- Scoping Review
- Open access
- Published: 14 November 2021

Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis
- Qiao Liu 1 na1 ,
- Chenyuan Qin 1 , 2 na1 ,
- Min Liu 1 &
- Jue Liu ORCID: orcid.org/0000-0002-1938-9365 1 , 2
Infectious Diseases of Poverty volume 10 , Article number: 132 ( 2021 ) Cite this article
58k Accesses
211 Citations
371 Altmetric
Metrics details
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
We searched PubMed, Embase and Web of Science from inception to July 22, 2021. Observational studies that examined the effectiveness and safety of SARS-CoV-2 vaccines among people vaccinated were included. Random-effects or fixed-effects models were used to estimate the pooled vaccine effectiveness (VE) and incidence rate of adverse events after vaccination, and their 95% confidence intervals ( CI ).
A total of 58 studies (32 studies for vaccine effectiveness and 26 studies for vaccine safety) were included. A single dose of vaccines was 41% (95% CI : 28–54%) effective at preventing SARS-CoV-2 infections, 52% (31–73%) for symptomatic COVID-19, 66% (50–81%) for hospitalization, 45% (42–49%) for Intensive Care Unit (ICU) admissions, and 53% (15–91%) for COVID-19-related death; and two doses were 85% (81–89%) effective at preventing SARS-CoV-2 infections, 97% (97–98%) for symptomatic COVID-19, 93% (89–96%) for hospitalization, 96% (93–98%) for ICU admissions, and 95% (92–98%) effective for COVID-19-related death, respectively. The pooled VE was 85% (80–91%) for the prevention of Alpha variant of SARS-CoV-2 infections, 75% (71–79%) for the Beta variant, 54% (35–74%) for the Gamma variant, and 74% (62–85%) for the Delta variant. The overall pooled incidence rate was 1.5% (1.4–1.6%) for adverse events, 0.4 (0.2–0.5) per 10 000 for severe adverse events, and 0.1 (0.1–0.2) per 10 000 for death after vaccination.
Conclusions
SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Graphical Abstract
Since its outbreak, coronavirus disease 2019 (COVID-19) has spread rapidly, with a sharp rise in the accumulative number of infections worldwide. As of August 8, 2021, COVID-19 has already killed more than 4.2 million people and more than 203 million people were infected [ 1 ]. Given its alarming-spreading speed and the high cost of completely relying on non-pharmaceutical measures, we urgently need safe and effective vaccines to cover susceptible populations and restore people’s lives into the original [ 2 ].
According to global statistics, as of August 2, 2021, there are 326 candidate vaccines, 103 of which are in clinical trials, and 19 vaccines have been put into normal use, including 8 inactivated vaccines and 5 protein subunit vaccines, 2 RNA vaccines, as well as 4 non-replicating viral vector vaccines [ 3 ]. Our World in Data simultaneously reported that 27.3% of the world population has received at least one dose of a COVID-19 vaccine, and 13.8% is fully vaccinated [ 4 ].
To date, COVID-19 become increasingly fierce due to the emergence of variants [ 5 , 6 , 7 ]. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance [ 6 , 8 ]. Several reviews systematically evaluated the effectiveness and/or safety of the three mainstream vaccines on the market (inactivated virus vaccines, RNA vaccines and viral vector vaccines) based on random clinical trials (RCT) yet [ 9 , 10 , 11 , 12 , 13 ].
In general, RNA vaccines are the most effective, followed by viral vector vaccines and inactivated virus vaccines [ 10 , 11 , 12 , 13 ]. The current safety of COVID-19 vaccines is acceptable for mass vaccination, but long-term monitoring of vaccine safety is needed, especially in older people with underlying conditions [ 9 , 10 , 11 , 12 , 13 ]. Inactivated vaccines had the lowest incidence of adverse events and the safety comparisons between mRNA vaccines and viral vectors were controversial [ 9 , 10 ].
RCTs usually conduct under a very demanding research circumstance, and tend to be highly consistent and limited in terms of population characteristics and experimental conditions. Actually, real-world studies differ significantly from RCTs in terms of study conditions and mass vaccination in real world requires taking into account factors, which are far more complex, such as widely heterogeneous populations, vaccine supply, willingness, medical accessibility, etc. Therefore, the real safety and effectiveness of vaccines turn out to be a major concern of international community. The results of a mass vaccination of CoronaVac in Chile demonstrated a protective effectiveness of 65.9% against the onset of COVID-19 after complete vaccination procedures [ 14 ], while the outcomes of phase 3 trials in Brazil and Turkey were 50.7% and 91.3%, reported on Sinovac’s website [ 14 ]. As for the Delta variant, the British claimed 88% protection after two doses of BNT162b2, compared with 67% for AZD1222 [ 15 ]. What is surprising is that the protection of BNT162b2 against infection in Israel is only 39% [ 16 ]. Several studies reported the effectiveness and safety of the COVID-19 vaccine in the real world recently, but the results remain controversial [ 17 , 18 , 19 , 20 ]. A comprehensive meta-analysis based upon the real-world studies is still in an urgent demand, especially for evaluating the effect of vaccines on variation strains. In the present study, we aimed to systematically evaluate the effectiveness and safety of the COVID-19 vaccine in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
Search strategy and selection criteria
Our methods were described in detail in our published protocol [PROSPERO (Prospective register of systematic reviews) registration, CRD42021267110]. We searched eligible studies published by 22 July 2021, from three databases including PubMed, Embase and Web of Science by the following search terms: (effectiveness OR safety) AND (COVID-19 OR coronavirus OR SARS-CoV-2) AND (vaccine OR vaccination). We used EndNoteX9.0 (Thomson ResearchSoft, Stanford, USA) to manage records, screen and exclude duplicates. This study was strictly performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We included observational studies that examined the effectiveness and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines among people vaccinated with SARS-CoV-2 vaccines. The following studies were excluded: (1) irrelevant to the subject of the meta-analysis, such as studies that did not use SARS-CoV-2 vaccination as the exposure; (2) insufficient data to calculate the rate for the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, or adverse events after vaccination; (3) duplicate studies or overlapping participants; (4) RCT studies, reviews, editorials, conference papers, case reports or animal experiments; and (5) studies that did not clarify the identification of COVID-19.
Studies were identified by two investigators (LQ and QCY) independently following the criteria above, while discrepancies reconciled by a third investigator (LJ).
Data extraction and quality assessment
The primary outcome was the effectiveness of SARS-CoV-2 vaccines. The following data were extracted independently by two investigators (LQ and QCY) from the selected studies: (1) basic information of the studies, including first author, publication year and study design; (2) characteristics of the study population, including sample sizes, age groups, setting or locations; (3) kinds of the SARS-CoV-2 vaccines; (4) outcomes for the effectiveness of SARS-CoV-2 vaccines: the number of laboratory-confirmed COVID-19, hospitalization for COVID-19, admission to the ICU for COVID-19, and COVID-19-related death; and (5) outcomes for the safety of SARS-CoV-2 vaccines: the number of adverse events after vaccination.
We evaluated the risk of bias using the Newcastle–Ottawa quality assessment scale for cohort studies and case–control studies [ 21 ]. and assess the methodological quality using the checklist recommended by Agency for Healthcare Research and Quality (AHRQ) [ 22 ]. Cohort studies and case–control studies were classified as having low (≥ 7 stars), moderate (5–6 stars), and high risk of bias (≤ 4 stars) with an overall quality score of 9 stars. For cross-sectional studies, we assigned each item of the AHRQ checklist a score of 1 (answered “yes”) or 0 (answered “no” or “unclear”), and summarized scores across items to generate an overall quality score that ranged from 0 to 11. Low, moderate, and high risk of bias were identified as having a score of 8–11, 4–7 and 0–3, respectively.
Two investigators (LQ and QCY) independently assessed study quality, with disagreements resolved by a third investigator (LJ).
Data synthesis and statistical analysis
We performed a meta-analysis to pool data from included studies and assess the effectiveness and safety of SARS-CoV-2 vaccines by clinical outcomes (rates of the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, and adverse events after vaccination). Random-effects or fixed-effects models were used to pool the rates and adjusted estimates across studies separately, based on the heterogeneity between estimates ( I 2 ). Fixed-effects models were used if I 2 ≤ 50%, which represented low to moderate heterogeneity and random-effects models were used if I 2 > 50%, representing substantial heterogeneity.
We conducted subgroup analyses to investigate the possible sources of heterogeneity by using vaccine kinds, vaccination status, sample size, and study population as grouping variables. We used the Q test to conduct subgroup comparisons and variables were considered significant between subgroups if the subgroup difference P value was less than 0.05. Publication bias was assessed by funnel plot and Egger’s regression test. We analyzed data using Stata version 16.0 (StataCorp, Texas, USA).
A total of 4844 records were searched from the three databases. 2484 duplicates were excluded. After reading titles and abstracts, we excluded 2264 reviews, RCT studies, duplicates and other studies meeting our exclude criteria. Among the 96 studies under full-text review, 41 studies were excluded (Fig. 1 ). Ultimately, with three grey literatures included, this final meta-analysis comprised 58 eligible studies, including 32 studies [ 14 , 15 , 17 , 18 , 19 , 20 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ] for vaccine effectiveness and 26 studies [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ] for vaccine safety. Characteristics of included studies are showed in Additional file 1 : Table S1, Additional file 2 : Table S2. The risk of bias of all studies we included was moderate or low.
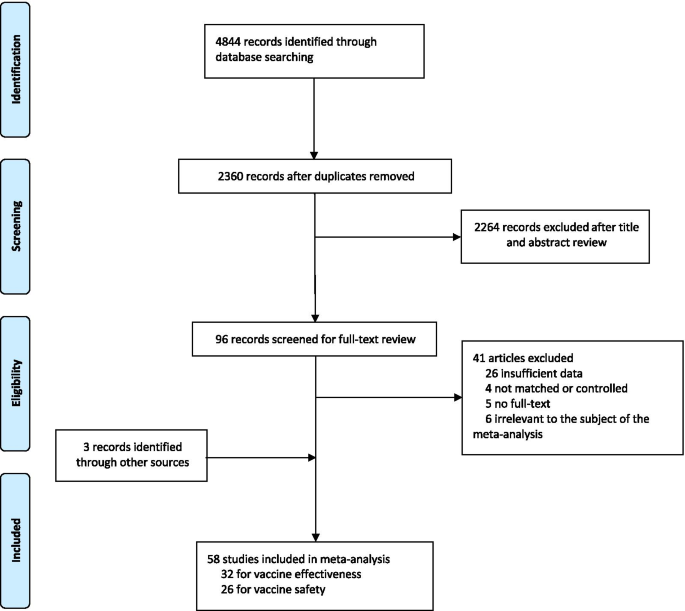
Flowchart of the study selection
Vaccine effectiveness for different clinical outcomes of COVID-19
We separately reported the vaccine effectiveness (VE) by the first and second dose of vaccines, and conducted subgroup analysis by the days after the first or second dose (< 7 days, ≥ 7 days, ≥ 14 days, and ≥ 21 days; studies with no specific days were classified as 1 dose, 2 dose or ≥ 1 dose).
For the first dose of SARS-CoV-2 vaccines, the pooled VE was 41% (95% CI : 28–54%) for the prevention of SARS-CoV-2 infection, 52% (95% CI : 31–73%) for the prevention of symptomatic COVID-19, 66% (95% CI : 50–81%) for the prevention of hospital admissions, 45% (95% CI : 42–49%) for the prevention of ICU admissions, and 53% (95% CI : 15–91%) for the prevention of COVID-19-related death (Table 1 ). The subgroup, ≥ 21 days after the first dose, was found to have the highest VE in each clinical outcome of COVID-19, regardless of ≥ 1 dose group (Table 1 ).
For the second dose of SARS-CoV-2 vaccines, the pooled VE was 85% (95% CI : 81–89%) for the prevention of SARS-CoV-2 infection, 97% (95% CI : 97–98%) for the prevention of symptomatic COVID-19, 93% (95% CI: 89–96%) for the prevention of hospital admissions, 96% (95% CI : 93–98%) for the prevention of ICU admissions, and 95% (95% CI : 92–98%) for the prevention of COVID-19-related death (Table 1 ). VE was 94% (95% CI : 78–98%) in ≥ 21 days after the second dose for the prevention of SARS-CoV-2 infection, higher than other subgroups, regardless of 2 dose group (Table 1 ). For the prevention of symptomatic COVID-19, VE was also relatively higher in 21 days after the second dose (99%, 95% CI : 94–100%). Subgroups showed no statistically significant differences in the prevention of hospital admissions, ICU admissions and COVID-19-related death (subgroup difference P values were 0.991, 0.414, and 0.851, respectively).
Vaccine effectiveness for different variants of SARS-CoV-2 in fully vaccinated people
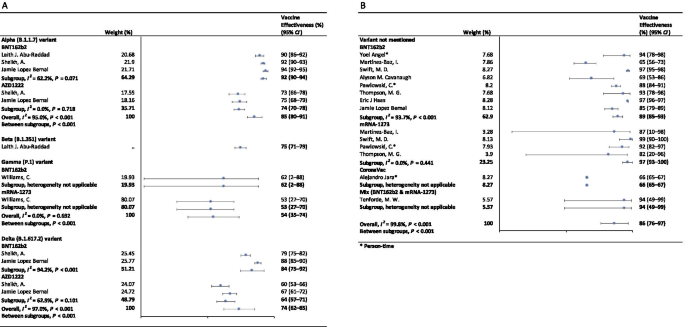
In the fully vaccinated groups (over 14 days after the second dose), the pooled VE was 85% (95% CI: 80–91%) for the prevention of Alpha variant of SARS-CoV-2 infection, 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. There was only one study [ 23 ] focused on the Beta variant, which showed the VE was 75% (95% CI : 71–79%) for the prevention of the Beta variant of SARS-CoV-2 infection. BNT162b2 vaccine had the highest VE in each variant group; 92% (95% CI : 90–94%) for the Alpha variant, 62% (95% CI : 2–88%) for the Gamma variant, and 84% (95% CI : 75–92%) for the Delta variant (Fig. 2 ).
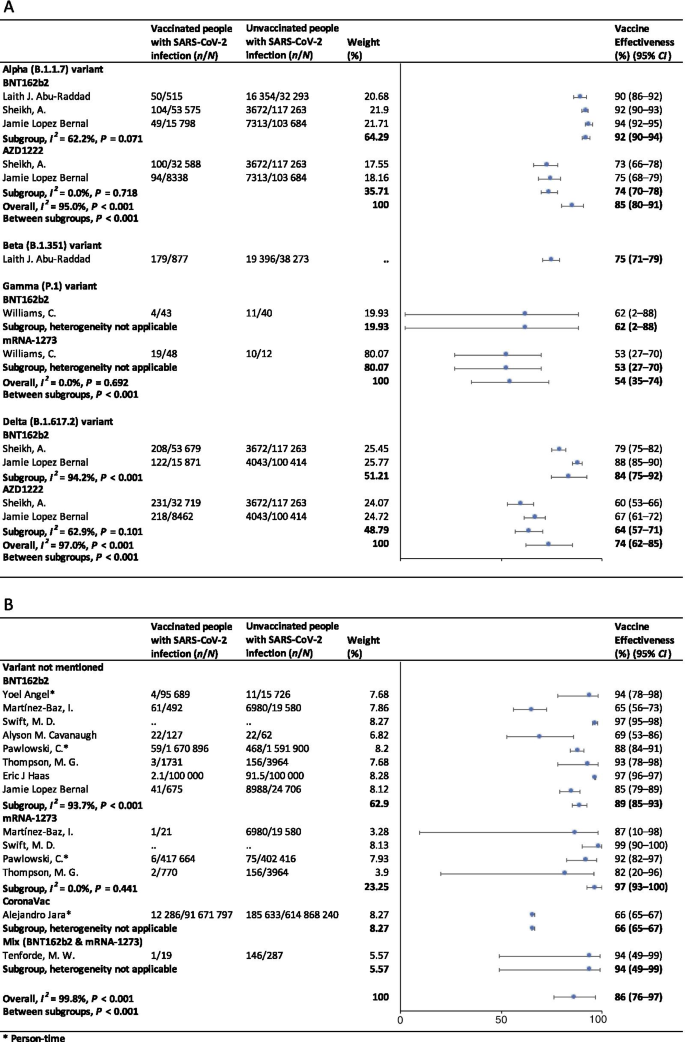
Forest plots for the vaccine effectiveness of SARS-CoV-2 vaccines in fully vaccinated populations. A Vaccine effectiveness against SARS-CoV-2 variants; B Vaccine effectiveness against SARS-CoV-2 with variants not mentioned. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, COVID-19 coronavirus disease 2019, CI confidence interval
For studies which had not mentioned the variant of SARS-CoV-2, the pooled VE was 86% (95% CI: 76–97%) for the prevention of SARS-CoV-2 infection in fully vaccinated people. mRNA-1273 vaccine had the highest pooled VE (97%, 95% CI: 93–100%, Fig. 2 ).
Safety of SARS-CoV-2 vaccines
As Table 2 showed, the incidence rate of adverse events varied widely among different studies. We conducted subgroup analysis by study population (general population, patients and healthcare workers), vaccine type (BNT162b2, mRNA-1273, CoronaVac, and et al.), and population size (< 1000, 1000–10 000, 10 000–100 000, and > 100 000). The overall pooled incidence rate was 1.5% (95% CI : 1.4–1.6%) for adverse events, 0.4 (95% CI : 0.2–0.5) per 10 000 for severe adverse events, and 0.1 (95% CI : 0.1–0.2) per 10 000 for death after vaccination. Incidence rate of adverse events was higher in healthcare workers (53.2%, 95% CI : 28.4–77.9%), AZD1222 vaccine group (79.6%, 95% CI : 60.8–98.3%), and < 1000 population size group (57.6%, 95% CI : 47.9–67.4%). Incidence rate of sever adverse events was higher in healthcare workers (127.2, 95% CI : 62.7–191.8, per 10 000), Gam-COVID-Vac vaccine group (175.7, 95% CI : 77.2–274.2, per 10 000), and 1000–10 000 population size group (336.6, 95% CI : 41.4–631.8, per 10 000). Incidence rate of death after vaccination was higher in patients (7.6, 95% CI : 0.0–32.2, per 10 000), BNT162b2 vaccine group (29.8, 95% CI : 0.0–71.2, per 10 000), and < 1000 population size group (29.8, 95% CI : 0.0–71.2, per 10 000). Subgroups of general population, vaccine type not mentioned, and > 100 000 population size had the lowest incidence rate of adverse events, severe adverse events, and death after vaccination.
Sensitivity analysis and publication bias
In the sensitivity analyses, VE for SARS-CoV-2 infections, symptomatic COVID-19 and COVID-19-related death got relatively lower when omitting over a single dose group of Maria et al.’s work [ 33 ]; when omitting ≥ 14 days after the first dose group and ≥ 14 days after the second dose group of Alejandro et al.’s work [ 14 ], VE for SARS-CoV-2 infections, hospitalization, ICU admission and COVID-19-related death got relatively higher; and VE for all clinical status of COVID-19 became lower when omitting ≥ 14 days after the second dose group of Eric et al.’s work [ 34 ]. Incidence rate of adverse events and severe adverse events got relatively higher when omitting China CDC’s data [ 74 ]. P values of Egger’s regression test for all the meta-analysis were more than 0.05, indicating that there might not be publication bias.
To our knowledge, this is a comprehensive systematic review and meta-analysis assessing the effectiveness and safety of SARS-CoV-2 vaccines based on real-world studies, reporting pooled VE for different variants of SARS-CoV-2 and incidence rate of adverse events. This meta-analysis comprised a total of 58 studies, including 32 studies for vaccine effectiveness and 26 studies for vaccine safety. We found that a single dose of SARS-CoV-2 vaccines was about 40–60% effective at preventing any clinical status of COVID-19 and that two doses were 85% or more effective. Although vaccines were not as effective against variants of SARS-CoV-2 as original virus, the vaccine effectiveness was still over 50% for fully vaccinated people. Normal adverse events were common, while the incidence of severe adverse events or even death was very low, providing reassurance to health care providers and to vaccine recipients and promote confidence in the safety of COVID-19 vaccines. Our findings strengthen and augment evidence from previous review [ 75 ], which confirmed the effectiveness of the BNT162b2 mRNA vaccine, and additionally reported the safety of SARS-CoV-2 vaccines, giving insight on the future of SARS-CoV-2 vaccine schedules.
Although most vaccines for the prevention of COVID-19 are two-dose vaccines, we found that the pooled VE of a single dose of SARS-CoV-2 vaccines was about 50%. Recent study showed that the T cell and antibody responses induced by a single dose of the BNT162b2 vaccine were comparable to those naturally infected with SARE-CoV-2 within weeks or months after infection [ 76 ]. Our findings could help to develop vaccination strategies under certain circumstances such as countries having a shortage of vaccines. In some countries, in order to administer the first dose to a larger population, the second dose was delayed for up to 12 weeks [ 77 ]. Some countries such as Canada had even decided to delay the second dose for 16 weeks [ 78 ]. However, due to a suboptimum immune response in those receiving only a single dose of a vaccine, such an approach had a chance to give rise to the emergence of variants of SARS-CoV-2 [ 79 ]. There remains a need for large clinical trials to assess the efficacy of a single-dose administration of two-dose vaccines and the risk of increasing the emergence of variants.
Two doses of SARS-CoV-2 vaccines were highly effective at preventing hospitalization, severe cases and deaths resulting from COVID-19, while the VE of different groups of days from the second vaccine dose showed no statistically significant differences. Our findings emphasized the importance of getting fully vaccinated, for the fact that most breakthrough infections were mild or asymptomatic. A recent study showed that the occurrence of breakthrough infections with SARS-CoV-2 in fully vaccinated populations was predictable with neutralizing antibody titers during the peri-infection period [ 80 ]. We also found getting fully vaccinated was at least 50% effective at preventing SARS-CoV-2 variants infections, despite reduced effectiveness compared with original virus; and BNT162b2 vaccine was found to have the highest VE in each variant group. Studies showed that the highly mutated variants were indicative of a form of rapid, multistage evolutionary jumps, which could preferentially occur in the milieu of partial immune control [ 81 , 82 ]. Therefore, immunocompromised patients should be prioritized for anti-COVID-19 immunization to mitigate persistent SARS-CoV-2 infections, during which multimutational SARS-CoV-2 variants could arise [ 83 ].
Recently, many countries, including Israel, the United States, China and the United Kingdom, have introduced a booster of COVID-19 vaccine, namely the third dose [ 84 , 85 , 86 , 87 ]. A study of Israel showed that among people vaccinated with BNT162b2 vaccine over 60 years, the risk of COVID-19 infection and severe illness in the non-booster group was 11.3 times (95% CI: 10.4–12.3) and 19.5 times (95% CI: 12.9–29.5) than the booster group, respectively [ 84 ]. Some studies have found that the third dose of Moderna, Pfizer-BioNTech, Oxford-AstraZeneca and Sinovac produced a spike in infection-blocking neutralizing antibodies when given a few months after the second dose [ 85 , 87 , 88 ]. In addition, the common adverse events associated with the third dose did not differ significantly from the symptoms of the first two doses, ranging from mild to moderate [ 85 ]. The overall incidence rate of local and systemic adverse events was 69% (57/97) and 20% (19/97) after receiving the third dose of BNT162b2 vaccine, respectively [ 88 ]. Results of a phase 3 clinical trial involving 306 people aged 18–55 years showed that adverse events after receiving a third dose of BNT162b2 vaccine (5–8 months after completion of two doses) were similar to those reported after receiving a second dose [ 85 ]. Based on V-safe, local reactions were more frequently after dose 3 (5323/6283; 84.7%) than dose 2 (5249/6283; 83.5%) among people who received 3 doses of Moderna. Systemic reactions were reported less frequently after dose 3 (4963/6283; 79.0%) than dose 2 (5105/6283; 81.3%) [ 86 ]. On August 4, WHO called for a halt to booster shots until at least the end of September to achieve an even distribution of the vaccine [ 89 ]. At this stage, the most important thing we should be thinking about is how to reach a global cover of people at risk with the first or second dose, rather than focusing on the third dose.
Based on real world studies, our results preliminarily showed that complete inoculation of COVID-19 vaccines was still effective against infection of variants, although the VE was generally diminished compared with the original virus. Particularly, the pooled VE was 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. Since the wide spread of COVID-19, a number of variants have drawn extensive attention of international community, including Alpha variant (B.1.1.7), first identified in the United Kingdom; Beta variant (B.1.351) in South Africa; Gamma variant (P.1), initially appeared in Brazil; and the most infectious one to date, Delta variant (B.1.617.2) [ 90 ]. Israel recently reported a breakthrough infection of SARS-CoV-2, dominated by variant B.1.1.7 in a small number of fully vaccinated health care workers, raising concerns about the effectiveness of the original vaccine against those variants [ 80 ]. According to an observational cohort study in Qatar, VE of the BNT162b2 vaccine against the Alpha (B.1.1.7) and Beta (B.1.351) variants was 87% (95% CI : 81.8–90.7%) and 75.0% (95% CI : 70.5–7.9%), respectively [ 23 ]. Based on the National Immunization Management System of England, results from a recent real-world study of all the general population showed that the AZD1222 and BNT162b2 vaccines protected against symptomatic SARS-CoV-2 infection of Alpha variant with 74.5% (95% CI : 68.4–79.4%) and 93.7% (95% CI : 91.6–95.3%) [ 15 ]. In contrast, the VE against the Delta variant was 67.0% (95% CI : 61.3–71.8%) for two doses of AZD1222 vaccine and 88% (95% CI : 85.3–90.1%) for BNT162b2 vaccine [ 15 ].
In terms of adverse events after vaccination, the pooled incidence rate was very low, only 1.5% (95% CI : 1.4–1.6%). However, the prevalence of adverse events reported in large population (population size > 100 000) was much lower than that in small to medium population size. On the one hand, the vaccination population in the small to medium scale studies we included were mostly composed by health care workers, patients with specific diseases or the elderly. And these people are more concerned about their health and more sensitive to changes of themselves. But it remains to be proved whether patients or the elderly are more likely to have adverse events than the general. Mainstream vaccines currently on the market have maintained robust safety in specific populations such as cancer patients, organ transplant recipients, patients with rheumatic and musculoskeletal diseases, pregnant women and the elderly [ 54 , 91 , 92 , 93 , 94 ]. A prospective study by Tal Goshen-lag suggests that the safety of BNT162b2 vaccine in cancer patients is consistent with those previous reports [ 91 ]. In addition, the incidence rate of adverse events reported in the heart–lung transplant population is even lower than that in general population [ 95 ]. On the other hand, large scale studies at the national level are mostly based on national electronic health records or adverse event reporting systems, and it is likely that most mild or moderate symptoms are actually not reported.
Compared with the usual local adverse events (such as pain at the injection site, redness at the injection site, etc.) and normal systemic reactions (such as fatigue, myalgia, etc.), serious and life-threatening adverse events were rare due to our results. A meta-analysis based on RCTs only showed three cases of anaphylactic shock among 58 889 COVID-19 vaccine recipients and one in the placebo group [ 11 ]. The exact mechanisms underlying most of the adverse events are still unclear, accordingly we cannot establish a causal relation between severe adverse events and vaccination directly based on observational studies. In general, varying degrees of adverse events occur after different types of COVID-19 vaccination. Nevertheless, the benefits far outweigh the risks.
Our results showed the effectiveness and safety of different types of vaccines varied greatly. Regardless of SARS-CoV-2 variants, vaccine effectiveness varied from 66% (CoronaVac [ 14 ]) to 97% (mRNA-1273 [ 18 , 20 , 45 , 46 ]). The incidence rate of adverse events varied widely among different types of vaccines, which, however, could be explained by the sample size and population group of participants. BNT162b2, AZD1222, mRNA-1273 and CoronaVac were all found to have high vaccine efficacy and acceptable adverse-event profile in recent published studies [ 96 , 97 , 98 , 99 ]. A meta-analysis, focusing on the potential vaccine candidate which have reached to the phase 3 of clinical development, also found that although many of the vaccines caused more adverse events than the controls, most were mild, transient and manageable [ 100 ]. However, severe adverse events did occur, and there remains the need to implement a unified global surveillance system to monitor the adverse events of COVID-19 vaccines around the world [ 101 ]. A recent study employed a knowledge-based or rational strategy to perform a prioritization matrix of approved COVID-19 vaccines, and led to a scale with JANSSEN (Ad26.COV2.S) in the first place, and AZD1222, BNT162b2, and Sputnik V in second place, followed by BBIBP-CorV, CoronaVac and mRNA-1273 in third place [ 101 ]. Moreover, when deciding the priority of vaccines, the socioeconomic characteristics of each country should also be considered.
Our meta-analysis still has several limitations. First, we may include limited basic data on specific populations, as vaccination is slowly being promoted in populations under the age of 18 or over 60. Second, due to the limitation of the original real-world study, we did not conduct subgroup analysis based on more population characteristics, such as age. When analyzing the efficacy and safety of COVID-19 vaccine, we may have neglected the discussion on the heterogeneity from these sources. Third, most of the original studies only collected adverse events within 7 days after vaccination, which may limit the duration of follow-up for safety analysis.
Based on the real-world studies, SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Abbreviations
Coronavirus disease 2019
Severe Acute Respiratory Syndrome Coronavirus 2
Vaccine effectiveness
Confidence intervals
Intensive care unit
Random clinical trials
Preferred reporting items for systematic reviews and meta-analyses
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021. https://coronavirus.jhu.edu/map.html . Accessed 20 Aug 2021.
Barranco R, Rocca G, Molinelli A, Ventura F. Controversies and challenges of mass vaccination against SARS-CoV-2 in Italy: medico-legal perspectives and considerations. Healthcare (Basel). 2021. https://doi.org/10.3390/healthcare9091163 .
Article Google Scholar
COVID-19 vaccine tracker. 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ . Accessed 20 Aug 2021.
Coronavirus (COVID-19) Vaccinations. 2021. https://ourworldindata.org/covid-vaccinations . Accessed 20 Aug 2021.
Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9(2):e20–1. https://doi.org/10.1016/s2213-2600(21)00005-9 .
Article CAS PubMed PubMed Central Google Scholar
Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021;589(7843):500–1. https://doi.org/10.1038/d41586-021-00121-z .
Article CAS PubMed Google Scholar
Reardon S. How the Delta variant achieves its ultrafast spread. Nature. 2021. https://doi.org/10.1038/d41586-021-01986-w .
Article PubMed Google Scholar
Li R, Liu J, Zhang H. The challenge of emerging SARS-CoV-2 mutants to vaccine development. J Genet Genomics. 2021;48(2):102–6. https://doi.org/10.1016/j.jgg.2021.03.001 .
Article PubMed PubMed Central Google Scholar
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, Zou H, Sun C. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94. https://doi.org/10.1186/s40249-021-00878-5 .
Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021. https://doi.org/10.1002/jmv.27203 .
Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, Turner RJ. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9050467 .
Sathian B, Asim M, Banerjee I, Roy B, Pizarro AB, Mancha MA, van Teijlingen ER, Kord-Varkaneh H, Mekkodathil AA, Subramanya SH, et al. Development and implementation of a potential coronavirus disease 2019 (COVID-19) vaccine: a systematic review and meta-analysis of vaccine clinical trials. Nepal J Epidemiol. 2021;11(1):959–82. https://doi.org/10.3126/nje.v11i1.36163 .
Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC. Safety, tolerability, and immunogenicity of COVID-19 vaccines: a systematic review and meta-analysis. medRxiv. 2020. https://doi.org/10.1101/2020.11.03.20224998 .
Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107715 .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2108891 .
Israel says Pfizer Covid vaccine is just 39% effective as delta spreads, but still prevents severe illness. 2021. https://www.cnbc.com/2021/07/23/delta-variant-pfizer-covid-vaccine-39percent-effective-in-israel-prevents-severe-illness.html . Accessed 20 Aug 2021.
Zacay G, Shasha D, Bareket R, Kadim I, Hershkowitz Sikron F, Tsamir J, Mossinson D, Heymann AD. BNT162b2 vaccine effectiveness in preventing asymptomatic infection with SARS-CoV-2 virus: a nationwide historical cohort study. Open Forum Infect Dis. 2021;8(6): ofab262. https://doi.org/10.1093/ofid/ofab262 .
Martínez-Baz I, Miqueleiz A, Casado I, Navascués A, Trobajo-Sanmartín C, Burgui C, Guevara M, Ezpeleta C, Castilla J. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.21.2100438 .
Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–9. https://doi.org/10.15585/mmwr.mm7018e1 .
Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan AJ, Niesen MJM, O’Horo JC, Virk A, Swift MD, Badley AD, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.007 .
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed 20 Aug 2021.
Rostom A, Dubé C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/ . Accessed 20 Aug 2021
Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–9. https://doi.org/10.1056/NEJMc2104974 .
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–65. https://doi.org/10.1001/jama.2021.7152 .
Azamgarhi T, Hodgkinson M, Shah A, Skinner JA, Hauptmannova I, Briggs TWR, Warren S. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers—a single centre cohort study. Nat Commun. 2021;12(1):3698. https://doi.org/10.1038/s41467-021-23927-x .
Bianchi FP, Germinario CA, Migliore G, Vimercati L, Martinelli A, Lobifaro A, Tafuri S, Stefanizzi P. BNT162b2 mRNA COVID-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection: a preliminary report. J Infect Dis. 2021. https://doi.org/10.1093/infdis/jiab262 .
Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396–401. https://doi.org/10.15585/mmwr.mm7011e3 .
Cavanaugh AM, Fortier S, Lewis P, Arora V, Johnson M, George K, Tobias J, Lunn S, Miller T, Thoroughman D, et al. COVID-19 outbreak associated with a SARS-CoV-2 R1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639–43. https://doi.org/10.15585/mmwr.mm7017e2 .
Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01446-y .
Chodick G, Tene L, Patalon T, Gazit S, Ben Tov A, Cohen D, Muhsen K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6): e2115985. https://doi.org/10.1001/jamanetworkopen.2021.15985 .
Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, Weil C, Goldshtein I, Twig G, Cohen D, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab438 .
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765 .
Flacco ME, Soldato G, Acuti Martellucci C, Carota R, Di Luzio R, Caponetti A, Manzoli L. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian Province. VacCInes (Basel). 2021. https://doi.org/10.3390/vaccines9060628 .
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. https://doi.org/10.1016/s0140-6736(21)00947-8 .
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. https://doi.org/10.1016/s0140-6736(21)00790-x .
Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Tuner A, Friedrich Z, Morrison L, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00330-3 .
Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021. https://doi.org/10.1053/j.gastro.2021.05.044 .
Knobel P, Serra C, Grau S, Ibañez R, Diaz P, Ferrández O, Villar R, Lopez AF, Pujolar N, Horcajada JP, et al. COVID-19 mRNA vaccine effectiveness in asymptomatic healthcare workers. Infect Control Hosp Epidemiol. 2021. https://doi.org/10.1017/ice.2021.287 .
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373: n1088. https://doi.org/10.1136/bmj.n1088 .
Mazagatos C, Monge S, Olmedo C, Vega L, Gallego P, Martín-Merino E, Sierra MJ, Limia A, Larrauri A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53, 2020 to 13 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.24.2100452 .
Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline HA, Hou PC, et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel—33 US Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–8. https://doi.org/10.15585/mmwr.mm7020e2 .
Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2. https://doi.org/10.1016/s0140-6736(21)01358-1 .
Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, Fuller C, Irwin-Singer A, Davies D, Tut G, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00289-9 .
Skowronski DM, Setayeshgar S, Zou M, Prystajecky N, Tyson JR, Galanis E, Naus M, Patrick DM, Sbihi H, El Adam S, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including Alpha and Gamma variants: a test-negative design in adults 70 years and older in British Columbia,Canada. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab616 .
Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, Murad MH, Berbari EF, Virk A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab361 .
Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107058 .
Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–57. https://doi.org/10.1016/s0140-6736(21)00677-2 .
Williams C, Al-Bargash D, Macalintal C, Stuart R, Seth A, Latham J, Gitterman L, Fedsin S, Godoy M, Kozak R, et al. COVID-19 outbreak associated with a SARS-CoV-2 P.1 lineage in a long-term care home after implementation of a vaccination program—Ontario, April–May 2021. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab617 .
Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, Algaissi A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060674 .
Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25(12):4418–21. https://doi.org/10.26355/eurrev_202106_26153 .
Baldolli A, Michon J, Appia F, Galimard C, Verdon R, Parienti JJ. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–3. https://doi.org/10.1016/j.vaccine.2021.06.054 .
Cherian S, Paul A, Ahmed S, Alias B, Manoj M, Santhosh AK, Varghese DR, Krishnan N, Shenoy P. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int. 2021;41(8):1441–5. https://doi.org/10.1007/s00296-021-04917-0 .
Chevallier P, Coste-Burel M, Le Bourgeois A, Peterlin P, Garnier A, Béné MC, Imbert BM, Drumel T, Le Gouill S, Moreau P, et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. EJHaem. 2021. https://doi.org/10.1002/jha2.242 .
Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, Garonzik-Wang J, Paik JJ. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220231 .
Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220647 .
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–8. https://doi.org/10.15585/mmwr.mm7008e3 .
Hashimoto T, Ozaki A, Bhandari D, Sawano T, Sah R, Tanimoto T. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese health care workers; a secondary analysis of initial post-approval safety data. J Travel Med. 2021. https://doi.org/10.1093/jtm/taab090 .
Lv G, Yuan J, Xiong X, Li M. Mortality rate and characteristics of deaths following COVID-19 vaccination. Front Med (Lausanne). 2021;8: 670370. https://doi.org/10.3389/fmed.2021.670370 .
McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, Venkatakrishnan AJ, Anand P, Agarwal V, O’Horo JC, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.006 .
Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. https://doi.org/10.1016/s1470-2045(21)00213-8 .
Pagotto V, Ferloni A, Mercedes Soriano M, Díaz M, Braguinsky Golde N, González MI, Asprea V, Staneloni MI, Zingoni P, Vidal G, et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. MediCIna (B Aires). 2021;81(3):408–14.
Google Scholar
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.04.003 .
Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. COVID-19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthc Qual Res. 2021. https://doi.org/10.1016/j.jhqr.2021.05.002 .
Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, Beyar-Katz O, Shefer G, Moshiashvili MM, Karni C, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single center prospective cohort study. Transplant Cell Ther. 2021. https://doi.org/10.1016/j.jtct.2021.06.024 .
Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X, André N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer. 2021;154:30–4. https://doi.org/10.1016/j.ejca.2021.06.002 .
Riad A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, Klugar M, Attia S. Safety of ChAdOx1 nCoV-19 vaccine: independent evidence from two EU states. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060673 .
Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, Klugar M. Prevalence and risk factors of CoronaVac Side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med. 2021. https://doi.org/10.3390/jcm10122629 .
Rosman Y, Lavi N, Meir-Shafrir K, Lachover-Roth I, Cohen-Engler A, Mekori YA, Confino-Cohen R. Safety of BNT162b2 mRNA COVID-19 vaccine in patients with mast cell disorders. J Allergy Clin Immunol Pract. 2021. https://doi.org/10.1016/j.jaip.2021.06.032 .
Signorelli C, Odone A, Gianfredi V, Capraro M, Kacerik E, Chiecca G, Scardoni A, Minerva M, Mantecca R, Musarò P, et al. Application of the “immunization islands” model to improve quality, efficiency and safety of a COVID-19 mass vaccination site. Ann Ig. 2021;33(5):499–512. https://doi.org/10.7416/ai.2021.2456 .
Vallée A, Chan-Hew-Wai A, Bonan B, Lesprit P, Parquin F, Catherinot É, Choucair J, Billard D, Amiel-Taieb C, Camps È, et al. Oxford-AstraZeneca COVID-19 vaccine: need of a reasoned and effective vaccine campaign. Public Health. 2021;196:135–7. https://doi.org/10.1016/j.puhe.2021.05.030 .
Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.04.026 .
Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021. https://doi.org/10.1080/14760584.2021.1925112 .
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–4. https://doi.org/10.15585/mmwr.mm7018e2 .
Prevention CCfDCa. Information analysis of COVID-19 vaccine adverse reaction monitoring in China. 2021-5-28. http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230908.html . Accessed 20 Aug 2021.
Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–90. https://doi.org/10.1007/s10787-021-00839-2 .
Angyal A, Longet S, Moore S, Payne RP, Harding A et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously SARS-CoV-2-Infected and Infection-Naive UK Healthcare Workers: A Multicentre, Prospective, Observational Cohort Study. Available at SSRN: https://ssrn.com/abstract=3820576 or https://doi.org/10.2139/ssrn.3820576 . Accessed 20 Aug 2021.
Pimenta D, Yates C, Pagel C, Gurdasani D. Delaying the second dose of covid-19 vaccines. BMJ. 2021;372: n710. https://doi.org/10.1136/bmj.n710 .
Tauh T, Mozel M, Meyler P, Lee SM. An updated look at the 16-week window between doses of vaccines in BC for COVID-19. BC Med J. 2021;63(3):102–3.
Kadire SR, Wachter RM, Lurie N. Delayed second dose versus standard regimen for COVID-19 vaccination. N Engl J Med. 2021;384(9): e28. https://doi.org/10.1056/NEJMclde2101987 .
Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2109072 .
Truong TT, Ryutov A, Pandey U, Yee R, Goldberg L, Bhojwani D, Aguayo-Hiraldo P, Pinsky BA, Pekosz A, Shen L, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021. https://doi.org/10.1101/2021.02.27.21252099 .
Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, et al. Persistence and evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383(23):2291–3. https://doi.org/10.1056/NEJMc2031364 .
Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–6. https://doi.org/10.1056/NEJMsb2104756 .
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. https://doi.org/10.1056/NEJMoa2114255 .
Hause AM, Baggs J, Gee J, Marquez P, Myers TR, Shimabukuro TT, Shay DK. Safety monitoring of an additional dose of COVID-19 vaccine—United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379–84. https://doi.org/10.15585/mmwr.mm7039e4 .
Furlow B. Immunocompromised patients in the USA and UK should receive third dose of COVID-19 vaccine. Lancet Rheumatol. 2021. https://doi.org/10.1016/s2665-9913(21)00313-1 .
Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–90. https://doi.org/10.1016/s0140-6736(21)01699-8 .
Peled Y, Ram E, Lavee J, Segev A, Matezki S, Wieder-Finesod A, Halperin R, Mandelboim M, Indenbaum V, Levy I, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.08.010 .
WHO. WHO press conference on coronavirus disease (COVID-19)—4 August 2021. 2021. https://www.who.int/multi-media/details/who-press-conference-on-coronavirus-disease-(covid-19)---4-august-2021 . Accessed 20 Aug 2021.
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.2675 .
Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, Krach MR, Jain VS, Werbel WA, Avery RK, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021. https://doi.org/10.1097/tp.0000000000003780 .
Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, Asraf K, Doolman R, Sapir E, Regev Yochay G, et al. Short-term outcome of pregnant women vaccinated by BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021. https://doi.org/10.1002/uog.23729 .
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. https://doi.org/10.1056/NEJMoa2104983 .
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–62. https://doi.org/10.1016/j.healun.2021.04.003 .
Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2110345 .
Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2105290 .
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2113017 .
Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç F, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. https://doi.org/10.1016/s0140-6736(21)01429-x .
Kumar S, Saurabh MK, Maharshi V. Efficacy and safety of potential vaccine candidates against coronavirus disease 2019: a systematic review. J Adv Pharm Technol Res. 2021;12(3):215–21. https://doi.org/10.4103/japtr.JAPTR_229_20 .
Burgos-Salcedo J. A rational strategy to support approved COVID-19 vaccines prioritization. Hum Vaccin Immunother. 2021;17(10):3474–7. https://doi.org/10.1080/21645515.2021.1922060 .
Download references
Acknowledgements
This study was funded by the National Natural Science Foundation of China (72122001; 71934002) and the National Science and Technology Key Projects on Prevention and Treatment of Major infectious disease of China (2020ZX10001002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. No payment was received by any of the co-authors for the preparation of this article.
Author information
Qiao Liu and Chenyuan Qin are joint first authors
Authors and Affiliations
Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, 100191, China
Qiao Liu, Chenyuan Qin, Min Liu & Jue Liu
Institute for Global Health and Development, Peking University, Beijing, 100871, China
Chenyuan Qin & Jue Liu
You can also search for this author in PubMed Google Scholar
Contributions
LQ and QCY contributed equally as first authors. LJ and LM contributed equally as correspondence authors. LJ and LM conceived and designed the study; LQ, QCY and LJ carried out the literature searches, extracted the data, and assessed the study quality; LQ and QCY performed the statistical analysis and wrote the manuscript; LJ, LM, LQ and QCY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Min Liu or Jue Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Additional file 1: table s1..
Characteristic of studies included for vaccine effectiveness.
Additional file 2: Table S2.
Characteristic of studies included for vaccine safety.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Q., Qin, C., Liu, M. et al. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty 10 , 132 (2021). https://doi.org/10.1186/s40249-021-00915-3
Download citation
Received : 07 September 2021
Accepted : 01 November 2021
Published : 14 November 2021
DOI : https://doi.org/10.1186/s40249-021-00915-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
- Meta-analysis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults
Affiliations.
- 1 Thibodaux Regional Health System, Thibodaux, LA, USA. Electronic address: [email protected].
- 2 Unit of Innovation and Organization, Navarre Health Service, Spain. Electronic address: [email protected].
- 3 Institute of Evidence-Based Healthcare, Bond University, Gold Coast, QLD, Australia. Electronic address: [email protected].
- 4 Fielding School of Public Health and College of Letters and Science, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 5 Geffen School of Medicine, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 6 Clinical Excellence Research Center, School of Medicine, Stanford University, CA, USA. Electronic address: [email protected].
- 7 School of Pharmacy, University of Maryland, Baltimore, MD, USA. Electronic address: [email protected].
- PMID: 36055877
- PMCID: PMC9428332
- DOI: 10.1016/j.vaccine.2022.08.036
Introduction: In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.
Methods: Secondary analysis of serious adverse events reported in the placebo-controlled, phase III randomized clinical trials of Pfizer and Moderna mRNA COVID-19 vaccines in adults ( NCT04368728 and NCT04470427 ), focusing analysis on Brighton Collaboration adverse events of special interest.
Results: Pfizer and Moderna mRNA COVID-19 vaccines were associated with an excess risk of serious adverse events of special interest of 10.1 and 15.1 per 10,000 vaccinated over placebo baselines of 17.6 and 42.2 (95 % CI -0.4 to 20.6 and -3.6 to 33.8), respectively. Combined, the mRNA vaccines were associated with an excess risk of serious adverse events of special interest of 12.5 per 10,000 vaccinated (95 % CI 2.1 to 22.9); risk ratio 1.43 (95 % CI 1.07 to 1.92). The Pfizer trial exhibited a 36 % higher risk of serious adverse events in the vaccine group; risk difference 18.0 per 10,000 vaccinated (95 % CI 1.2 to 34.9); risk ratio 1.36 (95 % CI 1.02 to 1.83). The Moderna trial exhibited a 6 % higher risk of serious adverse events in the vaccine group: risk difference 7.1 per 10,000 (95 % CI -23.2 to 37.4); risk ratio 1.06 (95 % CI 0.84 to 1.33). Combined, there was a 16 % higher risk of serious adverse events in mRNA vaccine recipients: risk difference 13.2 (95 % CI -3.2 to 29.6); risk ratio 1.16 (95 % CI 0.97 to 1.39).
Discussion: The excess risk of serious adverse events found in our study points to the need for formal harm-benefit analyses, particularly those that are stratified according to risk of serious COVID-19 outcomes. These analyses will require public release of participant level datasets.
Keywords: Adverse events of special interest; Brighton Collaboration; COVID-19; COVID-19 vaccines; Coalition for Epidemic Preparedness Innovations; Moderna COVID-19 vaccine mRNA-1273; NCT04368728 ; NCT04470427 ; Pfizer-BioNTech COVID-19 vaccine BNT162b2; SARS-CoV-2; Safety Platform for Emergency vACcines; Serious adverse events; Vaccines; mRNA vaccines.
Copyright © 2022 Elsevier Ltd. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Serious adverse events following mRNA vaccination in randomized trials in adults. Black S, Evans S. Black S, et al. Vaccine. 2023 May 26;41(23):3473-3474. doi: 10.1016/j.vaccine.2023.04.040. Epub 2023 Apr 28. Vaccine. 2023. PMID: 37121802 No abstract available.
Similar articles
- A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial. Akova M, Unal S. Akova M, et al. Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
- Use of COVID-19 Vaccines After Reports of Adverse Events Among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices - United States, July 2021. Rosenblum HG, Hadler SC, Moulia D, Shimabukuro TT, Su JR, Tepper NK, Ess KC, Woo EJ, Mba-Jonas A, Alimchandani M, Nair N, Klein NP, Hanson KE, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Mbaeyi SA, Oliver SE. Rosenblum HG, et al. MMWR Morb Mortal Wkly Rep. 2021 Aug 13;70(32):1094-1099. doi: 10.15585/mmwr.mm7032e4. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34383735 Free PMC article.
- Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. Buchan SA, Seo CY, Johnson C, Alley S, Kwong JC, Nasreen S, Calzavara A, Lu D, Harris TM, Yu K, Wilson SE. Buchan SA, et al. JAMA Netw Open. 2022 Jun 1;5(6):e2218505. doi: 10.1001/jamanetworkopen.2022.18505. JAMA Netw Open. 2022. PMID: 35749115 Free PMC article.
- COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. Meo SA, et al. Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
- Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, Hartling L. Pillay J, et al. BMJ. 2022 Jul 13;378:e069445. doi: 10.1136/bmj-2021-069445. BMJ. 2022. PMID: 35830976 Free PMC article. Review.
- Comparison of Transient and Persistent Adverse Events After COVID-19 Vaccination: A Retrospective Analysis. Hikichi H, Fujioka Y, Saga A, Watanabe K, Hasegawa R, Moritoki Y, Ueki S. Hikichi H, et al. Cureus. 2024 Jun 28;16(6):e63410. doi: 10.7759/cureus.63410. eCollection 2024 Jun. Cureus. 2024. PMID: 39070394 Free PMC article.
- Adverse Events of COVID-19 Vaccines in the United States: Temporal and Spatial Analysis. Li Y, Li J, Dang Y, Chen Y, Tao C. Li Y, et al. JMIR Public Health Surveill. 2024 Jul 15;10:e51007. doi: 10.2196/51007. JMIR Public Health Surveill. 2024. PMID: 39008362 Free PMC article.
- Decoding trends in mRNA vaccine research: A comprehensive bibliometric study. Zhang C, Wang Y, Peng J, Wen X, Zhang Y, Li K, Du H, Hu X. Zhang C, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2355037. doi: 10.1080/21645515.2024.2355037. Epub 2024 May 30. Hum Vaccin Immunother. 2024. PMID: 38813652 Free PMC article.
- From Detection to Protection: Antibodies and Their Crucial Role in Diagnosing and Combatting SARS-CoV-2. Kumar A, Tripathi P, Kumar P, Shekhar R, Pathak R. Kumar A, et al. Vaccines (Basel). 2024 Apr 25;12(5):459. doi: 10.3390/vaccines12050459. Vaccines (Basel). 2024. PMID: 38793710 Free PMC article. Review.
- Interchangeability of different COVID-19 vaccine platforms as booster doses: A phase 3 study mimicking real-world practice. Ann Costa Clemens S, Weckx L, Milan EP, Smolenov I, Clemens R. Ann Costa Clemens S, et al. Vaccine. 2024 Jul 25;42(19):3989-3998. doi: 10.1016/j.vaccine.2024.05.009. Epub 2024 May 17. Vaccine. 2024. PMID: 38762360 Free PMC article. Clinical Trial.
- Law B, Pim C. SO2-D2.1.3 Priority List of COVID-19 Adverse events of special interest [Internet]. 2021 Oct [cited 2022 Feb 17]. Available from: https://brightoncollaboration.us/wp-content/uploads/2021/11/SO2_D2.1.3_C... .
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. - PMC - PubMed
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. - PMC - PubMed
- Sadoff J., Gray G., Vandebosch A.n., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. - PMC - PubMed
- Health Canada. Search for clinical information on drugs and medical devices [Internet]. 2019 [cited 2021 Nov 9]. Available from: https://clinical-information.canada.ca/ .
Publication types
- Search in MeSH
Associated data
- Search in ClinicalTrials.gov
LinkOut - more resources
Full text sources.
- Elsevier Science
- Europe PubMed Central
- PubMed Central
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Lessons from the covid-19 pandemic: promoting vaccination and public health resilience, a narrative review.

1. Introduction
3. covid-19 vaccine hesitancy: addressing challenges beyond the pandemic, 4. enhancing vaccine acceptance: effective strategies for public health, 5. lessons learned from challenges and barriers of covid-19 vaccination campaigns, 6. overcoming inequalities in vaccination access: insights from the covid-19 campaign, 7. implications for future public health strategies, 8. conclusions, author contributions, conflicts of interest.
- Montero, D.A.; Vidal, R.M.; Velasco, J.; Carreño, L.J.; Torres, J.P.; Benachi, O.M.A.; Tovar-Rosero, Y.Y.; Oñate, A.A.; O’Ryan, M. Two centuries of vaccination: Historical and conceptual approach and future perspectives. Front. Public Health 2024 , 11 , 1326154. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022 , 12 , 1295. [ Google Scholar ] [ CrossRef ]
- Haileamlak, A. The impact of COVID-19 on health and health systems. Ethiop. J. Health Sci. 2021 , 31 , 1073–1074. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sachs, J.D.; Karim, S.S.A.; Aknin, L.; Allen, J.; Brosbol, K.; Colombo, F.; Barron, G.C.; Espinosa, M.F.; Gaspar, V.; Gaviria, A.; et al. The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet 2022 , 400 , 1224–1280. [ Google Scholar ] [ CrossRef ]
- Excler, J.L.; Saville, M.; Privor-Dumm, L.; Gilbert, S.; Hotez, P.J.; Thompson, D.; Abdool-Karim, S.; Kim, J.H. Factors, enablers and challenges for COVID-19 vaccine development. BMJ Glob. Health 2023 , 8 , e011879. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021 , 9 , 160. [ Google Scholar ] [ CrossRef ]
- Chavda, V.P.; Soni, S.; Vora, L.K.; Soni, S.; Khadela, A.; Ajabiya, J. mRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines 2022 , 10 , 2150. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Etowa, J.; Beauchamp, S.; Fseifes, M.; Osandatuwa, G.; Brenneman, P.; Salam-Alada, K.; Sulaiman, R.; Okolie, E.; Dinneh, I.; Julmisse, S.; et al. Understanding Low Vaccine Uptake in the Context of Public Health in High-Income Countries: A Scoping Review. Vaccines 2024 , 12 , 269. [ Google Scholar ] [ CrossRef ]
- European Centre for Disease Prevention and Control. Facilitating COVID-19 Vaccination Acceptance and Uptake in the EU/EEA ; ECDC: Stockholm, Sweden, 2021. [ Google Scholar ]
- Nuwarda, R.F.; Ramzan, I.; Weekes, L.; Kayser, V. Vaccine Hesitancy: Contemporary Issues and Historical Background. Vaccines 2022 , 10 , 1595. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cella, P.; Voglino, G.; Barberis, I.; Alagna, E.; Alessandroni, C.; Cuda, A.; D’aloisio, F.; Dallagiacoma, G.; De Nitto, S.; Di Gaspare, F.; et al. Resources for assessing parents’ vaccine hesitancy: A systematic review of the literature. J. Prev. Med. Hyg. 2020 , 61 , E340–E373. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rosenthal, S.; Cummings, C.L. Influence of rapid COVID-19 vaccine development on vaccine hesitancy. Vaccine 2021 , 39 , 7625–7632. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gianfredi, V.; Stefanizzi, P.; Berti, A.; D’Amico, M.; De Lorenzo, V.; Lorenzo, A.D.; Moscara, L.; Castaldi, S. A Systematic Review of Population-Based Studies Assessing Knowledge, Attitudes, Acceptance, and Hesitancy of Pregnant and Breastfeeding Women towards the COVID-19 Vaccine. Vaccines 2023 , 11 , 1289. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gianfredi, V.; Berti, A.; Stefanizzi, P.; D’Amico, M.; De Lorenzo, V.; Moscara, L.; Di Lorenzo, A.; Venerito, V.; Castaldi, S. COVID-19 Vaccine Knowledge, Attitude, Acceptance and Hesitancy among Pregnancy and Breastfeeding: Systematic Review of Hospital-Based Studies. Vaccines 2023 , 11 , 1697. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gianfredi, V.; Odone, A.; Fiacchini, D.; Rosselu, R.; Battista, T.; Signorelli, C. Trust and reputation management, branding, social media management nelle organizzazioni sanitarie: Sfide e opportunity per la comunita igienistica italiana. J. Prev. Med. Hyg. 2019 , 60 , E108–E109. [ Google Scholar ]
- Fitzpatrick, P.J. Improving health literacy using the power of digital communications to achieve better health outcomes for patients and practitioners. Front. Digit. Health 2023 , 5 , 1264780. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hong, S.-A. COVID-19 vaccine communication and advocacy strategy: A social marketing campaign for increasing COVID-19 vaccine uptake in South Korea. Humanit. Soc. Sci. Commun. 2023 , 10 , 109. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lanza, T.E.; Paladini, A.; Marziali, E.; Gianfredi, V.; Blandi, L.; Signorelli, C.; Odone, A.; Ricciardi, W.; Damiani, G.; Cadeddu, C. Training needs assessment of European frontline health care workers on vaccinology and vaccine acceptance: A systematic review. Eur. J. Public Health 2023 , 33 , 591–595. [ Google Scholar ] [ CrossRef ]
- Gianfredi, V.; Oradini-Alacreu, A.; Sá, R.; Blandi, L.; Cadeddu, C.; Ricciardi, W.; Signorelli, C.; Odone, A. Frontline health workers: Training needs assessment on immunisation programme. An EU/EEA-based survey. J. Public Health 2023 . [ Google Scholar ] [ CrossRef ]
- Odone, A.; Gianfredi, V.; Frascella, B.; Balzarini, F.; Alacreu, A.O.; Signorelli, C. Digitalization of immunization programmes in Europe: Results from the EUVIS project. Eur. J. Public Health 2020 , 30 , V7. [ Google Scholar ] [ CrossRef ]
- Gianfredi, V.; Moretti, M.; Lopalco, P.L. Countering vaccine hesitancy through immunization information systems, a narrative review. Hum. Vaccin. Immunother. 2019 , 15 , 2508–2526. [ Google Scholar ] [ CrossRef ]
- D’Ancona, F.; Gianfredi, V.; Riccardo, F.; Iannazzo, S. Immunisation Registries at regional level in Italy and the roadmap for a future Italian National Registry. Ann. Ig. 2018 , 30 , 77–85. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gianfredi, V.; D’Ancona, F.; Maraglino, F.; Cenci, C.; Iannazzo, S. Polio and measles: Reasons of missed vaccination in Italy, 2015–2017. Ann. Ig. 2019 , 31 , 191–201. [ Google Scholar ] [ CrossRef ]
- Fokoun, C. Strategies implemented to address vaccine hesitancy in France: A review article. Hum. Vaccines Immunother. 2018 , 14 , 1580–1590. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Desborough, J.; Wright, M.; Parkinson, A.; Hall Dykgraaf, S.; Ball, L.; Dut, G.M.; Sturgiss, E.; de Toca, L.; Kidd, M. What strategies have been effective in optimising COVID-19 vaccine uptake in Australia and internationally? Aust. J. Gen. Pr. 2022 , 51 , 725–730. [ Google Scholar ] [ CrossRef ]
- Scott, T.; Gutschow, B.; Ragavan, M.I.; Ho, K.; Massart, M.; Ripper, L.; Muthama, V.; Miller, E.; Bey, J.; Abernathy, R.P. A Community Partnered Approach to Promoting COVID-19 Vaccine Equity. Health Promot. Pr. 2021 , 22 , 758–760. [ Google Scholar ] [ CrossRef ]
- Ratzan, S.; Schneider, E.C.; Hatch, H.; Cacchione, J. Missing the Point—How Primary Care Can Overcome COVID-19 Vaccine “Hesitancy”. N. Engl. J. Med. 2021 , 384 , e100. [ Google Scholar ] [ CrossRef ]
- Sinuraya, R.K.; Nuwarda, R.F.; Postma, M.J.; Suwantika, A.A. Vaccine hesitancy and equity: Lessons learned from the past and how they affect the COVID-19 countermeasure in Indonesia. Glob. Health 2024 , 20 , 11. [ Google Scholar ] [ CrossRef ]
- Barry, V.; Dasgupta, S.; Weller, D.L.; Kriss, J.L.; Cadwell, B.L.; Rose, C.; Pingali, C.; Musial, T.; Sharpe, J.D.; Flores, S.A.; et al. Patterns in COVID-19 Vaccination Coverage, by Social Vulnerability and Urbanicity—United States, December 14, 2020–May 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021 , 70 , 818–824. [ Google Scholar ] [ CrossRef ]
- Cascini, F.; Pantovic, A.; Al-Ajlouni, Y.A.; Failla, G.; Puleo, V.; Melnyk, A.; Lontano, A.; Ricciardi, W. Social media and attitudes towards a COVID-19 vaccination: A systematic review of the literature. EClinicalMedicine 2022 , 48 , 101454. [ Google Scholar ] [ CrossRef ]
- Feifer, R.A.; Bethea, L.; White, E.M. Racial Disparities in COVID-19 Vaccine Acceptance: Building Trust to Protect Nursing Home Staff and Residents. J. Am. Med. Dir. Assoc. 2021 , 22 , 1853–1855.e1851. [ Google Scholar ] [ CrossRef ]
- Batteux, E.; Mills, F.; Jones, L.F.; Symons, C.; Weston, D. The Effectiveness of Interventions for Increasing COVID-19 Vaccine Uptake: A Systematic Review. Vaccines 2022 , 10 , 386. [ Google Scholar ] [ CrossRef ]
- Hernandez, R.G.; Hagen, L.; Walker, K.; O’Leary, H.; Lengacher, C. The COVID-19 vaccine social media infodemic: Healthcare providers’ missed dose in addressing misinformation and vaccine hesitancy. Hum. Vaccin. Immunother. 2021 , 17 , 2962–2964. [ Google Scholar ] [ CrossRef ]
- Budhwani, H.; Maragh-Bass, A.C.; Tolley, E.E.; Comello, M.L.G.; Stoner, M.C.D.; Adams Larsen, M.; Brambilla, D.; Muessig, K.E.; Pettifor, A.; Bond, C.L.; et al. Tough Talks COVID-19 Digital Health Intervention for Vaccine Hesitancy Among Black Young Adults: Protocol for a Hybrid Type 1 Effectiveness Implementation Randomized Controlled Trial. JMIR Res. Protoc. 2023 , 12 , e41240. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ngai, C.S.B.; Singh, R.G.; Yao, L. Impact of COVID-19 Vaccine Misinformation on Social Media Virality: Content Analysis of Message Themes and Writing Strategies. J. Med. Internet Res. 2022 , 24 , e37806. [ Google Scholar ] [ CrossRef ]
- Dube, E.; Labbe, F.; Malo, B.; Pelletier, C. Public health communication during the COVID-19 pandemic: Perspectives of communication specialists, healthcare professionals, and community members in Quebec, Canada. Can. J. Public Health 2022 , 113 (Suppl. S1), 24–33. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lowe, M.; Harmon, S.H.E.; Kholina, K.; Parker, R.; Graham, J.E. Public health communication in Canada during the COVID-19 pandemic. Can. J. Public Health 2022 , 113 (Suppl. S1), 34–45. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Krastev, S.; Krajden, O.; Vang, Z.M.; Perez-Gay Juarez, F.; Solomonova, E.; Goldenberg, M.; Weinstock, D.; Smith, M.J.; Turk, L.; Lin, X.; et al. Navigating the uncertainty: A novel taxonomy of vaccine hesitancy in the context of COVID-19. PLoS ONE 2023 , 18 , e0295912. [ Google Scholar ] [ CrossRef ]
- Pinaka, O.; Spanou, I.; Papadouli, V.; Papanikolaou, E.; Gioulekas, F.; Mouchtouri, V.A. The role of local primary healthcare units in increasing immunization uptake among children in vulnerable social groups through vaccination campaigns. Public Health Pr. 2021 , 2 , 100185. [ Google Scholar ] [ CrossRef ]
- Gianfredi, V.; Pennisi, F.; Lume, A.; Ricciardi, G.E.; Minerva, M.; Ricco, M.; Odone, A.; Signorelli, C. Challenges and Opportunities of Mass Vaccination Centers in COVID-19 Times: A Rapid Review of Literature. Vaccines 2021 , 9 , 574. [ Google Scholar ] [ CrossRef ]
- Pennisi, F.; Mastrangelo, M.; De Ponti, E.; Cuciniello, R.; Mandelli, A.; Vaia, F.; Signorelli, C. The role of pharmacies in the implementation of vaccination cover- age in Italy. Insights from the preliminary data of the Lombardy Region. Ann. Ig. 2024 , 36 , 363–369. [ Google Scholar ] [ CrossRef ]
- Gianfredi, V.; Nucci, D.; Salvatori, T.; Orlacchio, F.; Villarini, M.; Moretti, M. “PErCEIVE in Umbria”: Evaluation of anti-influenza vaccination’s perception among Umbrian pharmacists. J. Prev. Med. Hyg. 2018 , 59 , E14–E19. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lee, J.T.; Althomsons, S.P.; Wu, H.; Budnitz, D.S.; Kalayil, E.J.; Lindley, M.C.; Pingali, C.; Bridges, C.B.; Geller, A.I.; Fiebelkorn, A.P.; et al. Disparities in COVID-19 Vaccination Coverage Among Health Care Personnel Working in Long-Term Care Facilities, by Job Category, National Healthcare Safety Network—United States, March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021 , 70 , 1036–1039. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- World Health Organization and the United Nations Children’s Fund. Considerations for Integrating COVID-19 Vaccination into Immunization Programmes and Primary Health Care for 2022 and Beyond ; World Health Organization: Geneva, Switzerland, 2022. [ Google Scholar ]
- Albarracin, D.; Jung, H.; Song, W.; Tan, A.; Fishman, J. Rather than inducing psychological reactance, requiring vaccination strengthens intentions to vaccinate in US populations. Sci. Rep. 2021 , 11 , 20796. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- De Giorgio, A.; Kuvacic, G.; Males, D.; Vecchio, I.; Tornali, C.; Ishac, W.; Ramaci, T.; Barattucci, M.; Milavic, B. Willingness to Receive COVID-19 Booster Vaccine: Associations between Green-Pass, Social Media Information, Anti-Vax Beliefs, and Emotional Balance. Vaccines 2022 , 10 , 481. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alshahrani, S.M.; Dehom, S.; Almutairi, D.; Alnasser, B.S.; Alsaif, B.; Alabdrabalnabi, A.A.; Bin Rahmah, A.; Alshahrani, M.S.; El-Metwally, A.; Al-Khateeb, B.F.; et al. Acceptability of COVID-19 vaccination in Saudi Arabia: A cross-sectional study using a web-based survey. Hum. Vaccin. Immunother. 2021 , 17 , 3338–3347. [ Google Scholar ] [ CrossRef ]
- de Figueiredo, A.; Larson, H.J.; Reicher, S.D. The potential impact of vaccine passports on inclination to accept COVID-19 vaccinations in the United Kingdom: Evidence from a large cross-sectional survey and modeling study. EClinicalMedicine 2021 , 40 , 101109. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Natalia, Y.A.; Delporte, M.; De Witte, D.; Beutels, P.; Dewatripont, M.; Molenberghs, G. Assessing the impact of COVID-19 passes and mandates on disease transmission, vaccination intention, and uptake: A scoping review. BMC Public Health 2023 , 23 , 2279. [ Google Scholar ] [ CrossRef ]
- Radic, A.; Koo, B.; Kim, J.J.; Han, H. No jab, no international travel? Linking TRA, mass media, motivation, and experience. J. Vacat. Mark. 2023 , 29 , 365–385. [ Google Scholar ] [ CrossRef ]
- Wong, M.C.S.; Wong, E.L.Y.; Cheung, A.W.L.; Huang, J.; Lai, C.K.C.; Yeoh, E.K.; Chan, P.K.S. COVID-19 Vaccine Hesitancy in a City with Free Choice and Sufficient Doses. Vaccines 2021 , 9 , 1250. [ Google Scholar ] [ CrossRef ]
- Oliu-Barton, M.; Pradelski, B.S.R.; Woloszko, N.; Guetta-Jeanrenaud, L.; Aghion, P.; Artus, P.; Fontanet, A.; Martin, P.; Wolff, G.B. The effect of COVID certificates on vaccine uptake, health outcomes, and the economy. Nat. Commun. 2022 , 13 , 3942. [ Google Scholar ] [ CrossRef ]
- Kluver, H.; Hartmann, F.; Humphreys, M.; Geissler, F.; Giesecke, J. Incentives can spur COVID-19 vaccination uptake. Proc. Natl. Acad. Sci. USA 2021 , 118 , e2109543118. [ Google Scholar ] [ CrossRef ]
- Mills, M.C.; Ruttenauer, T. The effect of mandatory COVID-19 certificates on vaccine uptake: Synthetic-control modelling of six countries. Lancet Public Health 2022 , 7 , e15–e22. [ Google Scholar ] [ CrossRef ]
- Walkowiak, M.P.; Walkowiak, J.B.; Walkowiak, D. COVID-19 Passport as a Factor Determining the Success of National Vaccination Campaigns: Does It Work? The Case of Lithuania vs. Poland. Vaccines 2021 , 9 , 1498. [ Google Scholar ] [ CrossRef ]
- Khazanov, G.K.; Stewart, R.; Pieri, M.F.; Huang, C.; Robertson, C.T.; Schaefer, K.A.; Ko, H.; Fishman, J. The effectiveness of financial incentives for COVID-19 vaccination: A systematic review. Prev. Med. 2023 , 172 , 107538. [ Google Scholar ] [ CrossRef ]
- McCosker, L.K.; Ware, R.S.; Seale, H.; Hooshmand, D.; O’Leary, R.; Downes, M.J. The effect of a financial incentive on COVID-19 vaccination uptake, and predictors of uptake, in people experiencing homelessness: A randomized controlled trial. Vaccine 2024 , 42 , 2578–2584. [ Google Scholar ] [ CrossRef ]
- Terrell, R.; Alami, A.; Krewski, D. Interventions for COVID-19 Vaccine Hesitancy: A Systematic Review and Narrative Synthesis. Int. J. Environ. Res. Public Health 2023 , 20 , 6082. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barber, A.; West, J. Conditional cash lotteries increase COVID-19 vaccination rates. J. Health Econ. 2022 , 81 , 102578. [ Google Scholar ] [ CrossRef ]
- Alam, S.T.; Ahmed, S.; Ali, S.M.; Sarker, S.; Kabir, G. Challenges to COVID-19 vaccine supply chain: Implications for sustainable development goals. Int. J. Prod. Econ. 2021 , 239 , 108193. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tagoe, E.T.; Sheikh, N.; Morton, A.; Nonvignon, J.; Sarker, A.R.; Williams, L.; Megiddo, I. COVID-19 Vaccination in Lower-Middle Income Countries: National Stakeholder Views on Challenges, Barriers, and Potential Solutions. Front. Public Health 2021 , 9 , 709127. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fahrni, M.L.; Ismail, I.A.; Refi, D.M.; Almeman, A.; Yaakob, N.C.; Saman, K.M.; Mansor, N.F.; Noordin, N.; Babar, Z.U. Management of COVID-19 vaccines cold chain logistics: A scoping review. J. Pharm. Policy Pr. 2022 , 15 , 16. [ Google Scholar ] [ CrossRef ]
- Chakraborty, C.; Bhattacharya, M.; Dhama, K. SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic. Vaccines 2023 , 11 , 682. [ Google Scholar ] [ CrossRef ]
- Khan, W.H.; Hashmi, Z.; Goel, A.; Ahmad, R.; Gupta, K.; Khan, N.; Alam, I.; Ahmed, F.; Ansari, M.A. COVID-19 pandemic and vaccines update on challenges and resolutions. Front. Cell. Infect. Microbiol. 2021 , 11 , 690621. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gong, W.; Parkkila, S.; Wu, X.; Aspatwar, A. SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies. Int. Rev. Immunol. 2023 , 42 , 393–414. [ Google Scholar ] [ CrossRef ]
- Signorelli, C.; De Ponti, E.; Mastrangelo, M.; Pennisi, F.; Cereda, D.; Corti, F.; Beretta, D.; Pelissero, G. The contribution of the private healthcare sector during the COVID-19 pandemic: The experience of the Lombardy Region in Northern Italy. Ann. Ig. 2024 , 36 , 250–255. [ Google Scholar ] [ CrossRef ]
- McCready, J.L.; Nichol, B.; Steen, M.; Unsworth, J.; Comparcini, D.; Tomietto, M. Understanding the barriers and facilitators of vaccine hesitancy towards the COVID-19 vaccine in healthcare workers and healthcare students worldwide: An Umbrella Review. PLoS ONE 2023 , 18 , e0280439. [ Google Scholar ] [ CrossRef ]
- Nair, L.; Adetayo, O.A. Cultural Competence and Ethnic Diversity in Healthcare. Plast. Reconstr. Surg. Glob. Open 2019 , 7 , e2219. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Anderson, L.M.; Scrimshaw, S.C.; Fullilove, M.T.; Fielding, J.E.; Normand, J.; Task Force on Community Preventive, S. Culturally competent healthcare systems. A systematic review. Am. J. Prev. Med. 2003 , 24 , 68–79. [ Google Scholar ] [ CrossRef ]
- Ashfield, S.; Donelle, L.; Uppal, G.; Bauer, M.A.; Kothari, A. Community organization perspectives on COVID-19 vaccine hesitancy and how they increased COVID-19 vaccine confidence: A Canadian Immunization Research Network, social sciences and humanities network study. Front. Public Health 2023 , 11 , 1258742. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dube, E.; Gagnon, D.; MacDonald, N.E.; Sage Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: Review of published reviews. Vaccine 2015 , 33 , 4191–4203. [ Google Scholar ] [ CrossRef ]
- Opel, D.J.; Mangione-Smith, R.; Robinson, J.D.; Heritage, J.; DeVere, V.; Salas, H.S.; Zhou, C.; Taylor, J.A. The Influence of Provider Communication Behaviors on Parental Vaccine Acceptance and Visit Experience. Am. J. Public Health 2015 , 105 , 1998–2004. [ Google Scholar ] [ CrossRef ]
- Kaholokula, J.K.; Ing, C.T.; Look, M.A.; Delafield, R.; Sinclair, K. Culturally responsive approaches to health promotion for Native Hawaiians and Pacific Islanders. Ann. Hum. Biol. 2018 , 45 , 249–263. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- AuYoung, M.; Rodriguez Espinosa, P.; Chen, W.T.; Juturu, P.; Young, M.T.; Casillas, A.; Adkins-Jackson, P.; Hopfer, S.; Kissam, E.; Alo, A.K.; et al. Addressing racial/ethnic inequities in vaccine hesitancy and uptake: Lessons learned from the California alliance against COVID-19. J. Behav. Med. 2023 , 46 , 153–166. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sacre, A.; Bambra, C.; Wildman, J.M.; Thomson, K.; Bennett, N.; Sowden, S.; Todd, A. Socioeconomic inequalities in vaccine uptake: A global umbrella review. PLoS ONE 2023 , 18 , e0294688. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Flores-Landeros, H.; Pells, C.; Campos-Martinez, M.S.; Fernandez-Bou, A.S.; Ortiz-Partida, J.P.; Medellín-Azuara, J. Community perspectives and environmental justice in California’s San Joaquin Valley. Environ. Justice 2022 , 15 , 337–345. [ Google Scholar ] [ CrossRef ]
- Zhang, X.; Tulloch, J.S.P.; Knott, S.; Allison, R.; Parvulescu, P.; Buchan, I.E.; Garcia-Finana, M.; Piroddi, R.; Green, M.A.; Baird, S.; et al. Evaluating the impact of using mobile vaccination units to increase COVID-19 vaccination uptake in Cheshire and Merseyside, UK: A synthetic control analysis. BMJ Open 2023 , 13 , e071852. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fonseca, E.M.D.; Shadlen, K.C.; Achcar, H.M. Vaccine technology transfer in a global health crisis: Actors, capabilities, and institutions. Res. Policy 2023 , 52 , 104739. [ Google Scholar ] [ CrossRef ]
- Emanuel, E.J.; Persad, G.; Kern, A.; Buchanan, A.; Fabre, C.; Halliday, D.; Heath, J.; Herzog, L.; Leland, R.J.; Lemango, E.T.; et al. An ethical framework for global vaccine allocation. Science 2020 , 369 , 1309–1312. [ Google Scholar ] [ CrossRef ]
- Asundi, A.; O’Leary, C.; Bhadelia, N. Global COVID-19 vaccine inequity: The scope, the impact, and the challenges. Cell Host Microbe 2021 , 29 , 1036–1039. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- The, L. Access to COVID-19 vaccines: Looking beyond COVAX. Lancet 2021 , 397 , 941. [ Google Scholar ] [ CrossRef ]
- Gozzi, N.; Chinazzi, M.; Dean, N.E.; Longini, I.M., Jr.; Halloran, M.E.; Perra, N.; Vespignani, A. Estimating the impact of COVID-19 vaccine inequities: A modeling study. Nat. Commun. 2023 , 14 , 3272. [ Google Scholar ] [ CrossRef ]
- Kaiser, N.; Barstow, C.K. Rural Transportation Infrastructure in Low- and Middle-Income Countries: A Review of Impacts, Implications, and Interventions. Sustainability 2022 , 14 , 2149. [ Google Scholar ] [ CrossRef ]
- Oladimeji, D.; Gupta, K.; Kose, N.A.; Gundogan, K.; Ge, L.; Liang, F. Smart Transportation: An Overview of Technologies and Applications. Sensors 2023 , 23 , 3880. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gianfredi, V.; Filia, A.; Rota, M.C.; Croci, R.; Bellini, L.; Odone, A.; Signorelli, C. Vaccine Procurement: A Conceptual Framework Based on Literature Review. Vaccines 2021 , 9 , 1434. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Norman, G.; Kletter, M.; Dumville, J. Interventions to increase vaccination in vulnerable groups: Rapid overview of reviews. BMC Public Health 2024 , 24 , 1479. [ Google Scholar ] [ CrossRef ]
- Peters, M.D. Addressing vaccine hesitancy and resistance for COVID-19 vaccines. Int. J. Nurs. Stud. 2022 , 131 , 104241. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jabbar, F.; Kadhim, K.A.; Alhilfi, R.A.; Chitheer, A.; Rahi, A.; Hipgrave, D.B. Intensification of integrated immunization services to recover routine vaccination coverage and bring COVID-19 vaccine to the population of Iraq in 2022. Vaccine 2024 , 42 , 2036–2043. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Krzysztofowicz, S.; Osinska-Skotak, K. The Use of GIS Technology to Optimize COVID-19 Vaccine Distribution: A Case Study of the City of Warsaw, Poland. Int. J. Env. Res. Public Health 2021 , 18 , 5636. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-quality health systems in the Sustainable Development Goals era: Time for a revolution. Lancet Glob. Health 2018 , 6 , e1196–e1252. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ghaemmaghamian, Z.; Zarghami, R.; Walker, G.; O’Reilly, E.; Ziaee, A. Stabilizing vaccines via drying: Quality by design considerations. Adv. Drug Deliv. Rev. 2022 , 187 , 114313. [ Google Scholar ] [ CrossRef ]
- Gianfredi, V.; Monarca, S.; Moretti, M.; Villarini, M. Health education, what is the role for pharmacist? Results from a cross sectional study in Umbria, Italy. Recent. Prog. Med. 2017 , 108 , 433–441. [ Google Scholar ] [ CrossRef ]
- Lieneck, C.; Heinemann, K.; Patel, J.; Huynh, H.; Leafblad, A.; Moreno, E.; Wingfield, C. Facilitators and Barriers of COVID-19 Vaccine Promotion on Social Media in the United States: A Systematic Review. Healthcare 2022 , 10 , 321. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khoury, G.; Ward, J.K.; Mancini, J.; Gagneux-Brunon, A.; Luong Nguyen, L.B. Health Literacy and Health Care System Confidence as Determinants of Attitudes to Vaccines in France: Representative Cross-Sectional Study. JMIR Public Health Surveill. 2024 , 10 , e45837. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Pennisi, F.; Genovese, C.; Gianfredi, V. Lessons from the COVID-19 Pandemic: Promoting Vaccination and Public Health Resilience, a Narrative Review. Vaccines 2024 , 12 , 891. https://doi.org/10.3390/vaccines12080891
Pennisi F, Genovese C, Gianfredi V. Lessons from the COVID-19 Pandemic: Promoting Vaccination and Public Health Resilience, a Narrative Review. Vaccines . 2024; 12(8):891. https://doi.org/10.3390/vaccines12080891
Pennisi, Flavia, Cristina Genovese, and Vincenza Gianfredi. 2024. "Lessons from the COVID-19 Pandemic: Promoting Vaccination and Public Health Resilience, a Narrative Review" Vaccines 12, no. 8: 891. https://doi.org/10.3390/vaccines12080891
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
COVID-19 vaccine: A 2021 analysis of perceptions on vaccine safety and promise in a U.S. sample
Roles Conceptualization, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Global Health, Indiana University School of Medicine, Indianapolis, Indiana, United States of America
Roles Investigation, Methodology, Project administration, Validation, Visualization, Writing – review & editing
Affiliation Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia, United States of America
Roles Investigation, Methodology, Validation, Visualization, Writing – review & editing
Affiliation Department of Psychiatry, Harvard Medical School, Boston, Massachusetts, United States of America
Roles Data curation, Formal analysis, Software, Visualization, Writing – review & editing
Affiliation Department of Biostatistics and Health Data Science, Indiana University School of Medicine, Indianapolis, Indiana, United States of America
Roles Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing
Affiliation Department of Global Health, Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, Indiana, United States of America
- Vitalis C. Osuji,
- Eric M. Galante,
- David Mischoulon,
- James E. Slaven,
- Gerardo Maupome

- Published: May 19, 2022
- https://doi.org/10.1371/journal.pone.0268784
- Reader Comments
Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine.
Study participants in the U.S. (79% female, median age group 46–60 years) were recruited through an online Qualtrics survey distributed as a Facebook advertisement from 3/19/21–4/30/21. We assumed that every participant is at risk of COVID-19 infection and should be able to get the vaccine with proper access. Bivariate and multivariable models were performed. Collinearity between variables was assessed.
A total of 2,626 responses were generated and 2,259 were included in data analysis. According to our multivariate model analysis, vaccines were perceived as safe by those who had or planned to obtain full vaccination (adjusted odds ratio (aOR) (95% confidence interval) = 40.0 (19.0, 84.2); p< 0.0001) and those who indicated trust in science (aOR = 10.5 (5.1, 21.8); p< 0.0001); vaccines were perceived as not safe by those who self-identified as Republicans vs. self-identified Democrats (aOR = 0.2 (0.1, 0.5); p = 0.0020) and those with high school or lower education (aOR = 0.2 (0.1, 0.4); p = 0.0007). Similarly, according to our multivariate model analysis, the following groups were most likely to reject vaccination based on belief in vaccinations: those with lower income (aOR = 0.8 (0.6, 0.9); p = 0.0106), those who do not know anyone who had been vaccinated (aOR = 0.1 (0.1, 0.4); p< 0.0001), those who are unwilling to get vaccinated even if family and friends had done so (aOR = 0.1 (<0.1, 0.2); p< 0.0001), those who did not trust science (aOR < 0.1 (<0.1, 0.1); p< 0.0001), those who believe that vaccination was unnecessary if others had already been vaccinated (aOR = 2.8 (1.5, 5.1); p = 0.0007), and those who indicate refusal to vaccinate to help others (aOR = 0.1 (0.1, 0.2); p< 0.0001). An alpha of p<0.05 was used for all tests.
Level of education and partisanship, but not race/ethnicity, were the most likely factors associated with vaccine hesitancy or likelihood to vaccinate. Also, low vaccination rates among underrepresented minorities may be due to distrust for healthcare industries. Population sub-groups less likely to be vaccinated and/or receptive to vaccines should be targeted for vaccine education and incentives.
Citation: Osuji VC, Galante EM, Mischoulon D, Slaven JE, Maupome G (2022) COVID-19 vaccine: A 2021 analysis of perceptions on vaccine safety and promise in a U.S. sample. PLoS ONE 17(5): e0268784. https://doi.org/10.1371/journal.pone.0268784
Editor: Weijing He, University of Texas Health Science Center at San Antonio, UNITED STATES
Received: July 26, 2021; Accepted: May 8, 2022; Published: May 19, 2022
Copyright: © 2022 Osuji et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
In early 2020, the SARS-CoV-2 (COVID-19) pandemic unmasked the many flaws that healthcare systems faced worldwide. While some of these issues were difficult to predict, such as the feasibility of pandemic response protocols or federal government regulations to be activated [ 1 ], other healthcare issues were to be expected, especially in the United States. For example, disparities in healthcare treatment and outcomes derived from different socioeconomic factors. Studies published in 2020 showed that the pandemic had much higher infection rates in minority populations such as Black and Hispanic/Latinx compared to their white counterparts; American Indians/ Alaska Natives (AI/ ANs), Black and Hispanic/Latinx communities also experienced significantly higher mortality rates [ 2 , 3 ]. The Centers for Disease Control and Prevention (CDC) released information relating social determinants of health to poorer COVID-19 outcomes, stating that “factors such as discrimination, neighborhood and physical environment, housing, occupation, education, income, and wealth gaps put some racial and ethnic minority groups at increased risk of severe illness from COVID-19, including death” [ 4 ]. Many factors play a role in disparities relevant to the COVID-19 pandemic. These include limited access to health services, education, and transportation, which tend to affect more severely communities of color and people of low socioeconomic status [ 5 ].
Just under one year after the first identification of COVID-19 in China [ 6 , 7 ], the PfizerBioNTech and Moderna COVID-19 vaccines were approved by the US Food and Drug Administration (FDA) under Emergency Use Authorization [ 8 , 9 ]. Ultimately, the Pfizer vaccine was fully approved as of August 23 rd , 2021. These vaccines represented a major milestone in vaccine production history, as no other vaccine had ever been created so rapidly with such positive results [ 10 ]. Although mistrust of vaccines is not uncommon in American culture, hesitation regarding the COVID-19 vaccines may be among the strongest yet [ 11 ]. Despite substantial evidence-based research supporting the vaccines’ safety and efficacy, there are lay public concerns regarding the vaccine rollout. For instance, an analysis [ 12 ] from March 2021 in individuals getting vaccines showed that white Americans were receiving vaccinations at a rate two times that of Black Americans, and the gap for Hispanic/Latinx was even larger. The rationale behind these gaps between racial/ethnic groups remains uncertain and highlights the importance of characterizing the factors and mechanisms underlying potential associations amongst demographic and socioeconomic groups.
With the current vaccines showing 95% efficacy, the estimated percentage of Americans needing vaccination to reach herd immunity ranges from 60 to 72% [ 13 ]. However, according to a November 2020 survey [ 14 ], 40% of Americans said that they will “definitely not” or “probably not” get the COVID-19 vaccine when it becomes available to them. Therefore, more needs to be done to bolster interest and trust in the vaccines. While companies and governmental organizations attempt to convey the necessary strategies to ease vaccine uncertainty and hesitation, a large segment of the lay public remains skeptical. As of May 2021, there were state-level COVID-19 vaccine incentives developed to increase vaccination rates across the United States. Irrespective of these incentives, only 48.6% of the US population was fully vaccinated as of July 2021, while 56% had received at least one dose [ 15 ]. Given these data, reasons surrounding vaccination hesitancy needed to be further explored. We aimed to expand current knowledge about COVID-19 vaccine perceptions through a characterization of sociocultural, socioeconomic, and demographic features in the context of opinions about receiving a COVID-19 vaccine. The objectives of the present survey were to establish:
- What segments of the population believe the COVID-19 vaccines to be safe?
- What are the perceived barriers to obtaining the COVID-19 vaccine—for self and others?
- Is there an association between individual sociocultural characteristics and either acceptance or rejection of the vaccine?
- Is there an association between individual demographic characteristics and either acceptance or rejection of the vaccine?
Materials and methods
This research project was granted IRB approval by Indiana University (protocol #10670).
Data collection was done using an online survey distributed to the general public, and our methodology followed criteria from the CHERRIES checklist [ 16 ]. The survey was created using Qualtrics and piloted with 15 respondents. Based on responses and feedback from our iterative process to pilot the survey, questions were added, rephrased, or deleted. The final survey had 37 questions, with 1–6 questions per page. Question format included 28 multiple choices, with the remainder as yes/no questions. Both English and Spanish versions of the survey were available. A description of the ethical approval, anonymity, and data utilization was provided and acknowledged at the beginning of the survey. Personal information was not required, and participants were offered the option to enter an email address if they wished to participate in an optional raffle draw for five $20 Walmart gift cards. All data were stored in a secure password protected website, to which only study investigators had access. A completeness check prior to submission was not implemented, but a forced response feature on Qualtrics was used for all questions except those involving zip code and email address, to ensure that no significant questions were left unanswered. A link to the final version of the survey was posted to a Facebook page created for the study, and Facebook advertisements were used to promote the study. The survey was made available on March 19 th , 2021 and was closed on April 30 th , 2021. The final data collection survey is available as an attachment ( S1 File ).
This was a survey open to every Facebook user in the United States, based on the assumption that every adult was at risk of COVID-19 infection and should theoretically be able to get the vaccine. We limited responses to people stating they were at least 18-years old and able to read, understand, and agree to the terms of the online survey. Bivariate associations were evaluated using Mantel-Haenszel chi-square tests for questions where one or both variables had ordered categorical responses, and Pearson chi-square tests if both variables had nominal categories. Multivariable models were also performed, using an a priori p-value cut point of 0.20 for inclusion in the model. Collinearity between variables was assessed, leading to the exclusion of several variables from each multivariable model, retaining those based on statistical analysis and the team’s clinical experience. For ease of analysis, race was grouped into 2 categories: white and underrepresented minority. Low income was categorized based on respondents who indicated making less than $40,000 in annual income. The final level of significance for these multivariable models was set at p < 0.05.
All analytic assumptions were verified, and the analyses were performed using SAS/STAT software ® v9.4 [ 17 ].
A total of 2,626 responses were obtained. Based on a total of 3,743 potential participants who clicked our survey link on Facebook, our completion rate was 70.2%. Following data cleaning and exclusion of incomplete responses, a total of 2,259 responses were evaluable.
As outlined in Table 1 , most participants were under 60 years of age (61.5%; median age in the 46–60 years group), female (79.2%) and white (89.6%). Most had never been employed in the healthcare field (63.4%), some were employed full time (44.5%), many had at least some college education (93.1%), about half were affiliated with the Democratic party (54.7%), and many lived within family households (75.7%).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0268784.t001
To determine what groups perceived the vaccine as safe, bivariate and multivariable models were created. Table 2 shows that subjects who perceived the vaccination as being safe were more likely to have already obtained their second dose or planned on getting it (we allowed for single shot vaccines in our analyses) (97% vs. 12%; p< 0.0001), did not have a prior health condition (98% vs. 86%; p< 0.0001), trusted science (97.1% vs. 21%; p< 0.0001)/vaccines (97% vs. 17%; p< 0.0001)/doctors (97% vs. 21%; p< 0.0001), believed in the effectiveness of hand washing (94% vs. 88%; p = 0.0056)/social distancing (96% vs. 59%; p< 0.0001)/wearing a mask (95% vs. 43%; p< 0.0001), were female (88% vs. 66%; p = 0.0005), were white (90% vs. 82%; p = 0.0063), had higher levels of education (94% vs. 79%; p< 0.0001), and identified as Democrats (58% vs. 7%; p< 0.0001). In the multivariate model, subjects who were still independently associated with the perception of the vaccines being safe were those more likely to have received their second dose (or planned on it) (p< 0.0001), who trusted science (p< 0.0001), had higher levels of education (p = 0.0007), or were Democrats (p = 0.0020).
https://doi.org/10.1371/journal.pone.0268784.t002
To determine what groups were likely to perceive the most barriers to vaccination, bivariate and multivariable models were created ( Table 3 ). By analyzing subjects who were actively seeking vaccination versus those who were not, we found the former were more likely to have had their second dose (or were likely to get it) (92% vs. 20%; p< 0.0001), did not have a prior health condition (94% vs. 85%; p = 0.0283), trusted science (96% vs. 34%; p< 0.0001)/vaccines (95% vs. 31%; p< 0.0001)/doctors (93% vs. 35%; p< 0.0001), believed in the effectiveness of social distancing (91% vs. 68%; p< 0.0001)/wearing a mask (97% vs. 52%; p< 0.0001), were younger (p< 0.0001), were not male (72% vs. 68%; p = 0.0326), were an under-represented minority (40% vs. 23%; p = 0.0043), had a higher median income ($56,000 vs. $49,000; p = 0.0053), or were Democrats (48% vs. 12%; p< 0.0001). In the multivariate model, subjects that were still independently associated with actively seeking a vaccination were those with their second dose already received (or planned on it) (p< 0.0001) and who trusted in science (p = 0.0006).
https://doi.org/10.1371/journal.pone.0268784.t003
Data for the final two objectives were aggregated and analyzed together ( Table 4 ). For those who “do not believe in vaccines”, the variables more likely associated with such outcome included not having a high-risk medical condition (42% vs. 53%; p = 0.0111), not knowing someone who is vaccinated (87% vs. 98%; p< 0.0001), not trusting vaccines (21% vs. 97%; p< 0.0001)/science (26% vs. 97%; p< 0.0001)/doctors (28% vs. 97%; p< 0.0001), not believing in the effectiveness of hand washing (90% vs. 94%; p = 0.0410)/ social distancing (65% vs. 96%; p< 0.0001)/wearing a mask (51% vs. 94%; p< 0.0001), not receiving an annual flu shot (21% vs. 83%; p< 0.0001), thinking there is no need if others have been vaccinated (58% vs. 8%; p< 0.0001), and not wanting to get vaccinated to help others (27% vs. 96%; p< 0.0001). In the multivariate model, subjects that were still independently associated with not believing in vaccines did not know someone who was vaccinated (p< 0.0001), did not trust science (p< 0.0001), believed vaccination is unnecessary if others were vaccinated (p = 0.0007), and would not get vaccinated to help others (p< 0.0001).
https://doi.org/10.1371/journal.pone.0268784.t004
Additionally, the variables associated with subjects who “do not believe in vaccines” included not getting vaccinated even if friends and family had been vaccinated (26% vs. 89%; p< 0.0001), being male (30% vs. 19%; p = 0.0053), being an underrepresented minority (25% vs. 9%; p< 0.0001), not being employed full time (65% vs. 55%; p = 0.0260), having a lower median income ($ 49, 000 vs. $51, 000; p = 0.0020), having lower levels of educational attainment (21% vs. 6%; p< 0.0001), and not being a Democrat (89% vs. 43%; p< 0.0001). In the multivariate model, subjects who were still independently associated with not believing in vaccines were those not getting vaccinated even if friends and family had done so (p< 0.0001), and having a lower median income (p = 0.0106).
Our study is not the first to examine the relationship between various demographics and vaccine hesitancy. Kini and colleagues explored 39 studies regarding demographics of vaccine acceptance and hesitation. Their systematic review suggests that vaccine acceptance increases with age and is higher for males and white individuals [ 18 ]. While our study reports different significant findings (see below), this is likely attributed to the context and sample of the studies, along with possible confounding variables as discussed later. Our results pertain to the time when data were collected: given the long and haphazard evolution of the pandemic and associated perceptions, the relevance of our results must be contextualized to the time and the stage of the pandemic. Our data show some disparities in perception and opinions regarding the COVID-19 vaccines based on the following key variables: age, race, income, educational level, underlying health conditions, and political partisanship. Participants who had received the first of two doses of the COVID-19 vaccine at the time of our study may already have been convinced of the safety of the vaccines. Additionally, during the early stages of vaccine promotion, there was emphasis from the CDC on possible worsening of underlying pulmonary, cardiac, and other health conditions, such as chronic obstructive pulmonary disease, heart failure, and asthma [ 19 ]. This could explain why individuals with underlying health conditions were likely to regard the vaccines as protective and safe.
Our results also showed that those who identifies as white, compared to members of underrepresented minorities, were more likely to consider the vaccine as safe. Based on an assumption of a positive correlation between perceiving the vaccine as safe and actually getting the vaccine, the CDC has shown that as of July 4 th , 2021, of those who had received at least one dose of the vaccine, 59% were white, 9% were black, 16% were Hispanic/Latinx, and 6% were Asian Americans [ 20 ]. However, it is unclear whether such disparity is affected by the communities in which vaccines are most readily available, or if such disparity in fact represents an individual decision due to distrust that might exist between underrepresented minorities and the healthcare industry. As such, it is vital to review past literature as it pertains to recent findings during the pandemic. Regarding vaccine hesitancy of underrepresented minorities, there has been clear evidence of disparities in healthcare treatment for Black and white patients. Davidio et al reviewed multiple papers that describe physician perceptions and treatment of Black vs. white patients with clear significance regarding the negative handling of Black patients [ 21 ]. Armstrong et al point out that experience of discrimination was strongly associated with healthcare system distrust (HCSD) in their study comparing African American and white survey respondents [ 22 ]. Additionally, Balasuriya et al explored factors associated with COVID-19 acceptance and access among Black and Latinx communities, and identified the pervasive mistreatment of Black and Latinx communities, rooted in structural racism, to be a key influence on vaccine acceptance [ 23 ]. Results such as this provide a strong basis to argue why underrepresented minorities may have been less eager to seek out vaccinations. Regarding vaccine hesitancy and political affiliation, other studies corroborate these results. In one study, it was found that US Republican counties consistently had lower general vaccination rates than Democratic counties [ 24 ]. In a polling done by Kaiser Family Foundation in May 2020, it was found that Republicans were less likely to report wearing masks, social distancing or getting vaccinated against COVID-19 [ 25 ].
Level of education has a strong effect on willingness to receive a COVID-19 vaccine: having a college degree has been associated with a 43% increase in likelihood of getting the vaccine [ 26 ]. Assuming the likelihood of obtaining the COVID-19 vaccine is positively correlated with perception that the vaccine is safe, it is worthwhile posing the question whether level of education outweighs other effects of race, gender, political affiliation, and underlying health conditions. Delay in COVID-19 vaccination notwithstanding (earlier in 2021 when our data were collected), the CDC has pointed to a divide in communities based on political party affiliation. To ultimately determine the prime factors in safety perception, we conducted a multivariable analysis and found that the following groups were most likely to perceive vaccines as being safe: 99.3% Democrats (vs. 86.0% Republicans, specifically) and 93.1% with higher educational attainment (vs. 6.8% with high school level specifically). It is important to correlate these results with previous studies that examined similar topics. Regarding results about education impacting vaccine rates, previous studies would support this. Suryadevara and colleagues collaborated with their county health department to educate high-risk, resource-poor families regarding vaccination concerns. Their results showed a drastic increase for general vaccine completion and annual influenza vaccine rates [ 27 ]. Another study showed that when providing low-literacy educational materials to resource-poor families regarding the pneumococcal vaccine, the test group was four times more likely to discuss the vaccination in appointments and five times more likely to receive the vaccine than control group [ 28 ]. Even more recent studies with COVID-19 support our findings. For instance, a recent study indicated that lack of high school education positively correlates with increased vaccine hesitancy and decreased vaccination levels [ 29 ].
Our multivariable model outcome also suggests that race and ethnicity are not necessarily the primary determinants of vaccine hesitancy and likelihood of vaccination, because low vaccination rates among underrepresented populations may be explained by the historical distrust within some members of underrepresented minorities toward health care organizations and providers, as well as suspicion about clinical research studies, in view of past atrocities such as the Tuskegee Syphilis experiment [ 30 ], or similar experiments with STD infections in Guatemala [ 31 ]. Our multivariable results support this possibility by indicating those being potential factors in rejecting the COVID-19 vaccine. Specifically, after adjusting for variables, one of the groups found to be independently associated and most likely to reject vaccination according to socioeconomic and demographic factors were individuals with lower income. Considering that low-income populations usually consist of groups that identify as underrepresented minorities [ 32 ], slow rates of vaccination in these groups might reflect individual distrust of health care providers. However, this finding does not rule out the possibility of low distributions in low-income locations (e.g., rural), which could be a barrier by itself for vaccination opportunities. As pointed out by DeMaria-Ghalili and colleagues, “health inequalities are most acute among those living in rural and low resourced areas of the state, and among underrepresented populations (particularly Black/African American and Latino), who lack access to health care, experience digital divide, and face persistent local healthcare workforce shortages.” The report further discusses that people in areas of lower socio-economic status or fewer resources (usually rural areas) have a more difficult time scheduling and going to appointments for vaccinations, noting “pharmacy deserts” to be an issue in having access to appropriate healthcare resources such as vaccines [ 33 ]. Economic precarity and poor technological advancements may be obstacles to both registering for and getting the vaccine, possibly associated with sparse information among low-income populations [ 34 ]. Therefore, to bolster vaccination, efforts should be made to target groups who are most likely to encounter barriers to COVID-19 vaccination, through governmental incentives, including free childcare and rides to vaccination sites, lottery tickets or cash vouchers, complimentary food and drinks at the vaccination sites, and tax credit [ 35 ], rather than privately offered incentives that may vary greatly throughout the country.
Our successful recruitment for this survey was helped by the ever-increasing prevalence of social media in peoples’ lives. This highlights the need for proper, scientific-based information regarding the pandemic to reach the lay public before opinions appear on social media newsfeeds. On the other hand, only 2.1% of our sample thought that social media sites were reliable sources for vaccine information. While this would appear to suggest limited influence of social media with regard to COVID vaccines, we have to interpret this with caution in view of a small, self-selected sample that may not reflect the U.S. population as a whole. While some individuals may have legitimate reasons for declining vaccination, e.g. allergies to some ingredients in the preparation or other medical contraindications, misperceptions about vaccines as presented by some members of the media can lead to vaccine refusal for inappropriate reasons [ 36 ]. Therefore, it is important to disseminate the scientific basis for vaccines whenever possible. Negative press about variant viruses and the possibility of ineffective vaccines lead to further public distrust of the otherwise monumental feat of creating and distributing the COVID-19 vaccines [ 37 ]. Education of the public is essential for the continued success of vaccination efforts in general. As an example, in one study [ 38 ], Human Papilloma Virus (HPV) vaccine education sessions were held for parents, healthcare and school staff who had little knowledge regarding HPV vaccines. After the sessions, results showed that over 90% of respondents felt vaccine education was important and 85–97% were supportive of school-based vaccine clinics. In another study on flu vaccination during pregnancy [ 39 ], pregnant women refused flu vaccines due to likely susceptibility to influenza and concerns for vaccine safety. The study intervention was a brief educational video by the CDC, which addressed vaccination health beliefs in a clear and easy to understand format. The primary outcome was receipt of the flu vaccine on the next prenatal visit, and suggested that appropriate education and reassurance were influential in vaccination. We must do the same for the COVID-19 vaccine, seeing that our findings suggest that educational attainment is one of the two most important factors that determine the likelihood that one will perceive the vaccine as safe and be likely to accept vaccination. Given that an overwhelming majority of our respondents indicated that they considered doctors, nurses, and other healthcare workers as reliable sources of vaccination information, it is imperative to begin incorporating COVID-19 vaccine questions and education during health care visits. Moreover, training healthcare professionals in cultural competency, defined as “the ability of individuals and systems to work or respond effectively across cultures in a way that acknowledges and respects the culture of the person or organization being served” [ 40 ] would help them navigate this conversation with knowledge and transparency to promote mutual trust and possibly increased likelihood of vaccination [ 41 , 42 ]. Unfortunately, cultural competency training is still limited in medical schools and residency programs [ 43 ], and broader implementation is needed. This will be critical for engaging minority/underrepresented groups, though we acknowledge that these groups may have general difficulties accessing any medical care and this in turn may contribute to lower vaccination rates. Some respondents chose “no access” as a reason for not receiving the vaccine. The term “no access” is admittedly broad and could have included decreased vaccination distribution to impoverished neighborhoods, or it could mean that individuals do not know where to go to get their vaccine. We kept our questionnaire concise so as not to overburden respondents, and consequently could not necessarily qualify the specific reasons for perception about no or limited access. Further investigation is needed to characterize the specific obstacles experienced by people seeking the vaccination. As health literacy regarding the still relatively new COVID-19 pandemic remains a challenge [ 44 ], our present survey can hopefully act as a compass to inform providers on the underlying rationale that their patients have for being skeptical about vaccines or medical advice.
In addition, we need steps to encourage the population to get vaccinated irrespective of political affiliation. Per our findings, those who identify as Democrats are more likely to perceive the vaccine as being safe. Partisanship and vaccination status continue to play a role in both U.S. vaccination efforts and the government’s response to the pandemic in general. Other studies have shown similar results [ 45 ], where 65% of Democrats and 51% of vaccinated adults say that the surge in COVID cases makes them angry at people who have not gotten a vaccine, while 59% of Republicans and 56% of unvaccinated adults say that the federal government should be blamed. Our study shows that Republicans less likely to become vaccinated trust information that comes directly from their health care team, more than information that originates from the government. Therefore, ensuring that all personnel on the health care team are culturally competent to facilitate conversations brought on by patients regarding the COVID-19 vaccine will be instrumental in ensuring vaccination acceptance across spectra. Finally, incentives must be focused on core groups that we believe are more likely to reject the vaccine. These include underrepresented minorities, people with lower educational level, those who identify as young, males, and those with high risk underlying medical conditions.
Our study has limitations, especially regarding data collection. Given the current pandemic and difficulty with in-person survey distribution, it was decided that an online distribution would be preferable, based on the assumption that every individual is at risk of contracting the virus and becoming affected by the pandemic. We used Facebook due to its wide reach. However, we recognize that not everyone has access to computers or Facebook, so this survey may favor those of higher socioeconomic status. Likewise, we did not seek parity since the sample was largely one of convenience, based on who responded to the questionnaire. Although forced responses were used for our survey to ensure completion and prevent answers, we could not determine other potential factors that may have caused incomplete responses in cases where respondents were allowed to select up to three options, e.g. for trusted sources of information. Obstacles to completion might have included feeling pressed for time, concerns about privacy in view of the open nature of social media, or rejection based on personally held political views. This could result in a self-selection bias due to differences between respondents and non-respondents, therefore skewing the findings. For example, many participants were white, female, and/or Democrat voters, which is not representative of the U.S. population per se and could bias the results in favor of opting for vaccination, perhaps due to stronger belief in vaccines. Obviously, given the enormous number of Facebook users in the U.S., and the fact that users are allowed to protect their privacy by restricting access to personal data (including by omitting it in their profiles), it would be difficult to assess the “typical” Facebook user in the context of these factors. Along those lines, about 87% of respondents were already vaccinated, which suggests that most considered the benefits greater than the risks. This may therefore result in under-reporting and under-characterizing negative views of the vaccine that we sought to capture in the survey. Another limitation of this study is that it only represents a snapshot in time of opinions of COVID-19 vaccine perceptions, which can be fluid. Because the vaccine data are rapidly changing and information provided to the public may evolve as days progress, our results can only be applicable to this specific point in time. Ideally, the present study should be repeated in the future to ascertain trends over time. From a methodological standpoint, future studies should focus on obtaining a wider and more diverse set of respondents, including individuals that do not have access to computers or Facebook. One feasible alternative could be the distribution of both online and paper surveys to the same group of respondents during the same wave of data collection, thus allowing for estimation of changes across strategies for survey contact.
While our findings are in line with some existing perspectives in the field, as they relate to the role of socioeconomic factors [ 26 , 32 ], educational influence [ 38 , 39 ], and partisanship [ 45 ], we have contributed a more robust and elaborate perception of the U.S population on COVID vaccines, while identifying specific groups at risk for not getting a vaccine. In conclusion, level of education and partisanship, but not race/ethnicity, were the most likely factors associated with vaccine hesitancy or likelihood to vaccinate. This suggests that improved education, not just about vaccines per se, but with regard to formal schooling in general, may be at the heart of promoting greater acceptability of vaccination. Likewise, low vaccination rates among underrepresented minorities may be due to distrust for healthcare industries, but further research is needed to fully characterize the relative contributions of low access vs. distrust. Many white people and many with a Republican party affiliation also expressed reluctance about vaccination, suggesting that mistrust of the healthcare industry, vaccinations in general, and/or the government is not limited to minorities and/or economically challenged populations. Regardless, population sub-groups less likely to be vaccinated and/or receptive to vaccines should be targeted for vaccine education and incentives, and outcomes of these interventions need to be closely studied for determination of efficacy.
Supporting information
S1 file. qualtrics survey questionnaire..
https://doi.org/10.1371/journal.pone.0268784.s001
S1 Data. Inclusion criteria.
https://doi.org/10.1371/journal.pone.0268784.s002
Acknowledgments
The authors would like to thank our collaborators at Qualtrics and Facebook for helping facilitate the successful completion of this study.
- View Article
- PubMed/NCBI
- Google Scholar
- 4. Centers for Disease Control and Prevention (CDC): COVID-19 Racial and Ethnic Health Disparities . https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnic-disparities/index.html . Accessed on 10 th , Dec 2020.
- 5. Johnson, Akilah: Lack of Health Services and Transportation Impede Access to Vaccine in Communities of Color . https://www.washingtonpost.com/health/2021/02/13/covid-racial-ethnic-disparities/ . Accessed on 13 th , Feb 2021.
- 8. U.S Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine?s_cid=11700:covid%20vaccine%20fda%20approval:sem.ga:p:RG:GM:gen:PTN:FY22 Accessed on 11th, December, 2020.
- 9. U.S Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid Accessed on 18 th , December 2020
- 12. The New York Times: Pandemics Racial Disparities Persist in Vaccine Rollout . https://www.nytimes.com/interactive/2021/03/05/us/vaccine-racial-disparities.html?campaign_id=37&emc=edit_rr_20210306&instance_id=27792&nl=race%2Frelated®i_id=76131559&segment_id=52913&te=1&user_id=4fc6fb065b9e912a39a1a6408b1e81c2 . Accessed on 5 th , March, 2021.
- 14. Pew Research Center. https://www.pewresearch.org/science/2020/12/03/intent-toget-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-processincreases Accessed on 3 rd , December, 2020.
- 15. Mayo Clinic: US COVID-19 Vaccine Tracker. https://www.mayoclinic.org/coronavirus-covid-19/vaccine-tracker Accessed on: 17 th , July 2021.
- 17. All result output were created using SAS. Copyright © 2021 SAS Institute Inc., Cary, NC, USA.
- 19. Centers for Disease Control: Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19 . https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Accessed on: 18 th , October 2021.
- 20. Centers for Disease Control: Latest Data on COVID-19 vaccination by Race/ Ethnicity . https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/ Accessed on: 18 th , October 2021.
- 25. Hamel, L., Kearney, A., Kirzinger, A., Lopes, L., Munana, C., Brodie, M. KFF Health Tracking Poll — May 2020 . Kaiser Family Foundation . https://www.kff.org/coronavirus-covid-19/report/kff-health-tracking-poll-may-2020/ Accessed on 19 th , January 2022.
- 26. Thomas, K., Darling, J. Education is now a Bigger Factor than Race in Desire for COVID-19 Vaccine . https://healthpolicy.usc.edu/evidence-base/education-is-now-a-bigger-factor-than-race-in-desire-for-covid-19-vaccine/ Accessed on 17 th , July 2021.
- 30. Ada McVean. McGill. 40 Years of Human Experimentation in America : The Tuskegee Study . https://www.mcgill.ca/oss/article/history/40-years-human-experimentation-america-tuskegee-study Accessed on 19th January, 2022.
- 31. Amy Gutmann. Presidential Commission for the Study of Bioethical Issues. “Ethically Impossible” STD Research in Guatemala from 1946 to 1948. https://bioethicsarchive.georgetown.edu/pcsbi/node/654.html Accessed on 19 th January, 2022.
- 32. Simms, M. The Urban Institute. Racial and Ethnic Disparities among low-income Families . https://www.urban.org/sites/default/files/publication/32976/411936-racial-and-ethnic-disparities-among-low-income-families.pdf Accessed on 18th October, 2021
- 33. DiMaria-Ghalili, R., Foreshaw Rouse, A., Coates, M., Hathaway, Z., Hirsch, J., Wetzel, S., et al. Disrupting Disparities in Pennsylvania: Retooling for Geographic, Racial and Ethnic Growth [White paper]. https://aarp-states.brightspotcdn.com/6f/b6/de161f3a4a63a23e811693d90b68/aarp-drexel-pennsylvania-disrupting-disparities-design-0421-final.pdf Accessed on 19th January, 2022.
- 35. Devon Delfino. Incentives for CoVID-19 Vaccination : Food , Cash , & Other Perks . https://www.goodrx.com/health-topic/vaccines/covid-19-vaccination-incentives Accessed on 20th, January, 2022.
- 37. The Atlantic. 5 Pandemic Mistakes We Keep Repeating . https://www.theatlantic.com/ideas/archive/2021/02/how-public-health-messaging-backfired/618147/ Accessed on 26 th , February 2021.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 31 July 2024
Cohort study of cardiovascular safety of different COVID-19 vaccination doses among 46 million adults in England
- Samantha Ip ORCID: orcid.org/0000-0001-9162-6727 1 , 2 , 3 na1 ,
- Teri-Louise North 4 na1 ,
- Fatemeh Torabi ORCID: orcid.org/0000-0002-5853-4625 5 ,
- Yangfan Li 1 , 2 , 3 ,
- Hoda Abbasizanjani ORCID: orcid.org/0000-0002-9575-4758 5 ,
- Ashley Akbari ORCID: orcid.org/0000-0003-0814-0801 5 ,
- Elsie Horne 4 ,
- Rachel Denholm ORCID: orcid.org/0000-0002-8067-5440 4 , 6 , 7 ,
- Spencer Keene 1 , 3 ,
- Spiros Denaxas 8 , 9 , 10 , 11 , 12 ,
- Amitava Banerjee ORCID: orcid.org/0000-0001-8741-3411 9 ,
- Kamlesh Khunti 13 ,
- Cathie Sudlow ORCID: orcid.org/0000-0002-7725-7520 12 ,
- William N. Whiteley ORCID: orcid.org/0000-0002-4816-8991 12 , 14 ,
- Jonathan A. C. Sterne ORCID: orcid.org/0000-0001-8496-6053 4 , 6 , 7 na2 ,
- Angela M. Wood ORCID: orcid.org/0000-0002-7937-304X 1 , 3 , 12 , 15 , 16 , 17 , 18 na2 ,
- Venexia Walker ORCID: orcid.org/0000-0001-5064-446X 4 , 19 , 20 na2 ,
- the CVD-COVID-UK/COVID-IMPACT Consortium &
the Longitudinal Health and Wellbeing COVID-19 National Core Study
Nature Communications volume 15 , Article number: 6085 ( 2024 ) Cite this article
45k Accesses
1 Citations
2934 Altmetric
Metrics details
- Cardiovascular diseases
- Epidemiology
- Health policy
The first dose of COVID-19 vaccines led to an overall reduction in cardiovascular events, and in rare cases, cardiovascular complications. There is less information about the effect of second and booster doses on cardiovascular diseases. Using longitudinal health records from 45.7 million adults in England between December 2020 and January 2022, our study compared the incidence of thrombotic and cardiovascular complications up to 26 weeks after first, second and booster doses of brands and combinations of COVID-19 vaccines used during the UK vaccination program with the incidence before or without the corresponding vaccination. The incidence of common arterial thrombotic events (mainly acute myocardial infarction and ischaemic stroke) was generally lower after each vaccine dose, brand and combination. Similarly, the incidence of common venous thrombotic events, (mainly pulmonary embolism and lower limb deep venous thrombosis) was lower after vaccination. There was a higher incidence of previously reported rare harms after vaccination: vaccine-induced thrombotic thrombocytopenia after first ChAdOx1 vaccination, and myocarditis and pericarditis after first, second and transiently after booster mRNA vaccination (BNT-162b2 and mRNA-1273). These findings support the wide uptake of future COVID-19 vaccination programs.
Similar content being viewed by others

COVID-19 vaccination and major cardiovascular and haematological adverse events in Abu Dhabi: retrospective cohort study

Impact of vaccination on the association of COVID-19 with cardiovascular diseases: An OpenSAFELY cohort study
Effectiveness of inactivated COVID-19 vaccines among older adults in Shanghai: retrospective cohort study
Introduction.
SARS-CoV-2 vaccination prevented 14.4 million deaths from COVID-19 worldwide in the first year of the pandemic 1 . In England, which entered its third COVID-19 vaccine season in autumn 2023 2 , around 90% of people aged ≥12 years have been vaccinated at least once 3 . COVID-19 vaccines are associated with rare cardiovascular complications: mRNA-based brands (e.g. BNT-162b2, mRNA1273) with myocarditis and adenovirus-based brands (e.g. ChAdOx1) with vaccine-induced thrombotic thrombocytopenia (VITT), leading to intracranial venous thrombosis (ICVT) and thrombocytopenia 4 , 5 . It is important to understand the risk of thrombotic and cardiovascular complications arising from second and subsequent doses, within the general population and subpopulations 6 .
In this work, we use whole population longitudinal electronic health records from 45.7 million adults in England to quantify associations of first, second, and booster doses of mRNA and non-mRNA COVID-19 vaccine brands with subsequent thrombotic and cardiovascular events. Data were accessed within the NHS England Secure Data Environment (NHSE SDE) 7 , encompassing primary care, hospital admissions, COVID-19 testing and vaccination data, dispensed medication records in primary care data, and the Office of National Statistics death registrations. We use Cox regression to estimate adjusted hazard ratios (aHRs) and corresponding 95% confidence intervals (95% CIs) in time intervals since vaccination, adjusted for a wide range of co-morbidities, age, sex, and prior COVID-19. We show that the incidence of thrombotic and cardiovascular complications was generally lower after each dose of each vaccine brand, except for previously recognised rare complications of the ChAdOx1 vaccine and the mRNA vaccines.
Characteristics of the study populations for first, second and booster vaccination
Over the study period from 8th December 2020 to 23rd January 2022, 45.7 million individuals met the eligibility criteria for our first-dose analyses (Table 1 , Supplementary Fig. 1 ). Among these, 37.3 million people received a first ChAdOx1, BNT-162b2 or mRNA1273 vaccination and were eligible for the second dose analyses (Supplementary Table 1 ). We refer to the first and second dose vaccinations as the “primary course”, distinguishing them from booster vaccine doses. Third dose vaccination, which is distinct from the booster dose and is administered as part of an extended primary course, was not considered. Of those who received a first dose, 35.9 million people received ChAdOx1, BNT-162b2 or mRNA1273 primary course vaccinations and were eligible for analyses of booster vaccination (Supplementary Table 2 ).
Differences in characteristics of people in the three vaccination cohorts reflect known vaccination uptake profiles. Considering the strong similarity in characteristics of the second and booster dose cohorts, we compared characteristics of the second and booster dose cohorts with the first dose cohort. Compared to the first dose cohort (Table 1 ), people in the second and booster vaccine dose cohorts (Supplementary Tables 1 and 2 ), who had received at least one COVID-19 vaccine dose, were older (<40 years: 31% vs 38%), and less likely to have a non-White ethnicity (16% vs 20%), or be deprived (IMD deciles 1–4, 36% vs 39%). They were modestly more likely to have cancer (18–19% vs 14%), to have contracted COVID-19 previously (7–8% vs 6%), to take blood pressure (22% vs 18%) or lipid-lowering medication (17–18% vs 15%), or to be clinically vulnerable (25% vs 22%).
Characteristics of people receiving different vaccine brands reflect vaccine availability during the UK vaccine rollout, which was in order of priority groups specified by the Joint Committee on Vaccination and Immunisation (JCVI). People younger than 40 years old and not in a priority group were offered only mRNA (BNT-162b2 or mRNA1273) vaccine brands 8 .
Incidence of different cardiovascular events
From the start of vaccine rollout (8th December 2020) up to first vaccination, during approximately 21 million person-years there were 75,655 arterial and 21,230 venous incident thrombotic events (Table 2 and Supplementary Table 4 ). Arterial thromboses included acute myocardial infarction (AMI; 37,915) and ischaemic stroke (36,720). Venous thromboses included pulmonary embolism (PE; 11,835) and lower limb deep venous thrombosis (DVT; 9075), intracranial venous thrombosis (ICVT; 370) and portal vein thrombosis (PVT; 185). Other incident cardiovascular events included subarachnoid haemorrhage and haemorrhagic stroke (SAH; 5235), mesenteric thrombus (1515), thrombocytopenia (1885), myocarditis (590) and pericarditis (455). The numbers of events and incidence rates after first, second and booster vaccinations reflected the older age of vaccinated, compared with unvaccinated, people (Supplementary Tables 4 – 7 ).
COVID-19 vaccination and arterial, venous, and other thrombotic events
We used Cox models to estimate adjusted hazard ratios (aHRs) and corresponding 95% CIs, comparing the incidence of thrombotic and cardiovascular events after first, second and booster vaccine doses with the incidence before or without the corresponding vaccine dose, adjusting for a wide range of potential confounding factors (Supplementary Tables 8 – 25 ). Associations with second dose were estimated in people who had a first dose, and associations with booster vaccination in people who had both first and second vaccinations. The aHRs after first doses of mRNA-1273 were imprecisely estimated, because this brand was received by fewer people who were generally younger, so were reported in supplementary tables but omitted from the figures.
The incidence of composite arterial thrombotic events (AMI, ischaemic stroke and other arterial embolism) was similar or lower after first, second and booster doses of ChAdOx1 and BNT-162b2 vaccines and booster doses of mRNA-1273, compared to follow-up before or without the corresponding vaccine dose in eligible people (Fig. 1 , Supplementary Tables 8 – 25 ). After second and booster vaccine doses, the reduction in incidence of composite arterial thrombosis was greater than after first vaccination. For example, aHRs for arterial thrombotic events 13–24 weeks after first vaccine dose were 0.99 (95% CI 0.97–1.02) after ChAdOx1 and 0.90 (0.88–0.93) after BNT-162b2. Corresponding aHRs after second doses were 0.73 (0.70–0.76) and 0.80 (0.77–0.83) respectively. aHRs for mRNA booster vaccination after primary course of ChAdOx1 were 0.71 (0.66–0.76) 13–24 weeks after BNT-162b2 and 0.67 (0.62–0.72) 5–24 weeks after mRNA-1273 respectively. After a primary course of BNT-162b2, aHRs 13–24 weeks after booster vaccination were 0.73 (0.69–0.77) and 1.21 (0.38–3.86) after BNT-162b2 and mRNA-1273 respectively. For all vaccine brands and doses, aHRs in the first few weeks were lower than in later weeks. The aHR profiles for AMI and ischaemic stroke were similar to those of composite arterial thrombosis, for all vaccine brands and doses.

Vertical lines depict 95% CIs; these are not visible when they are very narrow. There were no AMI events during weeks 13–26 after mRNA-1273 booster vaccination, so follow-up is grouped as 1–4 and 5–26 weeks post-vaccination. The number of people eligible for first, second, and booster dose analyses were 45,673,965; 37,249,850 and 35,853,120, respectively. The number of people who received a first dose of ChAdOx1, BNT-162b2 and mRNA-1273 were 19,317,985, 16,846,995, and 1,084,865, respectively; a second dose of ChAdOx1, BNT-162b2 and mRNA-1273 were 18,920,225, 15,961,330, and 971,565, respectively; a booster dose of BNT-162b2 and mRNA-1273 following a primary course of ChAdOx1 were 11,964,635and 4,153,760 respectively; a booster dose of BNT-162b2 and mRNA-1273 following a primary course of BNT-162b2 were 9,821,835 and 1,914,925, respectively. The numerical values of hazard ratios and 95% CIs are displayed in Supplementary Tables 8 , 9 , 11 , 12 , 14 , 15 , 17 and 18 .
Similar to arterial events, the incidence of composite venous thrombotic events (PE, DVT, ICVT and PVT) was generally lower after first, second and booster doses vaccination, compared to follow-up before or without the corresponding vaccine dose (Fig. 2 , Supplementary Tables 8 – 25 ). After second and booster vaccine doses, the reduction in incidence of composite venous thrombotic events was greater than after first vaccination. For example, aHRs 13–24 weeks after first vaccine doses were 0.94 (95%CI 0.90–0.98) after ChAdOx1 and 0.85 (0.81–0.88) after BNT-162b2. Corresponding aHRs after second doses were 0.68 (0.63–0.73) and 0.77 (0.72–0.83) respectively. aHRs for mRNA booster vaccination after primary course of ChAdOx1 were 0.63 (0.54–0.74) 13–24 weeks after BNT-162b2 and 0.55 (0.47–0.65) 5–24 weeks after mRNA-1273. After a primary course of BNT-162b2, aHRs for booster vaccination were 0.56 (0.49–0.63) 13–24 weeks after BNT-162b2 and 0.58 (0.45–0.74) 5–24 weeks after mRNA-1273. The aHR profiles for common venous events PE and DVT were similar to those of composite venous thrombosis for all vaccine brands and doses. In contrast, there was a higher incidence of the known rare complication ICVT immediately after first dose of ChAdOx1 with greatest aHR 2 weeks after vaccination (5.92; 95% CI 4.07–8.63). There was no increase in incidence of ICVT after second dose of ChAdOx1 (Supplementary Table 8 ), or after any other vaccine brand.

Vertical lines depict 95% CIs; these are not visible when they are very narrow. The number of people eligible for first, second, and booster dose analyses were 45,673,965; 37,249,850 and 35,853,120, respectively. The number of people who received a first dose of ChAdOx1, BNT-162b2 and mRNA-1273 were 19,317,985, 16,846,995, and 1,084,865, respectively; a second dose of ChAdOx1, BNT-162b2 and mRNA-1273 were 18,920,225, 15,961,330, and 971,565, respectively; a booster dose of BNT-162b2 and mRNA-1273 following a primary course of ChAdOx1 were 11,964,635and 4,153,760 respectively; a booster dose of BNT-162b2 and mRNA-1273 following a primary course of BNT-162b2 were 9,821,835 and 1,914,925, respectively. The numerical values of hazard ratios and 95% CIs are displayed in Supplementary Tables 8 , 9 , 11 , 12 , 14 , 15 , 17 and 18 .
There was a higher incidence of thrombocytopenia after first dose of ChAdOx1 (Fig. 3 , Supplementary Table 8 ), compared with no vaccination, with greatest aHR 2 weeks after vaccination (2.07; 95% CI 1.67–2.58) but no increase in incidence after second dose of ChAdOx1 or after first or second dose of BNT-162b2, or after booster dose of mRNA-1273 following primary course of ChAdOx1 (Supplementary Tables 9 , 11 and 12 ). There was a higher incidence of thrombocytopenia 13–24 weeks after a booster dose of BNT-162b2 following primary course of ChAdOx1 (aHR 2.16; 95% CI 1.26–3.69) (Fig. 3 , Supplementary Table 15 ).

Vertical lines depict 95% CIs; these are not visible when they are very narrow. The number of people eligible for first, second, and booster dose analyses were 45,673,965; 37,249,850 and 35,853,120, respectively. The number of people who received a first dose of ChAdOx1, BNT-162b2 and mRNA-1273 were 19,317,985, 16,846,995, and 1,084,865, respectively; a second dose of ChAdOx1, BNT-162b2 and mRNA-1273 were 18,920,225, 15,961,330, and 971,565, respectively; a booster dose of BNT-162b2 and mRNA-1273 following a primary course of ChAdOx1 were 11,964,635and 4,153,760 respectively; a booster dose of BNT-162b2 and mRNA-1273 following a primary course of BNT-162b2 were 9,821,835 and 1,914,925, respectively. The numerical values of hazard ratios and 95% CIs are displayed in Supplementary Tables 8 , 9 , 11 , 12 , 14 , 15 , 17 , and 18 .
The incidence of SAH and mesenteric thrombus was similar or lower after all doses and brands of vaccination (Supplementary Tables 8 – 25 ), compared with before or without the corresponding dose. The incidence of mesenteric thrombus and SAH were markedly lower beyond 4 weeks after second dose of ChAdOx1 or BNT-162b2 vaccines. For example, aHRs for mesenteric thrombus 13–24 weeks after second dose were 0.64 (95% CI 0.49–0.83) for ChAdOx1 and 0.64 (0.49–0.83) for BNT-162b2. Corresponding aHRs for SAH 13–24 weeks after second dose were 0.64 (0.56–0.74) for ChAdOx1 and 0.84 (0.73–0.98) for BNT-162b2.
The incidence of myocarditis was higher after first dose of BNT-162b2 vaccine, with greatest aHR 1 week after vaccination (2.05; 95% CI 1.28–3.29) (Fig. 3 ; Supplementary Table 9 ), higher one week after second dose of BNT-162b2 (aHR 3.14; 95% CI 2.04–4.85) (Supplementary Table 12 ) and higher after some mRNA booster vaccinations (for example, the aHR 1 week after booster BNT-162b2 vaccination was 1.65; 95% CI 1.07–2.57 following primary course of any of ChAdOx1, BNT-162b2 or mRNA-1273) (Supplementary Tables 19 , 21 , 22 , 24 , and 25 ). Otherwise, the incidence of myocarditis after vaccination was similar to or lower than that before or without vaccination.
The incidence of pericarditis was higher after first dose of ChAdOx1, with greatest aHR 2 weeks after vaccination (1.74; 95% CI 1.04–2.91), after first dose of BNT-162b2 (for example, the aHR 13–24 weeks after vaccination was 1.50; 95% CI 1.17–1.92) (Supplementary Tables 8 and 9 ), after second dose of BNT-162b2 (aHR 3–4 weeks after vaccination 2.42; 95% CI 1.62–3.62) (Supplementary Table 12 ), and after mRNA-based booster vaccination (for example, the aHR 2 weeks after booster dose of BNT-162b2 vaccine following primary course of any vaccine brand was 1.73; 95% CI 1.05–2.83) (Supplementary Tables 22 and 25 ). Otherwise, the incidence of pericarditis after vaccination was similar to, or lower than, that before or without vaccination, with a halving in incidence beyond 4 weeks after second dose of ChAdOx1 (for example, the aHR after 13–24 weeks was 0.32; 95% CI 0.19–0.51) (Supplementary Table 11 ).
When follow-up was censored at the time of the US Centers for Disease Control (CDC) public announcement on myocarditis and pericarditis on 17th May 2021, the incidence of myocarditis and pericarditis after both first and second doses of ChAdOx1 and BNT-162b2 was similar to that before or without such vaccinations. One exception was a higher incidence of pericarditis 5–24 weeks after first dose BNT-162b2 vaccination (aHR 1.89; 95% CI 1.13–3.16) (Supplementary Fig. 8 ; Supplementary Table 30 ). Note that the smaller number of events in this sensitivity analyses meant that aHRs were less precisely estimated than in the main analyses.
COVID-19 vaccination and thrombotic events within population subgroups
Subgroup analyses by age group, ethnic group, previous history of COVID-19, history of the event of interest and sex were conducted for composite arterial and composite venous outcomes (Supplementary Figs. 2 – 7 ; Supplementary Tables 26 – 29 ). Associations between vaccination and thrombotic events were generally similar across subgroups, with the following exceptions. For composite arterial events after first doses of ChAdOx1 or BNT-162b2, aHRs in people with unknown ethnicity were higher than in people with known ethnicity (Supplementary Figs. 2 and 4 ; Supplementary Tables 26 and 27 ). In general, aHRs for composite arterial and composite venous events after first and second doses of ChAdOx1 or BNT-162b2 were higher in males than females (Supplementary Figs. 2 – 5 ).
This study used whole population longitudinal health records from over 45.7 million adults in England to quantify associations of first, second and booster doses of COVID-19 vaccine brands used during the first two years of the UK vaccine rollout with the incidence of arterial and venous thromboses, thrombocytopenia and myocarditis. Estimated hazard ratios were adjusted for a wide range of potential confounders. The incidence of thrombotic and cardiovascular complications was generally lower after each dose of each vaccine brand. Exceptions, consistent with previous findings that have been recognised by medicines regulators, included rare complications of the ChAdOx1 vaccine (ICVT and thrombocytopenia, due to vaccine-induced immune thrombocytopenia and thrombosis) and the mRNA vaccines (myocarditis and pericarditis). There were few differences between subgroups defined by demographic and clinical characteristics. These findings, in conjunction with the long-term higher risk of severe cardiovascular and other complications associated with COVID-19, offer compelling evidence supporting the net cardiovascular benefit of COVID vaccination.
The strengths of this study lie in the representativeness of the whole population data, offering an overview of thrombotic events after vaccination, as well as the comprehensive analyses of different vaccine dose and brand combinations in the general population. Consequently, the findings should apply to nations with comparable demographics and healthcare systems. The very large sample size facilitated estimation of associations with rare outcomes, within time periods after vaccination, and within population subgroups. The extensive longitudinal coverage of the health records also allowed examination of events after the first, second, and booster vaccinations. We addressed potential confounding by adjusting for a wide-range of demographic factors and prior diagnoses available in primary and secondary care records, defined using clinician-validated code lists that are accessible via our GitHub repository (available at https://github.com/BHFDSC/CCU002_06 ). Our analysis adhered to a pre-specified protocol with one deviation: we censored all analyses 26 weeks after vaccination to avoid interference from subsequent vaccinations 9 .
This study has several limitations. First, residual confounding, including that linked to delayed vaccination in high-risk individuals, may persist despite extensive adjustments for available covariates. We were able to identify some, but not all people who were clinically vulnerable (and hence might have been eligible for earlier vaccination): for example, younger adults in long-stay settings could not be reliably identified. Second, we did not adjust for potential confounding by time-varying post-baseline factors that may have influenced receipt of vaccination and the outcomes of interest: for example, development of respiratory symptoms or being admitted into hospital leading to postponement of vaccination. Such confounding may explain estimated lower hazard ratios soon after vaccination 10 . Third, ascertainment of some outcomes may have been influenced by public announcements from regulatory agencies, such as the European Medicines Agency Pharmacovigilance Risk Assessment Committee announcement 11 or the CDC announcement on myocarditis 12 , 13 . This was addressed in sensitivity analyses for myocarditis and pericarditis, censoring follow-up at the time of public announcements of these adverse effects of vaccination, although the shorter follow-up times and corresponding smaller numbers of events in the restricted analyses meant that aHRs were estimated with reduced precision. Fourth, outcomes may be underreported, particularly from people in nursing homes or among those with severe health conditions, due to diagnostic challenges; also, routine electronic health records, not intended for research, may under-ascertain less severe, non-hospitalised events. Both forms of potential underreporting, however, are expected to be uncommon for hospitalised thrombotic events 14 . Fifth, we restricted follow-up to 26 weeks after vaccination to prevent an influence of subsequent vaccinations on estimated associations and limit the impact of delayed vaccination on our findings. Horne et al. demonstrated selection bias in estimated HRs for non-COVID-19 death arising from deferred next-dose vaccination in people with a recent confirmed COVID-19 diagnosis or in poor health 9 . Sixth, we did not address long-term safety of vaccination, or the impact of subsequent booster doses.
The incidence of arterial and venous thrombotic events was generally lower after COVID-19 vaccination than before or without vaccination. The higher incidence of cardiovascular events after COVID-19 is well-established 14 , 15 , 16 , 17 and a plausible explanation tfor reductions in these events after vaccination is that vaccination prevents COVID-19, particularly severe COVID-19 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 . However, quantifying the mediating role of COVID-19 in cardioprotective effects of vaccination is beyond the scope of this paper.
The cohort design of our study allows estimation of incidence rates (see Table 2 ) and hence facilitates an understanding of the population-level impact of vaccination on common and rare cardiovascular events. However, our findings may be biased by unmeasured confounding, as discussed above. The self-controlled case series (SCCS) method aims to avoid the need to control for time-invariant confounders, but entails assumptions such as event-independent observation periods and medically-informed risk period definitions, and is particularly suited to short-term outcomes. Our results thus complement, and may be triangulated with, previous publications that examined cardiovascular implications of COVID-19 vaccination using the SCCS method 25 , 27 , 28 .
This England-wide study offers reassurance regarding the cardiovascular safety of COVID-19 vaccines, with lower incidence of common cardiovascular events outweighing the higher incidence of their known rare cardiovascular complications. We found no novel cardiovascular complications or new associations with subsequent doses. Our findings support the wide uptake of future COVID-19 vaccination programs. We hope this evidence addresses public concerns, supporting continued trust and participation in vaccination programs and adherence to public health guidelines.
This analysis was performed according to a pre-specified analysis plan published on GitHub, along with the code lists to define variables and analysis code: https://github.com/BHFDSC/CCU002_06 .
Study population
We analysed de-identified data, made available for the BHF Data Science Centre’s CVD-COVID-UK/COVID-IMPACT Consortium within the NHS England Secure Data Environment 7 , 29 , which is a secure, privacy-protecting platform. This data consists of linked datasets including General Practice Extraction Service Extract for Pandemic Planning and Research (GDPPR), hospital admission data from Secondary Uses Service (SUS), Hospital Episode Statistics for admitted patient care (HES-APC), national laboratory COVID-19 testing data from the UK Health Security Agency (UKHSA) Second Generation Surveillance System (SGSS), Office for National Statistics (ONS) Civil Registration of Deaths (ONS deaths registry), medicines dispensed in primary care data and COVID-19 vaccination data.
The primary course of vaccination consists of the first and second vaccinations and, for certain groups such as people with severe immunosuppression, a third vaccination: this is distinct from booster vaccination that is given some time after the primary course 30 .
People were included in the study if they were alive on 8th December 2020; aged 18–110 years inclusive; had a record in the GDPPR; recorded as male or female; and living in England. People with missing Lower-layer Super Output Area (LSOA) data were assumed to live in England. People were excluded if (1) they were vaccinated before 8th December 2020; (2) they were recorded as having a second dose and/or a booster or third dose, before or without records of first and second dose vaccinations respectively; (3) the interval between their first and second vaccination was less than 21 days 31 ; (4) they had mixed first and second vaccine brands where the second dose was given on or before 7th May 2021 32 , 33 , 34 ; (5) the interval between their second and booster vaccinations was less than 90 days 35 ; (6) they had conflicting vaccination records or a situation code attached to any vaccination indicating that the vaccination was not given 36 . We applied general quality checks, including removing people from the analysis who had nonsensical dates of birth or death (for details see https://github.com/BHFDSC/CCU002_06 ).
All eligible people were considered for the first dose analysis. Those who received a first dose of ChAdOx1, BNT-162b2, or mRNA1 were included in the second dose analyses. People who received the same vaccine brand for their first and second doses were included in the booster vaccination analyses.
Data for the study were extracted on 12th May 2022. The study spanned records from 8th December 2020, the start of the UK’s vaccine rollout, to 23rd January 2022, the latest available date to ensure completeness of records from across all datasets at data extraction. Follow-up for first dose vaccination analyses began on 8th December 2020 for all people. For the second and booster vaccination analyses, follow-up commenced on the date of the preceding-dose vaccination. The term “index date” will henceforth denote the start of follow-up for each individual in each analysis.
For the first and second dose analyses, the brands ChAdOx1, BNT-162b2 and mRNA-1273 were analysed separately. For the booster dose analyses, we analysed all combinations of primary courses (ChAdOx1, BNT-162b2, BNT-162b2/mRNA-1273) with booster vaccines (BNT-162b2, mRNA-1273, and BNT-162b2/mRNA-1273). (Supplementary Table 3 ).
Confounders
We considered the following confounders: age, sex, ethnic group, Index of Multiple Deprivation (2010 IMD deciles grouped into Deciles 1–4, 5–6, 7–10/missing), smoking status (never/ever, with missing classified as “never”), medical history (including acute myocardial infarction (AMI), diabetes, depression, obesity, cancer, chronic obstructive pulmonary disease (COPD), liver disease, chronic kidney disease, dementia, all stroke, all venous thromboembolic events and thrombophilia), major surgery in the last year, number of unique medical conditions in the last year, prior COVID-19 at index date, medications taken in the last 90 days (including antiplatelets, blood pressure lowering, lipid-lowering, oral anticoagulants, combined oral contraceptives (COCP) and hormone replacement therapy (HRT)) and clinical vulnerability (clinically extremely vulnerable/clinically vulnerable/neither). The medical history covariates were defined as a diagnosis of the condition before the index date except for diabetes which was additionally defined as a record of diabetic medication in the GDPPR data in the 90 days before the index date. Clinical vulnerability was defined on 8th December 2020. People were flagged as “clinically extremely vulnerable” using the SNOMED code 1300561000000107 37 , and “clinically vulnerable” by identifying component conditions as applied in Table 3 of the COVID-19 chapter of the Green Book 30 . Except for sex, clinical vulnerability and ethnic group, all other covariates were updated at individual-specific index dates for the dose 1, dose 2 and booster analyses. History of confirmed COVID-19 diagnosis was ascertained using established algorithms that combine information from SGSS, HES-APC, SUS, ONS deaths registry 38 .
Eleven cardiovascular outcomes were analysed: AMI, ischaemic stroke, lower limb deep venous thrombosis (DVT), pulmonary embolism (PE), intracranial venous thrombosis (ICVT), mesenteric thrombus, portal vein thrombosis (PVT), any thrombocytopenia, subarachnoid haemorrhage & haemorrhagic stroke (SAH & HS), myocarditis and pericarditis. In addition, two composite outcomes were analysed: composite arterial (AMI, ischaemic stroke and other arterial embolism) and composite venous (PE, DVT, ICVT and PVT). We selected the earliest date of outcome event on or after index date from GDPPR, SUS, HES-APC and ONS deaths registry. We considered only the first/primary position from HES-APC and SUS and used the underlying cause from the death data, to differentiate acute, new events, from prevalent conditions. Further, we had previously found that aHRs for outcomes recorded as primary or secondary reason for admission or death were consistent with those from analyses of outcomes in the primary position 5 .
Statistical analyses
Eligibility criteria and index dates for each vaccination course are detailed in Supplementary Table 3 . We censored follow-up at the earliest of death, outcome event of interest, receipt of another vaccine brand, 26 weeks since the vaccination under consideration 9 , or the study end date. Baseline demographic and clinical characteristics were detailed for each study cohort, and the number of outcome events and person-years of follow-up were quantified both before and after each vaccination, with incidence rates expressed per 100,000 person-years. Following the NHSE statistical disclosure control process, any counts less than 10 are presented as “10” and all numbers above 10 are rounded to the nearest 5. Percentages and incidence rates were calculated using rounded counts. Therefore, incidence rates corresponding to event counts less than 10 should be regarded as upper bounds and interpreted cautiously.
We analysed the time since vaccination to the first event for each outcome by fitting Cox models with a calendar time scale, with 8th December 2020 as time zero for the first dose analysis and the date of the previous dose as time zero for the second and booster dose analyses. We estimated aHRs and corresponding 95% CIs comparing follow-up after first, second and booster vaccine doses with follow-up before or without the corresponding vaccine dose for time intervals since vaccination: 1 weeks, 2 weeks, 3–4 weeks, 5–12 weeks, 13–24 weeks, and 25–26 weeks after vaccination. We expect that on average, and in the absence of bias, 5% of 95% CIs will exclude the true value of the aHR. Each comparison group included only individuals eligible to receive the vaccine brand and dose under consideration. For example, booster doses can only be received by individuals who have received a primary vaccine course. In the absence of any events in one of these intervals, we consolidated the periods into 1–4 weeks and 5–26 weeks after vaccination. For categorical confounders where fewer than two people in any category experienced an event, we combined categories where feasible and removed the confounder if not feasible. Furthermore, we stratified all models by region to account for between-region variation.
For computational feasibility, our analysis datasets comprised people who experienced the outcome (‘cases’) during follow-up and a randomly selected subsample with size twenty times the number of cases of those who did not experience the outcome. We applied inverse probability weights to account for this sampling method and used robust standard errors to compute confidence intervals. For each outcome and vaccination course, we estimated hazard ratios adjusting for (i) age and sex; and (ii) all measured confounders (maximally-adjusted).
We performed subgroup analyses for composite arterial and venous outcomes, by age group, ethnic group, prior history of the event (arterial: AMI, ischaemic stroke and other arterial embolism; venous: PE, DVT, ICVT, PVT and other DVT), prior history of confirmed COVID-19 diagnosis, and sex. We repeated the maximally-adjusted analyses for myocarditis and pericarditis, censoring follow-up on 17th May 2021, the date of the CDC’s public announcement of their potential associations with COVID-19 mRNA vaccines.
Analyses used SQL and Python (in Databricks, version 3.68), and RStudio (Professional) Version 1.3.1093.1 driven by R Version 4.0.3 (10th October 2020).
Ethical approval and information governance
The North East – Newcastle and North Tyneside 2 research ethics committee provided ethical approval for the CVD-COVID-UK/COVID-IMPACT research programme (REC No 20/NE/0161) to access, within secure trusted research environments, unconsented, whole-population, de-identified data from electronic health records collected as part of patients’ routine healthcare.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data used in this study are available in NHS England’s Secure Data Environment service for England for England, but as restrictions apply are not publicly available ( https://digital.nhs.uk/services/secure-data-environment-service ). The CVD-COVID-UK/COVID-IMPACT programme led by the BHF Data Science Centre ( https://bhfdatasciencecentre.org/ ) received approval to access data in the NHS England’s SDE service for England from the Independent Group Advising on the Release of Data (IGARD) ( https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/independent-group-advising-on-the-release-of-data ) via an application made in the Data Access Request Service (DARS) Online system (reference: DARS-NIC-381078-Y9C5K; https://digital.nhs.uk/services/data-access-request-service-dars/dars-products-and-services ). The CVD-COVID-UK/COVID-IMPACT Approvals & Oversight Board ( https://bhfdatasciencecentre.org/areas/cvd-covid-uk-covid-impact/ ) subsequently granted approval to this project to access the data within NHS England’s SDE service for England. The de-identified data used in this study were made available to accredited researchers only. Those wishing to access the data should contact [email protected].
Code availability
All code and code lists are shared openly for review and re-use under an MIT open license ( https://github.com/BHFDSC/CCU002_06/ ).
Watson, O. J. et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 22 , 1293–1302 (2022).
JCVI statement on the COVID-19 vaccination programme for autumn 2023, 26 May 2023. GOV.UK https://www.gov.uk/government/publications/covid-19-autumn-2023-vaccination-programme-jcvi-advice-26-may-2023/jcvi-statement-on-the-covid-19-vaccination-programme-for-autumn-2023-26-may-2023 (2023).
COVID-19 weekly announced vaccinations: Week ending Sunday 23rd January 2022. NHS England , https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2022/01/COVID-19-weekly-announced-vaccinations-27-January-2022.pdf (2022).
Vaccine Safety Publications | Research | Vaccine Safety | CDC. https://www.cdc.gov/vaccinesafety/research/publications/index.html (2023).
Whiteley, W. N. et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLOS Med. 19 , e1003926 (2022).
Article CAS PubMed PubMed Central Google Scholar
Demasi, M. FDA urged to publish follow-up studies on covid-19 vaccine safety signals. BMJ 379 , o2527 (2022).
Article PubMed Google Scholar
Secure Data Environment service for England. NHS Digital , https://digital.nhs.uk/services/secure-data-environment-service (2024).
JCVI advises on COVID-19 vaccine for people aged under 40. GOV.UK, https://www.gov.uk/government/news/jcvi-advises-on-covid-19-vaccine-for-people-aged-under-40 (2021).
Horne, E. M. F. et al. Challenges in estimating the effectiveness of 2 doses of COVID-19 vaccine beyond 6 months in England. American Journal of Epidemiology 193 , 227–231 (2024).
Hulme, W. J. et al. Challenges in Estimating the Effectiveness of COVID-19 Vaccination Using Observational Data. Ann. Intern. Med. 176 , 685–693 (2023).
Curtis, H. J. et al. Trends and clinical characteristics of COVID-19 vaccine recipients: a federated analysis of 57.9 million patients’ primary care records in situ using OpenSAFELY. Br. J. Gen. Pract. 72 , e51–e62 (2022).
COVID-19 VaST Technical Report May 17, 2021 | CDC. https://www.cdc.gov/vaccines/acip/work-groups-vast/report-2021-05-17.html (2021).
Clinical Considerations: Myocarditis after mRNA COVID-19 Vaccines | CDC. https://web.archive.org/web/20210528145419/https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html (2021).
Knight, R. et al. Association of COVID-19 With Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales. Circulation 146 , 892–906 (2022).
Nishiga, M., Wang, D. W., Han, Y., Lewis, D. B. & Wu, J. C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 17 , 543–558 (2020).
Davidson, J. A. et al. Acute Cardiovascular Events After COVID-19 in England in 2020: A Self-Controlled Case Series Study. Clin. Epidemiol. 15 , 911–921 (2023).
Article PubMed PubMed Central Google Scholar
Mafham, M. & Baigent, C. What is the association of COVID-19 with heart attacks and strokes? Lancet 398 , 561–563 (2021).
Andrews, N. et al. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. N. Engl. J. Med. 386 , 340–350 (2022).
Article CAS PubMed Google Scholar
Pritchard, E. et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat. Med. 27 , 1370–1378 (2021).
Lopez Bernal, J. et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 385 , 585–594 (2021).
Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet Lond. Engl. 397 , 99–111 (2021).
Article CAS Google Scholar
Horne, E. M. F. et al. Waning effectiveness of BNT162b2 and ChAdOx1 covid-19 vaccines over six months since second dose: OpenSAFELY cohort study using linked electronic health records. BMJ 378 , e071249 (2022).
Modin, D. et al. Acute COVID-19 and the Incidence of Ischemic Stroke and Acute Myocardial Infarction. Circulation 142 , 2080–2082 (2020).
Katsoularis, I., Fonseca-Rodríguez, O., Farrington, P., Lindmark, K. & Connolly, A.-M. F. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet 398 , 599–607 (2021).
Hippisley-Cox, J. et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 374 , n1931 (2021).
Bilaloglu, S. et al. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA 324 , 799–801 (2020).
Simpson, C. R. et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 27 , 1290–1297 (2021).
Patone, M. et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 28 , 410–422 (2022).
Wood, A. et al. Linked electronic health records for research on a nationwide cohort of more than 54 million people in England: data resource. BMJ 373 , n826 (2021).
Wayback Machine. https://web.archive.org/web/20210215140119/https:/assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961287/Greenbook_chapter_14a_v7_12Feb2021.pdf (2021).
Statement from the UK Chief Medical Officers on the prioritisation of first doses of COVID-19 vaccines. GOV.UK https://www.gov.uk/government/news/statement-from-the-uk-chief-medical-officers-on-the-prioritisation-of-first-doses-of-covid-19-vaccines (2020).
Wayback Machine. https://web.archive.org/web/20210702185339/https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/998309/Greenbook_chapter_14a_1July2021.pdf (2021).
Mahase, E. Covid-19: Vaccine brands can be mixed in “extremely rare occasions,” says Public Health England. BMJ 372 , n12 (2021).
COVID-19 vaccination programme: FAQs on second doses. NHS England , https://www.england.nhs.uk/coronavirus/documents/covid-19-vaccination-programme-faqs-on-second-doses/ (2021).
NHS booster bookings open to every eligible adult. NHS England , https://www.england.nhs.uk/2021/12/nhs-booster-bookings-open-to-every-eligible-adult/ (2021).
COVID-19 Vaccination Status. NHS Digital , https://digital.nhs.uk/services/data-access-request-service-dars/dars-products-and-services/data-set-catalogue/covid-19-vaccination-status (2024).
High Risk from COVID-19 code. OpenCodelists , https://www.opencodelists.org/codelist/primis-covid19-vacc-uptake/shield/v.1.5.3/#full-list (2024).
Thygesen, J. H. et al. COVID-19 trajectories among 57 million adults in England: a cohort study using electronic health records. Lancet Digit Health 4 , e542–e557 (2022).
Download references
Acknowledgements
This work was supported by the Longitudinal Health and Wellbeing COVID-19 National Core Study (UKRI Medical Research Council MC_PC_20030 and MC_PC_20059); and by the CONVALESCENCE long COVID study, funded by the UK National Institute for Health and Care Research (COVID-LT-009). This study was also supported by core funding from the: British Heart Foundation (RG/18/13/33946), NIHR Cambridge Biomedical Research Centre (BRC-1215-20014; NIHR203312) [*], Cambridge BHF Centre of Research Excellence (RE/18/1/34212), BHF Chair Award (CH/12/2/29428) and by Health Data Research UK, which receives its funding from HDR UK Ltd (HDR-9006), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and the Wellcome Trust. The British Heart Foundation (BHF) Data Science Centre, led by Health Data Research (HDR) UK (BHF Grant no. SP/19/3/34678, awarded to HDR UK) also supported this work. This study made use of de-identified data held in NHS England’s Secure Data Environment service for England and made available via the BHF Data Science Centre’s CVD-COVID-UK/COVID-IMPACT consortium. This work used data provided by patients and collected by the NHS as part of their care and support. We would also like to acknowledge all data providers who make health relevant data available for research. The BHF Data Science Centre funded co-development (with NHS England) of the Secure Data Environment service for England, provision of linked datasets, data access, user software licenses, computational usage, and data management and wrangling support, with additional contributions from the HDR UK Data and Connectivity component of the UK Government Chief Scientific Adviser’s National Core Studies programme to coordinate national COVID-19 priority research. Consortium partner organisations funded the time of contributing data analysts, biostatisticians, epidemiologists, and clinicians. Further support came from the Con-COV team funded by the Medical Research Council (grant number: MR/V028367/1) and the ADR Wales programme, part of the ADR UK investment, which unites expertise from Swansea University Medical School, WISERD at Cardiff University, and Welsh Government analysts. ADR UK is funded by the Economic and Social Research Council (ESRC), part of UK Research and Innovation. This research was also supported by ESRC funding, including Administrative Data Research Wales (ES/W012227/1). S.I. was funded by the International Alliance for Cancer Early Detection, a partnership between Cancer Research UK C18081/A31373, Canary Center at Stanford University, the University of Cambridge, OHSU Knight Cancer Institute, University College London and the University of Manchester. S.I. and Y.L. are supported by Cancer Research UK EDDPMA-May22\100062. A.B. has received funding from NIHR (COV-LT2-0043) as PI of the STIMULATE-ICP study. V.W. is supported by the Medical Research Council Integrative Epidemiology Unit at the University of Bristol [MC_UU_00032/03]. R.D. and J.A.C.S. are supported by the NIHR Bristol Biomedical Research Centre (NIHR203315) and by Health Data Research UK South-West (HDRUK2023.0022). A.M.W. and JACS are supported by the National Institute for Health Research (NIHR) (NIHR135073). A.M.W. is supported by the BHF Data Science Centre (HDRUK2023.0239) and as an NIHR Research Professor (NIHR303137). A.M.W. conducted this research whilst part of the BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking under grant agreement No 116074 and whilst supported by the BHF-Turing Cardiovascular Data Science Award (BCDSA\100005). The views expressed are those of the author(s) and not necessarily those of NIHR or the Department of Health and Social Care.
Author information
These authors contributed equally: Samantha Ip, Teri-Louise North.
These authors jointly supervised this work: Jonathan A. C. Sterne, Angela M. Wood, Venexia Walker.
Authors and Affiliations
British Heart Foundation Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK
Samantha Ip, Yangfan Li, Spencer Keene & Angela M. Wood
Centre for Cancer Genetic Epidemiology, University of Cambridge, Cambridge, UK
Samantha Ip & Yangfan Li
Victor Phillip Dahdaleh Heart and Lung Research Institute, University of Cambridge, Cambridge, UK
Department of Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK
Teri-Louise North, Elsie Horne, Rachel Denholm, Jonathan A. C. Sterne & Venexia Walker
Population Data Science, Swansea University Medical School, Faculty of Medicine, Health, and Life Science, Swansea University, Swansea, Wales, UK
Fatemeh Torabi, Hoda Abbasizanjani & Ashley Akbari
NIHR Bristol Biomedical Research Centre, Bristol, UK
Rachel Denholm & Jonathan A. C. Sterne
Health Data Research UK South-West, Bristol, UK
Health Data Research UK, London, UK
Spiros Denaxas
Institute of Health Informatics, University College London, London, UK
Spiros Denaxas & Amitava Banerjee
University College London Hospitals Biomedical Research Centre, University College London, London, UK
BHF Accelerator, London, UK
British Heart Foundation Data Science Centre, Health Data Research UK, London, UK
Spiros Denaxas, Cathie Sudlow, William N. Whiteley & Angela M. Wood
Diabetes Research Centre, University of Leicester, Leicester, UK
Kamlesh Khunti
Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK
William N. Whiteley
National Institute for Health and Care Research Blood and Transplant Research Unit in Donor Health and Behaviour, University of Cambridge, Cambridge, UK
Angela M. Wood
British Heart Foundation Centre of Research Excellence, University of Cambridge, Cambridge, UK
Health Data Research UK Cambridge, Wellcome Genome Campus and University of Cambridge, Cambridge, UK
Cambridge Centre for AI in Medicine, Cambridge, UK
MRC Integrative Epidemiology Unit, Bristol, UK
Venexia Walker
Department of Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA
You can also search for this author in PubMed Google Scholar
the CVD-COVID-UK/COVID-IMPACT Consortium
- Samantha Ip
- , Teri-Louise North
- , Fatemeh Torabi
- , Yangfan Li
- , Hoda Abbasizanjani
- , Ashley Akbari
- , Elsie Horne
- , Rachel Denholm
- , Spencer Keene
- , Spiros Denaxas
- , Amitava Banerjee
- , Kamlesh Khunti
- , Cathie Sudlow
- , William N. Whiteley
- , Jonathan A. C. Sterne
- , Angela M. Wood
- & Venexia Walker
Contributions
Author contributions are reported below in line with the Contributor Roles Taxonomy (CRediT). Conceptualisation: J.A.C.S., A.M.W., V.W., W.N.W., S.I., T.-L.N. Methodology: J.A.C.S., A.M.W., V.W., W.N.W., S.I., T.-L.N., A.A., E.H. Software: S.I., T.-L.N., E.H., S.K. Validation: S.I., T.-L.N., F.T. Formal analysis: S.I., T.-L.N. Investigation: S.I., T.-L.N. Resources: C.S. Data curation: S.I., T.-L.N. Writing - Original Draft: S.I., T.-L.N., Y.L., W.N.W., J.A.C.S., A.M.W., V.W. Writing - Review & Editing: S.I., T.-L.N., F.T., Y.L., H.A., A.A., E.H., R.D., S.K., S.D., A.B., K.K., C.S., W.N.W., J.A.C.S., A.M.W., V.W. Visualisation: S.I., T.-L.N., Y.L., W.N.W., J.A.C.S., A.M.W., V.W. Project administration: S.I., T.-L.N., J.A.C.S., A.M.W., V.W. Funding acquisition: J.A.C.S., A.M.W., C.S.
Corresponding author
Correspondence to Samantha Ip .
Ethics declarations
Competing interests.
K.K. was chair of the ethnicity subgroup of the UK Scientific Advisory Group for Emergencies (SAGE) and was a member of SAGE. C.S. is Director of the BHF Data Science Centre (whose main funding support comes from the British Heart Foundation) and Chief Scientist and Deputy Director at Health Data Research UK, the UK’s Institute for Health Data Science. She played a key role in co-developing NHS England’s national secure data environment and leads the CVD-COVID UK/COVID-IMPACT Consortium, which enabled this work. W.W. is supported by the Chief Scientists Office (CAF/01/17) and Stroke Association (SA CV 20100018). W.W. has given expert testimony to UK courts. The remaining authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Nick Andrews and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ip, S., North, TL., Torabi, F. et al. Cohort study of cardiovascular safety of different COVID-19 vaccination doses among 46 million adults in England. Nat Commun 15 , 6085 (2024). https://doi.org/10.1038/s41467-024-49634-x
Download citation
Received : 22 February 2024
Accepted : 11 June 2024
Published : 31 July 2024
DOI : https://doi.org/10.1038/s41467-024-49634-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
PERSPECTIVE article
Opportunities and challenges to implementing mrna-based vaccines and medicines: lessons from covid-19.

- 1 Moderna, Inc., Cambridge, MA, United States
- 2 London School of Hygiene and Tropical Medicine, University of London, London, United Kingdom
- 3 Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, United States
The messenger RNA (mRNA) platform emerged at the forefront of vaccine development during the COVID-19 pandemic, with two mRNA COVID-19 vaccines being among the first authorized globally. These vaccines were developed rapidly. Informed by decades of laboratory research, and proved to be safe and efficacious tools for mitigating the global impact of the COVID-19 pandemic. The mRNA platform holds promise for a broader medical application beyond COVID-19. Herein, we provide an overview of this platform and describe lessons learned from the COVID-19 pandemic to help formulate strategies toward enhancing uptake of future mRNA-based interventions. We identify several strategies as vital for acceptance of an expanding array of mRNA-based vaccines and therapeutics, including education, accurate and transparent information sharing, targeted engagement campaigns, continued investment in vaccine safety surveillance, inclusion of diverse participant pools in clinical trials, and addressing deep-rooted inequalities in access to healthcare. We present findings from the Global Listening Project (GLP) initiative, which draws on quantitative and qualitative approaches to capture perceptions and experiences during the COVID-19 pandemic to help design concrete action plans for improving societal preparedness for future emergencies. The GLP survey (>70,000 respondents in 70 countries) revealed tremendous disparities across countries and sociodemographic groups regarding willingness to accept novel mRNA vaccines and medicines. The comfort in innovations in mRNA medicines was generally low (35%) and was marginally lower among women (33%). The GLP survey and lessons learnt from the COVID-19 pandemic provide actionable insights into designing effective strategies to enhance uptake of future mRNA-based medicines.
1 Introduction
Tailored healthcare campaigns that engage the public and provide resources to address specific health needs are integral to enhancing health and preventing disease ( 1 ). The success of vaccination campaigns is predicated on a multitude of factors, including public trust in health authorities and political leadership, access to vaccines, and perceptions of vaccination ( 2 , 3 ). These factors vary across countries ( 2 , 4 – 8 ) and intersect with more dynamic influences (e.g., rapidly evolving policy recommendations, media coverage) ( 2 , 3 , 8 , 9 ). During the COVID-19 pandemic, the relationship between vaccine hesitancy, sociodemographic characteristics, and political leaning became notable ( 10 – 12 ). Social inequalities were associated with disparate access to care and differential health burden ( 11 , 13 ), influencing vaccine perceptions and potentially shaping future vaccine behavior. Quantitative measures and benchmarking can provide actionable insights on societal preparedness to mitigate the long-term impact of healthcare crises, e.g., by addressing the gaps between public perceptions and evidence-based information, and targeting trust-building interventions to appropriate demographic groups ( 14 ).
Messenger RNA-based vaccines (hereafter mRNA vaccines) were among the primary authorized vaccines against SARS-CoV-2 during the COVID-19 pandemic ( 15 ). Although mRNA research has been ongoing for several decades ( 16 ), the use of the mRNA platform for vaccines came into the limelight only during the COVID-19 pandemic ( 15 , 17 ). The novelty of this mode of producing vaccines generated concerns in the public regarding perceived lack of adequate testing of side-effects of mRNA vaccines ( 2 ). Herein, we provide an overview of how the mRNA platform works and discuss how lessons learned from the pandemic can inform strategies to enhance trust and facilitate uptake of mRNA-based vaccines and therapeutics beyond COVID-19. We present novel data from a Global Listening Project (GLP) survey ( 18 ) showcasing nationwide diversity in the pandemic-era experiences of mRNA-based vaccines and medicines. These data reveal a multifactorial basis underlying acceptance of mRNA-based medicines, highlighting the need for improved communication on this topic and equitable access to care in the time of crisis.
2 The mRNA vaccine platform
Messenger RNA is an essential molecule involved in relaying genetic information encoded in DNA to the production of proteins ( 19 – 21 ). Vaccines based on mRNA can be designed to selectively produce key proteins from pathogens that stimulate a specific immune response, thereby protecting from illness ( 16 , 22 , 23 ). mRNA contains a transcript that directs the production of highly immunogenic proteins by the cells that take up the vaccine and stimulate the immune system the same way as a natural infection ( 16 , 24 – 26 ). The protein encoded by mRNA represents one component of the pathogen, and therefore is unable to cause disease ( 20 ).
The constituents of mRNA vaccines are synthetic, non-replicating mRNA molecules that approximate the size and composition of naturally occurring mRNA ( 15 , 26 ), encapsulated by lipid nanoparticles (LNPs) that serve to protect the mRNA from degradation and enable targeted cellular delivery ( 19 , 24 , 27 , 28 ). Following administration, the mRNA is rapidly degraded by normal physiological processes ( 20 , 29 – 31 ), while the naturally occurring lipids in the LNP vehicles are assumed to be biologically degraded similar to their endogenous analogs ( 27 , 31 ). The synthetic amino lipid constituent of LNP is rapidly cleared from blood in rodent models ( 27 , 31 ). Notably, mRNAs do not enter the cell nucleus and therefore cannot integrate into the cellular genome ( 16 ).
The key advantages of mRNA over other vaccine platforms ( Table 1 ) are the precision in protein design, the flexibility to reconfigure protein formulations toward enhancing immunogenicity or developing combination vaccines to target multiple pathogens, and the speed at which vaccines can be manufactured and updated (e.g., allowing expeditious updates to target evolving or emerging strains) ( 24 , 28 ). The manufacturing of mRNA involves standardized chemical processes with reagents that can be rapidly repurposed independently of the encoded protein ( 28 ) without the need for adjuvants ( 26 ). The specificity and flexibility of the platform allow for iterative improvements in protein design and make the mRNA approach intrinsically faster and scalable up to hundreds of millions of doses ( 28 , 32 ).
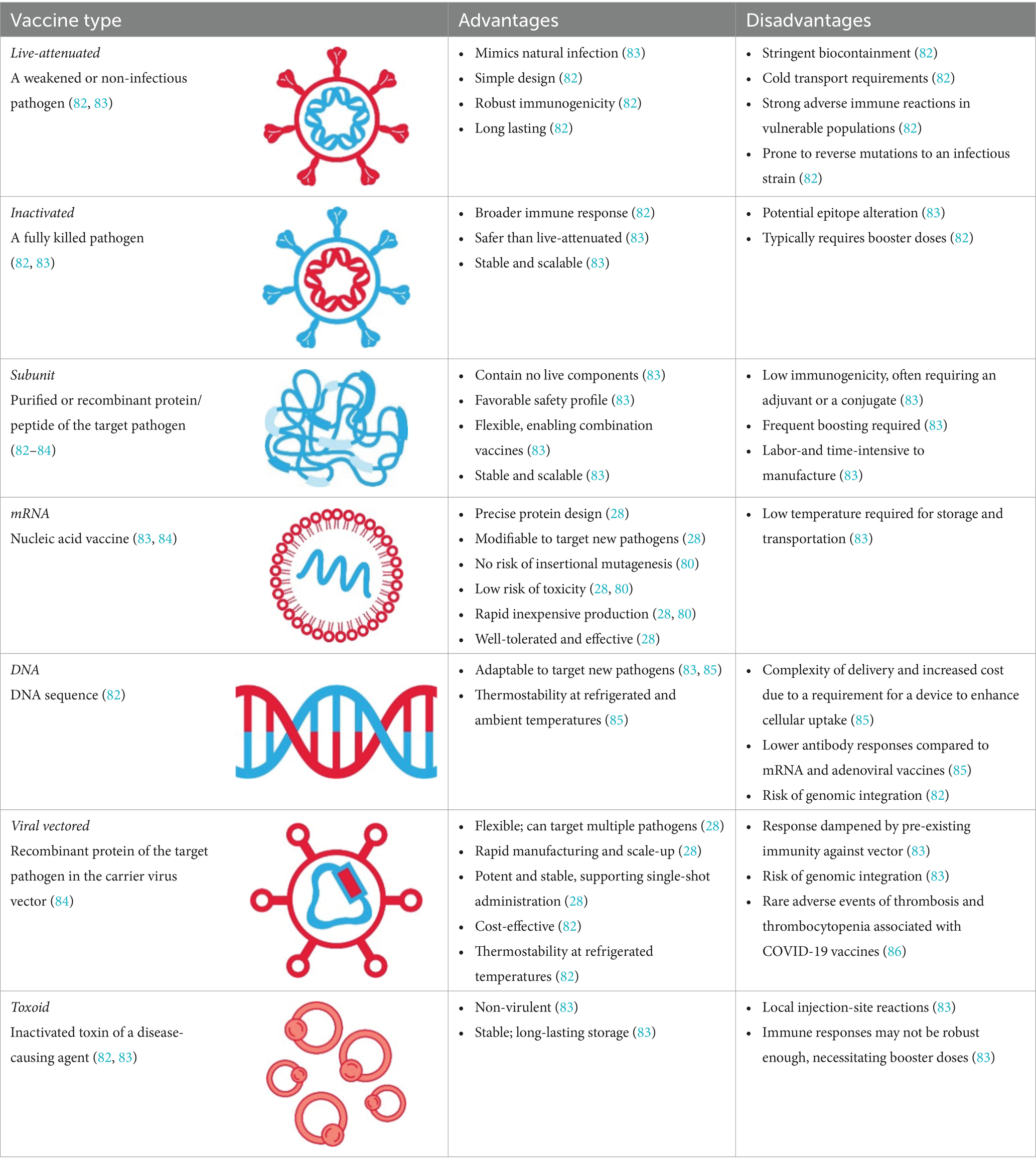
Table 1 . Summary of key differences between the mRNA platform and other vaccine technologies.
Since the discovery of mRNA in 1961, its medical application has been hampered by various factors, including short half-life and inflammatory properties ( 28 , 33 , 34 ). A breakthrough discovery in 2005 showing that replacing uridine with pseudouridine decreased the degree of mRNA-driven inflammation ( 28 , 34 , 35 ), and additional technological advancements in encapsulating mRNA in LNPs were the key milestones underlying the development of mRNA vaccines ( 16 , 31 ). With the declaration of the COVID-19 pandemic, two mRNA COVID-19 vaccines, mRNA-1273 (Spikevax, Moderna, Inc., Cambridge, MA, United States) and BNT162b2 (Comirnaty; Pfizer, Inc. New York, NY, United States) were among the first vaccines against SARS-CoV-2 authorized for emergency use worldwide ( 15 ). These approvals were based on the data from pivotal Phase 3 randomized clinical trials involving >30,000 participants, which demonstrated high efficacy (>90%) and a favorable risk–benefit profile ( 36 , 37 ). The mRNA platform was applied to the development of variant-adapted vaccines to target SARS-CoV-2 variants as they emerged ( 38 , 39 ). Extensive post-licensure real-world data attest to the safety and effectiveness of mRNA vaccines in curbing COVID-19–associated morbidity and mortality ( 40 , 41 ). These data were valuable for expanding the landscape of mRNA vaccines and therapeutics beyond COVID-19; numerous mRNA vaccines have entered clinical development for respiratory syncytial virus, Zika virus, HIV, influenza, cytomegalovirus, varicella-zoster, and rabies virus ( 42 ).

3 Implementation of the mRNA platform: lessons from COVID-19
Several lessons from the COVID-19 pandemic can be leveraged to improve on implementation of mRNA vaccines and medicines.
3.1 Promoting transparent and accurate information-sharing to enhance uptake of novel treatments
Early in the course of pandemic, only 50–60% of the surveyed global population reported willingness to receive a COVID-19 vaccine ( 43 ). Concerns about long-term effects, low confidence in efficacy, unprecedented speed of development, and lack of communication from trusted providers were identified as barriers to COVID-19 vaccine uptake ( 43 ). Vaccine hesitancy was more prevalent in certain demographic groups, including younger age, Black race, Hispanic ethnicity, and lower educational attainment ( 4 , 43 – 45 ). The degree of vaccine hesitancy among healthcare workers was concerning in some countries, as this population is regarded as a trusted source of information regarding COVID-19 ( 3 , 43 ). Public uncertainty around non-pharmaceutical interventions (e.g., masking) and frequent revisions to vaccine policy recommendations further fueled mistrust in COVID-19 vaccination ( 46 ). For example, at the beginning of vaccination campaigns, the advice was that only one or two doses (depending upon the vaccine brand) would be needed, and no booster ( 47 ). Subsequent recognition of the reduced vaccine effectiveness in the context of emerging variants led to the recommendation of booster shots ( 48 ). In addition, mixing of vaccine brands, initially discouraged, was ultimately encouraged after finding this improved the immune response ( 49 , 50 ).
The concerns about the short- and long-term side effects of the COVID-19 vaccine were echoed in parents of children aged 5–11 years following the authorization of COVID-19 vaccines for pediatric populations ( 3 , 46 ). Despite the established benefit–risk profile of mRNA COVID-19 vaccines ( 51 ), acceptable safety profiles ( 52 , 53 ), and the rarity of post-vaccination myocarditis in the general population ( 51 , 54 ), there were parental concerns about reactions to the vaccine, fertility issues, and myocarditis, while confusion around vaccine booster recommendations fueled vaccine hesitancy ( 46 ). Motivators among parents that drove vaccine uptake for children included protection from COVID-19 and multifaceted impact of disruptions to schooling (e.g., children missing school or falling behind) ( 46 ).
These findings underscore the importance of disseminating transparent, consistent, and evidence-based messaging, to ensure confidence in and enhanced uptake of novel treatments.
3.2 Supporting sectors that emerged as trusted sources of information during the COVID-19 pandemic
3.2.1 employers.
The Edelman Trust Barometer, a globally deployed online survey of the general population that included responses from ~33,000 individuals in 28 countries, revealed key shifts in public trust as the COVID-19 pandemic evolved ( 55 – 57 ). In May 2020, government was the institution most trusted by the public, compared with the media, non-government organizations (NGOs), and businesses, with increases in public trust of 5–24% since January 2020 in 10 of 11 countries surveyed, as determined by the Trust Barometer ( 55 ). By January 2021, trust in government had declined by an average of 8% globally; businesses emerged as the only institution trusted as both competent and ethical, with employers (76%) replacing other institutions (NGOs, 57%; government, 53%) as trusted sources of information ( 55 ). In 2022, trust in government and the media declined further, with a greater proportion of individuals perceiving these institutions as divisive (48 and 46%, respectively) rather than unifying force in society (36 and 35%); by contrast, businesses and NGOs were more frequently perceived as unifying (45 and 50%, respectively) than divisive (31 and 29%) ( 56 ).
A measurable impact of the role of employers during the COVID-19 pandemic was evidenced in a cross-sectional study of nursing and social-care employees in Austria, where employer recommendation affected the decision to vaccinate against COVID-19 in 19% of the 625 participants ( 58 ). These findings were echoed in a survey of 400 US-based companies, reporting that employer vaccine-adoption strategies centered on increasing conviction (e.g., sharing scientifically accurate resources), convenience (e.g., setting up onsite vaccination clinics), and reducing the cost (e.g., covering direct costs associated with vaccination) would encourage vaccination in the majority of employees ( 59 ). A viable strategy to enhance uptake is therefore to encourage vaccination through employers by disseminating evidence-based information and providing practical support. Notably, while employers appeared hesitant to mandate vaccination as a condition of employment ( 60 ), mandated vaccination seemed to have little impact on decision to vaccinate in unvaccinated employees, with 74.3% of participants responding they would rather lose their job than get vaccinated ( 58 ).
3.2.2 Healthcare providers
The global response to the COVID-19 pandemic was primarily led by government, who took on the role of recommending and implementing control measures ( 61 ). The government response was prone to politicization and divisiveness ( 56 , 62 , 63 ), and, due to the speed of the pandemic, HCPs were not necessarily involved in the traditional way during COVID-19 vaccination campaigns. Trust in HCPs was, however, reported to be greater than in government agencies ( 63 , 64 ), and was positively associated with COVID-19 vaccine behaviors in multiple studies ( 46 , 63 ). A qualitative study from the United States found that HCPs were the most trusted sources of information on COVID-19 vaccinations among parents ( 46 ). Furthermore, trust in physicians was associated with COVID-19 vaccine uptake among adults in the USA; it was estimated that increasing this trust could induce at least 10% increase in vaccine and booster uptake ( 63 ). The HCPs, therefore, seem to be uniquely positioned to educate communities and support uptake of novel vaccines.
3.3 Equitable healthcare requires expansion of health campaigns and clinical trials to be more inclusive
Enhancing inclusion of minority groups in healthcare and representation of historically marginalized communities in clinical trials is vital to ensure trust in the development of new vaccines and therapeutics, and ultimately, equitable healthcare.
Barriers to access COVID-19 vaccines were highlighted by the disproportionate burden of COVID-19 disease on certain ethnic and racial minority groups, arising from deep-rooted structural, social, and healthcare inequalities ( 65 – 68 ). Vaccine hesitancy in the United States was more prevalent in minority groups that were disproportionately affected by the pandemic, including African Americans (41.6%) and Hispanic individuals (30.2%), as compared with the general US population (26.3%) ( 10 , 66 ). Medical mistrust, lack of information on COVID-19 vaccines, and social disadvantage were among factors associated with increased vaccine hesitancy among these groups ( 10 ).
In 2021, 62% of the global population agreed with the statement that the pandemic was amplifying existing inequities worldwide ( 55 ). The well-documented disparity between high-and low-income populations on the Edelman Trust Barometer was especially notable in 2022 (62 vs. 47%) ( 56 ). Concerted efforts have been made to address some causes of inequity such as racial and ethnic disparities through targeted enrollments in clinical trials, including community outreach initiatives and careful monitoring of enrollment demographics to ensure rapid revision of recruitment strategies ( 67 ). Best practices which were built from past experience, with the participation of community and patient advocates in HIV research, were instrumental in driving positive change in the conduct of HIV trials in relation to participant recruitment, study design, and dissemination of findings ( 69 ). This highlights the importance of engaging community members in clinical research to raise the profile of novel therapies in the general public.
Targeted campaigns to increase healthcare availability for minority groups and improving diversity in clinical trials are viable strategies for building trust and ensuring equitable access to benefits of novel healthcare interventions, including mRNA vaccines and therapeutics.
The GLP is an initiative dedicated to generating insights into the key dimensions of societal preparedness as a way of building societal cohesion to better prepare society in times of crisis ( 18 ). The initiative draws upon quantitative and qualitative research to describe public perceptions and experiences of the COVID-19 pandemic in an effort to establish a foundational metric of public preparedness, a Societal Preparedness Index, for future emergencies ( 18 ).
The GLP survey (July 2023–September 2023) involved conducting interviews online, face-to-face, or via computer-assisted telephone in nationally representative samples in 70 countries. To be eligible for inclusion in the survey, respondents were required to be over the age of 18 years and a resident of the country where the survey was administered. To obtain a representative population, probability sampling was used for the face-to-face and computer-assisted telephone interviews. For online interviews, respondents from online panels were invited to participate, with quotas for age, gender, and region set to reflect the demographics of the national population.
The survey revealed stark geographic and demographic disparities in the experience of the COVID-19 pandemic and perceptions of mRNA vaccines and medicines. Among 70,781 participants who were interviewed on the mRNA vaccine acceptance, 66% affirmed that they would accept a newly approved mRNA vaccine to protect themselves; however, wide disparities were observed both by country and gender ( Figure 1 ). In the United States and United Kingdom, the percentage of participants who were willing to accept the new mRNA vaccine was higher than average (73 and 68%, respectively). Countries where less than half of the interviewed population expressed willingness to accept the new mRNA vaccine were South Africa (37%) and central/northeastern European states (41–49%), whereas the highest level of acceptance was observed in Sierra Leone (87%). Globally, more men (70%) than women (63%) were willing to accept the new mRNA vaccine, whereas no stark age disparities were observed (18–34 years, 67%; 35–54 years: 63%; ≥55 years: 69%). Among participants who have heard of vaccines or medicines that use mRNA ( n = 4,808), the majority agreed that mRNA vaccines were important (73%), effective (72%), and safe (68%); however, agreement was more prevalent among men (72–76%) than women (63–69%). Further, among those who reported being aware of mRNA, more than half (60%) reported that they had little knowledge about mRNA, whereas less than one-third (29%) reported they knew a lot, highlighting a discrepancy between the low prevalence of knowledge on mRNA vaccines/medicines and high prevalence of favorable perceptions on safety and efficacy of mRNA vaccines.
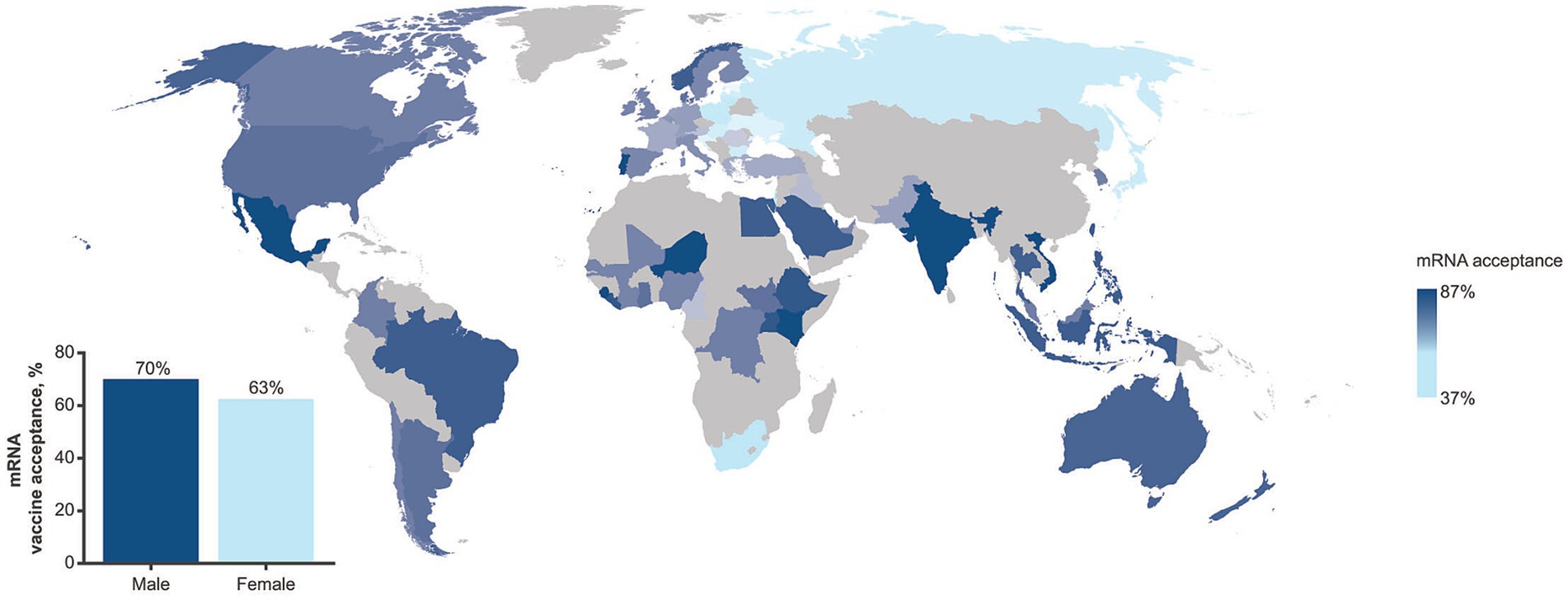
Figure 1 . Prevalence in mRNA vaccine acceptance as assessed in the GLP survey (July 2023 – September 2023) by geographic region. The inset shows prevalence by gender. The GLP survey involved more than 70,000 completed interviews in nationally representative samples from 70 countries.
Challenges with acceptance of novel therapeutics are not unique to mRNA-vaccines and have been observed globally in non-emergency situations including with stem cell and gene therapy. Since its nascency, public perception of the benefits and risks of stem cell therapy has varied ( 70 ); studies have reported varying levels of trust and acceptance between countries ( 71 ) and higher levels of trust among older adults (50 years of age or above) regardless of gender ( 72 ). Similarly, attitudes toward gene therapy and gene editing also have been met with varying and complex levels of public acceptance with concerns for this therapy found to be linked to a lack of trust, education, and knowledge of risks and benefits ( 73 – 75 ) suggesting that continuous engagement with the public is needed to address concerns with the adoption of new medicines. The Edelman Trust Barometer Global Report for 2024 indicated sex-based differences in the acceptance of gene-based medicine, with 31% of men and 26% of women supporting gene-based medicine ( 76 ); these observations are similar to those observed with the GLP survey regarding mRNA-based vaccines. Notably, vaccine hesitancy was a challenge prior to the COVID-19 pandemic with individuals, including HCPs, choosing to delay or refuse various vaccines, possibly influenced by concerns over vaccine safety, and a lack of knowledge and motivation to get vaccinated ( 77 , 78 ). Existing attitudes of vaccine hesitancy potentially influenced attitudes to COVID-19 vaccines since individuals are more likely to favor information that aligns with their existing beliefs ( 79 ). The data patterns emerging from the GLP global survey provide actionable insights to tailor strategies to increase awareness of mRNA-based vaccines and therapeutics for target populations. Among participants who were asked to report comfort with innovations in healthcare ( n = 9,651), fewer women (33%) than men (38%) reported being comfortable with mRNA-based innovations. The attributes deemed most important for accepting a new vaccine/medicine among interviewed participants ( n = 11,214) were proven safety (83%) and efficacy (82%), suggesting that campaigns designed to build confidence in those attributes could contribute to improving uptake. In addition, the GLP survey and related interviews revealed that the term “technology” in descriptions of mRNA-based medicines prompted negative perceptions. Public discourse and educational campaigns would therefore benefit from describing mRNA not in terms of a “technology” but as a new science-based approach to developing vaccines and therapeutics.
5 mRNA as a new class of medicine: application to therapeutic areas beyond infectious diseases
In addition to their application to infectious disease prevention, mRNA therapeutic approaches are being developed in oncology to induce immune-targeting responses by encoding proteins that attack and control tumors ( 42 ). Numerous mRNA therapeutic candidates against cancer are currently under investigation in clinical trials as monotherapies or combination therapies for a range of disease states, however, no mRNA-based cancer therapeutic has been approved to date ( 42 , 80 ).
The capacity of mRNA to induce therapeutically relevant expression of proteins that is suitable for substituting malfunctioning or absent proteins has applications in both rare and chronic disease ( 33 , 81 ). Several mRNA-based protein replacement therapies have entered phase 1 and 2 clinical trials, including LNP-encapsulated mRNA for the treatment of dysmetabolic disorders (Moderna) and cystic fibrosis (Translate Bio), and naked mRNAs for the treatment of ulcers in type 2 diabetes and heart failure (Moderna/AstraZeneca) ( 81 ). Application of mRNA vaccines in autoimmune disease is currently at the preclinical stage; however, the experimental data accrued thus far suggest that the mRNA platform is suitable for the delivery of proteins to modulate misguided immune responses in a range of autoimmune and allergic conditions ( 42 ).
Taken together, the attributes of mRNA-based products differ from other known approaches in medicine as they utilize innate biology to manufacture a broad range of preventive or therapeutic interventions, with the potential for rapid iteration. There is an urgency to apply the learnings on mRNA uptake from the pandemic and promote a broader level of confidence in this platform.
6 Conclusion
The cardinal feature of mRNA-based medicines is that they use intrinsic cellular mechanisms to generate proteins with therapeutic or prophylactic properties. Many decades of laboratory research in mRNA paved the way for the accelerated development of mRNA vaccines in response to the COVID-19 pandemic. However, despite favorable safety and efficacy profiles of approved mRNA COVID-19 vaccines, vaccine hesitancy was notable in the public, especially among minority and socially disadvantaged groups. As a trusted source of information, HCPs are well placed to take a greater role in building trust and discouraging the spread of misinformation. Employers are also uniquely positioned to support uptake of novel interventions during healthcare crises through transparent communication and provision of practical support to their workforce. Data from the GLP survey presented herein revealed tremendous disparities in willingness to accept new mRNA vaccines and medicines across countries, identifying women as a demographic group that should be prioritized for confidence-building strategies around mRNA vaccines and therapeutics. Concrete plans to enhance public trust and confidence in novel medicines, including the rapidly advancing field of mRNA-based therapeutics, are critical to improve clinical outcomes, reduce disease burden, and enhance the societal capacity to manage future healthcare crises.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Global Listening Project: http://www.global-listening.org .
Ethics statement
The studies involving humans were approved by the European Society for Opinion and Marketing Research. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because survey respondents had the opportunity to opt in/out of the survey and to exit the survey whenever they needed to. Respondents were also given the option to refuse to answer or state that they did not know an answer for all questions.
Author contributions
SMI: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis. AMR: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis. DE: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis. AB: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Conceptualization. HJL: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Conceptualization. MS: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis. MR: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis. AC: Writing – original draft, Writing – review & editing, Conceptualization. FC: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Moderna, Inc. This Global Listening Project 70-country study was supported by funding from GSK, the Bill & Melinda Gates Foundation, and the MacArthur Foundation. The Bill & Melinda Gates Foundation, and the MacArthur Foundation were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
Medical writing and editorial assistance were provided by Jessica Nepomuceno, PhD and Andreja Varjacic, PhD of MEDiSTRAVA in accordance with Good Publication Practice (GPP 2022) guidelines.
Conflict of interest
SMI, AMR, DE, MS, MR, AC, and FC are employees of Moderna, Inc., and hold stock/stock options in the company. HJL received research grants from GSK and Moderna related to the Global Listening Project.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Moderna, Inc. and GSK. Moderna, Inc. had the following involvement in the study: study design and analysis, preparation of the manuscript, and decision to publish. GSK had the following involvement in the study: study design.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wernette, R, Bhatnagar, B, and Bazant, E. Defining health campaigns and health campaign effectiveness . Decatur, GA: The Task Force for Global Health (2020).
Google Scholar
2. Ajana, B, Engstler, E, Ismail, A, and Kousta, M. Perceptions and attitudes towards COVID-19 vaccines: narratives from members of the UK public. Z Gesundh Wiss . (2022) 31:1699–715. doi: 10.1007/s10389-022-01728-w
PubMed Abstract | Crossref Full Text | Google Scholar
3. Iftekhar, EN, Priesemann, V, Balling, R, Bauer, S, Beutels, P, Vladez, AC, et al. A look into the future of the COVID-19 pandemic in Europe: an expert consultation. Lancet Region Health Eur . (2021) 8:100185. doi: 10.1016/j.lanepe.2021.100185
Crossref Full Text | Google Scholar
4. Fischer, R, and Karl, JA. Predicting behavioral intentions to prevent or mitigate COVID-19: a cross-cultural meta-analysis of attitudes, norms, and perceived behavioral control effects. Soc Psychol Personal Sci . (2022) 13:264–76. doi: 10.1177/19485506211019844
5. Galadima, AN, Zulkefli, NAM, Said, SM, and Ahmad, N. Factors influencing childhood immunisation uptake in Africa: a systematic review. BMC Public Health . (2021) 21:1475. doi: 10.1186/s12889-021-11466-5
6. Gallagher, KE, Kadokura, E, Eckert, LO, Miyake, S, Mounier-Jack, S, Aldea, M, et al. Factors influencing completion of multi-dose vaccine schedules in adolescents: a systematic review. BMC Public Health . (2016) 16:172. doi: 10.1186/s12889-016-2845-z
7. Kilich, E, Dada, S, Francis, MR, Tazare, J, Chico, RM, Paterson, P, et al. Factors that influence vaccination decision-making among pregnant women: a systematic review and meta-analysis. PLoS One . (2020) 15:e0234827. doi: 10.1371/journal.pone.0234827
8. Roy, DN, Biswas, M, Islam, E, and Azam, MS. Potential factors influencing COVID-19 vaccine acceptance and hesitancy: a systematic review. PLoS One . (2022) 17:e0265496. doi: 10.1371/journal.pone.0265496
9. Smith, LE, Amlot, R, Weinman, J, Yiend, J, and Rubin, GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine . (2017) 35:6059–69. doi: 10.1016/j.vaccine.2017.09.046
10. Khubchandani, J, and Macias, Y. COVID-19 vaccination hesitancy in Hispanics and African-Americans: a review and recommendations for practice. Brain Behav Immun Health . (2021) 15:100277. doi: 10.1016/j.bbih.2021.100277
11. Shearn, C, and Krockow, EM. Reasons for COVID-19 vaccine hesitancy in ethnic minority groups: a systematic review and thematic synthesis of initial attitudes in qualitative research. SSM Qual Res Health . (2023) 3:100210. doi: 10.1016/j.ssmqr.2022.100210
12. Alemi, F, and Lee, KH. Impact of political leaning on COVID-19 vaccine hesitancy: a network-based multiple mediation analysis. Cureus . (2023) 15:e43232. doi: 10.7759/cureus.43232
13. Quantin, C, and Tubert-Bitter, P. COVID-19 and social inequalities: a complex and dynamic interaction. Lancet Public Health . (2022) 7:e204–5. doi: 10.1016/s2468-2667(22)00033-0
14. The Global Listening Project. (2024). Societal preparedness insights . Available at: https://global-listening.org/societal-preparedness-insights/ (accessed March 21, 2024).
15. Chaudhary, N, Weissman, D, and Whitehead, KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov . (2021) 20:817–38. doi: 10.1038/s41573-021-00283-5
16. Parhiz, H, Atochina-Vasserman, EN, and Weissman, D. mRNA-based therapeutics: looking beyond COVID-19 vaccines. Lancet . (2024) 403:1192–204. doi: 10.1016/s0140-6736(23)02444-3
17. National Institutes of Health (NIH). (2023). Decades in the making: mRNA COVID-19 vaccines . Available at: https://covid19.nih.gov/nih-strategic-response-covid-19/decades-making-mrna-covid-19-vaccines (Accessed February 22, 2024).
18. The Global Listening Project. (2024). Home page 2024 . Available at: https://global-listening.org/ (Accessed February 1, 2024).
19. Edwards, DK, and Carfi, A. Messenger ribonucleic acid vaccines against infectious diseases: current concepts and future prospects. Curr Opin Immunol . (2022) 77:102214. doi: 10.1016/j.coi.2022.102214
20. Nance, KD, and Meier, JL. Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent Sci . (2021) 7:748–56. doi: 10.1021/acscentsci.1c00197
21. National Human Genome Research Institute (NIH). (2024). Messenger RNA (mRNA) . Available at: https://www.genome.gov/genetics-glossary/messenger-rna . (Accessed February 22, 2024).
22. Pardi, N, Hogan, MJ, Porter, FW, and Weissman, D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov . (2018) 17:261–79. doi: 10.1038/nrd.2017.243
23. Matsumura, T, Takano, T, and Takahashi, Y. Immune responses related to the immunogenicity and reactogenicity of COVID-19 mRNA vaccines. Int Immunol . (2023) 35:213–20. doi: 10.1093/intimm/dxac064
24. Park, JW, Lagniton, PNP, Liu, Y, and Xu, RH. mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci . (2021) 17:1446–60. doi: 10.7150/ijbs.59233
25. John, S, Yuzhakov, O, Woods, A, Deterling, J, Hassett, K, Shaw, CA, et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine . (2018) 36:1689–99. doi: 10.1016/j.vaccine.2018.01.029
26. Rijkers, GT, Weterings, N, Obregon-Henao, A, Lepolder, M, Dutt, TS, van Overveld, FJ, et al. Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2 vaccines. Vaccine . (2021) 9:848. doi: 10.3390/vaccines9080848
27. Ci, L, Hard, M, Zhang, H, Gandham, S, Hua, S, Wickwire, J, et al. Biodistribution of lipid 5, mRNA, and its translated protein following intravenous administration of mRNA-encapsulated lipid nanoparticles in rats. Drug Metab Dispos . (2023) 51:813–23. doi: 10.1124/dmd.122.000980
28. Gebre, MS, Brito, LA, Tostanoski, LH, Edwards, DK, Carfi, A, and Barouch, DH. Novel approaches for vaccine development. Cell . (2021) 184:1589–603. doi: 10.1016/j.cell.2021.02.030
29. Sharova, LV, Sharov, AA, Nedorezov, T, Piao, Y, Shaik, N, and Ko, MS. Database for mRNA half-life of 19 977 genes obtained by DNA microarray analysis of pluripotent and differentiating mouse embryonic stem cells. DNA Res . (2009) 16:45–58. doi: 10.1093/dnares/dsn030
30. Cheng, F, Wang, Y, Bai, Y, Liang, Z, Mao, Q, Liu, D, et al. Research advances on the stability of mRNA vaccines. Viruses . (2023) 15:668. doi: 10.3390/v15030668
31. Burdette, D, Ci, L, Shilliday, B, Slauter, R, Auerbach, A, Kenney, M, et al. Systemic exposure, metabolism, and elimination of [(14)C]-labeled amino lipid, lipid 5, after a single administration of mRNA encapsulating lipid nanoparticles to Sprague-Dawley rats. Drug Metab Dispos . (2023) 51:804–12. doi: 10.1124/dmd.122.001194
32. Urdaneta, V, Esposito, DB, Dharia, P, Moraga, MS, Anteyi, K, Oduyebo-Omotosho, T, et al. Global safety assessment of adverse events of special interest following 2 years of use and 772 million administered doses of mRNA-1273. Open forum. Infect Dis . (2024) 11:ofae067. doi: 10.1093/ofid/ofae067
33. Sahin, U, Karikó, K, and Türeci, Ö. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov . (2014) 13:759–80. doi: 10.1038/nrd4278
34. Karikó, K. Modified uridines are the key to a successful message. Nat Rev Immunol . (2021) 21:619. doi: 10.1038/s41577-021-00608-w
35. The Nobel Prize. (2023). The Nobel prize in physiology or medicine 2023 press release . Available at: https://www.nobelprize.org/prizes/medicine/2023/press-release/
36. Baden, LR, Essink, B, Kotloff, K, Frey, S, Novak, R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med . (2021) 384:403–16. doi: 10.1056/NEJMoa2035389
37. Polack, FP, Thomas, SJ, Kitchin, N, Absalon, J, Gurtman, A, Lockhart, S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med . (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
38. Echaide, M, Chocarro de Erauso, L, Bocanegra, A, Blanco, E, Kochan, G, and Escors, D. mRNA vaccines against SARS-CoV-2: advantages and caveats. Int J Mol Sci . (2023) 24:5944. doi: 10.3390/ijms24065944
39. Mir, S, and Mir, M. The mRNA vaccine, a swift warhead against a moving infectious disease target. Expert Rev Vaccines . (2024) 23:336–48. doi: 10.1080/14760584.2024.2320327
40. Xu, W, Ren, W, Wu, T, Wang, Q, Luo, M, Yi, Y, et al. Real-world safety of COVID-19 mRNA vaccines: a systematic review and meta-analysis. Vaccine . (2023) 11. doi: 10.3390/vaccines11061118
41. Zheng, C, Shao, W, Chen, X, Zhang, B, Wang, G, and Zhang, W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis . (2022) 114:252–60. doi: 10.1016/j.ijid.2021.11.009
42. Zhang, G, Tang, T, Chen, Y, Huang, X, and Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct Target Ther . (2023) 8:365. doi: 10.1038/s41392-023-01579-1
43. Razai, MS, Chaudhry, UAR, Doerholt, K, Bauld, L, and Majeed, A. Covid-19 vaccination hesitancy. BMJ . (2021) 373:n1138. doi: 10.1136/bmj.n1138
44. Al-Obaydi, S, Hennrikus, E, Mohammad, N, Lehman, EB, Thakur, A, and Al-Shaikhly, T. Hesitancy and reactogenicity to mRNA-based COVID-19 vaccines-early experience with vaccine rollout in a multi-site healthcare system. PLoS One . (2022) 17:e0272691. doi: 10.1371/journal.pone.0272691
45. Coustasse, A, Kimble, C, and Maxik, K. COVID-19 and vaccine hesitancy: a challenge the United States must overcome. J Ambul Care Manage . (2021) 44:71–5. doi: 10.1097/JAC.0000000000000360
46. Goulding, M, Ryan, GW, Minkah, P, Borg, A, Gonzalez, M, Medina, N, et al. Parental perceptions of the COVID-19 vaccine for 5-to 11-year-old children: focus group findings from Worcester Massachusetts. Hum Vaccin Immunother . (2022) 18:2120721. doi: 10.1080/21645515.2022.2120721
47. Bert, F, Scaioli, G, Vola, L, Accortanzo, D, Lo Moro, G, and Siliquini, R. Booster doses of anti COVID-19 vaccines: an overview of implementation policies among OECD and EU countries. Int J Environ Res Public Health . (2022) 19. doi: 10.3390/ijerph19127233
48. World Health Organization. WHO SAGE roadmap for prioritizing uses of COVID-19 vaccines . (2023). Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2023.1 (accessed November 10, 2023).
49. Mahase, E. Covid-19: vaccine brands can be mixed in “extremely rare occasions,” says Public Health England. BMJ . (2021) 372:n12. doi: 10.1136/bmj.n12
50. Capurro, G, Tustin, J, Jardine, CG, and Driedger, SM. When good messages go wrong: perspectives on COVID-19 vaccines and vaccine communication from generally vaccine accepting individuals in Canada. Hum Vaccin Immunother . (2022) 18:2145822. doi: 10.1080/21645515.2022.2145822
51. Klamer, TA, Linschoten, M, and Asselbergs, FW. The benefit of vaccination against COVID-19 outweighs the potential risk of myocarditis and pericarditis. Neth Heart J . (2022) 30:190–7. doi: 10.1007/s12471-022-01677-9
52. Anderson, EJ, Creech, CB, Berthaud, V, Piramzadian, A, Johnson, KA, Zervos, M, et al. Evaluation of mRNA-1273 vaccine in children 6 months to 5 years of age. N Engl J Med . (2022) 387:1673–87. doi: 10.1056/NEJMoa2209367
53. Creech, CB, Anderson, E, Berthaud, V, Yildirim, I, Atz, AM, Melendez Baez, I, et al. Evaluation of mRNA-1273 Covid-19 vaccine in children 6 to 11 years of age. N Engl J Med . (2022) 386:2011–23. doi: 10.1056/NEJMoa2203315
54. Marschner, CA, Shaw, KE, Tijmes, FS, Fronza, M, Khullar, S, Seidman, MA, et al. Myocarditis following COVID-19 vaccination. Heart Fail Clin . (2023) 19:251–64. doi: 10.1016/j.hfc.2022.08.012
55. Edelman Trust Barometer. (2021). Available at: https://www.edelman.com/trust/2021-trust-barometer (Accessed April 18, 2024).
56. Edelman Trust Barometer. (2022). Available at: https://www.edelman.com/trust/2022-trust-barometer (Accessed April 18, 2024).
57. About the Edelman Trust Barometer. (2024). Available at: https://www.edelman.com/trust/our-methodology (Accessed April 18, 2024).
58. Ruf, AK, Volkl-Kernstock, S, Eitenberger, M, Gabriel, M, Klager, E, Kletecka-Pulker, M, et al. Employer impact on COVID-19 vaccine uptake among nursing and social care employees in Austria. Front Public Health . (2022) 10:1023914. doi: 10.3389/fpubh.2022.1023914
59. Azimi, T, Heller, H, Latkovic, T, and Sabow, A. (2021). Pharmaceuticals and medical products practice. getting to work: employers’ role in COVID-19 vaccination . Available at: https://www.trsa.org/wp-content/uploads/2021/08/McKinsey-Employers-role-in-covid-19-vaccination_final.pdf (Accessed April 18, 2024)
60. Berkman, BE, Miner, SA, Wendler, DS, and Grady, C. The ethics of encouraging employees to get the COVID-19 vaccination. J Public Health Policy . (2022) 43:311–9. doi: 10.1057/s41271-022-00347-9
61. Tabari, P, Amini, M, Moghadami, M, and Moosavi, M. International public health responses to COVID-19 outbreak: a rapid review. Iran J Med Sci . (2020) 45:157–69. doi: 10.30476/ijms.2020.85810.1537
62. Gelfand, M, Li, R, Stamkou, E, Pieper, D, Denison, E, Fernandez, J, et al. Persuading republicans and democrats to comply with mask wearing: an intervention tournament. J Exp Soc Psychol . (2022) 101:104299. doi: 10.1016/j.jesp.2022.104299
63. Silver, D, Kim, Y, Piltch-Loeb, R, and Abramson, D. One year later: what role did trust in public officials and the medical profession play in decisions to get a booster and to overcome vaccine hesitancy? Prev Med Rep . (2024) 38:102626. doi: 10.1016/j.pmedr.2024.102626
64. Edelman Trust Barometer. Special report: Trust and health . (2023). Available at: https://www.edelman.com/trust/2023/trust-barometer/special-report-health (Accessed April 18, 2024).
65. Badalov, E, Blackler, L, Scharf, AE, Matsoukas, K, Chawla, S, Voigt, LP, et al. COVID-19 double jeopardy: the overwhelming impact of the social determinants of health. Int J Equity Health . (2022) 21:76. doi: 10.1186/s12939-022-01629-0
66. Evans, MK. Covid's color line - infectious disease, inequity, and racial justice. N Engl J Med . (2020) 383:408–10. doi: 10.1056/NEJMp2019445
67. Hill, J, Montross, D, and Ivarsson, M. Diversity and inclusion in clinical trials: evolution throughout the development of an mRNA COVID-19 vaccine. Front Public Health . (2023) 11:1113003. doi: 10.3389/fpubh.2023.1113003
68. Tai, DBG, Shah, A, Doubeni, CA, Sia, IG, and Wieland, ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis . (2021) 72:703–6. doi: 10.1093/cid/ciaa815
69. Karris, MY, Dubé, K, and Moore, AA. What lessons it might teach us? Community engagement in HIV research. Curr Opin HIV AIDS . (2020) 15:142–9. doi: 10.1097/coh.0000000000000605
70. Downey, R, and Geransar, R. Stem cell research, publics' and stakeholder views. Health L Rev. (2008) 16:2
71. Shineha, R, Inoue, Y, and Yashiro, Y. A comparative analysis of attitudes toward stem cell research and regenerative medicine between six countries – a pilot study. Regener Ther . (2022) 20:187–93. doi: 10.1016/j.reth.2022.04.007
72. Aboalola, D, Ramadan, M, Baadhaim, M, Alsiary, R, Badraiq, H, Alghamdi, T, et al. Public awareness and understanding of stem cell treatments available in Saudi Arabia and their trust in hospitals and research centers involved in stem cell research—a cross sectional study. Front Public Health . (2024) 12:1364809. doi: 10.3389/fpubh.2024.1364809
73. Delhove, J, Osenk, I, Prichard, I, and Donnelley, M. Public acceptability of gene therapy and gene editing for human use: a systematic review. Hum Gene Ther . (2020) 31:20–46. doi: 10.1089/hum.2019.197
74. McFadden, BR, Rumble, JN, Stofer, KA, and Folta, KM. U.S. public opinion about the safety of gene editing in the agriculture and medical fields and the amount of evidence needed to improve opinions. Front Bioeng Biotechnol . (2024) 12:1340398. doi: 10.3389/fbioe.2024.1340398
75. Li, Y, Zhang, X, Xiang, Z, Chen, T, Hu, Z, Yang, K, et al. Public attitudes about the use of gene therapy in mainland China. JAMA Netw Open . (2023) 6:e2328352–2. doi: 10.1001/jamanetworkopen.2023.28352
76. 2024 Edelman Trust Barometer Global Report. (2024). Available at: https://edelman.com/trust/2024/trust-barometer (Accessed April 18, 2024).
77. Paterson, P, Meurice, F, Stanberry, LR, Glismann, S, Rosenthal, SL, and Larson, HJ. Vaccine hesitancy and healthcare providers. Vaccine . (2016) 34:6700–6. doi: 10.1016/j.vaccine.2016.10.042
78. Larson, HJ, Jarrett, C, Eckersberger, E, Smith, DM, and Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine . 32:2150–9. doi: 10.1016/j.vaccine.2014.01.081
79. Lazarus, JV, White, TM, Wyka, K, Ratzan, SC, Rabin, K, Larson, HJ, et al. Influence of COVID-19 on trust in routine immunization, health information sources and pandemic preparedness in 23 countries in. Nat Med . (2023) 30:1559–63. doi: 10.1038/s41591-024-02939-2
80. Lorentzen, CL, Haanen, JB, Met, Ö, and Svane, IM. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol . (2022) 23:e450–8. doi: 10.1016/s1470-2045(22)00372-2
81. Vavilis, T, Stamoula, E, Ainatzoglou, A, Sachinidis, A, Lamprinou, M, Dardalas, I, et al. mRNA in the context of protein replacement therapy. Pharmaceutics . (2023) 15:166. doi: 10.3390/pharmaceutics15010166
82. Jain, S, Venkataraman, A, Wechsler, ME, and Peppas, NA. Messenger RNA-based vaccines: past, present, and future directions in the context of the COVID-19 pandemic. Adv Drug Deliv Rev . (2021) 179:114000. doi: 10.1016/j.addr.2021.114000
83. Kozak, M, and Hu, J. The integrated consideration of vaccine platforms, adjuvants, and delivery routes for successful vaccine development. Vaccine . (2023) 11:695. doi: 10.3390/vaccines11030695
84. Pollard, AJ, and Bijker, EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol . (2021) 21:83–100. doi: 10.1038/s41577-020-00479-7
85. Maslow, JN, Kwon, I, Kudchodkar, SB, Kane, D, Tadesse, A, Lee, H, et al. DNA vaccines for epidemic preparedness: SARS-CoV-2 and beyond. Vaccines . (2023) 11:1016. doi: 10.3390/vaccines11061016
86. Lamprinou, M, Sachinidis, A, Stamoula, E, Vavilis, T, and Papazisis, G. COVID-19 vaccines adverse events: potential molecular mechanisms. Immunol Res . (2023) 71:356–72. doi: 10.1007/s12026-023-09357-5
Keywords: mRNA vaccines and therapeutics, COVID-19, mRNA vaccine development, public trust, vaccine hesitancy, vaccine confidence
Citation: Iqbal SM, Rosen AM, Edwards D, Bolio A, Larson HJ, Servin M, Rudowitz M, Carfi A and Ceddia F (2024) Opportunities and challenges to implementing mRNA-based vaccines and medicines: lessons from COVID-19. Front. Public Health . 12:1429265. doi: 10.3389/fpubh.2024.1429265
Received: 07 May 2024; Accepted: 12 July 2024; Published: 08 August 2024.
Reviewed by:
Copyright © 2024 Iqbal, Rosen, Edwards, Bolio, Larson, Servin, Rudowitz, Carfi and Ceddia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shehzad M. Iqbal, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
COVID-19: Long-term effects
Some people continue to experience health problems long after having COVID-19. Understand the possible symptoms and risk factors for post-COVID-19 syndrome.
Most people who get coronavirus disease 2019 (COVID-19) recover within a few weeks. But some people — even those who had mild versions of the disease — might have symptoms that last a long time afterward. These ongoing health problems are sometimes called post- COVID-19 syndrome, post- COVID conditions, long COVID-19 , long-haul COVID-19 , and post acute sequelae of SARS COV-2 infection (PASC).
What is post-COVID-19 syndrome and how common is it?
Post- COVID-19 syndrome involves a variety of new, returning or ongoing symptoms that people experience more than four weeks after getting COVID-19 . In some people, post- COVID-19 syndrome lasts months or years or causes disability.
Research suggests that between one month and one year after having COVID-19 , 1 in 5 people ages 18 to 64 has at least one medical condition that might be due to COVID-19 . Among people age 65 and older, 1 in 4 has at least one medical condition that might be due to COVID-19 .
What are the symptoms of post-COVID-19 syndrome?
The most commonly reported symptoms of post- COVID-19 syndrome include:
- Symptoms that get worse after physical or mental effort
- Lung (respiratory) symptoms, including difficulty breathing or shortness of breath and cough
Other possible symptoms include:
- Neurological symptoms or mental health conditions, including difficulty thinking or concentrating, headache, sleep problems, dizziness when you stand, pins-and-needles feeling, loss of smell or taste, and depression or anxiety
- Joint or muscle pain
- Heart symptoms or conditions, including chest pain and fast or pounding heartbeat
- Digestive symptoms, including diarrhea and stomach pain
- Blood clots and blood vessel (vascular) issues, including a blood clot that travels to the lungs from deep veins in the legs and blocks blood flow to the lungs (pulmonary embolism)
- Other symptoms, such as a rash and changes in the menstrual cycle
Keep in mind that it can be hard to tell if you are having symptoms due to COVID-19 or another cause, such as a preexisting medical condition.
It's also not clear if post- COVID-19 syndrome is new and unique to COVID-19 . Some symptoms are similar to those caused by chronic fatigue syndrome and other chronic illnesses that develop after infections. Chronic fatigue syndrome involves extreme fatigue that worsens with physical or mental activity, but doesn't improve with rest.
Why does COVID-19 cause ongoing health problems?
Organ damage could play a role. People who had severe illness with COVID-19 might experience organ damage affecting the heart, kidneys, skin and brain. Inflammation and problems with the immune system can also happen. It isn't clear how long these effects might last. The effects also could lead to the development of new conditions, such as diabetes or a heart or nervous system condition.
The experience of having severe COVID-19 might be another factor. People with severe symptoms of COVID-19 often need to be treated in a hospital intensive care unit. This can result in extreme weakness and post-traumatic stress disorder, a mental health condition triggered by a terrifying event.
What are the risk factors for post-COVID-19 syndrome?
You might be more likely to have post- COVID-19 syndrome if:
- You had severe illness with COVID-19 , especially if you were hospitalized or needed intensive care.
- You had certain medical conditions before getting the COVID-19 virus.
- You had a condition affecting your organs and tissues (multisystem inflammatory syndrome) while sick with COVID-19 or afterward.
Post- COVID-19 syndrome also appears to be more common in adults than in children and teens. However, anyone who gets COVID-19 can have long-term effects, including people with no symptoms or mild illness with COVID-19 .
What should you do if you have post-COVID-19 syndrome symptoms?
If you're having symptoms of post- COVID-19 syndrome, talk to your health care provider. To prepare for your appointment, write down:
- When your symptoms started
- What makes your symptoms worse
- How often you experience symptoms
- How your symptoms affect your activities
Your health care provider might do lab tests, such as a complete blood count or liver function test. You might have other tests or procedures, such as chest X-rays, based on your symptoms. The information you provide and any test results will help your health care provider come up with a treatment plan.
In addition, you might benefit from connecting with others in a support group and sharing resources.
- Long COVID or post-COVID conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html. Accessed May 6, 2022.
- Post-COVID conditions: Overview for healthcare providers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Accessed May 6, 2022.
- Mikkelsen ME, et al. COVID-19: Evaluation and management of adults following acute viral illness. https://www.uptodate.com/contents/search. Accessed May 6, 2022.
- Saeed S, et al. Coronavirus disease 2019 and cardiovascular complications: Focused clinical review. Journal of Hypertension. 2021; doi:10.1097/HJH.0000000000002819.
- AskMayoExpert. Post-COVID-19 syndrome. Mayo Clinic; 2022.
- Multisystem inflammatory syndrome (MIS). Centers for Disease Control and Prevention. https://www.cdc.gov/mis/index.html. Accessed May 24, 2022.
- Patient tips: Healthcare provider appointments for post-COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/post-covid-appointment/index.html. Accessed May 24, 2022.
- Bull-Otterson L, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years — United States, March 2020 — November 2021. MMWR Morbidity and Mortality Weekly Report. 2022; doi:10.15585/mmwr.mm7121e1.
Products and Services
- A Book: Endemic - A Post-Pandemic Playbook
- Begin Exploring Women's Health Solutions at Mayo Clinic Store
- A Book: Future Care
- Antibiotics: Are you misusing them?
- COVID-19 and vitamin D
- Convalescent plasma therapy
- Coronavirus disease 2019 (COVID-19)
- COVID-19: How can I protect myself?
- Herd immunity and respiratory illness
- COVID-19 and pets
- COVID-19 and your mental health
- COVID-19 antibody testing
- COVID-19, cold, allergies and the flu
- COVID-19 tests
- COVID-19 drugs: Are there any that work?
- COVID-19 in babies and children
- Coronavirus infection by race
- COVID-19 travel advice
- COVID-19 vaccine: Should I reschedule my mammogram?
- COVID-19 vaccines for kids: What you need to know
- COVID-19 vaccines
- COVID-19 variant
- COVID-19 vs. flu: Similarities and differences
- COVID-19: Who's at higher risk of serious symptoms?
- Debunking coronavirus myths
- Different COVID-19 vaccines
- Extracorporeal membrane oxygenation (ECMO)
- Fever: First aid
- Fever treatment: Quick guide to treating a fever
- Fight coronavirus (COVID-19) transmission at home
- Honey: An effective cough remedy?
- How do COVID-19 antibody tests differ from diagnostic tests?
- How to measure your respiratory rate
- How to take your pulse
- How to take your temperature
- How well do face masks protect against COVID-19?
- Is hydroxychloroquine a treatment for COVID-19?
- Loss of smell
- Mayo Clinic Minute: You're washing your hands all wrong
- Mayo Clinic Minute: How dirty are common surfaces?
- Multisystem inflammatory syndrome in children (MIS-C)
- Nausea and vomiting
- Pregnancy and COVID-19
- Safe outdoor activities during the COVID-19 pandemic
- Safety tips for attending school during COVID-19
- Sex and COVID-19
- Shortness of breath
- Thermometers: Understand the options
- Treating COVID-19 at home
- Unusual symptoms of coronavirus
- Vaccine guidance from Mayo Clinic
- Watery eyes
Related information
- Post-COVID Recovery & COVID-19 Support Group - Related information Post-COVID Recovery & COVID-19 Support Group
- Rehabilitation after COVID-19 - Related information Rehabilitation after COVID-19
- Post-COVID-19 syndrome could be a long haul (podcast) - Related information Post-COVID-19 syndrome could be a long haul (podcast)
- COVID-19 Coronavirus Long-term effects
Help transform healthcare
Your donation can make a difference in the future of healthcare. Give now to support Mayo Clinic's research.
Along with Stanford news and stories, show me:
- Student information
- Faculty/Staff information
We want to provide announcements, events, leadership messages and resources that are relevant to you. Your selection is stored in a browser cookie which you can remove at any time using “Clear all personalization” below.
Researchers at Stanford Engineering have developed a nanoparticle platform that could make existing vaccines more effective, including those for influenza, COVID-19, and HIV. In addition to helping vaccine candidates produce stronger, longer-lasting immune responses, the platform will allow researchers to elicit and test different types of immune responses to determine what is most effective for protecting against specific pathogens.
“These nanoparticles elicit stronger, more robust immune responses, and the breadth of our platform allows us to readily tune the type of immune response in a way that just was not feasible with previous technologies,” said Eric Appel , an associate professor of materials science and engineering and senior author on the paper published Aug. 7 in Science Advances . “This can be a tool to understand how different types of immune responses give rise to better or worse protection – it was impossible to even ask that question before.”
A better adjuvant
Most modern vaccines teach our immune systems to recognize and fight off infections by introducing only a piece of a pathogen – such as the coronavirus’s now-infamous spike protein – instead of the whole virus. On their own, these fragments may not cause much of a reaction, so vaccines also contain adjuvants – additives that help stimulate and shape the body’s immune response. But there are currently only a handful of adjuvants available for clinical use and their effectiveness can vary widely.
“We wanted to create as potent of an adjuvant as possible,” said Ben Ou, a doctoral student in Appel’s lab and first author on the paper. “We combined two different adjuvant technologies to create a nanoparticle platform that will activate different immune pathways and improve vaccine responses.”
The researchers determined that they could attach molecules called toll-like receptor agonists, or TLR agonists, which interact with receptors on our innate immune cells, to a base nanoparticle made of saponin molecules, which have been used as effective adjuvants for decades, including in the Novavax COVID-19 vaccine. The result was an adjuvant that acted through multiple immune pathways, producing a broad, strong, long-lasting response.
Ou, Appel, and their colleagues tested their adjuvants, collectively called TLRa-SNP adjuvants, with both COVID-19 and HIV vaccine candidates. In both cases, the adjuvants greatly improved the effectiveness of the vaccines. In comparison to versions paired with an existing adjuvant, the vaccines were more potent and lasted longer. They also created immune responses that could detect and neutralize multiple versions of the pathogens – with the TLRa-SNP adjuvants, the COVID-19 vaccine candidate was effective against the original virus as well as Delta, Omicron, and other variants.
Finding the right immune response
There are multiple types of TLR agonists, each of which binds to a different immune receptor. The researchers created five different versions of their adjuvants using the saponin nanoparticle as a base platform and swapping the attached TLR agonists. While all the adjuvants were effective, each version created a slightly different type of immune response, activating different signaling proteins and prompting different actions from immune cells.
“All of our adjuvants improve overall vaccine responses, but the specific types of improvements are different,” Ou said. “If we know that a specific type of immune activation will confer better protection, we now have a platform that will allow you to pick the specific formulation that will drive that distinct response.”
With existing adjuvants, researchers can test which one creates the strongest immune response when paired with a particular vaccine, but the adjuvants are too different to allow investigations into which type of immune response would be most effective at protecting against infection for a given pathogen. The interchangeability of the TLR agonists in the TLRa-SNP adjuvants would allow researchers to tweak the nature of the immune response while maintaining the strong immune activation created by the saponin nanoparticle base.
There are other TLR agonists that could be paired with this platform, beyond the five that they tested in this paper, Ou said. He is interested in investigating others, as well as investigating the effects of using more than one type of TLR agonist at a time – the researchers have already shown that this is possible and hope to make additional bespoke nanoparticle adjuvants in the future, with the goal of developing the most effective adjuvants possible.
“This platform approach will open up opportunities for people in the field to ask more probing questions about what immunology works better in different contexts,” Appel said. “And it’s also making significantly better adjuvants.”
Related story
Drug delivery system could reduce daily diabetes shots to just three a year
Dietary management drugs have transformed Type 2 diabetes care, but daily injection routines are challenging for some patients. A new hydrogel could mean shots just three times a year.
For more information
Appel is a senior fellow of the Stanford Woods Institute for the Environment ; a member of Stanford Bio-X , the Stanford Cardiovascular Institute , the Wu Tsai Human Performance Alliance , the Maternal & Child Health Research Institute , the Stanford Cancer Institute , and the Wu Tsai Neurosciences Institute ; and a faculty fellow of Stanford Sarafan ChEM-H .
Additional Stanford co-authors of this research include Bali Pulendran , the Violetta L. Horton Professor in the School of Medicine; postdoctoral researcher Julie Baillet; and graduate students Maria V. Filsinger Interrante, Julia Z. Adamska, Xueting Zou, Olivia M. Saouaf, Jerry Yan, John H. Klich, Carolyn K. Jons, and Emily L. Meany.
Other co-authors on this work are from the University of Washington.
This work was funded by the Bill & Melinda Gates Foundation, the National Institute of Allergy and Infectious Disease, the Eastman Kodak Fellowship, the National Institutes of Health, the National Science Foundation, the Stanford Graduate Fellowship in Science and Engineering, and Sarafan ChEM-H.
Media contact: Jill Wu, School of Engineering: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Metabol Open
- v.12; 2021 Dec
COVID-19 vaccines: Current evidence and considerations
Alireza tavilani.
a Islamic Azad University, Hamedan Branch, Hamedan, Iran
Ebrahim Abbasi
b Department of Clinical Biochemistry, Hamadan University of Medical Sciences, Hamadan, Iran
Farhad Kian Ara
c Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
d Department of Nanotechnology, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran
Zahra Asefy
e School of Nursing and Allied Medical Sciences, Maragheh University of Medical Sciences, Maragheh, Iran
Associated Data
Data are available upon reasonable request.
The coronavirus disease 2019 (COVID-19) pandemic is a global crisis, with devastating health, business and social impacts. Vaccination is a safe, simple, and effective way of protecting a person against COVID-19. By the end of August 2021, only 24.6% of the world population has received two doses of a COVID-19 vaccine. Since the emergence of COVID-19, several COVID-19 vaccines have been developed and approved for emergency use. Current vaccines have shown efficacy with low risk of adverse effects. However, COVID-19 vaccines have been related to a relatively small number of cases of heart inflammation, anaphylaxis (allergic reactions), and blood clots formation. On the other hand, COVID-19 vaccination is not recommended for children less than 12 years of age. Furthermore, It has been proposed that some new variants (e.g., Lambda and Delta) are proficient in escaping from the antiviral immunity elicited by vaccination. Herein we present current considerations regarding the COVID-19 vaccines including: efficacy against new variants, challenges in distribution, disparities in availability, dosage gender and race difference, COVID-19 vaccine transport and storage, limitations in children and pregnant women. Long-time monitoring is essential in order to find vaccine efficacy and to rule out related side effects.
1. Introduction
Numerous medicines have been used for the treatment of coronavirus disease 2019 (COVID-19) during the past year. Although most of the medicines failed to show efficacy in treating COVID-19, researchers have encouraged herd immunity to control the current pandemic [ 1 , 2 ]. Vaccination is a safe, simple, and effective way of protecting a person against COVID-19. Although a massive number of experiments have been done since the virus was first recognized, there are still many unknowns about this COVID-19. Certain persons including pregnant women, breastfeeding individuals, autoimmune conditions and immunocompromised persons, diabetic patients, and people with respiratory and heart disease require special consideration for COVID-19 vaccination [ [3] , [4] , [5] , [6] ]. Having certain medical conditions can make a person more likely to get severely ill from COVID-19 [ 2 ].
The effects of vaccines on the COVID-19 pandemic depend on various factors, including the efficiency; how rapidly they are manufactured, approved, and delivered; the immunity against new variants and how many subjects get vaccinated. Various health organizations are working to help confirm that approved COVID-19 vaccines are as effective as possible, so that they can have the most significant effect on the COVID-19 pandemic. A vaccine is a vital tool in the battle against COVID-19 infection, and there are many lifesaving and public health benefits to using the tools we now have [ 7 , 8 ].
At present, 184 candidate vaccines were being evaluated in preclinical and 104 in clinical stages of development. Furthermore, there are 41 vaccines in phase 3 and 18 COVID-19 vaccines approved, and are currently in use worldwide. These vaccines are in four primary groups using various platforms: (1) viral vector vaccines, (2) whole virus vaccines, (3) nucleic acid vaccines, and (4) protein-based vaccines [ 9 ]. Table 1 depicts the main characteristics of the currently available vaccines.
Comparison of Pfizer/BioNTech, Moderna, Johnson & Johnson, and AstraZeneca vaccines [ 7 , 33 ].
| Name of vaccines | Pfizer-BioNTech vaccine | Moderna | Johnson & Johnson | AstraZeneca |
|---|---|---|---|---|
| mRNA vaccine | mRNA vaccine | Vector vaccine | Adenovirus vector vaccines | |
| Stored for 6 months at −70 °C. Undiluted vials can be stored at room temperature for no more than 2 h. | Stored for 30 days between 2 °C and 8 °C. | Stored for up to 3 months between 2 °C and 8 °C. | Store in a refrigerator (2–8 °C). Do not freeze. Preserve the vials from light. | |
| 95% in preventing the COVID-19 infection. | 94.5% in preventing the COVID-19 infection. | 85% in preventing the COVID-19 infection. | 70% in preventing the COVID-19 infection. | |
| 2 shots, given 21 days apart. | 2 shots, given 28 days apart. | One dose is needed. | 2 shots, given 28 days apart. | |
| People age 16 and older. | People age 18 and older. | People age 18 and older. | People age 18 and older. | |
| Quite effective against the South African, UK variant, and Latin American variants. | Quite effective against the South African, UK variant, and Latin American variants. | Less effective against the South African and Latin American strains. | Less effective against South African variant, but appears effective against Brazilian and UK variants. | |
| Swelling, pain, and redness at the site of vaccine. Fatigue, headache, fever, vomiting, chills, myalgia, urticarial, and arthralgia. Bell's palsy and facial swelling has also been reported. | Swelling, pain, and redness at the site of vaccine. Nausea, vomiting, tiredness, muscle pain, chills, headache, and fever. | Swelling, pain, warmth, itching or bruising, and redness at the site of vaccine. Fatigue, headache, fever, vomiting, diarrhea nausea, chills, joint pain, muscle ache. Vaccine induced thrombotic thrombocytopenia, which are estimated to occur in 1 in 100,000 vaccinated people. |
Although the striking amount of experiments carried out since the COVID-19 was first recognized, there are still a huge number of unknowns about this disease. Hence, there are multiple concerns about COVID-19 vaccines [ 8 ]. In the next section, we will discuss about vaccination in view of gender and race difference, new variants, efficacy and immunity, safety, dosage, transport and storage, distribution, vaccination in special groups, and virus transmission in vaccinated people.
2. Vaccination in view of gender difference
It has been shown that several factors, including the genetic, immune system, gut microbiome, and steroid hormones are varied between men and women that contribute to gender - and sex-specific vaccine responses and outcomes. Women produce more antibodies as a result of vaccination and respond more actively to infections. In women, a strong response of the immune system may increase the risk of autoimmune diseases and a good capability to fight against various infections. A higher level of COVID-19 antibody has been reported in women than in men after COVID-19 infection. Women display more strong cellular and humoral-mediated immune responses to vaccination and infection when compared to men [ 10 ]. Thus, the vaccine efficacy suggested for adults is potentially greater for women than men. Men, due to high levels of testosterone, show low levels of COVID-19 vaccine effectiveness. In this respect, males may need more doses of the COVID-19 vaccine compared with females [ 10 ].
3. Vaccination in view of race difference
Among those reported, the ethnic and racial distribution of the sample was not always stated, and methods are different, which may affect the results [ 11 ]. Asian, Hispanic, and Black people are infected with COVID-19 more than White ethnicity, with a possible relationship of higher risk of mortality and intensive care unit (ICU) admission in Asians [ 10 ]. Black females and males were about 4.2 times more likely to die from COVID-19 infection than White females and males [ 10 ]. However, in the UK, the mortality risks do not apply to Black ethnicity alone. Ethnicities of the people of Indian, Bangladeshi, Iranian, Pakistani, and Mixed had substantially increased risk of death by COVID-19 infection when compared with the White ethnicity [ 10 ].
4. COVID-19 vaccines and variants
RNA viruses such as the novel coronavirus are known for mutating and evolving quickly. RNA replication is more error-prone compared to DNA replication, so mutations happen commonly during copying. Sometimes the random mutation is beneficial for the virus, which helps it evade the host's immune system and infect new species or systems. A new variant of novel coronavirus emerged with a high number of mutations. The new variants are B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta), and C.37 (Lambda). The new variants are spread more easily, lead to severe disease, and may change the efficiency of COVID-19 vaccines [ 12 ].
These variants may be associated with a higher mortality rate. There is concern that the available COVID-19 vaccines may not provide sufficient immunity against new variants.
The vaccines are expected to protect subjects against new virus variants and effective at preventing severe respiratory disease and death. An update of vaccine composition may be necessary in order to maintain high efficacy against new variants. Furthermore, the revaccination schedule may also be essential if variants develop that are potentially different from the original coronavirus that the vaccines were produced against. Another variant, B.1.1.7, revealed in the UK, has been reported to have a high mortality rate and faster transmission speed. New variants reported in various countries can decrease the efficiency of the current COVID-19 vaccines. If the pandemic persists, the mutations of coronavirus will increase, and humanity must struggle for vaccination and worldwide distribution [ 13 ].
5. COVID-19 vaccines efficacy and immunity
No vaccine is 100% effective. There's no report so far that the COVID-19 vaccine can prevent transmission, but it can help protect against COVID-19 infection. Various countries have reported that the numbers of new cases and transmission rates of COVID-19 have reduced in many areas, probably due to the protective efficacy of vaccines and/or restrictions. However, the vaccine candidates have been evaluated in isolation, which makes it challenging to compare the efficiency of different vaccines. Therefore, it would be premature to hail the immunogenicity and safety observed in vaccine trials as a real achievement [ 14 ]. None of the approved COVID-19 vaccines contain the live virus that causes COVID-19. This means these vaccines cannot lead to COVID-19 infection. Generally a few weeks after vaccination, the body builds immunity against COVID-19 infection. Hence, it is possible for people to be infected with COVID-19 just before or after vaccination and yet get sick with COVID-19. This is because the COVID-19 vaccine has not yet had an adequate period to provide protection [ 15 ].
It has been reported that mRNA COVID-19 vaccines provide immunity for at least 6 months [ 16 ]. All COVID-19 vaccines have only been produced in the past months, It's too early to judge the duration of the immunity of these vaccines. Available findings [ 17 , 18 ] show that most patients who recover from disease develop an immune response against COVID-19 infection that provides about five to eight months of protection– although the exact immunity levels and protection period are not measured. Under normal conditions, phase 3 of vaccine studies could have continued for another few years, displaying how long protection lasts before the vaccine was distributed to the general community. The current COVID-19 vaccines are all two-dose vaccines (except for the vaccine from Johnson & Johnson). Appropriate immune response has been reported within about two weeks after the first dose. And the second dose then significantly increases the immune response and a shorter time after the second dose [ 15 ].
6. COVID-19 vaccines safety
The safety of the COVID-19 vaccine should be evaluated in participants of different ages and comorbidities a few months of follow-up after their first or second dose. We need a complete risk management and safety monitoring (pharmacovigilance) system, which determines the potential side effects. Similar to other vaccines, COVID-19 vaccines can cause mild or moderate side effects within a few days after injection. Some side effects such as headache, muscle pain, fatigue, fever, diarrhea, and chills have been reported, and most have happened during the first 48 h after vaccination. Therefore, subjects should continually monitor to distinguish adverse events [ 15 ].
WHO is aware that some people may show a severe allergic reaction to the vaccines (e.g., anaphylaxis). According to The United States Centers for Disease Control and Prevention (CDC) report, 11.1 per million cases of vaccinated people reported anaphylaxis in the USA [ 19 ]. If the subjects report a history of anaphylaxis with previous vaccines, they are advised not to take the new vaccine. Polyethylene glycol (PEG) and PEG derivatives (e.g., polysorbates) are probably responsible for anaphylaxis [ 13 ]. It has been recommended that before vaccination, people should notify the healthcare workers about any anaphylaxis they may have had previously. It has been proposed that all vaccinated cases remain at the vaccination site for 30 min to detect any serious side effects. It has been reported that the AstraZeneca and Johnson & Johnson/Janssen vaccines may have a possible link to a very rare side effect of unusual blood clots combined with low levels of platelet levels [ 7 , 20 ].
Various vaccines entered into clinical trials in a short time and were conditionally approved in less than one year. This unique speed was motivated by the timely detection of novel coronavirus genomic sequences, strong collaboration among the research centers, sufficient funding, and the urgent/huge market demand. Since the beginning of the COVID-19 pandemic, many countries are competing to develop vaccines. The development of the standard vaccine is a long process, and experiments are complete in sequential steps. However, the development of COVID-19 vaccines is being fast-tracked globally. Despite the significant progress, the safety and quality of various vaccines are the main concern. The UK, Germany, USA, and China have developed vaccines in phase 4 (post-market studies) [ 21 ].
7. COVID-19 vaccines dose
The Johnson & Johnson vaccine only requires one dose, while the Moderna, Pfizer-BioNTech, Oxford-AstraZeneca (in a 8–12 week interval), Sputnik V (in a 3 week interval), Novavax (in a 3 week interval), Coronavac (in a 1 month interval) need two doses. The CDC documented that while there's no priority for one vaccine over another, the vaccines aren't interchangeable.
Mixing two different vaccines can show long-lasting and strong immune responses when compared to the single vaccine. Scientists hope that mix-and-match COVID-19 vaccination regimens (e.g., e.g. AstraZeneca and Pfizer) can trigger stronger, more robust immune responses than two doses of a single vaccine. Mix-and-match COVID-19 vaccination is recognized by high levels of both T cells and antibodies, which kill infected cells and support other antiviral responses [ 22 , 23 ].
According to the CDC report the second dose should be injected as close to the suggested interval as possible. It may be injected up to 42 days after the first dose when a delay is inevitable. If the second dose is injected after the suitable interval, the series does not need to be restarted. Furthermore, the vaccine team should not inject second doses before the proposed interval or save or hold doses for cases who have not returned more than 42 days after their first dose [ 24 ]. The second dose of vaccine may be missed due to personal reasons or a fluctuating vaccine supply. If more than 3 weeks have passed since the first dose was received, the next dose can be injected as soon as possible [ 13 ].
8. COVID-19 vaccines transport and storage
Most of the available vaccines should be stored and transported in refrigeration to freezing temperatures (e.g., the Pfizer vaccine at −70 °C and Oxford-AstraZeneca 2–8 °C). Therefore, the storage and transport of mRNA vaccines is challenging. Some new vaccines can be stored at −15 to −25 °C for up to 14 days12 [ 25 ]. On the other hand, some other vaccines need ultra-cold storage (below −80 °C). That means they will be really challenging to administer effectively in poor countries or remote areas of the globe as they are far away from the central transport system. It can cause low COVID-19 immunization in these areas and, consequently, increase the endemicity of infections [ 25 ]. Care is necessary after transferring these vaccines to refrigerating to freezing temperatures or the following thawing to protect their quality. A regular schedule for temperature is vital for the preservation of stability, potency, and efficacy of COVID-19 vaccines [ 25 ]. Distribution and transportation of COVID-19 vaccines are difficult and complicated particularly in hot climate and low-income countries [ 26 ].
Stable and effective storage and transport of vaccines mean they need them at cold temperatures and transfer them quickly from the manufacturer to the medical centers. A previous report showed that 2.8 million vaccines were missed in 5 countries due to cold chain failures, and less than 10% of countries met WHO protocol for effective vaccine management [ 27 ]. Interestingly, nearly 80% of vaccine costs are related to the cold chain programme. Henceforth, the lyophilized vaccine has good stability compared with liquid form. Providing a cold chain for poor countries is the main concern. Proper preparation of lyophilized form is necessary, and powder should not be prepared until the administration. Liquid form loses its efficacy when kept at freezing temperatures because slow freezing leads to great stress to the colloids and increased aggregations [ 28 ]. Cold chain technology is needed for the liquid form, which can be challenging for use in poor countries. Appropriate cold chain infrastructure can prevent up to 25% vaccine loss in poor countries [ 8 ].
9. COVID-19 vaccine distribution
Many people in poor and middle-income countries may not be receiving vaccines; therefore, equitable COVID-19 vaccine distribution is essential. More than 700 million COVID-19 vaccines have been injected globally; low-income countries received only 0.2%, while wealthy countries have received more than 87%. On average, 1 in more than 500 people in poor countries has received COVID-19 vaccines, compared with 1 in 4 people in wealthy countries [ 13 ].
As of May 11, 2021, about 1.32 billion people had received the COVID-19 vaccine worldwide, equal to 17 doses for every 100 people. Some countries (e.g., Gibraltar and Israel) had vaccinated 78% of people, while Mauritius, Pakistan, Guyana, Cambodia, Albania, Bolivia, and Ecuador had less than 0.1 doses administered per 100 people. It is a disappointment that healthcare workers are dying in various countries, showing a global moral failure in these regions. Researchers believe that this uneven administration pattern can also cause virus mutations and new vaccine-resistant variants [ 25 ].
Many poor countries have low socioeconomic status (SES) with low income, high unemployment rates and poor education. These conditions may potentially influence the vaccine-accepting and purchasing processes of their people. The geographical landscape of some poor countries poses a substantial challenge to COVID-19 vaccine distribution. High altitude areas within Hindu-Kush Himalayan regions, such as Pakistan, Bhutan, Nepal, and Afghanistan, make it very difficult for health workers to distribute COVID-19 vaccines. The problematic condition may be aggravated in the desert, and remote areas participated in the war, conflict, and instability. In this respect, more than 160 million subjects have been expected to be at risk of COVID-19 vaccine inaccessibility in Syria, Yemen, Ethiopia, and South Sudan [ 25 ].
10. COVID-19 vaccine for children and pregnant women
COVID-19 infection has been a more dangerous and severe disease among older people. Most of the vaccines are commonly offered to adults first to avoid exposing children who are still growing and developing. Because of the high risk of severe disease in the children, elderly, immunocompromised subjects, and pregnant women, the vaccination programme should be conducted with care [ 10 ]. COVID-19 vaccine teams need to follow-up pregnancies long-term to recognize effects on infants and pregnancy.
The mRNA vaccines (Pfizer-BioNTech and Moderna) do not have the live coronavirus that leads to COVID-19 and, consequently, cannot infect. Moreover, the mRNA vaccines do not interact with an individual's DNA or lead to genetic alterations since the mRNA does not enter the cell's nucleus. The viral vector vaccines (J&J/Janssen vaccine) can be administered to pregnant women in all trimesters of pregnancy (like the Ebola vaccine). However, there are various types of COVID-19 vaccines, and our direct knowledge is currently limited about their effects during pregnancy. The efficacy and safety of COVID-19 vaccines in lactating women, the impact of COVID-19 vaccination on the breastfed infant, and effects on milk excretion or production have not been determined. However, non-replicating COVID-19 vaccines pose no risk for lactating women or their babies; hence lactating women may safely be vaccinated [ 29 ].
11. COVID-19 infection transmission in vaccinated people
The risks of COVID-19 in vaccinated subjects cannot be entirely eliminated as long as there is continued public transmission of the virus. Vaccinated subjects can still get COVID-19 and spread it to other people. Hence, the COVID-19 test and self-quarantine are required for travellers. Some vaccinated subjects later exposed to the coronavirus still get COVID-19. In this context, a fully vaccinated person should continue to wear a face mask, maintain social distance, and follow health care recommendations. Preliminary data from some countries showed that the viral load was 4–fold lower among those fully vaccinated with an effective vaccine. This finding suggests that viral transmission from fully vaccinated people is lower, as viral load has been recognized as the main factor for virus transmission [ 30 ]. So far, SARS-CoV-2 has not been detected in breast milk, and there are no recognized cases of transmission of virus to the infant through breast milk. However, infected women may select to breastfeed with protections to prevent transmission of the virus through respiratory droplets. Some newborns have shown COVID-19 shortly after birth. It is unknown if these newborns got the virus after, during, or before birth [ 31 ].
12. Low intends to take COVID-19 vaccine
It has been reported that about 15–20% of adults do not intend to take the COVID-19 vaccine. People who don't intend to get the COVID-19 vaccine are at higher risk of transmitting and contracting the virus. They can also enormously increase the pandemic period, contributing to spikes in COVID-19 cases and facilitating viral replication and the emergence of new viral variants. Common concerns among the people, who do not intend to get the COVID-19 vaccine, include the efficacy, safety, and the perceived hasty timeline for vaccine production. African American race, younger age, people with lower education, and conservative political ideology has lower intention to get COVID-19 vaccine. Receiving health care recommendations and having more fear of severe disease were both accompanied with more intention to vaccinate [ 11 , 32 ].
13. Conclusion
In conclusion, there are various types of vaccines worldwide. However, additional studies are necessary to determine the effectiveness of the COVID-19 vaccine against variants of concern. COVID-19 vaccines have obtained emergency use and there are various limitations such as vaccine distribution, variants of concern, vaccination willingness, herd immunity, vaccine efficacy, vaccine safety, and vaccine dose. To combat the current pandemic, manufacturers and healthcare authorities should work together to provide appropriate and adequate vaccinations for the prevention of COVID-19. Healthcare authorities should constantly update COVID-19-related information. Furthermore, vaccine booster doses may be required for several reasons; inadequate protection, reduced protection against new variants, and waning protection against disease or infection. However, the rationale for COVID-19 vaccine booster doses may vary by vaccine product, risk group epidemiological setting, and vaccine coverage rates.
This research did not receive any specific grant from funding agencies in the public, commercial, or Non-Profit sectors.
Ethics approval
Not applicable.
Availability of data and material
Credit authorship contribution statement.
Alireza Tavilani: Conceptualization, Visualization, Data curation, Validation, Writing – original draft. Ebrahim Abbasi: Project administration, Visualization, Validation, Supervision, Data curation, Writing – review & editing. Farhad Kian Ara: Software, Writing – review & editing. Ali Darini: Validation, Writing – original draft, Writing – review & editing. Zahra Asefy: Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
None to be declared.
Acknowledgement
We would like to thank Hamadan University of Medical Sciences.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
August 6, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Researchers create new treatment and vaccine for flu and various coronaviruses
by Laurie Fickman, University of Houston

A team of researchers, led by the University of Houston, has discovered two new ways of preventing and treating respiratory viruses. In back-to-back papers in Nature Communications , the team—from the lab of Navin Varadarajan, M.D. Anderson Professor of William A. Brookshire Chemical and Biomolecular Engineering—reports the development and validation of NanoSTING, a nasal spray, as a broad-spectrum immune activator for controlling infection against multiple respiratory viruses; and the development of NanoSTING-SN, a pan-coronavirus nasal vaccine, that can protect against infection and disease by all members of the coronavirus family.
NanoSTING is a special formula that uses tiny fat droplets to deliver an immune-boosting ingredient called cGAMP. This formula helps the body's cells stay on high alert to prevent attack from respiratory viruses .
"Using multiple models, the team demonstrated that a single treatment with NanoSTING not only protects against pathogenic strains of SARS-CoV-2 but also prevents transmission of highly transmissible variants like the Omicron variants," reports Varadarajan. "Delivery of NanoSTING to the nose ensures that the immune system is activated in the nasal compartment and this in turn prevents infection from viruses."
As the recent COVID19 pandemic illustrated, the development of off-the-shelf treatments that counteract respiratory viruses is a largely unsolved problem with a huge impact on human lives.
"Our results showed that intranasal delivery of NanoSTING, is capable of eliciting beneficial type I and type III interferon responses that are associated with immune protection and antiviral benefit," reports first author and postdoctoral associate, Ankita Leekha.
The authors further show that NanoSTING can protect against both Tamiflu sensitive and resistant strains of influenza, underscoring its potential as a broad-spectrum therapeutic.
"The ability to activate the innate immune system presents an attractive route to armoring humans against multiple respiratory viruses, viral variants and also minimizing transmission to vulnerable people," said Leekha. "The advantage of NanoSTING is that only one dose is required unlike the antivirals like Tamiflu that require 10 doses."
The mechanism of action of NanoSTING is complementary to vaccines, monoclonal antibodies and antivirals, the authors noted.
Nano STING-SN
Despite the successful implementation of multiple vaccines against SARS-CoV-2, these vaccines need constant updates due to viral evolution, plus the current generation of vaccines only offers limited protection against transmission of SARS-CoV-2.
Enter NanoSTING-SN, a multi-antigen, intranasal vaccine, that eliminates virus replication in both the lungs and the nostrils and has the ability to protect against multiple coronaviruses and variants.
"Using multiple preclinical models, the team demonstrated that the vaccine candidate protects the primary host from disease when challenged with highly pathogenic variants. Significantly, the vaccine also prevents transmission of highly transmissible variants like the Omicron variants to vaccine-naïve hosts," reports Varadarajan.
The authors further show that the nasal vaccine was 100% effective at preventing transmission of the Omicron VOCs to unvaccinated hosts.
"The ability to protect against multiple coronaviruses and variants provides the exciting potential towards a universal coronavirus vaccine ," said Leekha. "The ability to prevent infections and transmission might finally end this cycle of onward transmission and viral evolution in immunocompromised people."
The research was conducted by a collaborative team at UH including Xinli Liu, College of Pharmacy and Vallabh E. Das, College of Optometry along with Brett L. Hurst of Utah State University and consultation from AuraVax Therapeutics, a spinoff from Varadarajan's Single Cell Lab at UH, which is developing NanoSTING.
One article is titled " An intranasal nanoparticle STING agonist protects against respiratory viruses in animal models ," and the second article is titled " Multi-antigen intranasal vaccine protects against challenge with sarbecoviruses and prevents transmission in hamsters ." Both articles are published in Nature Communications .
Ankita Leekha et al, Multi-antigen intranasal vaccine protects against challenge with sarbecoviruses and prevents transmission in hamsters, Nature Communications (2024). DOI: 10.1038/s41467-024-50133-2
Explore further
Feedback to editors

The dengue vaccine is effective and safe: Confirmation from the first global meta-analysis
38 minutes ago

'PTNM' system provides new classification for Peyronie's disease and penile curvature

Researchers crack a key celiac mystery: Where the gluten reaction begins

How did mental health parity laws affect new moms?

Classical music lifts our mood by synchronizing our 'extended amygdala'
Pathways linking body and brain health and impacts to mental health revealed
4 hours ago

Alzheimer's disease: It's not only neurons—glial cells also produce harmful proteins

Serotonin changes how people learn and respond to negative information

A new way to measure bipolar disorder: Focus on the 'spikes'

Sequence of the day: Exploring how the mammalian brain represents multiple sequential experiences during sleep
5 hours ago
Related Stories
COVID-19 nasal vaccine candidate effective at preventing disease transmission
Sep 15, 2021
An intranasal COVID vaccine that works against variants in animals
Oct 24, 2022

Researchers develop a nasal vaccine that prevents COVID-19 in preclinical studies
Nov 6, 2023
Researchers examine the benefits of COVID-19 nasal spray vaccination
Feb 27, 2024

Single vaccine protects against three deadly strains of coronavirus
Oct 18, 2023

Nasal vaccine may aid fight against new viral variants
Dec 10, 2021
Recommended for you

New study shows a single experimental shot reduces HIV levels 1,000-fold
20 hours ago
Advanced MRI scans help identify one in three concussion patients with 'hidden disease'
18 hours ago

Intramuscular prime/intranasal boost vaccine strategy shows promise in porcine flu model
22 hours ago
Prioritizing the elderly for COVID boosters reduces overall deaths in range of socio-economic settings, study finds

New insights into antibody roles could improve malaria vaccines for children
Aug 8, 2024

Researchers develop a new vaccine additive that creates a stronger, tunable immune response
Aug 7, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Safety and adverse effects of current COVID-19 vaccines. As shown in Table I, current vaccines have demonstrated considerable efficacy in diminishing mild, moderate and severe cases with a low risk of adverse events 21.For some of these vaccines [such as Convidicea (AD5-nCoV), Janssen (Ad26.COV2.S), Sinopharm (BBIBP-CorV), Covaxin (BBV152) and Sinovac (CoronaVac)], there is the information ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
Discussion. A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with ...
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement ...
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
Although Covid-19 vaccines have been recommended for adults with chronic medical conditions, 22 ... The activity reported in this article was deemed not to be research as defined in 45 Code of ...
No vaccine was statistically significantly associated with a decreased risk for severe COVID-19 than other vaccines, although mRNA-1273 and Gam-COVID-Vac have the highest P-scores (0.899 and 0.816 ...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
Amid the staggering amount of suffering and death during this historic pandemic of COVID-19, a remarkable success story stands out. The development of several highly efficacious vaccines against a previously unknown viral pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in less than 1 year from the identification of the virus is unprecedented in the history of vaccinology.
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a ...
Our understanding of COVID-19 vaccinations and their impact on health and mortality has evolved substantially since the first vaccine rollouts. Published reports from the original randomized phase 3 trials concluded that the COVID-19 mRNA vaccines could greatly reduce COVID-19 symptoms. In the inter …
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
1. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) This clinical trial is designed to assess the safety, reactogenicity, and immunogenicity of mRNA-1273. It encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2.
Introduction: In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.
The COVID-19 pandemic has underscored the critical importance of adaptable and resilient public health systems capable of rapid response to emerging health crises. This paper synthesizes the lessons learned from the COVID-19 vaccination campaign and explores strategies to enhance vaccine uptake in the post-pandemic era. Key challenges identified include logistical, economic, sociocultural, and ...
The Coronavirus Efficacy (COVE) phase 3 trial was launched in late July 2020 to assess the safety and efficacy of the mRNA-1273 vaccine in preventing SARS-CoV-2 infection. An independent data and ...
WASHINGTON — A new report from the National Academies of Sciences, Engineering, and Medicine reviews evidence for 19 potential harms of the COVID-19 vaccines, and for nine potential shoulder injuries from intramuscular administration of vaccines more broadly. The committee that conducted the review identified sufficient evidence to draw 20 conclusions about whether these vaccines could cause ...
Background Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine. Methods Study participants in the U.S. (79% female, median age group 46-60 years) were ...
SARS-CoV-2 vaccination prevented 14.4 million deaths from COVID-19 worldwide in the first year of the pandemic 1. In England, which entered its third COVID-19 vaccine season in autumn 2023 2 ...
the authors showed the importance of booster doses in reducing severe COVID-19 outcomes, particularly in the older age groups. The first booster dose saved an estimated 798 376 of 1 560 661 lives (51%) in adults aged 25 years or older and the vast majority of lives saved were among those over 60 years old, with 60% of deaths averted (1 499 229 lives saved of 2 502 775 expected deaths).
Despite questions remain about the impact of virus variants and the duration of the immune response, messenger RNA (mRNA)-based and adenoviral vectored vaccines have demonstrated an overall efficacy from 70 to 95% in both phase III trials and real life. In addition, all these vaccines also reduce the severe forms of the disease and might ...
The messenger RNA (mRNA) platform emerged at the forefront of vaccine development during the COVID-19 pandemic, with two mRNA COVID-19 vaccines being among the first authorized globally. These vaccines were developed rapidly. Informed by decades of laboratory research, and proved to be safe and ...
Research suggests that between one month and one year after having COVID-19, 1 in 5 people ages 18 to 64 has at least one medical condition that might be due to COVID-19. Among people age 65 and older, 1 in 4 has at least one medical condition that might be due to COVID-19 .
This study evaluates the effectiveness of the novel BNT162b2 mRNA vaccine 1 against Covid-19 in a nationwide mass vaccination setting. Estimated vaccine effectiveness during the follow-up period ...
Ou, Appel, and their colleagues tested their adjuvants, collectively called TLRa-SNP adjuvants, with both COVID-19 and HIV vaccine candidates. In both cases, the adjuvants greatly improved the ...
Abstract. The coronavirus disease 2019 (COVID-19) pandemic is a global crisis, with devastating health, business and social impacts. Vaccination is a safe, simple, and effective way of protecting a person against COVID-19. By the end of August 2021, only 24.6% of the world population has received two doses of a COVID-19 vaccine.
In back-to-back papers in Nature Communications, ... COVID-19 nasal vaccine candidate effective at preventing disease transmission ... New COVID-19 research provides insights on variant spread in ...
Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet ...
Researchers have discovered new ways of preventing and treating respiratory viruses. In two new papers, the team reports the development and validation of NanoSTING, a nasal spray, as a broad ...