002147. doi: 10.1371/journal.ppat.1002147
Cohen, R., Orgil, G., Burger, Y., Saar, U., Elkabetz, M., Tadmor, Y., et al. (2015). Differences in the responses of melon accessions to fusarium root and stem rot and their colonization by F usarium oxysporum f. sp. radicis − cucumerinum . Plant Pathol. 64, 655–663. doi: 10.1111/ppa.12286
Coleman, J. J., Rounsley, S. D., Rodriguez-Carres, M., Kuo, A., Wasmann, C. C., Grimwood, J., et al. (2009). The genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genet. 5:e1000618. doi: 10.1371/journal.pgen.1000618
Correll, J. C. (1991). The relationship between formae speciales, races, and vegetative compatibility groups in Fusarium oxysporum . Phytopathology 81, 1061–1064.
Covey, P. A., Kuwitzky, B., Hanson, M., and Webb, K. M. (2014). Multilocus analysis using putative fungal effectors to describe a population of Fusarium oxysporum from sugar beet. Phytopathology 104, 886–896. doi: 10.1094/PHYTO-09-13-0248-R
Croll, D., and McDonald, B. A. (2012). The accessory genome as a cradle for adaptive evolution in pathogens. PLoS Pathog. 8:e1002608. doi: 10.1371/journal.ppat.1002608
Cuomo, C. A., Güldener, U., Xu, J. R., Trail, F., Turgeon, B. G., Di Pietro, A., et al. (2007). The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317, 1400–1402. doi: 10.1126/science.1143708
Czislowski, E., Fraser−Smith, S., Zander, M., O’Neill, W. T., Meldrum, R. A., Tran−Nguyen, L. T., et al. (2018). Investigation of the diversity of effector genes in the banana pathogen, Fusarium oxysporum f. sp. cubense , reveals evidence of horizontal gene transfer. Mol. Plant Pathol. 19, 1155–1171. doi: 10.1111/mpp.12594
Dangl, J. L., and Jones, J. D. G. (2001). Plant pathogens and integrated defence responses to infection. Nature 411, 826–833. doi: 10.1038/35081161
Davière, J. M., Langin, T., and Daboussi, M. J. (2001). Potential role of transposable elements in the rapid reorganization of the Fusarium oxysporum genome. Fungal Genet. Biol. 34, 177–192. doi: 10.1006/fgbi.2001.1296
de Lamo, F. J., and Takken, F. L. W. (2020). Biocontrol by Fusarium oxysporum Using Endophyte-Mediated Resistance. Front. Plant Sci. 11:37. doi: 10.3389/fpls.2020.00037
de Vega-Bartol, J. J., Martín-Dominguez, R., Ramos, B., García-Sánchez, M. A., and Díaz-Mínguez, J. M. (2011). New virulence groups in Fusarium oxysporum f. sp. phaseoli : the expression of the gene coding for the transcription factor ftf1 correlates with virulence. Phytopathology 101, 470–479. doi: 10.1094/PHYTO-09-10-0252
De Wit, P. J. (2016). Apoplastic fungal effectors in historic perspective; a personal view. N. Phytol. 212, 805–813. doi: 10.1111/nph.14144
Dean, R., Van Kan, J. A., Pretorius, Z. A., Hammond−Kosack, K. E., Di Pietro, A., Spanu, P. D., et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
DeIulio, G. A., Guo, L., Zhang, Y., Goldberg, J. M., Kistler, H. C., and Ma, L. J. (2018). Kinome expansion in the Fusarium oxysporum species complex driven by accessory chromosomes. Msphere. 3, 231–218e. doi: 10.1128/mSphere.00231-18
Di Pietro, A., García−Maceira, F. I., Méglecz, E., and Roncero, M. I. G. (2001). A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39, 1140–1152. doi: 10.1111/j.1365-2958.2001.02307.x
Di, X., Cao, L., Hughes, R. K., Tintor, N., Banfield, M. J., and Takken, F. L. (2017). Structure–function analysis of the Fusarium oxysporum Avr2 effector allows uncoupling of its immune−suppressing activity from recognition. N. Phytol. 216, 897–914. doi: 10.1111/nph.14733
Dobbs, J. T., Kim, M. S., Dudley, N. S., Klopfenstein, N. B., Yeh, A., Hauff, R. D., et al. (2020). Whole genome analysis of the koa wilt pathogen ( Fusarium oxysporum f. sp. koae ) and the development of molecular tools for early detection and monitoring. BMC Genomics 21:1–15. doi: 10.1186/s12864-020-07156-y
Dong, S., Raffaele, S., and Kamoun, S. (2015). The two-speed genomes of filamentous pathogens: waltz with plants. Curr. Opin. Genet. Dev. 35, 57–65. doi: 10.1016/j.gde.2015.09.001
Duan, Y., Qu, W., Chang, S., Li, C., Xu, F., Ju, M., et al. (2020). Identification of Pathogenicity Groups and Pathogenic Molecular Characterization of Fusarium oxysporum f. sp. sesami in China. Phytopathology 110, 1093–1104. doi: 10.1094/PHYTO-09-19-0366-R
Edel-Hermann, V., and Lecomte, C. (2019). Current status of Fusarium oxysporum f ormae speciales and races. Phytopathology 109, 512–530. doi: 10.1094/PHYTO-08-18-0320-RVW
Epstein, L., Kaur, S., Chang, P. L., Carrasquilla-Garcia, N., Lyu, G., Cook, D. R., et al. (2017). Races of the celery pathogen Fusarium oxysporum f. sp. apii are polyphyletic. Phytopathology 107, 463–473. doi: 10.1094/PHYTO-04-16-0174-R
Flor, H. H. (1971). Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 9, 275–296. doi: 10.1146/annurev.py.09.090171.001423
Fokkens, L., Shahi, S., Connolly, L. R., Stam, R., Schmidt, S. M., Smith, K. M., et al. (2018). The multi-speed genome of Fusarium oxysporum reveals association of histone modifications with sequence divergence and footprints of past horizontal chromosome transfer events. BioRxiv 465070. [preprint]. doi: 10.1101/465070
Fourie, G., Steenkamp, E. T., Gordon, T. R., and Viljoen, A. (2009). Evolutionary relationships among the Fusarium oxysporum f. sp. cubense vegetative compatibility groups. Appl. Environ. Microbiol. 75, 4770–4781. doi: 10.1128/AEM.00370-09
Fraser-Smith, S., Czislowski, E., Meldrum, R. A., Zander, M., O’neill, W., Balali, G. R., et al. (2014). Sequence variation in the putative effector gene SIX 8 facilitates molecular differentiation of Fusarium oxysporum f. sp. cubense . Plant Pathol. 63, 1044–1052. doi: 10.1111/ppa.12184
Fujinaga, M., Ogiso, H., Shinohara, H., Tsushima, S., Nishimura, N., Togawa, M., et al. (2005). Phylogenetic relationships between the lettuce root rot pathogen Fusarium oxysporum f. sp. lactucae races 1, 2, and 3 based on the sequence of the intergenic spacer region of its ribosomal DNA. J. Gen. Plant Pathol. 71, 402–407. doi: 10.1007/s10327-005-0226-z
Gawehns, F., Houterman, P. M., Ichou, F. A., Michielse, C. B., Hijdra, M., Cornelissen, B. J. C., et al. (2014). The Fusarium oxysporum effector Six6 contributes to virulence and suppresses I-2-mediated cell death. Mol. Plant Microbe Interact. 27, 336–348. doi: 10.1094/MPMI-11-13-0330-R
Gonzalez-Cendales, Y., Catanzariti, A. M., Baker, B., Mcgrath, D. J., and Jones, D. A. (2016). Identification of I−7 expands the repertoire of genes for resistance to Fusarium wilt in tomato to three resistance gene classes. Mol. Plant Pathol. 17, 448–463. doi: 10.1111/mpp.12294
Gordon, T. R. (2017). Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol. 55, 23–39. doi: 10.1146/annurev-phyto-080615-095919
Gordon, T. R., and Martyn, R. D. (1997). The evolutionary biology of Fusarium oxysporum . Annu. Rev. Phytopathol. 35, 111–128. doi: 10.1146/annurev.phyto.35.1.111
Gordon, W. L. (1965). Pathogenic strains of Fusarium oxysporum . Can. J. Bot. 43, 1309–1318. doi: 10.1139/b65-138
Guo, L., Han, L., Yang, L., Zeng, H., Fan, D., Zhu, Y., et al. (2014). Genome and transcriptome analysis of the fungal pathogen Fusarium oxysporum f. sp. cubense causing banana vascular wilt disease. PLoS One 9:e95543. doi: 10.1371/journal.pone.0095543
Guo, L., Yang, L., Liang, C., Wang, J., Liu, L., and Huang, J. (2016). The G-protein subunits FGA2 and FGB1 play distinct roles in development and pathogenicity in the banana fungal pathogen Fusarium oxysporum f. sp. cubense . Physiol. Mol. Plant Pathol. 93, 29–38. doi: 10.1016/j.pmpp.2015.12.003
Henry, P., Kaur, S., Pham, Q. A. T., Barakat, R., Brinker, S., Haensel, H., et al. (2020). Genomic differences between the new Fusarium oxysporum f. sp. apii (Foa) race 4 on celery, the less virulent Foa races 2 and 3, and the avirulent on celery f. sp. coriandrii. BMC Genomics 21:1–23. doi: 10.1186/s12864-020-07141-5
Hill, A. L., Reeves, P. A., Larson, R. L., Fenwick, A. L., Hanson, L. E., and Panella, L. (2011). Genetic variability among isolates of Fusarium oxysporum from sugar beet. Plant Pathol. 60, 496–505. doi: 10.1111/j.1365-3059.2010.02394.x
Hogenhout, S. A., Van der Hoorn, R. A., Terauchi, R., and Kamoun, S. (2009). Emerging concepts in effector biology of plant-associated organisms. Mol. Plant Microbe Interact. 22, 115–122. doi: 10.1094/MPMI-22-2-0115
Houterman, P. M., Cornelissen, B. J., and Rep, M. (2008). Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog. 4:e1000061. doi: 10.1371/journal.ppat.1000061
Houterman, P. M., Ma, L., Van Ooijen, G., De Vroomen, M. J., Cornelissen, B. J., Takken, F. L., et al. (2009). The effector protein Avr2 of the xylem-colonizing fungus Fusarium oxysporum activates the tomato resistance protein I-2 intracellularly. Plant J. 58, 970–978. doi: 10.1111/j.1365-313X.2009.03838.x
Houterman, P. M., Speijer, D., Dekker, H. L., de Koster, C. G., Cornelissen, B. J., and Rep, M. (2007). The mixed xylem sap proteome of Fusarium oxysporum −infected tomato plants. Mol. Plant Pathol. 8, 215–221. doi: 10.1111/j.1364-3703.2007.00384.x
Hu, G., de Hart, A. K., Li, Y., Ustach, C., Handley, V., Navarre, R., et al. (2005). EDS1 in tomato is required for resistance mediated by TIR−class R genes and the receptor−like R gene Ve. Plant J. 42, 376–391. doi: 10.1111/j.1365-313X.2005.02380.x
Husaini, A. M., Sakina, A., and Cambay, S. R. (2018). Host–pathogen interaction in Fusarium oxysporum infections: Where do we stand? Mol. Plant Microbe Interact. 31, 889–898. doi: 10.1094/MPMI-12-17-0302-CR
Imazaki, I., Kurahashi, M., Iida, Y., and Tsuge, T. (2007). Fow2, a Zn (II) 2Cys6−type transcription regulator, controls plant infection of the vascular wilt fungus Fusarium oxysporum . Mol. Microbiol. 63, 737–753. doi: 10.1111/j.1365-2958.2006.05554.x
Jain, S., Akiyama, K., Kan, T., Ohguchi, T., and Takata, R. (2003). The Gprotein beta subunit FGB1 regulates development and pathogenicity in Fusarium oxysporum . Curr. Genet. 43, 79–86. doi: 10.1007/s00294-003-0372-9
Jain, S., Akiyama, K., Mae, K., Ohguchi, T., and Takata, R. (2002). Targeted disruption of a G protein alpha subunit gene results in reducedpathogenicity in Fusarium oxysporum . Curr. Genet. 41, 407–413. doi: 10.1007/s00294-002-0322-y
Jain, S., Akiyama, K., Takata, R., and Ohguchi, T. (2005). Signaling via the G protein α subunit FGA2 is necessary for pathogenesis in Fusarium oxysporum . FEMS Microbiol. Lett. 243, 165–172. doi: 10.1016/j.femsle.2004.12.009
Jiménez-Gasco, M. M., Milgroom, M. G., and Jiménez-Díaz, R. M. (2002). Gene genealogies support Fusarium oxysporum f. sp. ciceris as a monophyletic group. Plant Pathol. 51, 72–77. doi: 10.1046/j.0032-0862.2001.00610.x-i1
Jones, J. D., and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329.
Joobeur, T., King, J. J., Nolin, S. J., Thomas, C. E., and Dean, R. A. (2004). The Fusarium wilt resistance locus FOM-2 of melon contains a single resistance gene with complex features. Plant J. 39, 283–297. doi: 10.1111/j.1365-313X.2004.02134.x
Joshi, R. (2018). A review of Fusarium oxysporum on its plant interaction and industrial use. J. Med. Plant. 6, 112–115. doi: 10.22271/plants.2018.v6.i3b.07
Kang, S., Demers, J., del Mar, Jimenez-Gasco, M., and Rep, M. (2014). “Fusarium oxysporum,” in Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens , eds A. Lichens-Park, C. Kole, and R. A. Dean (Berlin: Springer), 99–119.
Kashiwa, T., Inami, K., Fujinaga, M., Ogiso, H., Yoshida, T., Teraoka, T., et al. (2013). An avirulence gene homologue in the tomato wilt fungus Fusarium oxysporum f. sp. lycopersici race 1 functions as a virulence gene in the cabbage yellows fungus F. oxysporum f. sp. conglutinans . J. Gen. Plant Pathol. 79, 412–421. doi: 10.1007/s10327-013-0471-5
Kawabe, M., Kobayashi, Y., Okada, G., Yamaguchi, I., Teraoka, T., and Arie, T. (2005). Three evolutionary lineages of tomato wilt pathogen, Fusarium oxysporum f. sp. lycopersici , based on sequences of IGS, MAT1, and pg1, are each composed of isolates of a single mating type and a single or closely related vegetative compatibility group. J. Gen. Plant. Pathol. 71, 263–272. doi: 10.1007/s10327-005-0203-6
Kistler, H. C. (1997). Genetic diversity in the plant-pathogenic fungus Fusarium oxysporum . Phytopathology 87, 474–479. doi: 10.1094/PHYTO.1997.87.4.474
Kistler, H. C., Benny, U., Boehm, E. W. A., and Katan, T. (1995). Genetic duplication in Fusarium oxysporum . Curr. Genet. 28, 173–176. doi: 10.1007/BF00315784
Kuang, H., Padmanabhan, C., Li, F., Kamei, A., Bhaskar, P. B., Ouyang, S., et al. (2009). Identification of miniature inverted-repeat transposable elements (MITEs) and biogenesis of their siRNAs in the Solanaceae: new functional implications for MITEs. Genome Res. 19, 42–56. doi: 10.1101/gr.078196.108
Kuldau, G. A., and Yates, I. E. (2000). “Evidence for Fusarium endophytes,” in Microbial Endophytes , eds C. W. Bacon and J. F. White (New York, NY: Marcel Dekker, Inc.), 85–117.
Laurence, M. H., Summerell, B. A., and Liew, E. C. Y. (2015). Fusarium oxysporum f. sp. canariensis : evidence for horizontal gene transfer of putative pathogenicity genes. Plant Pathol. 64, 1068–1075. doi: 10.1111/ppa.12350
Leslie, J. F., and Summerell, B. A. (2008). The Fusarium laboratory manual. Hoboken, NJ: John Wiley & Sons.
Li, E., Wang, G., Xiao, J., Ling, J., Yang, Y., and Xie, B. (2016). A SIX1 homolog in Fusarium oxysporum f. sp. conglutinans is required for full virulence on cabbage. PLoS One 11:e0152273. doi: 10.1371/journal.pone.0152273
Li, J., Fokkens, L., and Rep, M. (2021). A single gene in Fusarium oxysporum limits host range. Mol. Plant Pathol. 22, 108–116. doi: 10.1111/mpp.13011
Lievens, B., Claes, L., Vakalounakis, D. J., Vanachter, A. C., and Thomma, B. P. (2007). A robust identification and detection assay to discriminate the cucumber pathogens Fusarium oxysporum f. sp. cucumerinum and f. sp. radicis − cucumerinum . Environ. Microbiol. 9, 2145–2161. doi: 10.1111/j.1462-2920.2007.01329.x
Lievens, B., Houterman, P. M., and Rep, M. (2009a). Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales . FEMS Microbiol. Lett. 300, 201–215. doi: 10.11a11/j.1574-6968.2009.01783z.x
Lievens, B., Rep, M., and Thomma, B. P. (2008). Recent developments in the molecular discrimination of formae speciales of Fusarium oxysporum . Pest Manag. Sci. 64, 781–788. doi: 10.1002/ps.1564
Lievens, B., Van Baarlen, P., Verreth, C., Van Kerckhove, S., Rep, M., and Thomma, B. P. (2009b). Evolutionary relationships between Fusarium oxysporum f. sp. lycopersici and F. oxysporum f. sp. radicis-lycopersici isolates inferred from mating type, elongation factor-1α and exopolygalacturonase sequences. Mycol. Res. 113, 1181–1191. doi: 10.1016/j.mycres.2009.07.019
Lim, G. T. T., Wang, G. P., Hemming, M. N., Basuki, S., McGrath, D. J., Carroll, B. J., et al. (2006). Mapping the I-3 gene for resistance to Fusarium wilt in tomato: application of an I-3 marker in tomato improvement and progress towards the cloning of I-3. Australas. Plant Pathol. 35, 671–680. doi: 10.1071/AP06073
Lim, G. T. T., Wang, G. P., Hemming, M. N., McGrath, D. J., and Jones, D. A. (2008). High resolution genetic and physical mapping of the I-3 region of tomato chromosome 7 reveals almost continuous microsynteny with grape chromosome 12 but interspersed microsynteny with duplications on Arabidopsis chromosomes 1, 2 and 3. Theor. Appl. Genet. 118, 57–75. doi: 10.1007/s00122-008-0876-2
Liu, Z., Zhang, X., Liu, X., Fu, C., Han, X., Yin, Y., et al. (2016). The chitin synthase FgChs2 and other FgChss co-regulate vegetative development and virulence in F. graminearum . Sci. Rep. 6, 1–12. doi: 10.1038/srep34975
Lyons, R., Czislowski, E., Zeil-Rolfe, I., Kaur, S., Chen, A., et al. (2019). Unique Secreted in Xylem Genes in Banana-Infecting Endophytic Fusarium oxysporum . MDPI 36:180. doi: 10.3390/proceedings2019036180
Ma, L. J., Geiser, D. M., Proctor, R. H., Rooney, A. P., O’Donnell, K., Trail, F., et al. (2013). Fusarium pathogenomics. Annu. Rev. Microbiol. 67, 399–416. doi: 10.1146/annurev-micro-092412-155650
Ma, L., Cornelissen, B. J. C., and Takken, F. L. W. (2013). A nuclear localization for Avr2 from Fusarium oxysporum is required to activate the tomato resistance protein I-2. Front. Plant Sci. 4:94. doi: 10.3389/fpls.2013.00094
Ma, L. J., Shea, T., Young, S., Zeng, Q., and Kistler, H. C. (2014). Genome sequence of Fusarium oxysporum f. sp. melonis strain NRRL 26406, a fungus causing wilt disease on melone. Genome Announc. 2, 730–714e. doi: 10.1128/genomeA.00730-14
Ma, L. J., Van Der Does, H. C., Borkovich, K. A., Coleman, J. J., Daboussi, M. J., Di Pietro, A., et al. (2010). Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium . Nature 464, 367–373. doi: 10.1038/nature08850
Ma, L., Houterman, P. M., Gawehns, F., Cao, L., Sillo, F., Richter, H., et al. (2015). The AVR2–SIX5 gene pair is required to activate I−2−mediated immunity in tomato. N. Phytol. 208, 507–518. doi: 10.1111/nph.13455
Maldonado, B. L. D., Villarruel, O. J. L., Calderón, O. M. A., and Sánchez, E. A. C. (2018). Secreted in Xylem (Six) Genes in Fusarium oxysporum f. sp. cubense and Their Potential Acquisition by Horizontal Transfer. Adv. Biotech. Micro. 10:555779. doi: 10.19080/AIBM.2018.10.555778
Mandeel, Q., and Baker, R. (1991). Mechanisms involved in biological control of Fusarium wilt of cucumber with strains of nonpathogenic Fusarium oxysporum . Phytopathology 81, 462–469. doi: 10.1094/phyto-81-462
Mandeel, Q., Ayub, N., and Gul, J. (2005). Survey of Fusarium species in an environment of Bahrain. VI. Biodiversity of the genus Fusarium in root-soil ecosystem of halophytic date palm ( Phoenix dactylifera ) community. Cryptogamie Mycol. 26, 365–404.
McGrath, D., Gillespie, D., and Vawdrey, L. (1987). Inheritance of resistance to Fusarium oxysporum f. sp. lycopersici races 2 and 3 in Lycopersicon pennellii . Aust. J. Agric. Res. 38, 729–733. doi: 10.1071/AR9870729
Meldrum, R. A., Fraser-Smith, S., Tran-Nguyen, L. T. T., Daly, A. M., and Aitken, E. A. B. (2012). Presence of putative pathogenicity genes in isolates of Fusarium oxysporum f. sp. cubense from Australia. Australas. Plant Pathol. 41, 551–557. doi: 10.1007/s13313-012-0122-x
Mes, J. J., van Doorn, A. A., Wijbrandi, J., Simons, G., Cornelissen, B. J., and Haring, M. A. (2000). Expression of the Fusarium resistance gene I-2 colocalizes with the site of fungal containment. Plant J. 23, 183–193. doi: 10.1046/j.1365-313x.2000.00765.x
Michielse, C. B., and Rep, M. (2009). Pathogen profile update: Fusarium oxysporum . Mol. Plant Pathol. 10:311. doi: 10.1111/j.1364-3703.2009.00538.x
Michielse, C. B., van Wijk, R., Reijnen, L., Cornelissen, B. J., and Rep, M. (2009a). Insight into the molecular requirements for pathogenicity of Fusarium oxysporum f. sp. lycopersici through large-scale insertional mutagenesis. Genome Biol. 10:R4. doi: 10.1186/gb-2009-10-1-r4
Michielse, C. B., van Wijk, R., Reijnen, L., Manders, E. M., Boas, S., Olivain, C., et al. (2009b). The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog. 5:e1000637. doi: 10.1371/journal.ppat.1000637
Moore, N. Y., Pegg, K. G., Allen, R. N., and Irwin, J. A. G. (1993). Vegetative compatibility and distribution of Fusarium oxysporum f. sp. cubense in Australia. Aus. J. Exp. Agric. 33, 797–802. doi: 10.1071/EA9930797
Namiki, F., Shiomi, T., Kayamura, T., and Tsuge, T. (1994). Characterization of the formae speciales of Fusarium oxysporum causing wilts of cucurbits by DNA fingerprinting with nuclear repetitive DNA sequences. Appl. Environ. Microbiol. 60, 2684–2691. doi: 10.1128/aem.60.8.2684-2691.1994
Niño-Sánchez, J., Casado-Del Castillo, V., Tello, V., De Vega-Bartol, J. J., Ramos, B., Sukno, S. A., et al. (2016). The FTF gene family regulates virulence and expression of SIX effectors in Fusarium oxysporum . Mol. Plant Pathol. 17, 1124–1139. doi: 10.1111/mpp.12373
Niño-Sánchez, J., Tello, V., Casado-del Castillo, V., Thon, M. R., Benito, E. P., and Díaz-Mínguez, J. M. (2015). Gene expression patterns and dynamics of the colonization of common bean ( Phaseolus vulgaris L.) by highly virulent and weakly virulent strains of Fusarium oxysporum . Front. Microbiol. 6:234. doi: 10.3389/fmicb.2015.00234
Nirmaladevi, D., Venkataramana, M., Srivastava, R. K., Uppalapati, S. R., Gupta, V. K., Yli-Mattila, T., et al. (2016). Molecular phylogeny, pathogenicity and toxigenicity of Fusarium oxysporum f. sp. lycopersici . Sci. Rep. 6, 1–14. doi: 10.1038/srep21367
Niu, X., Zhao, X., Ling, K. S., Levi, A., Sun, Y., and Fan, M. (2016). The FonSIX6 gene acts as an avirulence effector in the Fusarium oxysporum f. sp. niveum -watermelon pathosystem. Sci. Rep. 6, 1–7. doi: 10.1038/srep28146
O’Donnell, K., and Cigelnik, E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7, 103–116. doi: 10.1006/mpev.1996.0376
O’Donnell, K., Kistler, H. C., Cigelnik, E., and Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. U S A. 95, 2044–2049. doi: 10.1073/pnas.95.5.2044
O’Donnell, K., Rooney, A. P., Proctor, R. H., Brown, D. W., McCormick, S. P., Ward, T. J., et al. (2013). Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria . Fungal Genet. Biol. 52, 20–31. doi: 10.1016/j.fgb.2012.12.004
O’Donnell, K., Gherbawy, Y., Schweigkofler, W., Adler, A., and Prillinger, H. (1999). Phylogenetic analyses of DNA sequence and RAPD data compared in Fusarium oxysporum and related species from maize. J. Phytopathol. 147, 445–452. doi: 10.1111/j.1439-0434.1999.tb03849.x
Ogiso, H., Fujinaga, M., Saito, H., Takehara, T., and Yamanaka, S. (2002). Physiological races and vegetative compatibility groups of Fusarium oxysporum f. sp. lactucae isolated from crisphead lettuce in Japan. J. Gen. Plant Pathol. 68, 292–299. doi: 10.1007/PL00013094
Olivain, C., and Alabouvette, C. (1999). Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici in comparison with a non−pathogenic strain. N. Phytol. 141, 497–510. doi: 10.1046/j.1469-8137.1999.00365.x
Palmero, D., Iglesias, C., de Cara, M., Lomas, T., Santos, M., and Tello, J. C. (2009). Species of Fusarium isolated from river and sea water of southeastern Spain and pathogenicity on four plant species. Plant Dis. 93, 377–385. doi: 10.1094/PDIS-93-4-0377
Pasquali, M., Dematheis, F., Gilardi, G., Gullino, M. L., and Garibaldi, A. (2005). Vegetative compatibility groups of Fusarium oxysporum f. sp. lactucae from lettuce. Plant Dis. 89, 237–240. doi: 10.1094/PD-89-0237
Pietro, A. D., Madrid, M. P., Caracuel, Z., Delgado-Jarana, J., and Roncero, M. I. G. (2003). Fusarium oxysporum : exploring the molecular arsenal of a vascular wilt fungus. Mol. Plant Pathol. 4, 315–325. doi: 10.1046/j.1364-3703.2003.00180.x
Pinaria, A. G., Laurence, M. H., Burgess, L. W., and Liew, E. C. Y. (2015). Phylogeny and origin of Fusarium oxysporum f. sp. vanillae in Indonesia. Plant Pathol. 64, 1358–1365. doi: 10.1111/ppa.12365
Ploetz, R. C., and Correll, J. C. (1988). Vegetative compatibility among races of Fusarium oxysporum f.sp. cubense . Plant Dis. 72, 325–328. doi: 10.1094/pd-72-0325
Ponukumati, S. V., Elliott, M. L., and Des Jardin, E. A. (2019). Comparison of Secreted in Xylem (SIX) genes in two fusarium wilt pathogens of ornamental palms. Plant Pathol. 68, 1663–1681. doi: 10.1111/ppa.13090
Pradhan, A., Ghosh, S., Sahoo, D., and Jha, G. (2020). Fungal effectors, the double edge sword of phytopathogens. Curr. Genet. 67, 27–40. doi: 10.1007/s00294-020-01118-3
Puhalla, J. E. (1985). Classification of strains of Fusarium oxysporum on the basis of vegetative compatibility. Can. J. Bot. 63, 179–183. doi: 10.1139/b85-020
Punja, Z. K., and Parker, M. (2000). Development of fusarium root and stem rot, a new disease on greenhouse cucumber in British Columbia, caused by Fusarium oxysporum f. sp. radicis-cucumerinum . Can. J. Plant Pathol. 22, 349–363. doi: 10.1080/07060660009500453
Raffaele, S., and Kamoun, S. (2012). Genome evolution in filamentous plant pathogens: why bigger can be better. Nat. Rev. Microbiol. 10, 417–430. doi: 10.1038/nrmicro2790
Rana, A., Sahgal, M., and Johri, B. N. (2017). “ Fusarium oxysporum : genomics, diversity and plant–host interaction,” in Developments in Fungal Biology and Applied Mycology , eds S. K. Deshmukh, T. Satyanarayana, and B. N. Johri (Singapore: Springer), 159–199. doi: 10.1007/978-981-10-4768-8_10
Rao, V. S., Srinivas, K., Sujini, G. N., and Kumar, G. N. (2014). Protein-protein interaction detection: methods and analysis. Int. J. Proteom. 2014:147648. doi: 10.1155/2014/147648
Recorbet, G., Steinberg, C., Olivain, C., Edel, V., Trouvelot, S., Dumas-Gaudot, E., et al. (2003). Wanted: pathogenesis−related marker molecules for Fusarium oxysporum . N. Phytol. 159, 73–92. doi: 10.1046/j.1469-8137.2003.00795.x
Rep, M. (2005). Small proteins of plant-pathogenic fungi secreted during host colonization. FEMS Microbiol. Lett. 253, 19–27. doi: 10.1016/j.femsle.2005.09.014
Rep, M., and Kistler, H. C. (2010). The genomic organization of plant pathogenicity in Fusarium species. Curr. Opin. Plant Biol. 13, 420–426. doi: 10.1016/j.pbi.2010.04.004
Rep, M., Dekker, H. L., Vossen, J. H., de Boer, A. D., Houterman, P. M., Speijer, D., et al. (2002). Mass spectrometric identification of isoforms of PR proteins in xylem sap of fungus-infected tomato. Plant Physiol. 130, 904–917. doi: 10.1104/pp.007427
Rep, M., Meijer, M., Houterman, P. M., van der Does, H. C., and Cornelissen, B. J. (2005). Fusarium oxysporum evades I-3-mediated resistance without altering the matching avirulence gene. Mol. Plant Microbe Interact. 18, 15–23. doi: 10.1094/MPMI-18-0015
Rep, M., Van Der Does, H. C., Meijer, M., Van Wijk, R., Houterman, P. M., Dekker, H. L., et al. (2004). A small, cysteine−rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I−3−mediated resistance in tomato. Mol. Microbiol. 53, 1373–1383. doi: 10.1111/j.1365-2958.2004.04177.x
Rocha, L. O., Laurence, M. H., Ludowici, V. A., Puno, V. I., Lim, C. C., Tesoriero, L. A., et al. (2016). Putative effector genes detected in Fusarium oxysporum from natural ecosystems of Australia . Plant Pathol. 65, 914–929. doi: 10.1111/ppa.12472
Sasaki, K., Nakahara, K., Tanaka, S., Shigyo, M., and Ito, S. I. (2015). Genetic and pathogenic variability of Fusarium oxysporum f. sp. cepae isolated from onion and Welsh onion in Japan. Phytopathology 105, 525–532. doi: 10.1094/PHYTO-06-14-0164-R
Schlechtendal, D. F. L. (1824). Flora Berolinensis: Pars secunda: cryptogamia 2. Berolini: Sumtibus Ferdinandi Dümmler.
Schmidt, S. M., Houterman, P. M., Schreiver, I., Ma, L., Amyotte, S., Chellappan, B., et al. (2013). MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum . BMC Genomics 14:119. doi: 10.1186/1471-2164-14-119
Schmidt, S. M., Lukasiewicz, J., Farrer, R., van Dam, P., Bertoldo, C., and Rep, M. (2016). Comparative genomics of Fusarium oxysporum f. sp. melonis reveals the secreted protein recognized by the Fom−2 resistance gene in melon. N. Phytol. 209, 307–318. doi: 10.1111/nph.13584
Scott, J. W., and Jones, J. P. (1989). Monogenic resistance in tomato to Fusarium oxysporum f. sp. lycopersici race 3. Euphytica 40, 49–53. doi: 10.1007/BF00023296
Simbaqueba, J., Catanzariti, A. M., González, C., and Jones, D. A. (2018). Evidence for horizontal gene transfer and separation of effector recognition from effector function revealed by analysis of effector genes shared between cape gooseberry and tomato−infecting formae speciales of Fusarium oxysporum . Mol. Plant Pathol. 19, 2302–2318. doi: 10.1111/mpp.12700
Simons, G., Groenendijk, J., Wijbrandi, J., Reijans, M., Groenen, J., Diergaarde, P., et al. (1998). Dissection of the Fusarium I-2 gene cluster in tomato reveals six homologs and one active gene copy. Plant Cell 10, 1055–1068. doi: 10.1105/tpc.10.6.1055
Skovgaard, K., Nirenberg, H. I., O’Donnell, K., and Rosendahl, S. (2001). Evolution of Fusarium oxysporum f. sp. vasinfectum races inferred from multigene genealogies. Phytopathology 91, 1231–1237. doi: 10.1094/PHYTO.2001.91.12.1231
Snyder, W. C., and Hansen, H. N. (1940). The species concept in Fusarium . Am. J. Bot. 27, 64–67. doi: 10.2307/2436688
Stall, R. E., and Walter, J. M. (1965). Selection and inheritance of resistance in tomato to isolates of races 1 and 2 of the Fusarium wilt organism. Phytopathology 55, 1213–1215.
Takken, F., and Rep, M. (2010). The arms race between tomato and Fusarium oxysporum . Mol. Plant Pathol. 11, 309–314. doi: 10.1111/j.1364-3703.2009.00605.x
Taylor, A., Armitage, A. D., Handy, C., Jackson, A. C., Hulin, M. T., Harrison, R. J., et al. (2019). Basal Rot of Narcissus: Understanding Pathogenicity in Fusarium oxysporum f. sp. naricissi. Front. Microbiol. 10:2905. doi: 10.3389/fmicb.2019.02905
Taylor, A., Vágány, V., Jackson, A. C., Harrison, R. J., Rainoni, A., and Clarkson, J. P. (2016). Identification of pathogenicity−related genes in Fusarium oxysporum f. sp. cepae . Mol. Plant Pathol. 17, 1032–1047. doi: 10.1111/mpp.12346
Thatcher, L. F., Gardiner, D. M., Kazan, K., and Manners, J. M. (2012). A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis . Mol. Plant Microbe Interact. 25, 180–190. doi: 10.1094/MPMI-08-11-0212
Vakalounakis, D. J. (1996). Root and Stem Rot of Cucumber Caused by Fusarium oxysporum f. sp. radicis-cucumerinum f. sp. nov . Plant Dis. 80, 313–316.
van Dam, P., and Rep, M. (2017). The Distribution of Miniature Impala Elements and SIX Genes in the Fusarium Genus is Suggestive of Horizontal Gene Transfer. J. Mol. Evol. 85, 14–25. doi: 10.1007/s00239-017-9801-0
van Dam, P., Fokkens, L., Ayukawa, Y., van der Gragt, M., Ter Horst, A., Brankovics, B., et al. (2017). A mobile pathogenicity chromosome in Fusarium oxysporum for infection of multiple cucurbit species. Sci. Rep. 7:9042. doi: 10.1038/s41598-017-07995-y
van Dam, P., Fokkens, L., Schmidt, S. M., Linmans, J. H., Kistler, H. C., Ma, L. J., et al. (2016). Effector profiles distinguish formae speciales of Fusarium oxysporum . Environ. Microbiol. 18, 4087–4102. doi: 10.1111/1462-2920.13445
Van der Biezen, E. A., and Jones, J. D. G. (1998). Plant disease resistance proteins and the gene-for-gene concept. Trends Plant Sci. 23, 454–456. doi: 10.1016/s0968-0004(98)01311-5
van der Does, H. C., and Rep, M. (2007). Virulence genes and the evolution of host specificity in plant-pathogenic fungi. Mol. Plant Microbe Interact. 20, 1175–1182. doi: 10.1094/mpmi-20-10-1175
Van Der Does, H. C., Fokkens, L., Yang, A., Schmidt, S. M., Langereis, L., Lukasiewicz, J. M., et al. (2016). Transcription Factors Encoded on Core and Accessory Chromosomes of Fusarium oxysporum Induce Expression of Effector Genes. PLoS Genet. 12:e1006401. doi: 10.1371/journal.pgen.1006401
Van Der Does, H. C., Lievens, B., Claes, L., Houterman, P. M., Cornelissen, B. J., and Rep, M. (2008). The presence of a virulence locus discriminates Fusarium oxysporum isolates causing tomato wilt from other isolates. Environ. Microbiol. 10, 1475–1485. doi: 10.1111/j.1462-2920.2007.01561.x
van der Hoorn, R. A., and Kamoun, S. (2008). From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell 20, 2009–2017. doi: 10.1105/tpc.108.060194
VanderMolen, G. E., Beckman, C. H., and Rodehorst, E. (1987). The ultrastructure of tylose formation in resistant banana following inoculation with Fusarium oxysporum f. sp. cubense . Physiol. Mol. Plant Pathol. 31, 185–200. doi: 10.1016/0885-5765(87)90063-4
Vlaardingerbroek, I., Beerens, B., Rose, L., Fokkens, L., Cornelissen, B. J., and Rep, M. (2016a). Exchange of core chromosomes and horizontal transfer of lineage-specific chromosomes in Fusarium oxysporum . Environ. Microbiol. 18, 3702–3713. doi: 10.1111/1462-2920.13281
Vlaardingerbroek, I., Beerens, B., Schmidt, S. M., Cornelissen, B. J., and Rep, M. (2016b). Dispensable chromosomes in Fusarium oxysporum f. sp. lycopersici . Mol. Plant Pathol. 17, 1455–1466. doi: 10.1111/mpp.12440
Weimer, J. L. (1944). Some root rots and a foot rot of lupines in the Southeastern part of the Unites States. J. Agric. Res. 68:441.
Widinugraheni, S., Niño-Sánchez, J., Van Der Does, H. C., Van Dam, P., García-Bastidas, F. A., Subandiyah, S., et al. (2018). A SIX1 homolog in Fusarium oxysporum f. sp. cubense tropical race 4 contributes to virulence towards Cavendish banana. PLoS One 13:e0205896. doi: 10.1371/journal.pone.0205896
Williams, A. H., Sharma, M., Thatcher, L. F., Azam, S., Hane, J. K., Sperschneider, J., et al. (2016). Comparative genomics and prediction of conditionally dispensable sequences in legume-infecting Fusarium oxysporum formae speciales facilitates identification of candidate effectors. BMC Genomics 17:191. doi: 10.1186/s12864-016-2486-8
Yadeta, K. A., and Thomma, B. P. J. (2013). The xylem as battleground for plant hosts and vascular wiltpathogens. Front. Plant Sci. 4:97. doi: 10.3389/fpls.2013.00097
Zhang, J. X., Mace, M. E., Stipanovic, R. D., and Bell, A. A. (1993). Production and fungitoxicity of the terpenoid phytoalexins in cotton inoculated with Fusarium oxysporum f. sp. vasinfectum . J. Phytopathol. 139, 247–252. doi: 10.1111/j.1439-0434.1993.tb01423.x
Zhou, E., Jia, Y., Singh, P., Correll, J. C., and Lee, F. N. (2007). Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genet. Biol. 44, 1024–1034. doi: 10.1016/j.fgb.2007.02.003
Zipfel, C., and Rathjen, J. P. (2008). Plant Immunity: AvrPto targets the frontline. Curr. Biol. 18, R218–R220. doi: 10.1016/j.cub.2008.01.016
Zitter, T. A. (1998). Fusarium diseases of cucurbits. New York, NY: cornell university library.
Keywords : Fusarium oxysporum , Fusarium oxysporum species complex, Secreted in Xylem, effectors, pathogenicity, host specificity
Citation: Jangir P, Mehra N, Sharma K, Singh N, Rani M and Kapoor R (2021) Secreted in Xylem Genes: Drivers of Host Adaptation in Fusarium oxysporum . Front. Plant Sci. 12:628611. doi: 10.3389/fpls.2021.628611
Received: 12 November 2020; Accepted: 01 March 2021; Published: 22 April 2021.
Reviewed by:
Copyright © 2021 Jangir, Mehra, Sharma, Singh, Rani and Kapoor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rupam Kapoor, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
COMMENTS
To move forward, the research should now focus on unravelling how water transport through the xylem network is regulated using ingenious combinations of advanced techniques that probe the structure-function relationships of this fascinating transport system.
Effective point-of-use devices for providing safe drinking water are urgently needed to reduce the global burden of waterborne disease. Here we show that plant xylem from the sapwood of coniferous trees - a readily available, inexpensive, biodegradable, and disposable material - can remove bacteria from water by simple pressure-driven filtration. Approximately 3 cm3 of sapwood can filter ...
In this review we highlight current research in plant hydraulics in the context of plant physiology, ecology, and evolution. We address methodological issues, and focus on current and future research opportunities. However, we first explain the physics of xylem water transport in plants and the limits of the system.
Employing plant xylem for household water treatment is highly advantageous, as it is a local and cheap material, easily available in many rural areas. A filtration system based on xylem involves forcing of water through the cross-section of a cut plant stem.
Abstract. Xylem, as a unique organizational structure of vascular plants, bears water transport and supports functions necessary for plant survival. Notably, secondary xylem in the stem (i.e., wood) also has important economic and ecological value. In view of this, the regulation of xylem development has been widely concerned.
Our work enhances the understanding of xylem as a filtration material, and opens opportunities for engineering a diverse range of low-cost, biodegradable xylem-based filtration products on a ...
Based on the review of driving force and microstructure for xylem water trans- port, up-to-date understanding of the mechanism of xylem water transport was briefly summarized in this paper, and ...
This second edition provides detailed techniques used for the study and characterization of the plant vascular system, with a central focus on the xylem tissue. Chapters are organized in three main sections covering; analysis of xylem development, xylem characterization though imaging techniques, and analysis of the xylem composition.
Xylem is the specialised tissue of vascular plants that transports water and nutrients from the plant-soil interface to stems and leaves, and provides mechanical support and storage. The water-conducting function of xylem is one of the major distinguishing features of vascular plants.
Abstract • Background and Aims The xylem, or water transport system, in vascular plants adopts different morphologies that appear sequentially during growth phases. This paper proposes an explanation of these morphologies based on engineering design principles.
Xylem is the tissue of vascular plants that transports water and nutrients from the soil to the stems and leaves. Xylem plays an essential 'supporting' role providing strength to tissues and organs, to maintain plant architecture and resistance to bending. The water-transporting cells of mature xylem are dead, and therefore the transport of ...
Xylem density, strength, and stiffness were greater in shoots than roots. Along the main root-to-shoot axis, xylem strength and stiffness were greatest at shoot tips, and the tissue became linearly weaker and less stiff down the plant and through the root. Roots had greater water storage with lower biomechanical support, and shoots had ...
Abstract Xylem is a complex tissue that forms the bulk of tree bodies and has several functions, including to conduct water, store water and nutrients, and biomechanically support the plant body. We examined how xylem functional traits varied at different positions within 9-year-old Populus balsamifera subsp. trichocarpa. Whole trees were excavated, and xylem samples were collected at 1-m ...
Developmental initiation of plant vascular tissue, including xylem and phloem, from the vascular cambium depends on environmental factors, such as temperature and precipitation. Proper formation of vascular tissue is critical for the transpiration stream, ...
Abstract and Figures Effective point-of-use devices for providing safe drinking water are urgently needed to reduce the global burden of waterborne disease. Here we show that plant xylem from the ...
This review covers mainly papers which appeared from 1983 to 1985. Especial consideration was given to the differentiation of tracheary elements, vascularization of organs, functional xylem anatomy, water conduction, and translocation in xylem sap. Download to read the full chapter text
Main characteristics of the xylem network. Organization and characteristics of the xylem network: water flow throughout the plants depends on characteristics of the xylem in different organs.
In this paper, we present and use a coupled xylem/phloem mathematical model of passive water and solute transport through a reticulated vascular system of an angiosperm leaf. We evaluate the effect of leaf width-to-length proportion and orientation of ...
We summarize the advances in the research on the patterns of water flow into and out of the fruit over development and under different environmental conditions and cultural practices. We review the key findings on fruit transpiration, xylem transport, phloem transport, and the coordination of water flows in maintaining fruit water balance.
Application of xylem tissue inside plant stem has the potential as a filter for water filtration. This research focuses on xylem tissue in Malaysian tropical plants from cassava stem.
The cambium and procambium generate the majority of biomass in vascular plants. These meristems constitute a bifacial stem cell population from which xylem and phloem are specified on opposing sides by positional signals. The PHLOEM INTERCALATED WITH XYLEM (PXY) receptor kinase promotes vascular cel …
Why a xylem filter? In 2013, Rohit Karnik, Associate Professor in the Mechanical Engineering department at MIT, came up with the idea of using the natural filtration capabilities of xylem tissue in wood to develop low-cost water filters. Further experiments showed that the sapwood xylem tissue from coniferous trees can be used to effectively remove E. coli bacteria and other microbes from water.
Secreted in Xylem Genes: Drivers of Host Adaptation in. Fusarium oxysporum. Fusarium oxysporum ( Fo) is a notorious pathogen that significantly contributes to yield losses in crops of high economic status. It is responsible for vascular wilt characterized by the browning of conductive tissue, wilting, and plant death.